Abstract
Study Design
Retrospective cohort study
Objective
Given changes in bone density induced by degenerative disease, general measures of bone health (ie DEXA) are inadequate to evaluate bone density in surgical areas of interest. Regional differences in HU in the cervical spine may influence surgical strategies. The purposes of our study were to determine whether cervical Hounsfield units (HU) vary by level, examine their relationship with age, comorbidities, and alignment, and propose a technique to measure HU in the lateral masses.
Methods
Two hundred twenty-four patients with degenerative spine pathology with a cervical computed tomography were included (2015-2019). Measurements were performed in each vertebral body (C2-T1; mid-axial, anterior-axial, posterior-axial, mid-coronal, and mid-sagittal) and 2 regions of the lateral masses (C3-C6; mid-cor, mid-sag). To evaluate reliability, 6 observers each measured 355 HU values, inter-relater reliability assessed with intraclass correlation coefficients Correlations of HU with age, BMI, comorbidities, and cervical alignment were evaluated.
Results
Bone density differed by level, with the lowest HU scores in the lower cervical spine (C6-T1) (P < .001). No correlations were found between LM HU and age, BMI, CCI, or alignment (P > .05). Increased kyphosis was weakly correlated with VB HU, while age and CCI showed moderate correlations with VB HU at all levels (P<.001). ICC for HU measurements were good to excellent for the VBs, but poor to moderate for the LMs.
Conclusion
Bone is least dense in the lower cervical spine. HU scoring is not reliable in the lateral masses. We recommend that a level-specific approach to bone density is considered in surgical planning.
Keywords: cervical, hounsfield units, bone density, computed tomography, fusion
Introduction
The density of tissue on computed tomography (CT) reconstructions can be measured in Hounsfield units (HU), which are a measure of tissue attenuation. The clinical prevalence of CT scans has fostered interest in the use of HU as a marker of bone density. 1 Certain authors have advocated for the use of cervical or lumbar CT scans as an “opportunistic” manner of screening for osteoporosis. 2 However, while the relationship between osteoporosis (diagnosed with DEXA) and low HU values on CT scan is well-established,1-3 the patient’s general bone health may not correlate with the bone density in the surgical zone of interest. In accordance with Wolff’s law, bone remodels under stress. Thus, patients with degenerative lumbar spine disease have been shown to have higher bone density in the lumbar spine, despite having femoral neck DEXA T-scores consistent with osteoporosis.4,5 In this context, the measurement of HU in the surgical region of interest (ie the levels for which a surgical procedure is planned) may be a more accurate method of evaluating regional bone density and predicting complications. Indeed, several authors have found that HU in the surgical region of interest correlate with mechanical and clinical outcomes, such as pseudarthrosis, cage subsidence, and proximal junctional kyphosis.6-8
In the lumbar spine, HU have been shown to vary by vertebral level. 3 Similar studies have not been performed for the cervical spine. Understanding trends in bone density of the cervical spine (as measured by HU scores) may be of clinical importance given the associations between HU and mechanical complications. 9 Furthermore, HU scores have not been attempted in the lateral masses, which may be of interest for posterior cervical fusion constructs. Thus, the purposes of this analysis were to (1) define trends in bone density of the degenerative cervical spine, (2) examine the relationship between cervical bone density, age, BMI, comorbidities, and alignment, and (3) develop a reliable technique to measure HU scores in the lateral masses.
Methods
Patient Sample
This study was approved by the institutional review board. A retrospective chart review was performed to identify all consecutive adult patients (>18 years old) who underwent a cervical spine CT by one of six spine surgeons (May 2015 – December 2019) for evaluation of cervical-spine related complaints. Patients with outside cervical spine CTs, previous cervical spine instrumentation, pathophysiologic cervical spine conditions (eg, Klippel-Feil), osteoporosis (defined as a medical history of osteoporosis or current or past treatment with osteoporosis medications [excluding supplements]) were excluded.
Data Collection
Demographics and medical history were retrieved from the electronic medical record. All components of the Charlson Comorbidity Index (CCI) were collected from the medical record and used to calculate the CCI for each patient as a composite measure of their chronic health status. 10 The American Society of Anesthesiologists Class was collected for each patient as an additional composite measure of pre-anesthesia medical co-morbidities. 11
Each patient underwent presurgical AP and lateral cervical spine radiographs in neutral alignment position, from which the C2-C7 sagittal cobb angle, the T1 slope [T1S], and the C2-C7 sagittal vertical axis [cSVA] were measured by an independent research technician using a dedicated measurement software (Surgimap, Nemaris Inc, New York, NY). 12
CT scans were performed using a 16-MDCT scanner (MX8000 Philips Healthcare, Andover, MA). For the assessment of bone mineral density, we modified a previously published protocol by Schreiber et al.1,6 As described by this protocol, HU scores were obtained on circular areas of medullary bone that excluded cortical bone or areas of sclerosis on three thin-cut (1.25 mm) axial CT slices. The protocol was developed in the lumbar spine and consisted of measurements on three axial CT slices (inferior, mid, and superior). Given that the vertebral bodies in the cervical spine are smaller and shorter, we modified this protocol by obtaining HU scores from axial, coronal, and sagittal multiplanar reconstructions. 3 Furthermore, the assessment of trabeculae microstructure over three axes gives a more accurate picture of the overall bone density.13-15
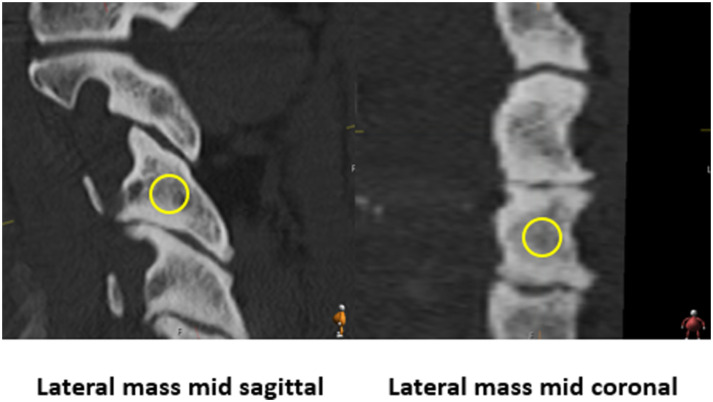
Hounsfield units were measured in 5 regions of each vertebral body (C2-T1; mid-axial, anterior-axial, posterior-axial, mid-coronal, and mid-sagittal). Mid-axial was defined as the CT slice at the region halfway between the inferior and superior endplates. Mid-coronal was defined as the CT slice halfway between the anterior and posterior aspects of the vertebral body, and mid-sagittal was defined as the slice halfway between the lateral aspects of the vertebral body (Figure 1). Similarly, the lateral masses (left and right, C3-C6) were measured at two regions: mid-coronal and mid-sagittal. The mid-coronal region was defined using the CT slice halfway between the anterior and posterior aspect of the lateral mass, along an anterior-posterior axis in line with the inferior and superior facet joints. The mid-sagittal region was defined as the slice halfway between the medial and lateral aspects of the lateral mass (Figure 2). Notably, mid-axial LM measurements were excluded as the relative thickness of the medial/lateral and anterior/posterior cortical walls of the LM made it near-impossible to measure the HU values at these regions without including cortical or sclerotic bone.
Figure 1.
Measurement regions for vertebral bodies.
Figure 2.
Measurement regions for the lateral masses.
The intraobserver reliability for HU measurements using the described technique has already been well established.1,6 Measurements were performed by six members of the research team (orthopaedic residents and spine surgery fellows). Six persons were tasked with performing measurements as we aimed to find an HU method with high interrater reliability, especially with regards to the lateral masses. Thus, before all CTs were assessed, the first five patients were independently measured by the six observers, consisting 355 independently measured HU regions (71 regions per patient, 5 patients). After reliability was established, the rest of the CT scans were assessed.
Statistical Analysis
Given that a major purpose of this study was to determine level-specific average HU scores in the cervical spine, we chose to eliminate outliers. For each set of measurements, the top and bottom five values were excluded and analyses were performed. The 3 measured regions in each VB and the 2 measured regions in each LM were combined and averaged to obtain the “Total VB” and “Total LM” HU values, respectively (ie, Total C2, Total LM C5, etc.). These means were utilized for correlation and comparison analyses. One-way ANOVA was used to compare HU measurements between vertebral levels and to compare HU means across age groups. Post-hoc pairwise comparisons were also performed between HU values between vertebral levels, with Bonferroni corrections applied. Correlations of HU values with age, body mass index, radiographic alignment, and medical comorbidities were assessed using Spearman’s coefficients. Inter-observer reliability for the measurement technique was assessed using two-way random-effects models to calculate intraclass correlation coefficients (ICCs). A value of P < .05 was considered significant. Portions of the data were managed using REDCap (Research Electronic Data Capture) hosted by [BLINDED] Medicine Clinical and Translational Science Center under the following grant: [BLINDED]. 16 Statistical analyses were performed using IBM SPSS Version 25.0 (Armonk, NY).
Results
Patient Sample
201 patients were included in the final analysis (Figure 3 & Table 1). The sample had a mean C2-C7 sagittal cobb angle of −4.88±14.2 (negative values correspond to lordosis), cSVA 26.2±15.6 mm, and mean T1S of 28.2±10.5 (positive values correspond to forward tilt).
Figure 3.
Patient selection diagram.
Table 1.
Description of patient characteristics. BMI, body mass index; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease (Stage 3 or greater); CCI, Charlson Comorbidity Index.
| Demographics and Comorbidities | ||
|---|---|---|
| n=201 | Mean/N | Sd/% |
| Age (years) | 56.7 | 12.3 |
| BMI (kg/m 2 ) | 28.6 | 5.6 |
| Gender | ||
| Male | 129 | 63.5% |
| Female | 74 | 36.5% |
| Race | ||
| white | 183 | 90.1% |
| Black | 4 | 2.0% |
| Asian | 3 | 1.5% |
| Other | 13 | 6.4% |
| Visit diagnosis | ||
| Myelopathy | 77 | 37.9% |
| Radiculopathy | 93 | 45.8% |
| Facet arthropathy | 94 | 46.3% |
| Other | 6 | 3.0% |
| ASA class | ||
| I | 15 | 7.5% |
| II | 163 | 81.1% |
| III | 23 | 11.4% |
| Comorbidities | ||
| Diabetes | 19 | 9.5% |
| Current smoker | 18 | 9.0% |
| Rheumatologic disease | 7 | 3.5% |
| Copd | 18 | 9.0% |
| Ckd | 3 | 1.5% |
| CCI | 1.9 | 1.7 |
Reliability of Measurement Method
Measurement of vertebral bodies was performed with moderate to excellent inter-relater reliability. 17 The intraclass correlation coefficients for the three VB regions as follows: .82 mid-ax (good), .88 mid-cor (excellent), .88 mid-sag (excellent). In contrast, the reliability of the lateral mass measurements was fair to good: .46 mid-sag (poor), .61 mid-cor (moderate).
Bone Density Trends in the Cervical Spine
Mean bone density was different at each level (P < .001, Figure 4). While HU values were relatively close from C2-C5 (ranging between 370.2 [C2]to 389.0 [C4]), density gradually decreased through T1, which had the minimum average HU (232.3). Post-hoc comparisons showed that C2 VB was significantly different (P < .05 with Bonferroni correction applied) compared to C6-T1 and all LMs, C3 was significantly different compared to C6-T1 and LM3-5, C4 significantly differently compared to C6-T1 and all LMs, C5 significantly differently compared to C6-T1 and LM3-5. The lower VBs (C6-T1) were significantly different compared to all VBs and LMs.
Figure 4.
Total HU scores of vertebral bodies. HU scores are significantly different, with a maximum score at C4 and minimum score at T1 (P<.001). Post-hoc comparisons showed that C2 VB was significantly different (P<.05 with Bonferroni correction applied) compared to C6-T1 and all LMs, C3 was significantly different compared to C6-T1 and LM3-5, C4 significantly differently compared to C6-T1 and all LMs, C5 significantly differently compared to C6-T1 and LM3-5. The lower VBs (C6-T1) were significantly different compared to all VBs and LMs. LM3 was significantly different compared to LM4 and LM6, LM4 significant different compared to all LMs, LM5 significantly different compared to LM4, and LM6 was significantly differently compared to LM3 and 4. Standard deviations represented by error bars.
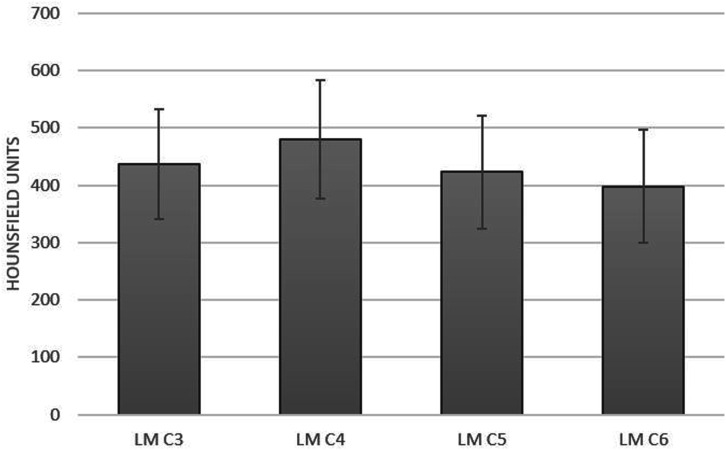
Regarding the lateral masses, the mean HU differed by level but fell within a narrow range (480.0-383.3) (Figure 5). On one-way ANOVA, the lateral masses had significantly higher HU scores compared to the vertebral bodies (P < .001). Post-hoc comparisons showed that LM3 was significantly different compared to LM4 and LM6, LM4 significant different compared to all LMs, LM5 significantly different compared to LM4, and LM6 was significantly differently compared to LM3 and 4
Figure 5.
Total HU scores of lateral masses. The HU scores of the lateral masses were higher than those of the vertebral bodies (P<.001).
Relationship Between Clinical Factors and Bone Density
Significant negative correlations were found between VB HU, age, and CCI (P < .05) (Table 2). The largest negative correlations between age and VB HU were found in the uppermost and lowermost portions of the cervical spine. A similar correlation pattern was found between VB HU and CCI (Table 2). When analyzed categorically, a significant decrease in mean bone density was seen with age for all vertebral bodies, with the exception of C4 (P < .05 for all other comparisons). Increasing ASA class was also associated with a significant decrease in bone density with the exception of T1 (P < .05 for all other comparisons).
Table 2.
Correlation coefficients between HU scores, demographics, CCI, and alignment. Bolded values indicate significance (P<.05).
| Spearman Coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | T1 Slope | C2-7 SVA | C2-C7 Cobb | Age | BMI | CCI | |
| TotalC2 | 368.0 | 72.0 | −.07 | −.09 | .15 | −.28 | .10 | −.28 |
| TotalC3 | 372.6 | 64.4 | −.09 | −.01 | .16 | −.26 | .05 | −.19 |
| TotalC4 | 383.6 | 71.9 | −.11 | −.03 | .17 | −.20 | −.03 | −.17 |
| TotalC5 | 371.3 | 85.1 | −.10 | −.03 | .24 | −.22 | −.07 | −.19 |
| TotalC6 | 329.0 | 82.0 | −.09 | −.06 | .23 | −.24 | −.15 | −.19 |
| TotalC7 | 277.1 | 56.7 | −.13 | −.07 | .25 | −.30 | −.13 | −.28 |
| TotalT1 | 232.3 | 46.5 | −.12 | −.09 | .10 | −.35 | −.05 | −.28 |
| LatMassC3 | 437.9 | 95.8 | −.04 | .09 | .14 | .02 | .10 | .06 |
| LatMassC4 | 480.0 | 103.2 | −.05 | .001 | .11 | −.02 | .01 | −.05 |
| LatMassC5 | 423.1 | 98.8 | −.05 | −.038 | .08 | −.07 | −.01 | −.04 |
| LatMassC6 | 398.3 | 98.2 | −.10 | −.15 | −.02 | −.13 | −.05 | −.11 |
Bone density in the lateral masses (at any level) was not associated with age, BMI, CCI or ASA class on correlation or categorical analyses.
Relationship Between Alignment and Bone Density
A more kyphotic cervical spine was weakly correlated with higher bone density in the mid-cervical spine (Table 2). ] T1S and cSVA were not correlated with bone density. Alignment was not correlated with HU scores in the lateral masses (Table 2).
Discussion
Our retrospective review of 201 CT scans in patients with symptomatic cervical spine pathology found that vertebral body HU score varies by level, with the lowest scores in the inferior cervical spine. Age and comorbidity burden were inversely correlated with the density of the vertebral bodies, especially in the upper and lower cervical spine. Regarding alignment, we found that a more kyphotic cervical spine was associated with higher HU scores, with the strongest correlations in the mid-lower cervical spine. In contrast, HU scores in the lateral masses were not correlated with any factor, including alignment. Finally, we showed that the density of the lateral masses is higher than the that of the vertebral bodies, however, the lateral mass analyses must be interpreted cautiously in the context of poor inter-observer reliability.
We used a novel technique in our measurement of HU scores in cervical vertebral bodies, which was found to have similar inter-observer reliability compared to traditional measurement techniques. 4 While the majority of HU investigations have exclusively used axial cuts,1,2,6,9 we incorporated sagittal, coronal, and regional axial HU scores into our protocol. Given that bony trabeculae form a complex, three-dimensional network, we believe that a three-dimensional assessment gives the most accurate picture of local bone quality.13-15 Previous authors have attempted similar analyses in the lumbar spine, finding that at certain levels, sagittal HU scores were more strongly correlated to DEXA than axial scores (though the differences in correlation were minimal). 3 For the purposes of measuring HU scores, whether one CT reformat is superior to another remains to be seen.
Our HU values are similar to previous investigations of HU scores in the cervical spine.2,7 Wang et al 7 investigated a smaller cohort (91 patients) who underwent one-level ACDF, reporting mean C3-C7 HU values remarkably similar to those reported in our study (C2 and T1 were not measured). Colantonio and colleagues examined 149 cervical spine CTs in the United States Department of Defense database, finding a mean C4 axial HU value of 452±116 in healthy subjects, compared to 320±82 in those with osteoporosis (as diagnosed by DEXA). Notably, they performed their analyses in all patients who had a previous cervical CT scan, not necessarily patients presenting with cervical spine pathology. Thus, we believe that our investigation currently represents the best possible “reference values” for HU in patients with degenerative cervical spine pathology. However, there is a caveat to this statement—given that the primary purpose of our study was to establish average level-specific HU values in the cervical spine, we chose to eliminate the top 5 and bottom 5 measures of bone density. While this may have made our average HU values more accurate, it also increased our inter-observer reliability. To this end, we caution readers that very high or low HU scores (ie several standard deviations lower or higher than the average values we report) may not be reliable. 1
A similar statement can be made about using HU scoring for the lateral masses. Ours is the first study to attempt HU scoring of the lateral masses. Anecdotally, we found these measurements to be quite difficult given that the high ratio of cortical to medullary bone in the lateral masses. The difficulty in assessing lateral mass bone density was evident in the poor to moderate inter-observer reliability of our measurements. Thus, we do not believe HU scores should be used to evaluate the density of the lateral masses. However, there is still a clinical need to evaluate local bone quality in the lateral masses (eg to prevent instrumentation failure). 18 This may be especially relevant for long posterior fusion constructs extending into the cervical spine. Future avenues of research could analyze new modes of assessing bone quality, such as cortical thickness or cortical to medullary ratios. The bone quality of the cervical pedicle may also be an area of research to allow for comparisons of cervical posterior fixation methods.
In our study, we found that the least dense bone was found in the lower cervical spine. Notably, the relationship of HU scores with mechanical outcomes is a well-researched topic, with studies showing correlations between HU scores and cage subsidence in ACDF and lateral lumbar fusions,7,9 loss of pedicle screw fixation,18,19 proximal junctional kyphosis,6,20 and lumbar pseudarthrosis. 8 Thus, the fact that HU scores vary by level has several potential clinical consequences. First, anterior constructs ending in the lower cervical spine (the majority of 3- and 4- level ACDFs) are seated in the least dense region of bone. This may help to explain the higher rate of pseudarthrosis in multilevel constructs,21,22 and especially why multilevel fusions in the lower cervical spine demonstrate a larger loss of lordosis than those in the mid-upper cervical spine. 23 Second, while age and a higher comorbidity burden were correlated with decreased bone density at nearly all cervical levels, the fact that density varied shows that a level-specific (and not necessarily patient-specific) approach should be taken when determining the risk for mechanical complications.7,24 For example, the C6-T1 region in a healthy 50 year-old may have similar HU scores as the C3-C5 region in a sick 70 year old, which may in turn influence surgical strategy or the risk-benefit discussion. We recommend that surgeons evaluate the bone density in the surgical regional of interest when planning fusions of the cervical spine.
These considerations become even more complex when the effect of alignment is taken into account. Wolff’s law states that bone adapts to stress, organizing its trabeculae to support load. In this sense, the fact that posterior-axial HU scores were higher than anterior-axial HU scores in our population (who had an average lordotic alignment of 4°) should not be surprising. Wolff’s law could also explain why patients with kyphosis began to show a reversal of this relationship, with similar HU values in the anterior-axial and posterior-axial regions of C5 and C6. Kyphotic cervical spines were also associated with higher HU scores overall—correlations strongest in the C5-C7 regions, where degenerative disc disease is most likely to occur. 25 This finding only strengthens our recommendation that surgeons consider the HU scores in the surgical region of interest, and avoid assuming that bone density throughout the spine is the same.
This study has several limitations. First, we did not have gold-standard measures of bone density (DEXA or quantitative CT scans) available for all patients, which precludes our ability to relate cervical spine HU scores with these metrics. However, several lumbar spine studies have shown that DEXA may overestimate the bone density at the surgical region of interest. 4 Thus, we do not believe that the inclusion of DEXA scores in our analysis would affect our conclusions. Second, while we excluded patients with a clinical history of osteoporosis or history of taking osteoporosis medications, we could not account for patients with undiagnosed osteoporosis. Regardless, our patient population is a real-world example of patients presenting for evaluation by a spine surgeon, many of who will present without a diagnosis of osteoporosis. Third, while the purpose of this study was not to evaluate risk factors for decreased bone density in the cervical spine, the small number of smokers, patients with rheumatologic disease, and non-white subjects precluded meaningful analysis of these risk factors. Thus, our conclusions may not apply to patients dissimilar to our cohort. Fourth, as stated above, given that we eliminated outliers in order to obtain the most accurate HU values, we caution readers that very high or low HU scores (ie several standard deviations lower or higher than the average values we report) may not be reliable. Fifth, our findings only apply to patients with degenerative spine pathology presenting for cervical-spine related complaints, we cannot comment on HU values or trends in patients with healthy cervical spines. Furthermore, we did not include the “degree of degeneration” in the cervical spine. While we did note that kyphosis was associated with an increase in HU, this is an imperfect surrogate for disc degeneration. Further study on the specific relationship between HU values and disc degeneration is merited.
In conclusion, our investigation of 201 CT scans in patients with cervical pathology demonstrates that cervical HU scores vary by vertebral level. The lowest density was found in the cervical spine, which may partially explain the high rate of mechanical complications in multilevel anterior fusions. Further investigation on preventing these complications should incorporate HU scoring. Age, comorbidities, and alignment were all found to influence HU scores of the vertebral bodies, which demonstrates that a level-specific approach to bone density should be considered when evaluating a patient for surgery. Future studies will be needed to determine if such an approach will influence clinical and mechanical outcomes. Finally, we encourage continued research on methods to measure regional bone density in the lateral masses, as HU scoring may not be appropriate.
Acknowledgments
We would like to thank Dr Jingyan Yang for her assistance in the statistical methodology in preparing this manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval Statement: This study was approved by the Institutional Review Board of the Hospital for Special Surgery (#2020-0945). Given the study design, the board deemed the study exempt from the requirement of informed consent.
ORCID iDs
Francis Lovecchio, MD https://orcid.org/0000-0001-5236-1420
Bryan Ang https://orcid.org/0000-0002-4174-997X
Philip K. Louie https://orcid.org/0000-0002-4787-1538
Virginie Lafage, PhD https://orcid.org/0000-0002-0119-7111
References
- 1.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J Bone Jt Surg - Ser A. 2011;93:1057-1063. [DOI] [PubMed] [Google Scholar]
- 2.Colantonio DF, Saxena SK, Vanier A, Rodkey D, Tintle S, Wagner SC. Cervical spine computed tomography hounsfield units accurately predict low bone mineral density of the femoral neck. Clin Spine Surg. 2020;33:E58-E62. [DOI] [PubMed] [Google Scholar]
- 3.Elarjani T, Warner T, Nguyen K, Nguyen S, Urakov TM. Quantifying bone quality using computed tomography hounsfield units in the mid-sagittal view of the lumbar spine. World Neurosurg. 2021;151:e418-e425. [DOI] [PubMed] [Google Scholar]
- 4.Zaidi Q, Danisa OA, Cheng W. Measurement techniques and utility of hounsfield unit values for assessment of bone quality prior to spinal instrumentation: a review of current literature. Spine (Phila Pa. 2019;44:E239-E244. [DOI] [PubMed] [Google Scholar]
- 5.Pappou I, Girardi FP, Sandhu H, et al. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa. 2006;31:1614-1620. [DOI] [PubMed] [Google Scholar]
- 6.Yao YC, Elysee J, Lafage R, et al. Preoperative hounsfield units at the planned upper instrumented vertebrae may predict proximal junctional kyphosis in adult spinal deformity. Spine (Phila Pa. 2021;46:E174-E180. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Mummaneni PV, Xi Z, et al. Lower hounsfield units on ct are associated with cage subsidence after anterior cervical discectomy and fusion. J Neurosurg Spine. 2020;33:425-432. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber JJ, Hughes AP, Taher F, Girardi FP. An Association Can Be Found Between Hounsfield Units and Success of Lumbar Spine Fusion. HSS J. 2014;10:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xi Z, Mummaneni PV, Wang M, et al. The Association between Lower Hounsfield Units on Computed Tomography and Cage Subsidence after Lateral Lumbar Interbody Fusion. Epub ahead of print 2020. doi: 10.3171/2020.5.FOCUS20169 [DOI] [PubMed]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373-383. [DOI] [PubMed] [Google Scholar]
- 11.Di Capua J, Somani S, Kim JS, et al. Analysis of Risk Factors for Major Complications Following Elective Posterior Lumbar Fusion; 1976. 2017:1.Spine (Phila Pa [DOI] [PubMed] [Google Scholar]
- 12.Champain S, Benchikh K, Nogier a, Mazel C, Guise JD, Skalli W. Validation of new clinical quantitative analysis software applicable in spine orthopaedic studies. Eur Spine J. 2006;15:982-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaron JE, Shore PA, Shore RC, Beneton M, Kanis J. Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: II. three-dimensional histology. Bone. 2000;27:277-282. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Graffy PM, Zea RD, et al. Future Osteoporotic Fracture Risk Related to Lumbar Vertebral Trabecular Attenuation Measured at Routine Body CT. Epub ahead of print 2018. doi: 10.1002/jbmr.3383 [DOI] [PMC free article] [PubMed]
- 15.Kim HJ, Dash A, Cunningham M, et al. Patients with abnormal microarchitecture have an increased risk of early complications after spinal fusion surgery. Bone. 2021;143:115731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu F, Zou D, Li W, et al. Hounsfield Units of the Vertebral Body and Pedicle as Predictors of Pedicle Screw Loosening after Degenerative Lumbar Spine Surgery. doi: 10.3171/2020.5.FOCUS20249 [DOI] [PubMed]
- 19.Zou D, Muheremu A, Sun Z, Zhong W, Jiang S, Li W. Computed tomography Hounsfield unit–based prediction of pedicle screw loosening after surgery for degenerative lumbar spine disease. J Neurosurg Spine. 2020;32:716-721. [DOI] [PubMed] [Google Scholar]
- 20.Duan P-G, Mummaneni PV, Rivera J, et al. The association between lower Hounsfield units of the upper instrumented vertebra and proximal junctional kyphosis in adult spinal deformity surgery with a minimum 2-year follow-up. doi: 10.3171/2020.5.FOCUS20192 [DOI] [PubMed]
- 21.Laratta JL, Reddy HP, Bratcher KR, McGraw KE, Carreon LY, Owens II RK. Outcomes and revision rates following multilevel anterior cervical discectomy and fusion. J Spine Surg. 2018;4:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wewel JT, Kasliwal MK, Adogwa O, Deutsch H, O'Toole JE, Traynelis VC. Fusion rate following three- and four-level ACDF using allograft and segmental instrumentation: A radiographic study. J Clin Neurosci. 2019;62:142-146. [DOI] [PubMed] [Google Scholar]
- 23.Louie PK, Sexton AC, Bohl DD, et al. Original Article Corresponding Author Rigid-Plating and Cortico-Cancellous Allograft Are Effective for 3-Level Anterior Cervical Discectomy and Fusion: Radiographic and Clinical Outcomes. doi: 10.14245/ns.1836052.026 [DOI] [PMC free article] [PubMed]
- 24.Wang H, Sun Z, Wang L, et al. Proximal fusion level above first coronal reverse vertebrae: an essential factor decreasing the risk of adjacent segment degeneration in degenerative lumbar scoliosis. Global Spine J. Epub ahead of print 2021. doi: 10.1177/2192568221994082 [DOI] [PMC free article] [PubMed]
- 25.Tao Y, Galbusera F, Niemeyer F, Samartzis D, Vogele D, Wilke HJ. Radiographic cervical spine degenerative findings: a study on a large population from age 18 to 97 years. Eur Spine J. 2021;30:431-443. [DOI] [PubMed] [Google Scholar]