Abstract
The methods section of a manuscript is one of the most important parts of a research paper because it provides information on the validity of the study and credibility of the results. Inadequate description of the methods has been reported as one of the main reasons for manuscript rejection. The methods section must include sufficient detail so that others could repeat the study and reproduce the results. The structure of the methods section should flow logically and chronologically. There are multiple components of methods sections, including study design, materials used, study procedures, and data analysis. Each element must be adequately described and thoroughly detailed to provide an understanding of how the results were obtained and how to interpret the findings. Studies that involved humans or animals must include an ethics statement of approval from the appropriate governing body. The methods section should explain how subjects were identified and should state inclusion and exclusion criteria. All materials used to complete the study should be described in detail, including equipment, drugs, gases, chemicals, treatments, interventions, or other items. Study procedures should outline all steps taken to obtain the results and clearly state the outcome measures. Subheadings might be helpful for organizing the methods section into subsections when there is a considerable amount of information to report. A well-written methods section will guide the reader through the research process and provide adequate information to evaluate study validity and reproduce the work. The purpose of this paper is to provide guidance for writing the methods section of a manuscript.
Keywords: manuscript, research paper, science writing, publication, research, study protocol, methods
Introduction
Dissemination of research findings occurs through abstracts, posters, presentations, and manuscripts.1-3 Writing the manuscript is considered the last step of the research process because it provides a detailed account of the research from start to finish.4,5 The main components of a research paper include an abstract, the introduction, methods, results, discussion, and conclusions.3,4 Each section of the manuscript is important and has a specific role in describing the research story. However, the methods are one of the most critical sections of a manuscript because the details are used to evaluate and determine the validity of the study and credibility of the results.6 Validity in research refers to reliability of the measured results: the extent to which the study accurately measured what it intended (internal validity) and how the results can be applied to the general population beyond the study (external validity).6,7
The methods section describes what was done to answer the research question.8 This section specifies how the research was done, the rationale for the procedures, what materials were used, and how the results were analyzed, all in a clear, concise, and organized manner.6 The description of the research should provide enough detail so others could repeat the study and reproduce the results.6,9,10 Much of the methods section should be written before the study is initiated. Indeed, for funded research, a detailed methods section is written as part of the grant application. There are several aspects of the methods sections, and the essential elements will vary, depending on the type of study. Submission requirements differ among journals; therefore, it is important to consult the instructions for authors for the specific journal to ensure that all necessary elements are included.11 The purpose of this paper is to describe the different components of the methods section and provide guidance for writing the methods section of a research paper.
General Considerations
An inadequate description of methods has been identified as one of the top reasons for manuscript rejection.12 It has been suggested that including too much information is better than having insufficient detail because irrelevant content can later be omitted.12 The methods section of a research paper is analogous to a recipe.10,13 A recipe is composed of multiple elements, including the list and quantity of ingredients, equipment and tools needed and applicable settings, and the detailed instructions for how to create the recipe. Similar to a recipe, there are different elements of methods to describe in a manuscript. In general, common components of the methods section include a description of the study design, materials used, study procedures, measurements or calculations, and the statistical tests used to analyze the results. Materials used to conduct research are comparable with the ingredients, tools, and equipment for a recipe. Materials represent what was studied, including subjects, equipment or devices, and treatments or interventions.6,14 The steps to create a recipe are akin to study procedures such as the process for data collection, measurements, calculations, and statistical analysis. A summary of the different elements of the methods section is included in Table 1. The individual components for each element may vary, depending on the nature of the study.
Table 1.
Methods Section Elements

Although similarities exist between a recipe and the methods section of a research paper, the methods section should not be formatted to read like a recipe.13 Use past tense for writing the methods section because the study has been completed and describes what was already done.6,9,10,13,14 The methods section should be structured for logical and chronological flow.6,14,15 Use of subheadings can be helpful for organizing the different components for the methods section when there is a substantial amount of detail to describe.6,13 However, subheadings may not be required by some journals. An excessive use of subheadings can be distracting to the reader by interrupting the flow of the manuscript. There should not be a subheading for every paragraph. This is particularly distracting when each subheading is followed by a short 1- or 2-sentence paragraph. Paragraphs with fewer than 3 sentences should be avoided; combine the information with another paragraph unless the journal to which the paper will be submitted requires specific subheadings. Subheadings can be useful as an outline when writing the methods section but then might be omitted in the final manuscript.
A common error in manuscript writing is reporting results in the methods section and vice versa. A frequently occurring example is including the number of subjects who participated in the research in the methods section when it was unknown how many met inclusion criteria before study initiation and subject screening. The methods section should only include information available during the planning phase, before study initiation.10,16 There are instances in which study procedures may have changed after the study commencement. This information would be reported in the methods section but the outcomes stated in the results section. The results section should reflect the data obtained from study procedures because this information would be unknown before the study was completed.
Study Design
The methods section often begins with an overall description of the study design and key attributes, including the type of study, setting, time frame, and procedures.14,15 This provides an overview and context for how the study was conducted with further details and specifics described in subsequent subsections. Study design has been described as a road map for the methods section to provide information for how to understand the approach and interpret the results.14
Common study designs include observational, bench evaluation, systematic review, randomized controlled trial, survey, and others. Guidelines for writing the manuscript include the Consolidated Standards of Reporting Trials (CONSORT) checklist for randomized controlled trials and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews.17,18 Registration is another consideration for clinical trials and systematic reviews. The International Committee of Medical Journal Editors (ICMJE)16 requires registration of clinical trials on a public trials registry. Many journals, including Respiratory Care, follow the recommendations for publication set forth by this group. A randomized controlled trial should also include the blinding mechanism and different treatment groups as applicable.17 Although registration of a systematic review is often not a prerequisite for publication, registering the protocol supports transparency, decreases potential bias, and can help prevent duplication of reviews.18 An observational study should report if the design was retrospective, prospective, a secondary or post hoc analysis, or other category of observational design.7
The setting where the study occurred, if it included data from a single-center or multiple centers, and the time frame in which it took place must be included because these factors have implications for clinical practice, generalizability, and validity.7 Potential study settings might include an ICU (or specific ICU type), medical surgical ward, emergency department, out-patient clinic, home-care environment, or simulation laboratory. The time frame is an essential element for context because practices and trends change over time. A prime example of this is prone positioning for treatment of hypoxemic respiratory failure as use substantially increased during the COVID-19 pandemic.19
Ethics Statement
The United States Department of Health and Human Services defines a human subject “as a living individual about whom an investigator (whether professional or student) conducting research obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the biospecimens; or obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”20 The methods section must include a statement regarding approval from an institutional review board (IRB) or ethics committee for research that included human subjects.16 Quality improvement studies and certain types of surveys are often not considered human subject research and therefore may not require IRB oversight but the decision is made by the IRB. Quality improvement projects, depending on requirements of the institution or organization, can be performed without IRB approval in some cases; however, IRB approval is needed before publication or presentation outside of the institution, and human subject determination is made by the IRB, not the investigators.
Animal studies also require ethics approval to be reported in the methods section. Research that involves animals is subject to approval from the local Institutional Animal Care and Use Committee and must be conducted in accordance with national guidelines, for example, the National Institute of Health Public Health Service Policy on Humane Care and Use of Laboratory Animals.21 For journals that do not have a specific requirement for where to include the ethics statement within the methods section, many authors typically incorporate it in the initial general description of the study or with the detailed description of the subjects. Some studies have included it at the beginning of the methods section.
Subjects
Characteristics of the study population should be described. This includes basic demographics (eg, adults or children, age, sex) and general health status such as if the individuals were healthy volunteers or had a specific diagnosis or condition. This information is also needed for control groups.4 Inclusion criteria for how subjects were identified and selected should be detailed as well as reasons for exclusion. For example, an evaluation of a disease management program included adults ages ≥ 65 years and with COPD who were admitted to 1 of 5 hospitals during a specified time frame.22 Patients were excluded if they left against medical advice, died during admission, transferred to a hospital outside of the health system, entered hospice care, refused home care, or were unable to participate in education.22 In this example, subject characteristics (adults with COPD), selection and identification (hospital admission during defined time frame), and exclusion criteria are clearly stated.
When referring to human subjects in research, the terms subject and patient are often used interchangeably, but there is a difference.23 A patient receives care to improve health, and care is individualized in each particular case. When a patient participates in research, he or she becomes a subject. In research, care is designed to create information and is the same for all subjects based on the study protocol. The individual conducting the research is not always involved in the patient care provided, thus also making the distinction between subject and patient. A common error is to use the word subjects exclusively when writing the manuscript. However, individuals are patients before enrollment. When referring to the broader population of individuals who might benefit from the research findings, the word patients is likely more correct. Participant and volunteer are other terms that can be used in place of subject. Individuals who participated in survey research are typically referred to as respondents.24
In addition to humans, research subjects may also involve animals or organisms such as cells. When animals are studied, the methods should describe the species, weight, age, and sex of the animals.6 Ring et al25 used ex vivo porcine lungs to evaluate the effect of breathing pattern and nebulization on exhaled viral content during mechanical ventilation. The authors reported that the lungs were sourced from a retail processing facility and were from 6-month-old Yorkshire hybrid pigs that weighed 118 kg. In addition, it was noted that approval to conduct the study was granted by the local Institutional Animal Care and Use Committee. This publication demonstrates an appropriate description of animal subjects, including an ethics statement.
Equipment and Other Materials
Identify all equipment and other materials used in the study, including devices, related accessories, drugs, or chemicals. At first mention of any device, provide the specific name of the item, model number if applicable, and manufacturer information. Many scientific journals do not usually allow use of trademark or registration symbols.10 The ICMJE recommends that manufacturer name and location be included in parentheses.16 For example, a study that evaluated the safety and feasibility of breathing high-dose nitric oxide in healthy volunteers used a Sievers 280i nitric oxide analyzer (GE Analytical Instruments, Boulder, Colorado) to measure nitric oxide gas concentration.26 Subsequent mentions of equipment should be noted by generic name versus trade name when possible. It is important that the methods section does not project any bias that an author may have for a specific device or manufacturer.
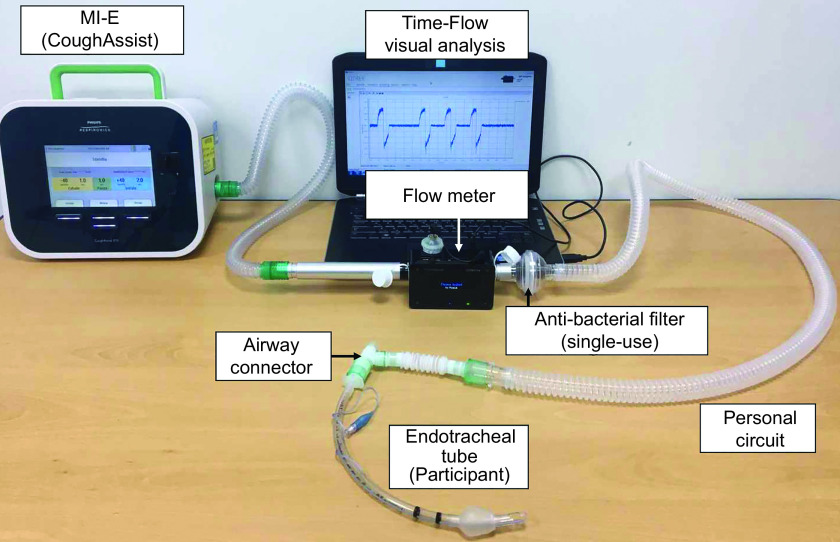
Use of figures can be an effective means of providing a visual description of the equipment setup, especially when there are many components involved. This can also help reduce the amount of text and improve understanding of how the equipment was assembled. Figures can be either a photograph of the equipment or a graphic illustration (line drawing), but all components should be clearly labeled. An illustration of the setup used to deliver high-dose nitric oxide in the aforementioned study is provided in Figure 1.26 Use of a photograph to depict the experimental setup for measuring peak expiratory flow during mechanical insufflation-exsufflation is demonstrated in Figure 2.27 Photographs should be of good quality and include all relevant items. In both examples, all components are clearly identified and labeled.
Fig. 1.
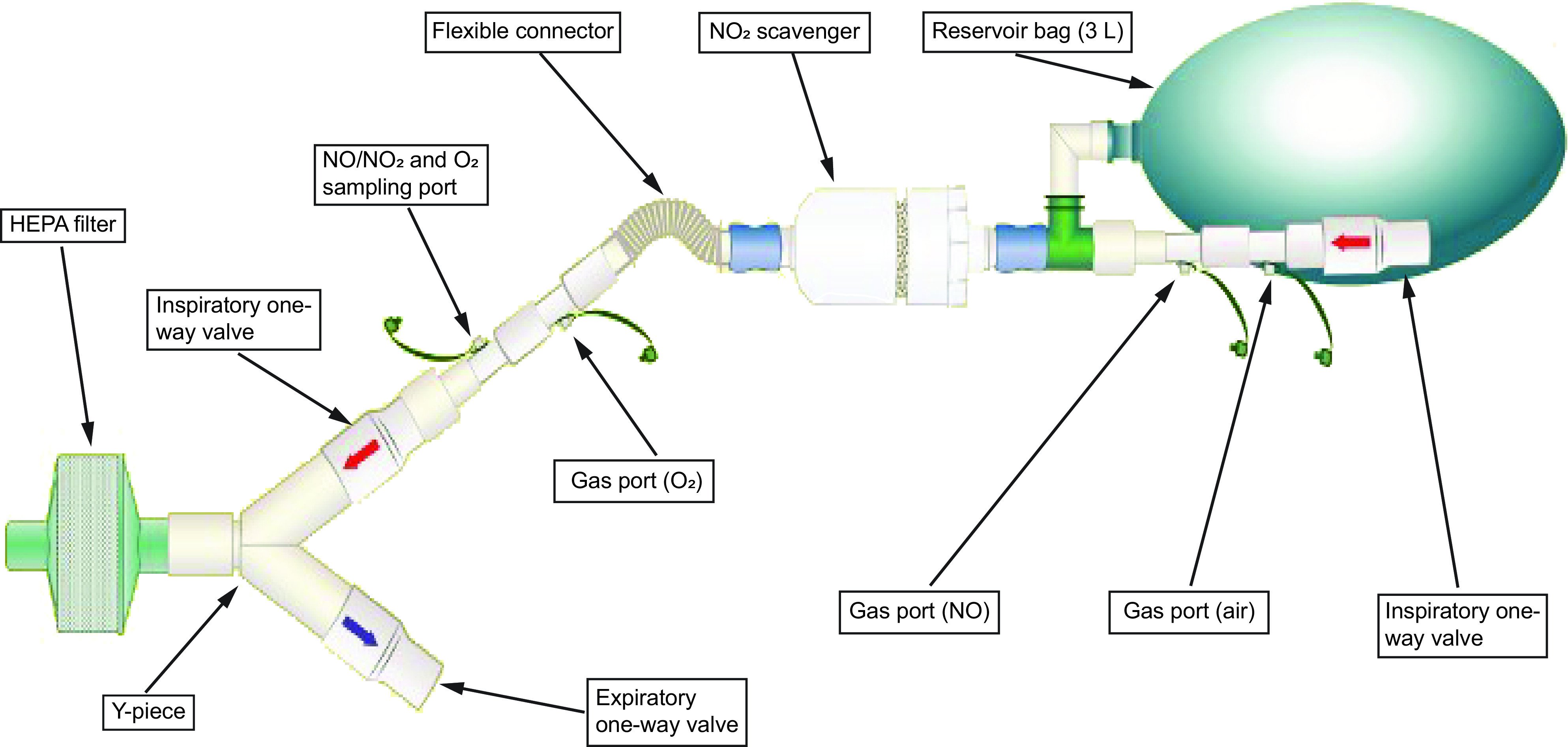
Graphic illustration of an experimental setup. From Reference 26.
Fig. 2.
Photograph of an experimental setup. From Reference 27.
In addition to naming the specific equipment used in the study, settings should also be included in the methods section because these details are highly relevant for duplication of the study. For example, the evaluation of peak expiratory flow during mechanical insufflation-exsufflation provided the pressure settings used during therapy.27 Not only is this important for repeating the study, but it is also essential for assessing the validity of the results. If the settings were not typical of those used in the study population or in clinical practice, then this would introduce limitations to interpreting and understanding the results.
Equipment preparation is another consideration for the methods section. Describe the calibration process and the frequency for equipment that requires calibration. The flow meter used to measure peak expiratory flow during mechanical insufflation-exsufflation was calibrated and validated annually by the manufacturer.27 If manufacturer standards for calibration are not followed, then the accuracy of the results may be affected. It should be noted that calibration and validation represent two different processes.4 Both should be described as applicable.
In addition to equipment, identify all drugs, chemicals, gases, or other materials used specifically for the study. The details for drugs and gases should include the concentration, dose, frequency, and route of administration. Gases should also note the flow used. Chemicals should be noted with the name and concentration as applicable. Use the generic name for drugs. If the trade name for a drug is relevant to the study, then follow the same process for identifying equipment brands and manufacturer information and use the generic name after initial identification. Preparation information may be needed in some cases. For example, detailed preparation information was provided for the bacteriophage used in the animal study conducted by Ring et al.25 The process for how the bacteriophage was prepared was described in detail as well as the amounts used for the study.
Study Procedures
The methods section should explicitly detail all procedures, treatments, or interventions used in the study. This portion of the methods section describes how study procedures were performed, the chronological order of procedures, measurements or calculations made, and the specific data elements collected. A rationale may be needed for some procedures, depending on the audience.6 Outcome measures are often included in the subsection for study procedures, but some authors report them in the overall description for study design.
A comprehensive explanation of the procedures is vital for providing adequate details for reproducibility and validity regardless of the study design. A retrospective cohort study investigated outcomes of children treated with continuous albuterol that contains benzalkonium chloride and preservative-free solutions.28 Collected data were clearly stated and included subject demographics, diagnosis, mortality risk score, albuterol dose and duration, use of adjunctive therapies, and respiratory support. The methods section for this paper also reported the source of the data extraction (electronic medical records, database, manual chart review) and the process for how therapies were initiated, escalated, and de-escalated (intensivist discretion).28 The basis for the use of therapies in this study is an important consideration for generalizability because practices vary among institutions and some care might involve the use of protocols.
Diagrams and flow charts can be helpful for illustrating processes or workflow. An evaluation of sputum volume obtained with different cough augmentation techniques outlined the protocol in an illustrated timeline for the sequence of interventions and data collection (Fig. 3).29 The timeline provides clear information for the procedures that were done, when they were done, and the data elements collected. Data were collected at baseline, at the end of the intervention, and then 1 h after the intervention, followed by a minimum 4-h washout period before the second intervention and data collection.29 Details with regard to who performed the interventions (5 experienced respiratory clinicians), how they were administered (cough augmentation technique and settings), and subject information (positioning) were comprehensively described.
Fig. 3.
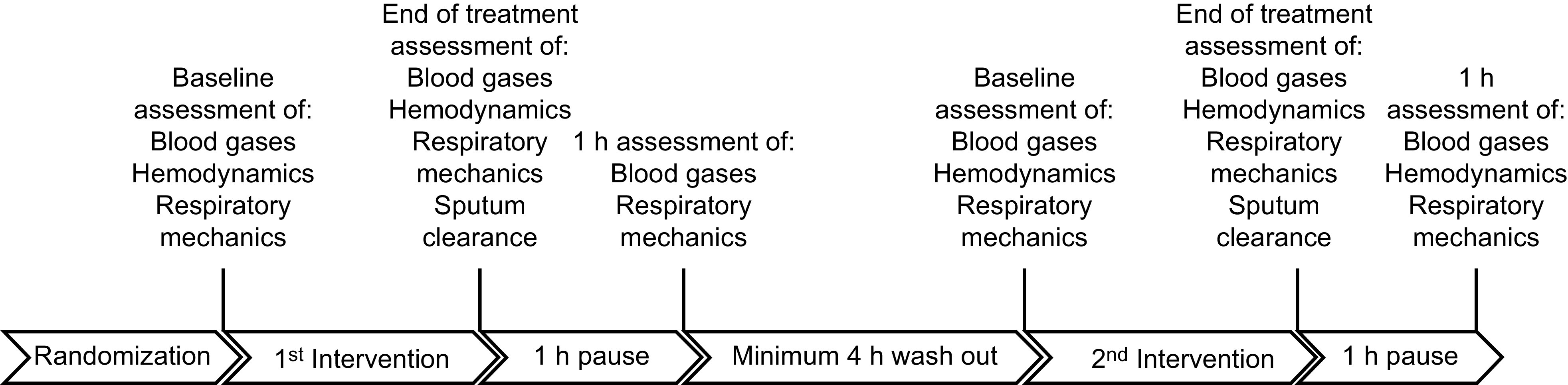
Illustrated timeline of study protocol that depicts chronological order. From Reference 29.
Measurements obtained during study procedures should be identified along with a description of how they were obtained and the devices used. For example, the same study measured ventilator parameters before, during, and after interventions by using a Fluxmed GrH monitor (MBMED, Buenos Aires, Argentina).29 Procedures for measurements or techniques with established references do not have to be described in detail and can be omitted if the procedure could be repeated without the specific details.6,12,14 This is a common practice for measurements obtained during spirometry. In those instances, provide the reference for the previous work without providing all of the additional details. In a study that aimed to correlate baseline spirometry with airway hyper-responsiveness in methacholine challenge, the reported testing was performed according to published guidelines.30 The guideline was referenced without providing all the specifics. On the contrary, studies that used novel methods would need to be further described.6
The outcome measures that address the research question should be clearly stated. Outcome measures are the dependent or response variables assessed to evaluate the impact of the research that is established before beginning the study.6,8 Outcome measures may include both primary and secondary outcomes. The primary outcome is the main measure of the research question, and secondary outcomes provide additional information for interpreting results. The retrospective evaluation of different albuterol solutions used ICU and hospital length of stay as primary outcomes and duration of continuous albuterol, use and duration of adjunctive therapies, and need for mechanical ventilation as secondary outcomes.28 The primary outcome was sputum volume for the trial that assessed cough augmentation techniques, and secondary outcomes were respiratory mechanics and hemodynamics.29
Statistical Analysis
The statistical analysis component is typically included as the last part of the methods section. This subsection describes how the collected data were analyzed through identification of the statistical tests that were used and the P value threshold for statistical significance. A clinical trial that evaluated the effect of endotracheal tube scraping during mechanical ventilation reported that categorical variables were analyzed with the chi-square or Fisher exact test, and continuous variables were presented as mean ± SD or median (interquartile range) based on distribution and analyzed with t test or Mann-Whitney test.31 P < .05 was considered significant. The statistical analysis section of this paper distinctly identified the tests used to analyze specific data points and provided an explanation for when mean or median was reported.
The statistical analysis should also describe how the power analysis was conducted to determine the appropriate sample size. Justification for the approach should be provided when needed. For example, the study that evaluated the effect of endotracheal tube scraping calculated sample size for each treatment group based on previous institutional data for the mean duration of mechanical ventilation and determined that each group needed 136 subjects with an alpha of 0.05 and power of 0.80.31 Citing references for the rationale and justification for the selected statistical tests is also an approach to support the choice of test. The previously noted evaluation of methacholine reactivity used a reference to support the use of partition analysis.30 The software package and version used for data analysis should also be specified in the data analysis portion of the methods section.16
A Methods Model
Several publications were used throughout this paper to demonstrate the different elements of the methods section of a research paper. A summary of each of those elements and the individual components comprised within each subsection adapted from the endotracheal tube scraping clinical trial are included in Table 2.31 It is important to note how some items were further described in the text, such as the technique for airway suctioning, the definition of a successful spontaneous breathing trial, an explanation for extubation outcome, the elements of the ventilator-associated event prevention bundle, and how ventilator-associated events were defined. These specifics provide additional information to help determine validity and generalizability, and highlight the importance of including enough detail to duplicate the study.
Table 2.
Summary of Methods Elements and Details from a Published Paper


Summary
The methods section is an important part of a manuscript because it provides information on the validity of the study. One of the main reasons for manuscript rejection is an inadequate description of the methods. Enough detail must be provided so others could repeat the study and reproduce the results, similar to following a recipe. The methods section should be structured for logical and chronological flow, and be written in past tense. There are multiple components of the methods section that must be adequately described and thoroughly detailed to provide an understanding of how the results were obtained to interpret the findings. Subheadings can be helpful for organizing the methods section into subsections when there is a considerable amount of information to report, but subheadings should be used judiciously. A well-written methods section will guide the reader through the research process and provide adequate information to evaluate study validity and credibility of the results as well as reproduce the work.
Footnotes
Ms Willis is a Section Editor for Respiratory Care.
Ms Willis presented a version of this paper at the symposium Research in Respiratory Care at AARC Congress 2022 held November 8, 2022, in New Orleans, Louisiana.
REFERENCES
- 1.Miller AG. How to write an abstract for presentation at a scientific meeting. Respir Care 2023;68(11):1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willis LD. How to present your research findings at a scientific meeting. Respir Care 2023;68(11):1598-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AG. Moving from abstract to manuscript. Respir Care 2023. [Epub ahead of print] doi: 10.4187/respcare.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branson RD. Anatomy of a research paper. Respir Care 2004;49(10):1222-1228. [PubMed] [Google Scholar]
- 5.Hess DR. Research and publication in respiratory care. Respir Care 2023;68(8):1171-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallet RH. How to write the methods section of a research paper. Respir Care 2004;49(10):1229-1232. [PubMed] [Google Scholar]
- 7.Hess DR. Observational studies. Respir Care 2023;68(11):1585-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis LD. Formulating the research question and framing the hypothesis. Respir Care 2023;68(8):1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenson JF, Foltz C, Lendner M, Vaccaro AR. How to write an effective materials and methods section for clinical studies. Clin Spine Surg 2019;32(5):208-209. [DOI] [PubMed] [Google Scholar]
- 10.Foote M. Materials and methods: a recipe for success. Chest 2008;133(1):291-293. [DOI] [PubMed] [Google Scholar]
- 11.Moore S. Submitting a manuscript to a scientific journal. Respir Care 2023;68(9):1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierson DJ. The top 10 reasons why manuscripts are not accepted for publication. Respir Care 2004;49(10):1246-1252. [PubMed] [Google Scholar]
- 13.Annesley TM. Who, what, when, where, how, and why: the ingredients in the recipe for a successful methods section. Clin Chem 2010;56(6):897-901. [DOI] [PubMed] [Google Scholar]
- 14.Ghasemi A, Bahadoran Z, Zadeh-Vakili A, Montazeri SA, Hosseinpanah F. The principles of biomedical scientific writing: materials and methods. Int J Endocrinol Metab 2019;17(1):e88155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azevedo LF, Canário-Almeida F, Almeida Fonseca J, Costa-Pereira A, Winck JC, Hespanhol V. How to write a scientific paper—writing the methods section. Rev Port Pneumol 2011;17(5):232-238. [DOI] [PubMed] [Google Scholar]
- 16.International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Updated May 2022. https://www.icmje.org/recommendations/. Accessed July 6, 2023 [PubMed]
- 17.Kaur R, Li J. How to conduct a randomized controlled trial. Respir Care 2023. [Epub ahead of print] doi: 10.4187/respcare.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaccagnini M, Li J. How to conduct a systematic review and meta-analysis: a guide for clinicians. Respir Care 2023;68(9):1295-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott JB, Weiss TT, Li J. COVID-19 lessons learned: prone positioning with and without invasive ventilation. Respir Care 2022;67(8):1011-1021. [DOI] [PubMed] [Google Scholar]
- 20.United States Department of Health and Human Services. 45 Code of Federal Regulations Part 46. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. Accessed July 6, 2023
- 21.National Institutes of Health Office of Laboratory and Animal Welfare. Public Health Service Policy on Humane Care and Use of Laboratory Animals. https://olawnihgov/policies-laws/phs-policyhtm 2015. Accessed July 6, 2023.
- 22.Truumees M, Kendra M, Tonzola D, Chiu S, Cerrone F, Zimmerman D, et al. The impact of a home respiratory therapist to reduce 30-day readmission rates for exacerbation of COPD. Respir Care 2022;67(6):631-637. [DOI] [PubMed] [Google Scholar]
- 23.Paal E. The difference between a “patient” and a “subject.” University of Arkansas for Medical Sciences Institutional Review Board. May 19, 2016. https://research.uams.edu/irb/irb-blog/irb-research-news/the-difference-between-a-patient-and-a-subject/. Accessed July 7, 2023.
- 24.Goodfellow LT. An overview of survey research. Respir Care 2023;68(9):1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ring BJ, Pestana K, Sombatsaphay V, Huet Y, Steck T. Impact of breathing pattern and nebulization on expelled viral content during mechanical ventilation using an ex vivo porcine lung system. Respir Care 2022;67(10):1217-1225. [DOI] [PubMed] [Google Scholar]
- 26.Gianni S, Fenza RD, Morais CCA, Fakhr BS, Mueller AL, Yu B, et al. High-dose nitric oxide from pressurized cylinders and nitric oxide produced by an electric generator from air. Respir Care 2022;67(2):201-208. [DOI] [PubMed] [Google Scholar]
- 27.Hyun SE, Lee S-M, Shin H-I. Peak expiratory flow during mechanical insufflation-exsufflation: endotracheal tube versus face mask. Respir Care 2021;66(12):1815-1823. [DOI] [PubMed] [Google Scholar]
- 28.Al-Subu AM, Friestrom E, Terry E, Langkamp MR, Adams CK, Yngsdal-Krenz RA, et al. Effectiveness and safety of albuterol solutions with and without benzalkonium chloride. Respir Care 2023;68(6):734-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Alejos R, Marti J-D, Li Bassi G, Gonzalez-Anton D, Pilar-Diaz X, Reginault T, et al. Effects of mechanical insufflation-exsufflation on sputum volume in mechanically ventilated critically ill subjects. Respir Care 2021;66(9):1371-1379. [DOI] [PubMed] [Google Scholar]
- 30.Hunninghake JC, McCullough SB, Aden JK, Hayes JA, McCann ET, Morris MJ. Baseline spirometry as a predictor of positive methacholine challenge testing for exertional dyspnea. Respir Care 2022;67(6):694-701. [DOI] [PubMed] [Google Scholar]
- 31.Kaur R, Scott JB, Weiss TT, Klein A, Charlton ME, Villanueva KA, et al. Evaluation of a closed suction system with integrated tube-scraping technology. Respir Care 2023;68(8):1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]