Abstract
Prostate cancer is the second most common cancer in men and affects 1 in 9 men in the United States. Early screening for prostate cancer often involves monitoring levels of prostate-specific antigen (PSA) and performing digital rectal exams. However, a prostate biopsy is always required for definitive cancer diagnosis. The Early Detection Research Network (EDRN) is a consortium within the National Cancer Institute aimed at improving screening approaches and early detection of cancers. As part of this effort, the Weill Cornell EDRN Prostate Cancer has collected and biobanked specimens from men undergoing a prostate biopsy between 2008 and 2017. In this report, we describe blood metabolomics measurements for a subset of this population. The dataset includes detailed clinical and prospective records for 580 patients who underwent prostate biopsy, 287 of which were subsequentially diagnosed with prostate cancer, combined with profiling of 1,482 metabolites from plasma samples collected at the time of biopsy. We expect this dataset to provide a valuable resource for scientists investigating prostate cancer metabolism.
Subject terms: Prostate cancer, Cancer
Background & Summary
Prostate Cancer (PCa) is the second most common malignancy (after lung cancer) in men, with more than 1.4 million new cases and more than 375,000 deaths worldwide in 20201. When detected early, PCa can usually be treated and managed without impacting the patient’s lifespan, and the 5-year survival rate for patients diagnosed with early-stage, localized PCa is virtually 100%2. However, when detected after the appearance of symptoms, PCa is typically at a more advanced stage, often after the development of metastases. For this group of patients, the 5-year survival rate drops to 32%3. Therefore, early detection of this disease is essential for patient survival.
Serum Prostate-Specific Antigen (PSA) quantification has been at the core of screening practices for the past 35 years4. However, recent studies have shown how PSA, while being informative in the prediction of recurrence5,6, has demonstrated very limited accuracy in discriminating PCa patients from controls (only ~25% of men with elevated PSA are found to have prostate cancer after biopsy7), and in discriminating indolent from aggressive disease (Area-under-the-curve, or AUC, less than 65%8). This has led to substantial overdiagnosis and overtreatment of patients with indolent disease9,10, with significant impact on the quality of life of these patients11. Therefore, better biomarkers for the early detection of PCa are urgently needed.
The National Cancer Institute Early Detection Research Network (NCI-EDRN) Prostate Cancer Cohort has been created to collect patient specimens and develop new clinically applicable molecular biomarkers to replace PSA testing for the diagnosis and risk assessment of PCa12. As part of these efforts, Weill Cornell Medicine recruited 1,144 patients with no prior history of prostate cancer, who underwent a prostate biopsy between 2008 and 2017. Extensive clinal data were collected for these patients, including patient’s demographic information and medical history, as well as 12- and 24-month clinical follow-up. For cancer patients, this included details on patient treatment and response, additional longitudinal PSA measurements, as well as the results of any relevant clinical tests, such as CTs or bone scans, MRIs, or repeat biopsies. For controls, information regarding longitudinal PSA measurements, digital rectal exam (DRE) results, and repeat biopsy results were recorded where available.
Recent studies have identified several blood metabolites as potential biomarkers for PCa diagnosis and prognosis. Among these, elevated sarcosine levels and decreased levels of phosphatidylcholines (PCs) and citrate have been observed in plasma of PCa patients compared to healthy controls13,14. Moreover, increased sarcosine abundance has been linked to prostate cancer metastasis15. A notable study employed 1H NMR-based metabolomics to discern characteristic metabolic panels for different stages of prostate cancer, demonstrating the potential of metabolomics in cancer research16. The discovery of these biomarkers is crucial for more precise PCa diagnosis13,17. However, further validation is needed to ensure their clinical effectiveness18.
Here, we present a new dataset related to 580 individuals from the Weill Cornell EDRN Prostate Cancer Cohort, for which metabolomic profiling was performed on plasma samples collected prior to biopsy (Fig. 1). The metabolite quantification included four profiling modes: (1–2) two separate reverse phase (RP)/Ultrahigh Performance Liquid Chromatography (UPLC) – Tandem Mass Spectroscopy (MS/MS) methods with positive ion mode electrospray ionization (ESI), (3) one RP/UPLC-MS/MS with negative ion mode ESI, and (4) one Hydrophilic Interaction Chromatography (HILIC) UPLC-MS/MS with negative ion mode ESI, allowing for the detection of more than 1,400 compounds, spanning 9 molecular classes and almost 120 molecular pathways. Here we make these data, together with the corresponding rich clinical annotations, publicly available to the scientific community.
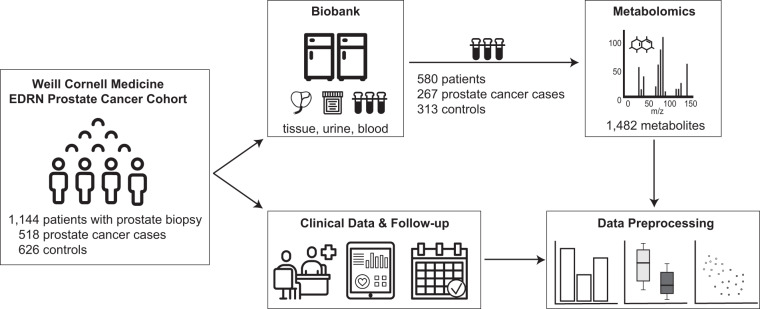
Fig. 1.
Study overview. The Weill Cornell Medicine EDRN Prostate Cancer Cohort included 1,144 patients undergoing prostate biopsy. For each patient, biopsy tissue, as well as urine and blood plasma were collected and biobanked. Clinical annotations included demographic information and medical history, as well as 12- and 24-months follow-up including biopsy pathology reports, PSA measurements, patients’ treatment, and clinical exams. From this cohort, plasma samples of 580 patients (267 cases and 313 controls) were profiled metabolically by Metabolon, Inc.
Methods
Weill cornell early detection research network (edrn) prostate cancer cohort
The Weill Cornell EDRN Prostate Cancer Cohort was consented and recruited 1,144 patients (518 cases and 626 controls) over 9 years (from 2008 to 2017). In order to be eligible for the study, patients needed to be adult males, have no prior history of prostate cancer or prostate biopsies, and be scheduled to undergo prostate biopsy at Weill Cornell Medicine in New York City. Need of prostate biopsy was determined based on suspicion of prostate cancer, which primarily included elevated PSA levels, abnormal digital rectal exam (DRE) results, or suspicious findings based on imaging. Prostate biopsies included at least 10 cores taken in a laterally directed fashion, and a fasting blood sample was collected prior to prostate biopsy from all recruited patients. Biopsy tissue, together with urine and blood samples were processed and biobanked at Weill Cornell Medicine.
For each patient, extensive clinical parameters were collected, including biopsy results, prostate cancer diagnosis and 2-year follow-up clinical information. EDTA-plasma samples for metabolomics profiling were selected to include patients with complete 12- and 24-months follow-up information available (see Data Records section for more details). This subset included a total of 580 patients, with 267 men diagnosed with PCa and 313 controls (Fig. 1). An overview of the main clinical and demographic parameters for this cohort is provided in Table 1.
Table 1.
Demographics of the profiled cohort.
| Cases (N = 267) | Controls (N = 313) | p-value | |
|---|---|---|---|
| Age in years | 0.109 | ||
| median (IQR) | 66.0 (59.0–71.5) | 64.0 (58.0–70.0) | |
| [min, max] | [39, 85] | [33, 84] | |
| BMI | 0.981 | ||
| median (IQR) | 26.3 (24.4–29.0) | 26.3 (24.2–19.3) | |
| PSA in ng/ml | 0.010 | ||
| median (IQR) | 5.3 (4.0–7.9) | 4.9 (3.6–6.7) | |
| Elevated PSA | N = 242 | N = 276 | 0.490 |
| Abnormal DRE | N = 40 | N = 21 | 0.001 |
| Gleason score (GS) |
GS 6 N = 113 GS 7 N = 121 GS 8 N = 15 GS 9 N = 17 GS 10 N = 1 |
— | — |
BMI = Body Mass Index; PSA = Prostate-Specific Antigen; DRE = Digital Rectal Exam; IQR = Interquartile Range. A Wilcoxon test was performed for continuous variables (Age, BMI, PSA), and a Fisher’s exact test for dichotomous variables (Elevated PSA, Abnormal DRE).
This study has been approved by the Weill Cornell Internal Review Board (IRB protocol number 0711009545). All patients provided informed consent for the collection, storage, and de-identified sharing of their data for research purposes. The IRB has approved the release of data in the provided format.
Sample collection processing
Fasting blood was collected prior to biopsy in 6 ml BD Vacutainer® EDTA tubes. Immediately after blood draw, the tube was inverted 8–10 times to mix the additive with the blood and placed immediately on ice. Tubes were centrifuged at 2,500 rpm for 15 minutes at 4 °C within two hours of blood collection. Plasma aliquots of 100ul or 200 ul were extracted from the top layer, transferred into 0.5 ml polypropylene micro tubes and stored at −80C until shipment for metabolomic profiling.
Metabolomics analysis
Metabolomic profiling of samples and quality control was performed by Metabolon Inc. (Morrisville, NC).
Sample preparation
Upon receipt, samples were catalogued and instantly stored at −80oC until they were processed. Each acquired sample was allocated a unique identifier for tracking all handling, tasks, and outcomes. Both the original samples and any resultant aliquots were monitored continuously through an automated system.
On the extraction day, the frozen samples were thawed while on ice. Sample preparation was conducted using the automated MicroLab STAR® mechanism (Hamilton Company, Reno, NV). A volume of 100 µl from each sample was relocated to a well within a deepwell plate. Before the extraction, several isotopically labeled standards were introduced to each specimen to ensure precise extraction. For the purpose of removing proteins or disengaging small molecules bound to proteins or encapsulated in the precipitated protein matrix, proteins were sedimented with 500 µl of methanol via rigorous shaking for 2 minutes using a GenoGrinder 2000 (Glen Mills, Inc, Clifton, NJ), succeeded by a 10-minute centrifugation at 680 g.
Ultrahigh performance liquid chromatography – tandem mass spectroscopy (UPLC-MS/MS)
The sample was partitioned into five aliquots: two for examination using dual independent reverse phase (RP)/Ultrahigh Performance Liquid Chromatography (UPLC) – Tandem Mass Spectroscopy (MS/MS) approaches with positive ion mode electrospray ionization (ESI), one for testing through RP/UPLC-MS/MS with negative ion mode ESI, one for evaluation via Hydrophilic Interaction Chromatography (HILIC) UPLC-MS/MS with negative ion mode ESI, and a single sample was set aside for backup. Samples were momentarily situated on a TurboVap® (Zymark, Inc., Hopkinton, MA) to eliminate the methanol solvent. Extracts were subsequently dried under liquid nitrogen cooling prior to analysis.
Chromatography was performed using an ACQUITY (Waters, Milford, MA) ultra-performance liquid chromatography (UPLC) held at 40 °C–50 °C, and mass spectrometry was performed using a Q-Exactive (Thermo Scientific, Waltham, MA) high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. The linearity of the instrument performance standards has been shown previously19. Immediately prior to analysis, dried samples were reconstituted in 40 µL ›of the “A” mobile phase solvent, as specified for each analytical method in the supplementary data of Ford et al.20. Each reconstitution solvent contained several instrument performance standards at fixed concentrations to ensure injection and chromatographic consistency and to aid in peak alignment. After reconstitution, samples were centrifuged at 2800 rpm for 5 minutes. The injection volume was 5 mL with a 2x loop overfill.
For the RP/UPLC - MS/MS with positive ion mode ESI arm, the two aliquots were analyzed using acidic positive ion conditions; one was chromatographically optimized for more hydrophilic compounds and the other for more hydrophobic compounds. In the first case, the extract was gradient-eluted from a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µm) using water and methanol, containing 0.05% perfluoropentanoic acid (PFPA) and 0.1% formic acid (FA), while in the second, the extract was gradient-eluted from the same C18 column mentioned before, this time using methanol, acetonitrile, water, 0.05% PFPA and 0.01% FA and was operated at an overall higher organic content (for details, see Ford et al.20).
The aliquot for profiling with RP/UPLC-MS/MS with negative ion mode ESI was analyzed using basic negative ion optimized conditions, using a separate dedicated C18 column. The basic extracts were gradient-eluted from the column using methanol and water, with 6.5 mM Ammonium Bicarbonate at pH 8.
The aliquot for profiling with HILIC/UPLC-MS/MS with negative ion mode ESI was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 µm) using a gradient consisting of water and acetonitrile with 10 mM Ammonium Formate, pH 10.8. The MS analysis alternated between MS and data-dependent multistage (MSn) scans using dynamic exclusion. The scan range varied slighted between methods but covered 70–1000 m/z.
All MS2 spectra were collected with a data dependent acquisition (DDA) method using an exclusion list as described in Ford et al. previously20.
Detailed instrument parameters, reagents, standards, and chromatography run times are listed in Supplementary Material 1.
Data extraction and compound identification
Proprietary, in-house software by Metabolon was used to perform the detection and integration of MS peaks. The extracted chromatograms were binned by mass in a specified range; moreover, for each sample, a noise baseline was determined. All samples were aligned using the retention index (RI)21, a parameter computed from retention time (RT), of internal standards spiked into every analyzed sample. The internal standards were isotopically labelled metabolites that elute approximately every 30 s of chromatography, therefore spanning the whole chromatographic range, providing a reference for alignment across samples.
Compound identification was performed by matching the ion chromatographic features to a curated library of more than 5,000 purified standards or recurrent unknown entities22. Biochemical identifications were based on three criteria: (1) retention index within the RI window of the proposed identification, where the window is based both on the behavior of standards over a concentration range as well as historic behavior, (2) accurate mass match to library entries within 10 ppm m/z of the measured compound, and (3) a forward and reverse fragmentation scores23. Briefly, the forward score indicates how well the ions in the experimental spectrum were present in the library at the correct ratios, while the reverse score indicates how well the ions in the library were present in the experimental spectrum at the correct ratios. Compounds that satisfied criteria 1 and 2 and with forward and reverse fragmentation scores above 80% were automatically approved, while compounds with fragmentation scores below 35% were automatically rejected. Compounds with intermediate scores were marked for manual review and approval22. Metabolites were assigned confidence tiers according to the following guidelines: Biochemical Name (no asterisk) corresponds to Metabolomics Standards Initiative Tier 1 identification, indicating a compound confirmed based on an authentic chemical standard with high confidence in its identity. Biochemical Name * indicates a compound that has not been confirmed based on a standard, but for which there is high confidence in its identity (Not Tier 1). Biochemical Name ** represents a compound for which a standard is not available, but for which there is reasonable confidence in its identity or the information provided (Not Tier 1).
Peaks that could not be matched to any entry in the library were individually analyzed across injections, to identify strong correlations likely arising from isotope or adduct relationships. Recurrent peaks that could not be related to known compounds but that strongly correlated across multiple injections were considered likely to represent authentic biological entities that are not yet included in the library entries. These compounds were manually analyzed and reviewed, and, if considered to likely represent a novel undocumented biochemical, were assigned a numerical designation (e.g., “X-12345”) and were added as a new library entry for future analyses and classification. Importantly, these unknown metabolite names are consistent across studies, allowing for comparability with other Metabolon datasets.
Curation
All identification and quantification tasks underwent QC to confirm the quality of the identification and the integrity of peak integration. This concluding verification stage eliminated procedural artifacts, substances with inferior peak forms and thus flawed integration, compounds showing a systematic upward or downward trend in area, and fragmentation scores that led to rejected library identifications. Procedural artifacts were categorized as biochemicals existing in the biological samples at levels < = 3× those in the water process blanks. For compounds that were detected in both positive and negative modes, one mode was selected to represent the substance, based on the relative standard deviation among samples and the percentage of samples where the metabolite was observed in each mode.
Data normalization
Since the samples in this study were profiled over multiple days (run-days), a data normalization step was performed to correct variation resulting from inter-day instrument tuning differences. The integrated area-under-the-curve data for each metabolite was first divided by the corresponding run-day median for that metabolite and then multiplied by the overall dataset median for that metabolite.
Missing values
Missing values can result from chromatographical issues, software processing errors, when metabolite levels are below the limit of instrument detection (LOD), or simply when a metabolite is not present in a particular sample. Alternatively, sometimes missing values arise due to technical problems such as a temporary reduction in electrospray performance due to particulate material in the spray nozzle (Payne et al. 2009). These values are represented by NAs (not a number) in the data matrix.
Data preprocessing
To facilitate statistical analysis, we provide a preprocessed version of the metabolomics data in addition to the raw metabolite ion counts. The preprocessing workflow involved the following steps: (1) Metabolites with more than 50% missing values were filtered out. (2) Metabolite abundances were normalized using the probabilistic quotient approach24, using only variables with less than 20% missing values to construct the reference sample. (3) Normalized values were log-scaled to improve normality, as metabolite abundances are typically log-normally distributed. (4) The remaining missing values were imputed using a k-Nearest-Neighbors-based algorithm (knn with variable preselection and k = 10)25.
Clinical annotations
This cohort has rich clinical annotations, which range from global demographic details and patients’ medical history to detailed pathology and treatment information, as well as 12- and 24-month follow-up for both cases and controls, for a total of 420 unique parameters. Importantly, the follow-up information includes longitudinal PSA measurements pre and post biopsy, detailed biopsy pathology reports, patients’ treatment, as well as any additional prostate cancer related clinical test, such as CTs or bone scans, MRIs, or repeat biopsies.
An overview of the types of annotations available is provided in Table 2. A detailed description of all clinical parameters is included in the clinical annotation data file (see Data Records).
Table 2.
Overview of clinical annotations.
| Category | Number of parameters | Examples |
|---|---|---|
| Demographics | 14 | Age |
| Ethnicity | ||
| Smoking | ||
| Cancer history | 8 | Family history of prostate cancer |
| Previous cancer diagnoses | ||
| Medical history/Comorbidities | 12 | Arthritis |
| Diabetes | ||
| Inflammatory bowel disease | ||
| Pre-biopsy clinical data | 43 | Digital rectal exam results |
| Longitudinal PSA measurements | ||
| Low % free PSA | ||
| Prostate-related conditions | 10 | Prostatitis |
| Benign prostatic hyperplasia | ||
| Urethritis | ||
| Prostate-related medications | 10 | 5-alpha reductase inhibitors |
| Alpha-blockers | ||
| Androgens | ||
| Past surgical history | 10 | TURP |
| TUIP | ||
| TUMT | ||
| Biopsy procedure details | 7 | Biopsy date |
| Number of cores taken | ||
| Prostate size | ||
| Prostate cancer diagnosis | 14 | Cancer diagnosis |
| Gleason score | ||
| TNM stages | ||
| Per-biopsy-core pathology report | 97 | Core position |
| Primary Gleason score | ||
| Secondary Gleason score | ||
| Perineural invasion | ||
| Percent of cancer in core | ||
| Core length | ||
| Negative biopsy reporting | 5 | High grade PIN |
| Atypia | ||
| Atrophy | ||
| 12-month follow-up clinical data | 60 | Bone scan results |
| CT scan results | ||
| MRI results | ||
| 12-month follow-up prostate cancer treatment details | 31 | Treatment Type and Duration |
| Radical prostatectomy results | ||
| 24-month follow-up clinical data | 62 | Bone scan results |
| CT scan results | ||
| MRI results | ||
| 24-month follow-up prostate cancer treatment details | 48 | Treatment type and duration |
| Radical prostatectomy results |
Data Records
The integrated metabolite ion counts, the preprocessed metabolomics data, the metabolite annotations, and the deidentified clinical data are available for download on Metabolomics Workbench, 10.21228/M86H7K26. The data is available as a zip file (EDRN_Data.zip) and contains two data files (EDRN_MetabolomicsData.xlsx and EDRN_ClinicalData.xlsx) and one text document (preprocessing_workflow.docx) detailing the data preprocessing steps.
The unprocessed ion count data (tab “Data” in file EDRN_MetabolomicsData.xlsx) include metabolite abundances of 1,482 biochemicals, of which 1,155 are compounds of known identity (named biochemicals) covering 119 biochemical pathways across 9 molecular classes (tab “MetaboliteAnnotations” in file EDRN_MetabolomicsData.xlsx), and 327 are compounds of unknown structural identity (unnamed biochemicals). The preprocessed data (tab “PreprocessedData” in file EDRN_MetabolomicsData.xlsx) were generated as described in Section 2.4 and only account for metabolites with less than 50% missing values. The data file includes 1,169 metabolites (915 known and 254 unknowns). To protect patients’ privacy, in the clinical annotation file (tab “ClinicalAnnotations” in file EDRN_ClinicalData.xlsx) the age of individuals older than 90 years is reported as 90 and all dates have been reported as number of days prior to or since biopsy. A detailed description of each clinical variable is provided in the tab “Legend”.
Technical Validation
In order to avoid systematic effects in any of the comparison groups, samples were randomized both across profiling days and within each profiling run and plate.
Moreover, several types of control samples were analyzed in parallel with the experimental samples to assess instrument and process variability. First, a pool of well-characterized EDTA human plasma (“MTRX”) served as a technical replicate throughout the data set. These matrix samples were used to estimate process variability by calculating the median relative standard deviation (RSD) for all measured metabolites (i.e., non-instrument standards) present in 100% of the pooled matrix samples. The median RDS for process variability was around 9%.
Additionally, a mixture of QC standards was spiked into every analyzed sample and used to aid chromatographic alignment and to monitor instrument performance. In this case, instrument variability was determined by calculating the median RDS for the QC standards that were added to each sample prior to injection into the mass spectrometers. The median RDS for instrument variability was roughly 6%. Note that the QC standards were carefully chosen not to interfere with the measurement of compounds in the biological samples.
Water samples (“PRCS”) were additionally included in each run and served as process blanks for the assessment and removal of process artifacts. A schematic representation of the plate layout is provided in Fig. 2.
Fig. 2.
Plate layout and quality control samples. Each plate included four ultra-pure water samples that served as process blanks (PRCS) for the assessment and removal of process artifacts, four samples of pooled EDTA human plasma (MTRX) that served as technical replicates throughout the dataset, and 36 biological samples. For metabolomic analysis, each plate is generated in four replicates, one for each of the four platform arms: two separate reverse phase (RP)/Ultrahigh Performance Liquid Chromatography (UPLC) – Tandem Mass Spectroscopy (MS/MS) methods with positive ion mode electrospray ionization (ESI), one RP/UPLC-MS/MS with negative ion mode ESI, and one Hydrophilic Interaction Chromatography (HILIC) UPLC-MS/MS with negative ion mode ESI.
Supplementary information
Acknowledgements
This work was funded by the SPORE CEP grant from Weill Cornell Medicine (WCM) SPORE in Prostate Cancer (PCa). The EDRN Cornell Clinical Biobank for Prostate Cancer Research was supported by the NIH grants 1R01CA179100-01A1 and 7U01CA113913-09.
Author contributions
T.F. and D.S.S. were involved in the E.D.R.N. study design, patient recruitment, and extracted the clinical patient annotation for this paper. E.B., J.K., C.E.B. and M.L. designed the metabolomics study and selected the patient sampes for metabolomic profiling. E.B. performed all data processing and analysis. E.B. and J.K. wrote the primary manuscript. All authors approved the final manuscript.
Code availability
The R code used to preprocess the metabolomics data was based on R 4.0.1 and the R package maplet27. The code is available at https://github.com/krumsieklab/prostate-cancer-edrn. In the same script, we also provide an example to illustrate how to use the maplet R package to load the data and run differential analyses based on the available clinical parameters. This can serve as a template for users to build their own analysis pipelines.
Competing interests
JK holds equity in Chymia LLC and iollo, and IP in PsyProtix.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-023-02750-7.
References
- 1.Wang, L. et al. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health10, (2022). [DOI] [PMC free article] [PubMed]
- 2.Sosnowski R, et al. Active surveillance for low-risk prostate cancer – in pursuit of a standardized protocol. Cent. Eur. J. Urol. 2020;73:123–126. doi: 10.5173/ceju.2020.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer of the Prostate - Cancer Stat Facts. SEERhttps://seer.cancer.gov/statfacts/html/prost.html.
- 4.Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM. Beyond PSA: The Next Generation of Prostate Cancer Biomarkers. Sci. Transl. Med. 2012;4:127rv3-127rv3. doi: 10.1126/scitranslmed.3003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartor CI, et al. Rate of PSA rise predicts metastatic versus local recurrence after definitive radiotherapy. Int. J. Radiat. Oncol. 1997;38:941–947. doi: 10.1016/S0360-3016(97)00082-5. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Urology. 2003;61:365–369. doi: 10.1016/S0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 7.Crawford ED, Ventii K, Shore ND. New biomarkers in prostate cancer. Oncol. Williston Park. 2014;28:135–142. [PubMed] [Google Scholar]
- 8.Lojanapiwat B, Anutrakulchai W, Chongruksut W, Udomphot C. Correlation and diagnostic performance of the prostate-specific antigen level with the diagnosis, aggressiveness, and bone metastasis of prostate cancer in clinical practice. Prostate Int. 2014;2:133–139. doi: 10.12954/PI.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bangma CH, Roemeling S, Schröder FH. Overdiagnosis and overtreatment of early detected prostate cancer. World J. Urol. 2007;25:3–9. doi: 10.1007/s00345-007-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heijnsdijk EaM, et al. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br. J. Cancer. 2009;101:1833–1838. doi: 10.1038/sj.bjc.6605422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizer AA, et al. Cost Implications and Complications of Overtreatment of Low-Risk Prostate Cancer in the United. States. J. Natl. Compr. Canc. Netw. 2015;13:61–68. doi: 10.6004/jnccn.2015.0009. [DOI] [PubMed] [Google Scholar]
- 12.Liss MA, Leach RJ, Sanda MG, Semmes OJ. Prostate Cancer Biomarker Development: National Cancer Institute’s Early Detection Research Network Prostate Cancer Collaborative Group Review. Cancer Epidemiol. Biomarkers Prev. 2020;29:2454–2462. doi: 10.1158/1055-9965.EPI-20-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Cebrián N, et al. Metabolomics Contributions to the Discovery of Prostate Cancer Biomarkers. Metabolites. 2019;9:48. doi: 10.3390/metabo9030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Lv H, Qiu S, Gao L, Ai H. Plasma metabolic profiling and novel metabolite biomarkers for diagnosing prostate cancer. RSC Adv. 2017;7:30060–30069. doi: 10.1039/C7RA04337F. [DOI] [Google Scholar]
- 15.McDunn JE, et al. Metabolomic signatures of aggressive prostate cancer. The Prostate. 2013;73:1547–1560. doi: 10.1002/pros.22704. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, et al. Identification of characteristic metabolic panels for different stages of prostate cancer by 1H NMR-based metabolomics analysis. J. Transl. Med. 2022;20:275. doi: 10.1186/s12967-022-03478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salciccia S, et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021;22:4367. doi: 10.3390/ijms22094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kdadra M, Höckner S, Leung H, Kremer W, Schiffer E. Metabolomics Biomarkers of Prostate Cancer: A Systematic Review. Diagnostics. 2019;9:21. doi: 10.3390/diagnostics9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridgewater BR, E. A. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. J. Postgenomics Drug Biomark. Dev. 04, (2014).
- 20.Ford L, et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J. Appl. Lab. Med. 2020;5:342–356. doi: 10.1093/jalm/jfz026. [DOI] [PubMed] [Google Scholar]
- 21.The IUPAC Compendium of Chemical Terminology: The Gold Book. 10.1351/goldbook (International Union of Pure and Applied Chemistry (IUPAC), 2019).
- 22.DeHaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminformatics. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 24.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in1H NMR metabonomics. Anal. Chem. 2006;78:4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 25.Do KT, et al. Characterization of missing values in untargeted MS-based metabolomics data and evaluation of missing data handling strategies. Metabolomics. 2018;14:128. doi: 10.1007/s11306-018-1420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krumsiek J. 2023. Plasma Metabolomics Profiling of 580 Patients from the Weill Cornell Medicine Early Detection Research Network Prostate Cancer Cohort. Project PR001613. Metabolomics Workbench. [DOI] [PubMed]
- 27.Chetnik K, et al. maplet: An extensible R toolbox for modular and reproducible metabolomics pipelines. Bioinformatics. Oxf. Engl. 2021;38:1168–1170. doi: 10.1093/bioinformatics/btab741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Supplementary Materials
Data Availability Statement
The R code used to preprocess the metabolomics data was based on R 4.0.1 and the R package maplet27. The code is available at https://github.com/krumsieklab/prostate-cancer-edrn. In the same script, we also provide an example to illustrate how to use the maplet R package to load the data and run differential analyses based on the available clinical parameters. This can serve as a template for users to build their own analysis pipelines.