Abstract
Study Design
Meta-analysis.
Objectives
This study aimed to summarize the clinical efficacy and safety of the various annular defect repair methods that have emerged in recent years.
Methods
A meta-analysis of randomized and non-randomized controlled trials was conducted. Articles from PubMed, Embase, and the Cochrane Library (CENTRAL) on Lumbar disc herniation treatment with annular repair published from inception to April 2, 2022 were included. We summarized the clinical efficacy and safety of annular repair techniques based on a random-effects model meta-analysis.
Results
7 randomized controlled studies and 8 observational studies with a total of 2161 participants met the inclusion criteria. The pooled data analysis showed that adding the annular repair technique reduced postoperative recurrence rate, reoperation rate, and loss of intervertebral height compared with lumbar discectomy alone. Subgroup analysis based on different annular repair techniques showed that the Barricaid Annular Closure Device (ACD) was effective in preventing re-protrusion and reducing reoperation rates, while there was no significant difference between the other subgroups. The annulus fibrosus suture (AFS) did not improve the postoperative Oswestry Disability Index (ODI). No statistically significant difference was observed in the incidence of adverse events between the annular repair and control groups.
Conclusions
Lumbar discectomy combined with ACD can effectively reduce postoperative recurrence and reoperation rates in patients with LDH. AFS alone was less effective in reducing recurrence and reoperation rates and did not improve postoperative pain and function.
Keywords: annular closure device, annular fibrous suture, lumbar discectomy, lumbar disc herniation, recurrence, reoperation
Introduction
Lumbar disc herniation (LDH) involves degenerative alterations in various parts of the lumbar intervertebral disc (nucleus, annulus fibrosus, and cartilage plate) and annulus fibrosus ruptures, causing the nucleus pulposus to protrude (or prolapse) from the ruptured area to the spinal canal. Consequently, the adjacent nerve roots are stimulated and/or compressed, resulting in low back and leg pain, which are the main clinical symptoms of LDH. LDH affects approximately 9% of the global population. 1 Strict conservative treatment fails in 10%–20% of patients, subsequently warranting surgery. 2 Lumbar discectomy is a standard surgical treatment method for LDH, and its efficacy and safety have been confirmed. 3 By removing the disc tissue that is compressing the nerve roots, the surgery relieves symptoms in most patients. However, sustained clinical improvement may not have been achieved in up to 20% of patients at the long-term follow-up.4,5 Postoperative reherniation and further degeneration of the disc are important prognostic factors, with the former symptom having an incidence rate of 2%–18%.6-8 Patients with severe symptoms require reoperation. Additionally, a decrease in intervertebral height, as a typical manifestation of disc degeneration, generally manifests after discectomy, probably leads to hyperostosis and spinal stenosis, and is not conducive to improved pain and patient function. 9 Although minimally invasive discectomy assisted by a tubular retractor (tubular discectomy), microscope (microdiscectomy) or endoscope (endoscopic discectomy) have emerged in recent years, they have no significant advantage over the open procedure in terms of postoperative pain, function, and reoperation rate. 10 In that simple lumbar discectomy, regardless of whether it is open or minimally invasive surgery, requires the intraoperative incision of the annulus fibrosus or through the original annulus fibrosus defect to remove the nucleus pulposus tissue; no further treatment of the annulus fibrosus defect is required. Afterwards, the annulus fibrosus defect relies on local scar tissue filling for healing,11,12 a limited and slow self-healing process. 13 Studies have shown that the recurrence and reoperation rate of patients with postoperative large annular defects (>6 mm wide) are significantly higher. 14 Furthermore, it is currently believed that an intact annulus fibrosus assists in maintaining intradiscal pressure, thereby maintaining adequate disc tissue height and stiffness under axial compression. 15 In recent years, several annular repair technologies have been proposed and applied to prevent reherniation; however, their clinical efficacy has not been conclusively confirmed.10,16,17
In this meta-analysis, we compared the clinical outcomes of discectomy with combinations of lumbar discectomy and annulus fibrosus repair. We further investigated the differences between various annular repair techniques, aiming to demonstrate the current development of these techniques and explore their clinical value.
Methods
We followed a PRISMA checklist-guided protocol for development and reporting. The protocol was registered on PROSPERO (registration number: CRD42022344031).
Search Strategy
We searched PubMed, Embase, and the Cochrane Library (CENTRAL) from inception to April 2, 2022 without any language restrictions. To achieve the highest possible sensitivity, we referred to the entry terms of “annular repair” used in previous reviews and meta-analyses. The full methods used for our search are detailed in Supplementary Table 1. To supplement the identified citations, we searched the reference lists of key reviews and meta-analyses.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) the study type was a clinical randomized controlled study or an observational controlled study; (2) the target population was diagnosed with LDH; (3) the treatment group received discectomy combined with any type or combination of annular repair techniques, and the control group received discectomy alone; and (4) the provision of data on the incidence of reherniation.
The exclusion criteria were as follows: (1) non-human subjects; (2) duplicates of target populations; (3) not being a controlled study; and (4) other instances that did not meet the inclusion criteria.
Two independent investigators (Yangbin Wang and Xiaoyu He) filtered the literature based on the inclusion and exclusion criteria using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) and used the following process: titles and abstracts were screened first (including the use of filters to exclude duplicates and any result that was not a controlled study) followed by the full manuscript. Disagreements were resolved through discussion or by a third investigator (Haiming Yu and Shu Lin). The screening process included random checks by a third investigator (Hanshi Wang) to check the reliability of the data.
Data Extraction and Quality Assessment
The following data were extracted from the included articles by two independent investigators (Yangbin Wang and Xiaoyu He): (1) study characteristics, including first author name, year, country or region, study institution, study design, number of patients, duration of follow-up, type of discectomy, and involvement of annular repair techniques; (2) the baseline characteristics of patients, including age, weight, body mass index (BMI), and sex; (3) reherniation and related reoperation rate; (4) pre- and post-operative Oswestry Disability Index (ODI); (5) pre- and post-operative back and leg visual analogue scores (VAS); (6) pre- and post-operative intervertebral height; and (7) incidence of serious complications, including dural injury or spinal fluid leak, epidural hematoma, and wound infection, dehiscence, or delayed healing. For data that could not be obtained directly, we used the statistical formula of the required data to calculate it. For missing data due to missing visits, we extracted per-protocol results unless the literature reported intention-to-treat results, and the original data was not available.
Two independent investigators (Yangbin Wang and Xiaoyu He) used the Cochrane risk-of-bias assessment tool to evaluate the bias of the included randomized controlled studies. Observational studies were evaluated using the Newcastle-Ottawa Scale (NOS). Two other investigators (Shupeng Chen and Yiyong Weng) sorted and checked the extracted data and the quality assessment results. Disagreements were resolved through internal discussions within the research team, and if differences persisted, a decision was made by a third investigator (Haiming Yu and Shu Lin). A third investigator (Qunlong Pan) randomly checked the assessments of the two investigators to assess their reliability.
Statistical Analysis
Data entry and statistical analyses were performed using Stata SE 15.1 (Stata Corp, College Station, TX). Reherniation incidence, reoperation incidence, and serious adverse events were considered dichotomous variables using odds ratio (OR) as an effect size indicator; 95% confidence intervals (CI), P-values, and forest plots were used. Postoperative ODI, VAS score for low back pain and leg pain, and intervertebral height were considered continuous variables using mean difference (MD) and 95% CI, P-value, and forest plots. A P-value <.05 was considered statistically significant. Owing to differences in patient characteristics and surgical techniques, we developed a random-effects meta-analysis model using inverse variance weighting. The P-values of the Q-test and I2 testing were used to quantitatively demonstrate heterogeneity between studies. For example, P-value <.1 or I2 >50% indicated a high heterogeneity between studies.
Subgroup analyses and Galbraith plots were used to explore possible sources of heterogeneity. Subgroup analyses of ethnicity, annular repair method, and whether there were randomized controls were planned. The differences between annular repair techniques were explored based on the results of the subgroup analysis. In the sensitivity analysis, the Metaninf command was used to examine the effect of individual studies on the total combined effect. Reporting bias was identified by funnel plots and quantified using Begg’s test and Egger’s test, and the trim-fill method was used to examine the effect of reporting bias on pooled results.
Results
Search Results
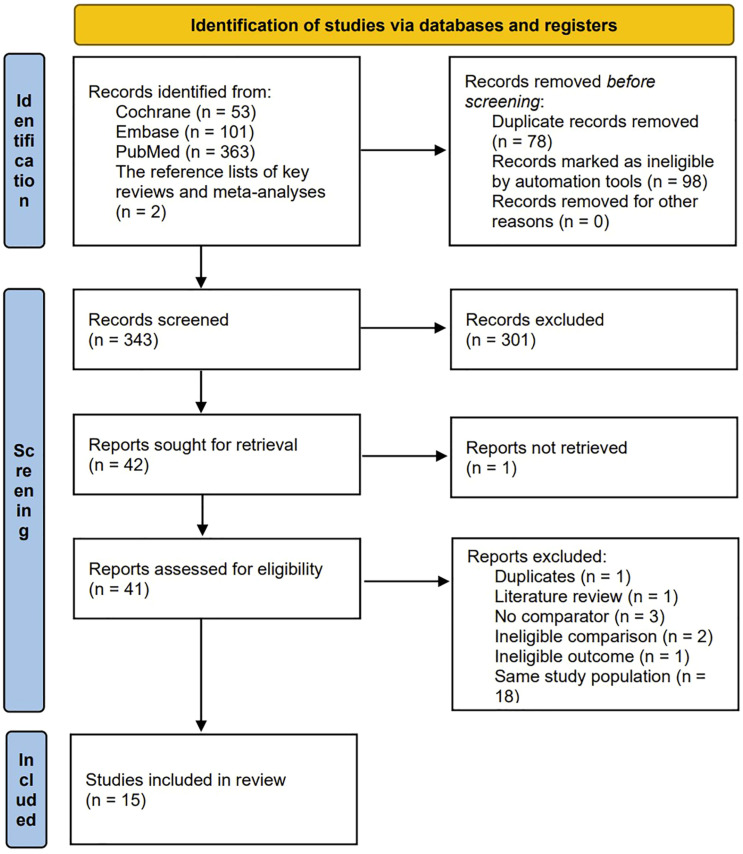
We obtained 363 publications from the PubMed search, 101 from Embase, and 53 from Cochrane. After reviewing previous relevant reviews and meta-analyses, we included two additional references, totalling 519 papers. Seventy-eight duplicate documents and 98 documents including reviews, metadata, case reports, meeting summaries, comments, notes, books, and protocols were filtered out. After screening the titles and abstracts, 301 articles were excluded. Among the remaining 42 articles, one was excluded because the full text could not be found. Subsequently, 1 article that was repeated, 1 article was a review, three articles that did not have a control group, two articles that were unqualified for comparison, 1 article that was unqualified for results, and 18 articles involving the same study populations were excluded. Ultimately, 7 randomized controlled studies18-24 and 8 observational studies25-32 matched the inclusion criteria. A flowchart is shown in Figure 1.
Figure 1.
The graph shows the flow chart of article filtering.
Study Characteristics
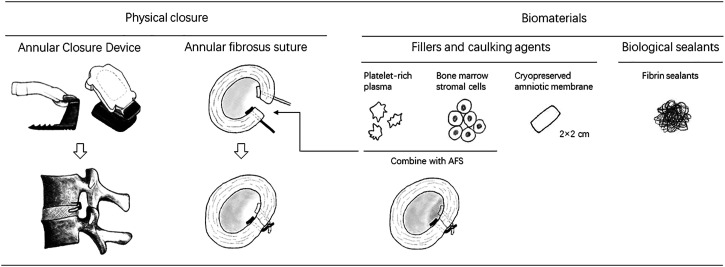
Fifteen studies included a total of 2161 participants with a median age of 43 (range, 16-87) years; 42% of patients were women, and the median BMI was 26.2 (16.5-38.5) kg/m2. The median follow-up duration was 21 (6-60) months. A total of 1165 patients underwent lumbar discectomy combined with annular repair. The annular repair techniques involved were divided into the following 4 subgroups: (1) the Barricaid Annular Closure Device (ACD) (Intrinsic Therapeutics, Woburn, MA, USA); (2) the annular fibrosus suture (AFS) systems, including the Xclose system (Anulex Technologies, Inc Minnetonka, MN, USA) and the Annular Stapler (2020 Medical Technology, Beijing, China); (3) biomaterials (BM), including cryopreserved amniotic membrane (CAM), platelet-rich plasma (PRP), fibrin sealants, and bone mesenchymal stem cells (BMSCs); and (4) the combined use of AFS and BM. The principle of both the Xclose system 19 and annular stapler system21,22,24,28,30 included the suturing of the annular defect. The Barricaid ACD is a composite implant device that is anchored to the adjacent cone by a titanium bone anchor and a polymer mesh then fills the annular defect to form a mechanical barrier. 33 CAMs have been used in ophthalmology and wound healing34-38 due to their anti-inflammatory and anti-scar properties. 18 Fibrin sealant, a blood-derived tissue binder, not only has efficient haemostatic properties but is also considered a scaffold for fibroblasts, stimulates mesenchymal stem cells, and promotes angiogenesis.31,39,40 In a three-arm study by Li et al, 22 patients in the trial group received both autologous conditioned plasma (ACP) and AFS. ACP, a type of PRP, was also applied in an observational study by Jiang et al. 26 Activated platelets release growth factors and downregulate several inflammatory factors that can promote the repair of different injuries. 22 In a three-arm study by Xu et al, 24 the second trial group received AFS and bone mesenchymal stem cells (BMSCs), which can effectively promote tissue repair. Figure 2 shows various annular repair techniques. In the control group, 996 patients underwent simple lumbar discectomy, including open discectomy, tubular discectomy, microdiscectomy, and endoscopic discectomy. For three-arm studies, including those by Li et al and Xu et al, we used a common control group. Both “Li-2” and “Xu-2” were second control groups. Table 1 summarizes the main characteristics of these studies. Supplementary Table 2 summarizes the population baseline values of patients for all articles.
Figure 2.
The graph shows various annular repair techniques.
Table 1.
Summary of Study Characteristics.
| Reference | Number of Institutions | Country or Region | Study Type | Annular Repair | Lumbar Discectomy | AR | No AR | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|
| Anderson 2017 | 1 | America | R | BM | Tubular discectomy | 40 | 40 | 24 months |
| Bailey 2013 | 34 | America | R | AFS | Microdiscectomy | 478 | 249 | 24 months |
| Barth 2016 | 1 | Europe | NR | ACD | Microdiscectomy | 42 | 29 | 18 months |
| Cho 2019 | 2 | Korea | R | ACD | Open discectomy | 30 | 30 | 24 months |
| Jiang, Y 2022 | 1 | China | NR | BM | Endoscopic discectomy | 51 | 57 | 12 months |
| Jinag, X 2017 | 1 | China | R | AFS | Tubular discectomy | 25 | 23 | 12 months |
| Kurzbuch 2022 | 1 | Europe | NR | ACD | Microdiscectomy | 12 | 41 | 12 months |
| Li 2021 | 1 | China | R | AFS & BM | Endoscopic discectomy | 50 | 25 | 6 months |
| Li 2016 | 1 | China | NR | AFS | Open discectomy | 12 | 14 | 12 months |
| Parker 2016 | 7 | Europe | NR | ACD | Open discectomy | 30 | 46 | 12 months |
| Ren 2020 | 1 | China | NR | AFS | Endoscopic discectomy | 51 | 54 | 36 months |
| Thome 2021 | 21 | Europe | R | ACD | Microdiscectomy | 272 | 278 | 5 years |
| Torkian 2020 | 1 | Iran | NR | BM | Endoscopic discectomy | 17 | 18 | 6 months |
| Vukas 2013 | 2 | Croatia | NR | ACD | Open discectomy | 30 | 72 | 24 months |
| Xu 2021 | 1 | China | R | AFS & BM | Endoscopic discectomy | 30 | 15 | 24 months |
R, randomized; NR, non-randomized; AR, annular repair; AFS, annular fibrosus suture; ACD, barricaid annular closure device; BM, biomaterial.
Study Quality and Bias
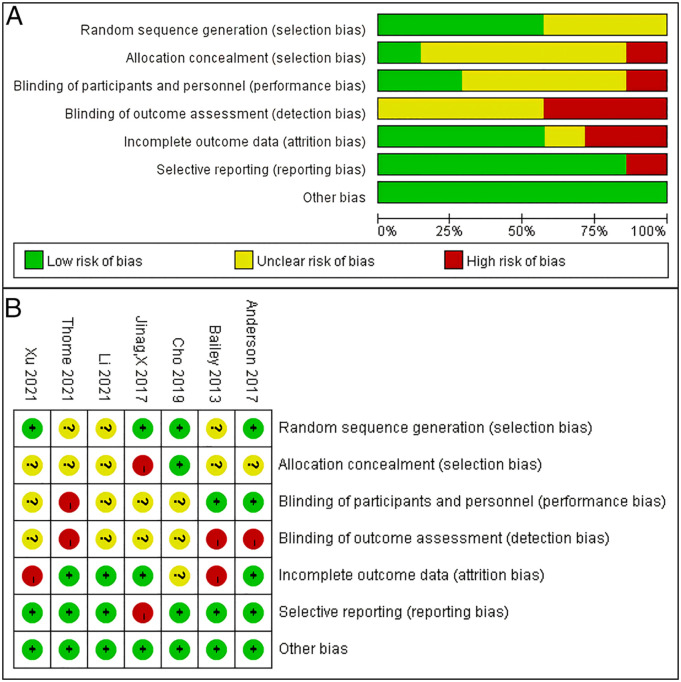
All 7 randomized controlled studies reported adequate randomization, but only 1 explicitly mentioned allocation concealment. 20 2 articles explicitly mentioned a single-blind setting.18,19 2 randomized controlled studies19,24 may have had a high level of follow-up bias. There may have been reporting bias in 1 article that planned to collect preoperative and postoperative VAS scores for lower back and leg pain in the study methodology, but only 1 VAS result was reported in the results. 21 All 8 observational studies had NOS scores of ≥6 stars. The trial group in 1 of the studies may have been underrepresented because the study population comprised adolescents. 28 3 studies selected control groups of different populations at different periods.25,29,32 4 studies had varying degrees of missing visits.25,29-31 Figure 3(A) and 3(B) show a summary of the risk of bias for the 7 randomized controlled studies. Table 2 shows the NOS results of the 8 observational studies.
Figure 3.
A-B. The (A) graph and (B) summary show the risk of bias for the 7 randomized controlled studies.
Table 2.
Results of the Quality Assessment Using the Newcastle-Ottawa Scale for Cohort Studies.
| Selection | Comparability | Exposure/Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that the outcome of interest was not present at the start of the study | Comparability of cohorts based on design or analysis a | Assessment of outcome | Follow-up was long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total |
| Barth 2016 | ⭐ | ⭐ | ⭐ | ⭐⭐ | ⭐ | ⭐ | 7 | ||
| Jiang, Y 2022 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 8 |
| Kurzbuch 2022 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐⭐ | ⭐ | ⭐ | ⭐ | 9 |
| Li 2016 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 7 | |
| Parker 2016 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 6 | ||
| Ren 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 7 | |
| Torkian 2020 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 7 | |
| Vukas 2013 | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | ⭐ | 7 | |
aA maximum of 2 stars can be allotted in this category: 1 for age and the other for other controlled factors.
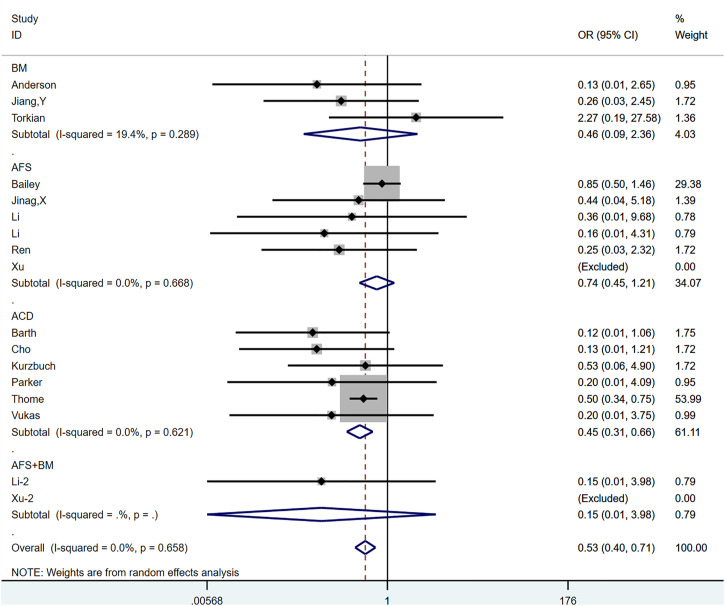
Reherniation
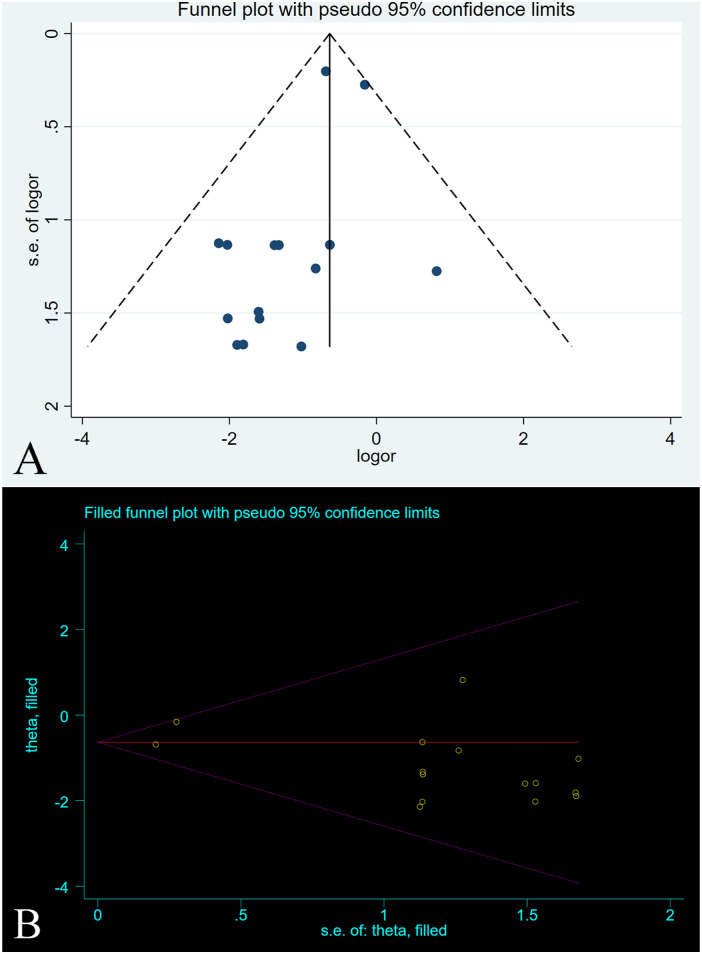
All 15 studies, comprising a total of 17 control groups, reported reherniation rates at the follow-up endpoint. The OR after the combined treatment was .529 (95% CI, .395-.708; P < .0001) (Figure 4), suggesting that the postoperative recurrence rate in the annular repair group was significantly lower than that in the control group. Between-group heterogeneity was not significant (P = .658; I2 = .0%). We used the Metaninf command to test the effect of single studies on combined results, suggesting that the combined results were robust (Supplementary Figure 1). The results of the Egger test suggested publication bias (P = .028). However, further trimming analysis showed that publication bias did not affect results; no trimming was performed because the data remained unchanged (Figure 5(A) and 5(B)).
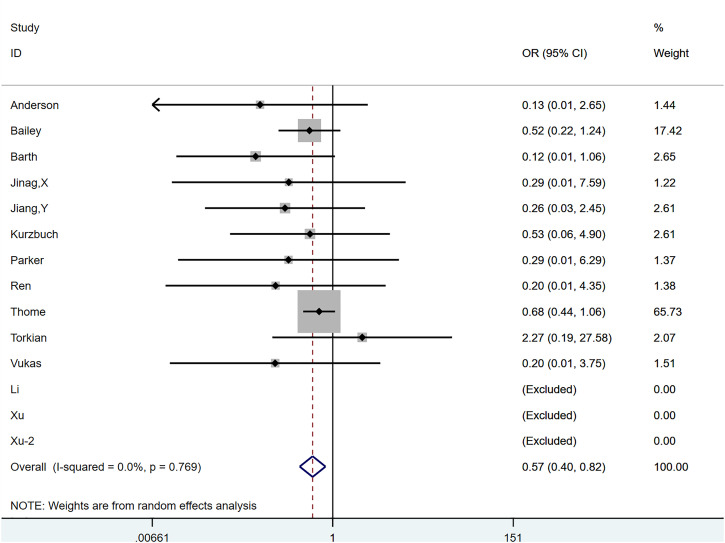
Figure 4.
The graph shows the merge result of reherniation.
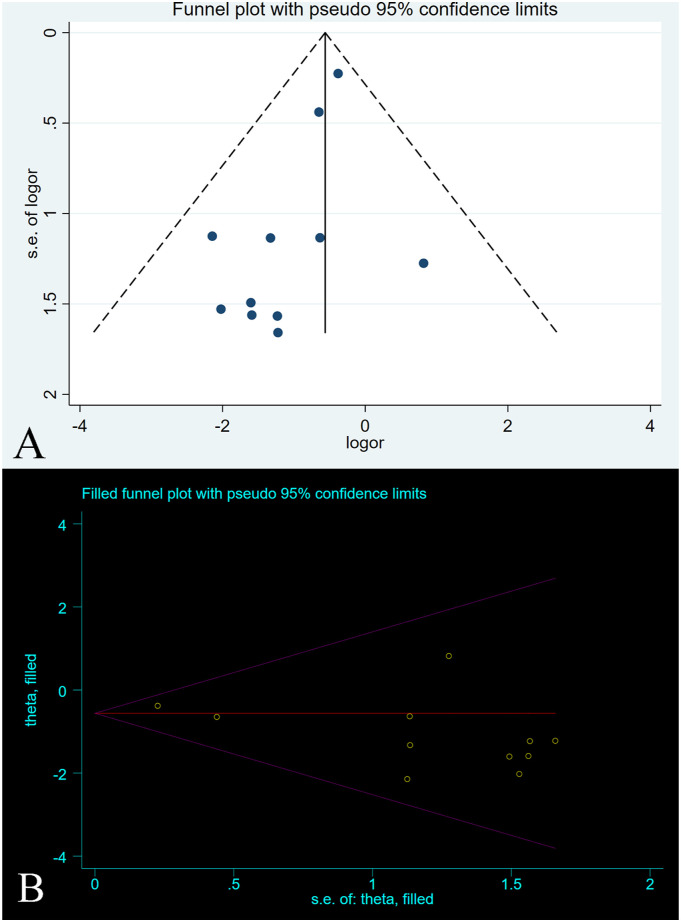
Figure 5.
A-B. The graph shows the (A) funnel plot and (B) trimming analysis of reherniation.
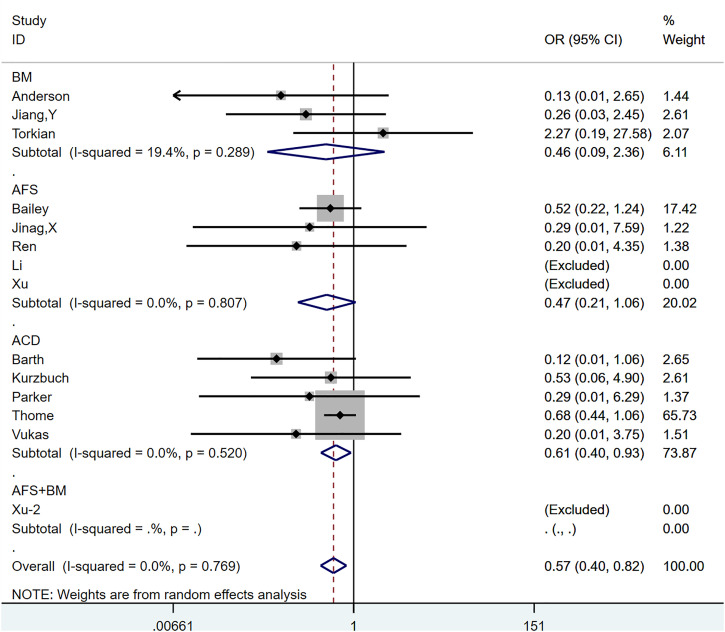
Reherniation-Related Reoperation
Thirteen articles,18,19,21,23-32 with a total of 14 control groups, reported reoperation rates associated with reherniation as the end point of follow-up.
The OR after combined treatment was .571 (95% CI, .398-.817; P = .002) (Figure 6), indicating that the postoperative recurrence rate in the annular repair group was significantly lower than that in the control group. Between-group heterogeneity was not significant (P = .769; I2 = .0%). We used the Metaninf command to examine the effect of each study on results (Supplementary Figure 2). We found that the study of Thome et al 23 may have had a greater impact on the combined results, but when we temporarily removed articles, the reanalysed results were not significantly different from the original results, suggesting that the original results were robust. The results of Egger’s test indicated a publication bias (P = .037). However, further trimming analysis showed that publication bias did not affect results; no trimming was performed because the data remained unchanged (Figure 7).
Figure 6.
The graph shows the merge result of reherniation-related reoperation.
Figure 7.
A-B. The graph shows the (A) funnel plot and (B) trimming analysis of reherniation-related reoperation.
Oswestry Index (ODI)
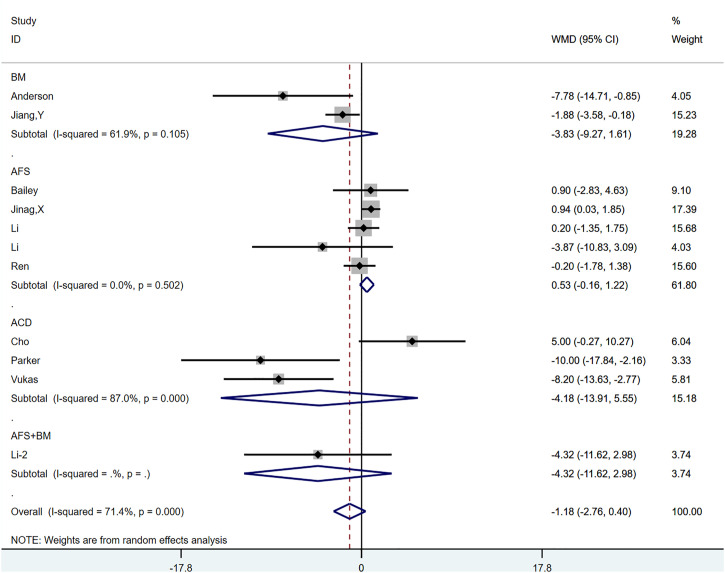
All 10 studies reported postoperative ODI scores,18-22,26,28-30,32 and there was a total of 11 control groups. For literature using standard errors and 95% CI, we performed the corresponding data transformation using relevant statistical methods. The weighted MD (WMD) after incorporation was −1.181 (95% CI, −2.761, .399; P = .143) (Supplementary Figure 3). Significant heterogeneity was observed between groups (P < .0001; I2 = 71.4%). None of the ethnicity-based subgroup analysis (Supplementary Figure 4(A)), randomized controls (Supplementary Figure 4(B)), or annular repair modalities (Figure 8) were able to explain the source of heterogeneity. Notably, the combined results of the AFS subgroup showed low heterogeneity (P = .502, I2 = .0%), and the combined results (.527; 95% CI, −.162, 1.216; P = .134) suggested that the addition of the AFS technique may have failed to further improve pain and function in patients who underwent lumbar discectomy. Stata’s Galbraith plots also indicated considerable heterogeneity between studies (Supplementary Figure 5). We used the Metaninf command to examine the effects of individual studies on the overall pooled results, which showed that all studies had a small effect on the combined effect measure (Supplementary Figure 6). Both the Begg and Egger tests suggested a publication bias (P = .087 and P = .042, respectively). However, further trimming analysis revealed that publication bias did not affect result; no trimming was performed because the data did not change (Supplementary Figures 7(A) and 7(B)).
Figure 8.
The graph shows the result of the subgroup analysis based on annular repair modalities for the Oswestry index (ODI).
VAS of Low Back Pain and Leg Pain
Twelve articles reported postoperative VAS scores,18-22,26,28-32 and there were 13 control groups. For studies using standard errors, 95% CI, and medians, we used relevant statistical formulas for conversion. Most studies reported VAS scores for low back and leg pain. Two studies only reported separate VAS scores.28,31 Although the study by Jiang et al 21 planned to collect postoperative VAS scores for lower back and leg pain in the methodological design, it ended up reporting only separate VAS scores. We combined the reported VAS results for low back and leg pain separately, and if only 1 VAS result was reported in the literature, it was used in both analyses. The combined WMD VAS scores for low back and leg pain were −.303 (95% CI, −.637, .031, P = .075) and -.261 (95% CI: −.435, −.088; P = .003), respectively, with significant heterogeneity between groups (lower back pain: P < .0001, I2 = 93.9%; leg pain: P < .0001; I2 = 74.8%) (Supplementary Figures 8(A) and 8(B)). Subgroup analyses based on ethnicity (Supplementary Figures 9(A) and 9(B)), randomized controls (Supplementary Figures 9(C) and 9(D)), and mode of repair (Supplementary Figures 9(E) and 9(F)) failed to identify valid sources of heterogeneity. Galbraith plots further indicated significant heterogeneity among the study groups (Supplementary Figures 10(A) and 10(B)). Funnel plots were quantified by Begg’s and Egger’s tests and showed no significant publication bias (lower back VAS: P = .193 and P = .454, respectively; leg VAS: P = .537 and P = .577, respectively), and the results of the trimming analysis were unchanged (Supplementary Figures 11(A)–(D)). The Metaninf order to test the effect of a single study on the combined effect size also showed robust results (Supplementary Figures 12(A) and 12(B)).
Intervertebral Height
Intervertebral height at the end of follow-up was reported in 1 randomized controlled study 20 and two non-randomized controlled studies.29,30 Of these studies, two used ACD devices, and 1 used AFS technology. The combined WMD was .857 (95% CI, .485, 1.229; P < .0001), suggesting that the addition of annular repair techniques can reduce intervertebral height loss after lumbar discectomy. Heterogeneity between the groups was not significant (P = .658; I2 = .0%) (Figure 9). Stata’s Metaninf command suggested robust results (Supplementary Figure 13).
Figure 9.
The graph shows the merge result of intervertebral height.
Serious Adverse Events
We investigated three main adverse event rates: dural injury or spinal fluid leakage, epidural hematoma, and wound-related adverse events, including infection, dehiscence, and delayed healing. Serious adverse events were reported in 12 studies,18-21,23,24,26-30,32 and none of the patients in 8 of these studies18,20,21,24,26-28,30 experienced serious adverse events.
The combined OR of dural injury or spinal fluid leakage was .549 (95% CI: .128, 2.345; P = .418) (Figure 10). There was no significant heterogeneity between groups (P = .261; I2 = 25.1%). Stata’s Metaninf command suggested robust results (Supplementary Figure 14).
Figure 10.
The graph shows the merge result of dural injury or spinal fluid leakage.
The combined OR of epidural hematoma was .247 (95% CI: .026, 2.380; P = .226) (Figure 11), and there was no significant difference or heterogeneity between groups (P = .759, I2 = .0%). Stata’s Metaninf command suggested robust results (Supplementary Figure 15).
Figure 11.
The graph shows the merge result of epidural hematoma.
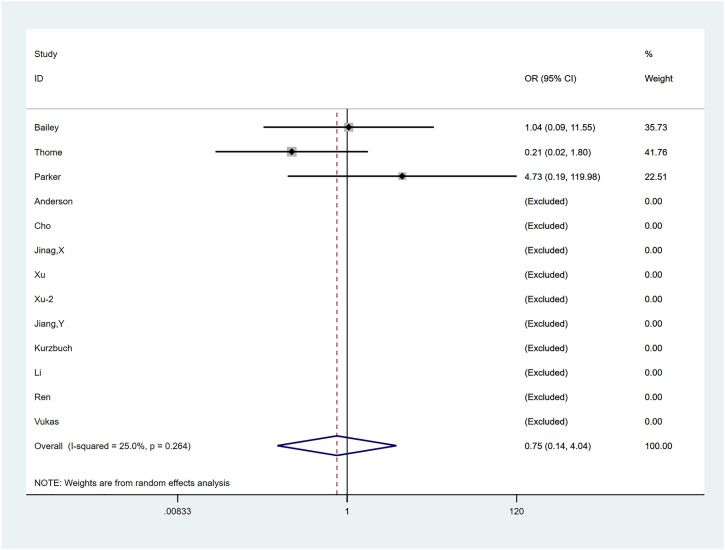
The combined OR of wound-related adverse events was .749 (95% CI: .139, 4.039; P = .737) (Figure 12), with no significant difference or heterogeneity between groups (P = .264, I2 = 25.0%). Stata’s Metaninf command suggested robust results (Supplementary Figure 16).
Figure 12.
The graph shows the merge result of wound-related adverse events.
Subgroup Analysis of Annular Repair
We performed a subgroup analysis based on the pattern of annular repair and associated reherniation and reoperation rates. The result of reherniation was noteworthy; the most statistically significant combined results were from the ACD subgroup (P < .0001), while the combined results for the other subgroups, including the BM (P = .353), AFS (P = .229), and AFS + BM (P = .257) subgroups, suggested a lack of statistical significance. The results of reherniation-related reoperation were like those of re-protrusion, and only the ACD subgroup combined results were statistically significant. There was no significant heterogeneity among subgroups. Figures 13 and 14 illustrate the results of the subgroup analysis.
Figure 13.
The graph shows the subgroup analysis based on the pattern of annular repair for reherniation rate.
Figure 14.
The graph shows the subgroup analysis based on the pattern of annular repair for reoperation rates.
Discussion
Our results showed that patients who underwent lumbar discectomy combined with annular repair had lower postoperative recurrence and reoperation rates than those who underwent lumbar discectomy alone. However, only the ACD subgroup showed true efficacy in the subgroup analysis based on annular repair modality. The AFS technique did not improve postoperative ODI. Annular repair helped maintain the postoperative disc height, and all annular repair techniques had a good safety profile. Further studies to determine whether these techniques help improve pain and functional recovery in patients postoperatively are warranted. There were previously published meta-analyses similar to ours, but they all had less than 5 papers and a lacked subgroup analysis or discussion of different annular repair modalities.17,41,42 Rickers et al 10 conducted a large network meta-analysis of interventions for LDH, including 4 randomized controlled studies of annular repair; however, contrary to our findings, there was no difference in reoperation rates between multiple surgical interventions, including combined lumbar discectomy with annular repair. The authors offered two explanations for this result: The actual differences that existed may have been lost in the high-intensity composite comparison, and the authors did not perform subgroup analyses of different repair modalities, which may have revealed differences.
AFS is a general term used for mainstream physical suture methods, and its safety in clinical practice has been confirmed. 24 Several animal and in vitro experiments have shown that suturing the annulus promotes faster healing, enhances its resistance to tension, effectively restores intradiscal disc pressure, stabilizes lumbar segmental motion, and prevents reherniation.43,44 However, the clinical results of these techniques are unsatisfactory. Although the recurrence rate in the trial group was lower than that in the control group, the difference lacked statistical support.19,21,22,24,28,30 The lack of dominance of AFS was also suggested in our subgroup analysis based on different repair modalities. Limited by the avascularity of the annular fibrous region, 33 AFS merely facilitates the scar healing process after rupture of the annulus fibrosus, 45 but the mechanical strength of the scar tissue is still lower than that of the original normal annulus fibrosus,46,47 meaning that reherniation is still a possibility. Moreover, for larger annular defects, AFS alone did not produce better closure. It was difficult to achieve an ideal suture because of the limited surgical space and the presence of tension in the annular defect, and some annular defects were not completely closed after suturing. Further investigation into the ability of AFS to reduce postoperative disc height loss is required; we retrieved only 1 observational study 30 and included it in our meta-analysis. Furthermore, adding AFS failed to achieve better postoperative pain relief and functional recovery not only in the short-term postoperative period but also in the long-term in both individual studies and our AFS subgroup analysis.
ACD is another implantable device used after lumbar discectomy for annular defects that are 4-6 mm high and 6-10 mm wide. It had a good clinical effect in reducing recurrence and reoperation rates, which is consistent with the results of our subgroup analysis. In a 5-year, multicentre, randomized, controlled study reported by Thome et al, 23 the recurrence and reoperation rates were significantly lower in the ACD group than in the control group. Moreover, ACD appears to be helpful in maintaining postoperative intervertebral space height, but the evidence for this is sparse, and our meta-analysis included only two studies.20,29 The ability of ACD to improve pain and function remains questionable, with several studies reaching different conclusions.20,23,25,29 Moreover, as rigid implants, dura mater and nerve root damage during the implantation of ACD remain noteworthy. Thome et al 23 found no significant difference in the incidence of related complications between the ACD and control groups; however, some patients have been reported to suffer from an abandoned implantation because of difficulties or a high risk of dural and nerve root injury. Moreover, Krutko et al reported that the ACD group had longer operative times, additional blood loss, and some possibility of postoperative loosening. 48 Lange et al reported a case in which the contact of the ACD device with the spinal nerve caused significant pain. 49 Benedikt et al reported the case of a 28-year-old patient with symptom relapse three years after ACD; the second surgery confirmed that the titanium arm of the ACD had broken, and the recurrent protruding nucleus pulposus was attached to the polymer mesh occlusion component through scar tissue, which compressed the nerve root. 50 Therefore, ACD, similar to most endografts and implants, still has a risk of loosening, displacing, breaking, and damaging the surrounding tissues, deserving the attention of clinicians.
Unlike physical closures, biomaterials tend to exhibit multiple properties. For example, fibrin sealants not only provides a certain mechanical strength against the occurrence of re-protrusion but also promotes the coagulation process. Fibrin sealants is a scaffold for coordinating fibroblasts, stimulating mesenchymal stem cells, and promoting angiogenesis.31,39,40 However, the mechanical strength provided by bio-adhesives alone is insufficient. 51 Furthermore, most other biomaterials currently in clinical use are only used as fillers and tamponades and cannot provide sufficient mechanical strength. Notably, these biomaterials can inhibit scarring, downregulate inflammatory factors, and promote tissue repair. In several of the included studies herein, biomaterials reportedly helped improve postoperative pain and function.18,26 In Li and Xu’s study, in all patients who also underwent lumbar discectomy, postoperative pain and function were significantly better among patients who were treated with a combination of biomaterials and AFS than among patients treated with AFS alone.22,24 Therefore, AFS and biomaterials may have complementary advantages. On the one hand, biomaterials, including adhesives, can further enhance the anti-protrusion ability after mechanical closure. 51 On the other hand, the addition of biomaterials can reduce the formation of scar tissue, downregulate the level of local inflammation, achieve better healing of the annulus fibrosus, and mesenchymal stem cells can repair the disc tissue. Currently, some manufacturers are trying to develop biological patches combined with AFS, which are expected to compensate for the shortcomings of AFS alone. These combined repair methods may be an alternative approach before the development of ideal biomimetic closure materials. Currently, most current bioremediation strategies or tissue engineering methods cannot reach the standards of ideal biomaterials; 52 consequently, the use of biodegradable or bioresorbable implants alone to close annular defects may still lack practicality. Nevertheless, it is reasonable to expect that, with the continuous development of tissue engineering, materials science, and 3D printing, biomaterials with better performance may be the main direction of future development.
Our meta-analysis is not without limitations. First, due to the current level of technical development and research, there is a lack of high-quality randomized controlled studies. We included randomized controlled and observational studies, which may reduce the level of evidence of this study. Second, some confounding factors, including BMI and the male-to-female ratio, were not sufficiently reported in some studies. Similarly, there was a high degree of heterogeneity in some of the pooled results, and we were unable to identify the source of heterogeneity. Moreover, owing to a lack of adequate literature and large heterogeneity, we were unable to further explore some comprehensive results for the subgroup analysis based on annular repair. Finally, as for the possible differences between studies, we did not perform a net meta-analysis to further compare the advantages and disadvantages of different interventions.
Conclusion
Lumbar discectomy combined with ACD can effectively reduce the postoperative recurrence and reoperation rates in patients with LDH. AFS alone was less effective in reducing recurrence and reoperation rates and did not improve postoperative pain and function. Annular repair may help maintain postoperative disc height; further studies to confirm this are warranted. Currently, biomaterials lack application value but can improve postoperative pain and function. Combining them with AFS may be an adequate alternative at this stage; further studies to confirm these findings. All current annular repair technologies are safe, and biomaterials with better performance will be the main direction of future development.
Supplemental Material
Supplemental Material for Annulus Fibrosus Repair for Lumbar Disc Herniation: A Meta-Analysis of Clinical Outcomes From Controlled Studies Yangbin Wang, MB, Xiaoyu He, MB, Shupeng Chen, MB, Yiyong Weng, MB, Zhihua Liu, MB, Qunlong Pan, MB, Rongmou Zhang, MM, Yizhong Li, MM, Hanshi Wang, MD, Shu Lin, and Haiming Yu, MM in Global Spine Journal.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the two authors (Shu Lin and Haiming Yu) have separately received funding from the Science and Technology Bureau of Quanzhou (Grant No. 2020CT003) and the Natural Science Foundation of Fujian Province, China (Grant No. 2021J01268).
Data Sharing: The analytic dataset is available upon request by contacting the corresponding author.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Yangbin Wang https://orcid.org/0000-0003-0846-7599
Xiaoyu He https://orcid.org/0000-0002-7829-8025
Shupeng Chen https://orcid.org/0000-0003-1608-0124
Yiyong Weng https://orcid.org/0000-0002-8408-5149
Qunlong Pan https://orcid.org/0000-0002-4056-8333
Rongmou Zhang https://orcid.org/0000-0002-6011-0039
Shu Lin https://orcid.org/0000-0002-4239-2028
Haiming Yu https://orcid.org/0000-0002-9280-2371
References
- 1.Hoy D, March L, Brooks P, et al. The global burden of low back pain: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968-974. doi: 10.1136/annrheumdis-2013-204428 [DOI] [PubMed] [Google Scholar]
- 2.Yuan C, Wang J, Zhou Y, Pan Y. Endoscopic lumbar discectomy and minimally invasive lumbar interbody fusion: A contrastive review. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques. 2018;13(4):429-434. doi: 10.5114/wiitm.2018.77744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan M, Li Q, Li S, et al. Percutaneous Endoscopic Lumbar Discectomy: Indications and Complications. Pain Physician. 2020;23(1):49-56. [PubMed] [Google Scholar]
- 4.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine patient outcomes research trial (SPORT): A randomized trial. Jama. 2006;296(20):2441-2450. doi: 10.1001/jama.296.20.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenger M, Mariani L, Kalbarczyk A, Gröger U. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49(2):329-334; discussion 334-335. doi: 10.1097/00006123-200108000-00013 [DOI] [PubMed] [Google Scholar]
- 6.Guo JJ, Yang H, Tang T. Long-term outcomes of the revision open lumbar discectomy by fenestration: A follow-up study of more than 10 years. Int Orthop. 2009;33(5):1341-1345. doi: 10.1007/s00264-008-0648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moliterno JA, Knopman J, Parikh K, et al. Results and risk factors for recurrence following single-level tubular lumbar microdiscectomy. J Neurosurg Spine. 2010;12(6):680-686. doi: 10.3171/2009.12.Spine08843 [DOI] [PubMed] [Google Scholar]
- 8.Shriver MF, Xie JJ, Tye EY, et al. Lumbar microdiscectomy complication rates: a systematic review and meta-analysis. Neurosurg Focus. 2015;39(4):E6. doi: 10.3171/2015.7.Focus15281 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Sandhu HS, Vargas Castillo J, Diwan AD, The association between pain scores and disc height change following discectomy surgery in lumbar disc herniation patients: A systematic review and meta-analysis. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2021;30(11):3265-3277. doi: 10.1007/s00586-021-06891-4 [DOI] [PubMed] [Google Scholar]
- 10.Rickers KW, Pedersen PH, Tvedebrink T, Eiskjær SP. Comparison of interventions for lumbar disc herniation: A systematic review with network meta-analysis. Spine J. 2021;21(10):1750-1762. doi: 10.1016/j.spinee.2021.02.022 [DOI] [PubMed] [Google Scholar]
- 11.Mo F, Yue J, Zhang J, Howk K, Williams A. Evaluation of perivascular adhesion formation in New Zealand white rabbits using oxiplex and duraseal xact adhesion barrier system. SAS journal. 2009;3(2):68-76. doi: 10.1016/sasj-2009-0006-nt [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fransen P. Safety of carboxymethylcellulose/polyethylene oxide for the prevention of adhesions in lumbar disc herniation--consecutive case series review. Ann Surg Innovat Res. 2008;2:2. doi: 10.1186/1750-1164-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakir B, Richter M, Käfer W, Puhl W, Schmidt R. The impact of total lumbar disc replacement on segmental and total lumbar lordosis. Clin Biomech. 2005;20(4):357-364. doi: 10.1016/j.clinbiomech.2004.11.019 10.1016/j.clinbiomech.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 14.Miller LE, McGirt MJ, Garfin SR, Bono CM. Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: Systematic review and meta-analysis of comparative studies. Spine. 2018;43(5):E308-e315. doi: 10.1097/brs.0000000000002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vergroesen PPA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057-1070. doi: 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 16.Murphy TP, Panarello NM, Baird MD, Helgeson MD, Wagner SC. Should annular closure devices be utilized to reduce the risk of recurrent lumbar disk herniation? Clin Spine Surg. 2022;35:187-189. doi: 10.1097/bsd.0000000000001104 [DOI] [PubMed] [Google Scholar]
- 17.Choy WJ, Phan K, Diwan AD, Ong CS, Mobbs RJ. Annular closure device for disc herniation: meta-analysis of clinical outcome and complications. BMC Musculoskelet Disord. 2018;19(1):290. doi: 10.1186/s12891-018-2213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson DG, Popov V, Raines AL, O’Connell J. Cryopreserved amniotic membrane improves clinical outcomes following microdiscectomy. Clin Spine Surg. 2017;30(9):413-418. doi: 10.1097/bsd.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 19.Bailey A, Araghi A, Blumenthal S, Huffmon GV, Anular Repair Clinical Study Group . Prospective, multicenter, randomized, controlled study of anular repair in lumbar discectomy: Two-year follow-up. Spine. 2013;38(14):1161-1169. doi: 10.1097/BRS.0b013e31828b2e2f [DOI] [PubMed] [Google Scholar]
- 20.Cho PG, Shin DA, Park SH, Ji GY. Efficacy of a novel annular closure device after lumbar discectomy in Korean patients: A 24-Month Follow-Up of a randomized controlled trial. J Korean Neurosurg Soc. 2019;62(6):691-699. doi: 10.3340/jkns.2019.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Li F, Pan HS, Huo XT, Xiao QB, Yang GX. Clinical effectiveness of annulus repair after discectomy under mast quadrant system for lumbar disc herniation. Chinese Journal of Tissue Engineering Research. 2017;21(24):3912-3917. doi: 10.3969/j.issn.2095-4344.2017.24.024 [DOI] [Google Scholar]
- 22.Li J, Yuan X, Li F, et al. A novel full endoscopic annular repair technique combined with autologous conditioned plasma intradiscal injection: A new safe serial therapeutic model for the treatment of lumbar disc herniation. Ann Palliat Med. 2021;10(1):292-301. doi: 10.21037/apm-20-2257 [DOI] [PubMed] [Google Scholar]
- 23.Thome C, Kuršumovic A, Klassen PD, et al. Effectiveness of an annular closure device to prevent recurrent lumbar disc herniation: A secondary analysis with 5 years of follow-up. JAMA Netw Open. 2021;4(12):e2136809. doi: 10.1001/jamanetworkopen.2021.36809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Zhang H, Du L, et al. Selective retention of bone marrow stromal cells with gelatin sponge for repair of intervertebral disc defects after microendoscopic discectomy: A prospective controlled study and 2-year follow-up. BioMed Res Int. 2021;2021:4822383. doi: 10.1155/2021/4822383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barth M, Fontana J, Thomé C, Bouma GJ, Schmieder K. Occurrence of discal and non-discal changes after sequestrectomy alone versus sequestrectomy and implantation of an anulus closure device. J Clin Neurosci. 2016;34:288-293. doi: 10.1016/j.jocn.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Zuo R, Yuan S, et al. Transforaminal endoscopic lumbar discectomy with versus without platelet-rich plasma injection for lumbar disc herniation: A prospective cohort study. Pain Research & Management. 2022;2022:6181478. doi: 10.1155/2022/6181478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzbuch AR, Tuleasca C, Fournier JY. Lumbar discectomy with annulus fibrosus closure: A retrospective series of 53 consecutive patients. Neurochirurgie. 2022;68:393-397. doi: 10.1016/j.neuchi.2021.12.009 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Ma C, Li YM, et al. Comparison of results between fenestration discectomy associated with annulus repair and fenestration discectomy for lumbar disc herniation in the adolescents. Zhonghua Yixue Zazhi. 2016;96(32):2573-2577. doi: 10.3760/cma.j.issn.0376-2491.2016.32.012 [DOI] [PubMed] [Google Scholar]
- 29.Parker SL, Grahovac G, Vukas D, et al. Effect of an annular closure device (Barricaid) on same-level recurrent disk herniation and disk height loss after primary lumbar discectomy: Two-year results of a multicenter prospective cohort study. Clin Spine Surg. 2016;29(10):454-460. doi: 10.1097/BSD.0b013e3182956ec5 [DOI] [PubMed] [Google Scholar]
- 30.Ren C, Qin R, Li Y, Wang P. Microendoscopic discectomy combined with annular suture versus percutaneous transforaminal endoscopic discectomy for lumbar disc herniation: A prospective observational study. Pain Physician. 2020;23(6):E713-e721. [PubMed] [Google Scholar]
- 31.Torkian P, Daneshvar K, Taherian E, Rezaeifar Y, Akhlaghpoor S. Fibrin sealants in lumbar annuloplasty after endoscopic discectomy as a method to prevent recurrent lumbar disc herniation. Eur J Transl Myol. 2020;30(2):8748. doi: 10.4081/ejtm.2019.8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vukas D, Ledić D, Grahovac G, Kolić Z, Rotim K, Vilendecić M. Clinical outcomes in patients after lumbar disk surgery with annular reinforcement device: Two-year follow up. Acta Clin Croat. 2013;52(1):87-91. [PubMed] [Google Scholar]
- 33.Guardado AA, Baker A, Weightman A, Hoyland JA, Cooper G. Lumbar intervertebral disc herniation: Annular closure devices and key design requirements. Bioengineering (Basel, Switzerland). 2022;9(2):47. doi: 10.3390/bioengineering9020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He H, Zhang S, Tighe S, Son J, Tseng SCG. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288(36):25792-25803. doi: 10.1074/jbc.M113.479584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JS. II. skin grafting at the Johns Hopkins hospital. Ann Surg. 1909;50(3):542-549. doi: 10.1097/00000658-190909000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellington JK, Ferguson CM. The use of amniotic membrane/umbilical cord in first metatarsophalangeal joint cheilectomy: A comparative bilateral case study. Surg Technol Int. 2014;25:63-67. [PubMed] [Google Scholar]
- 37.DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: A consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261. [PubMed] [Google Scholar]
- 38.Warner M, Lasyone L. An Open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255. [PubMed] [Google Scholar]
- 39.Dickneite G, Metzner H, Pfeifer T, Kroez M, Witzke G. A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thromb Res. 2003;112(1-2):73-82. doi: 10.1016/j.thromres.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 40.Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15-56. doi: 10.1166/jnn.2014.9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arts MP, Kuršumović A, Miller LE, et al. Comparison of treatments for lumbar disc herniation: Systematic review with network meta-analysis. Medicine. 2019;98(7):e14410. doi: 10.1097/md.0000000000014410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller LE, Allen RT, Duhon B, Radcliff KE. Expert review with meta-analysis of randomized and nonrandomized controlled studies of Barricaid annular closure in patients at high risk for lumbar disc reherniation. Expet Rev Med Dev. 2020;17(5):461-469. doi: 10.1080/17434440.2020.1745061 [DOI] [PubMed] [Google Scholar]
- 43.Chiang CJ, Cheng CK, Sun JS, Liao CJ, Wang YH, Tsuang YH. The effect of a new anular repair after discectomy in intervertebral disc degeneration: An experimental study using a porcine spine model. Spine. 2011;36(10):761-769. doi: 10.1097/BRS.0b013e3181e08f01 [DOI] [PubMed] [Google Scholar]
- 44.Bartlett A, Wales L, Houfburg R, Durfee WK, Griffith SL, Bentley I. Optimizing the effectiveness of a mechanical suture-based anulus fibrosus repair construct in an acute failure laboratory simulation. J Spinal Disord Tech. 2013;26(7):393-399. doi: 10.1097/BSD.0b013e31824c8224 [DOI] [PubMed] [Google Scholar]
- 45.Li ZZ, Cao Z, Zhao HL, Shang WL, Hou SX. A pilot study of full-endoscopic annulus fibrosus suture following lumbar discectomy: Technique notes and one-year follow-up. Pain Physician. 2020;23(5):E497-e506. [PubMed] [Google Scholar]
- 46.Freeman AL, Buttermann GR, Beaubien BP, Rochefort WE. Compressive properties of fibrous repair tissue compared to nucleus and annulus. J Biomech. 2013;46(10):1714-1721. doi: 10.1016/j.jbiomech.2013.03.034 [DOI] [PubMed] [Google Scholar]
- 47.Fujita N, Imai Ji, Suzuki T, et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. 2008;372(2):367-372. doi: 10.1016/j.bbrc.2008.05.044 [DOI] [PubMed] [Google Scholar]
- 48.Krutko AV, Baykov ES, Sadovoy MA. Reoperation after microdiscectomy of lumbar herniation: Case report. Int J Surg Case Rep. 2016;24:119-123. doi: 10.1016/j.ijscr.2016.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lange N, Meyer B, Shiban E. Symptomatic annulus-repair-device loosening due to a low-grade infection. Acta Neurochir. 2018;160(1):199-203. doi: 10.1007/s00701-017-3371-1 [DOI] [PubMed] [Google Scholar]
- 50.Burkhardt BW, Oertel JM. Annular closure device breakage due to recurrent lumbar disc herniation: A case report. Acta Neurochir. 2021;163(1):269-273. doi: 10.1007/s00701-020-04651-9 [DOI] [PubMed] [Google Scholar]
- 51.Heuer F, Ulrich S, Claes L, Wilke HJ. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J Neurosurg Spine. 2008;9(3):307-313. doi: 10.3171/spi/2008/9/9/307 [DOI] [PubMed] [Google Scholar]
- 52.Tavakoli J. Tissue engineering of the intervertebral disc’s annulus fibrosus: A scaffold-based review study. Tissue Eng Regen Med. 2017;14(2):81-91. doi: 10.1007/s13770-017-0024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Annulus Fibrosus Repair for Lumbar Disc Herniation: A Meta-Analysis of Clinical Outcomes From Controlled Studies Yangbin Wang, MB, Xiaoyu He, MB, Shupeng Chen, MB, Yiyong Weng, MB, Zhihua Liu, MB, Qunlong Pan, MB, Rongmou Zhang, MM, Yizhong Li, MM, Hanshi Wang, MD, Shu Lin, and Haiming Yu, MM in Global Spine Journal.