Abstract
Current antithrombotic therapies used in clinical settings target either the coagulation pathways or platelet activation receptors (P2Y12 or GPIIb/IIIa), as well as the cyclooxygenase (COX) enzyme through aspirin. However, they are associated with bleeding risk and are not suitable for long-term use. Thus, novel strategies which provide broad protection against platelet activation with minimal bleeding risks are required. Regardless of the nature of agonist stimulation, platelet activation is an energy-intensive and ATP-driven process characterized by metabolic switching toward a high rate of aerobic glycolysis, relative to oxidative phosphorylation (OXPHOS). Consequently, there has been considerable interest in recent years in investigating whether targeting metabolic pathways in platelets, especially aerobic glycolysis and OXPHOS, can modulate their activation, thereby preventing thrombosis. This review briefly discusses the choices of metabolic substrates available to platelets that drive their metabolic flexibility. We have comprehensively elucidated the relevance of aerobic glycolysis in facilitating platelet activation and the underlying molecular mechanisms that trigger this switch from OXPHOS. We have provided a detailed account of the antiplatelet effects of targeting vital metabolic checkpoints such as pyruvate dehydrogenase kinases (PDKs) and pyruvate kinase M2 (PKM2) that preferentially drive the pyruvate flux to aerobic glycolysis. Furthermore, we discuss the role of fatty acids and glutamine oxidation in mitochondria and their subsequent role in driving OXPHOS and platelet activation. While the approach of targeting metabolic regulatory mechanisms in platelets to prevent their activation is still in a nascent stage, accumulating evidence highlights its beneficial effects as a potentially novel antithrombotic strategy.
Keywords: Platelets • Aerobic glycolysis • OXPHOS • Thrombosis
Graphical Abstract
Graphical Abstract.
1. Introduction
Platelets ensure a normal hemostatic response to prevent blood loss during injury. However, pathological activation of platelets, for instance, due to the rupture of an atherosclerotic plaque can induce the formation of an occlusive thrombus or distal embolism, a disease process known as atherothrombosis.1 This can lead to potentially fatal ischemic events such as coronary heart disease (angina or myocardial infarction), peripheral arterial disease, or stroke.2 Currently used antiplatelet drugs combat thrombosis by targeting either a specific platelet receptor (such as P2Y12 or GPIIb/IIIa), a molecular signaling pathway, or through aspirin-mediated irreversible inactivation of the cyclooxygenase (COX) enzyme. However, physiologically, platelet aggregation is an outcome of platelet stimulation facilitated concomitantly by multiple activation pathways triggered mainly by collagen or soluble agonists (such as thrombin, ADP, and thromboxane A2) that bind the GPVI receptor or G-protein-coupled receptors (GPCRs), respectively.3 This limits the ability of a single antiplatelet drug (such as aspirin or P2Y12 inhibitors) to provide broad protection against thrombosis, making patients vulnerable to thrombotic events elicited by other potential triggers and related platelet activation pathways. Notably, bleeding is the most common side effect associated with increasingly potent antiplatelet therapies (GPIIb/IIIa inhibitors such as tirofiban and eptifibatide), which aggravates even more when dual- or triple-antiplatelet therapies are implemented to provide broad protection against thrombotic episodes.4 Therefore, novel therapeutic strategies that provide global protection against atherothrombosis with minimal bleeding complications are required.
The generation of ATP to meet the energy requirement is essential to both quiescent and activated platelets. However, in contrast to the resting state, platelet activation is characterized by high-energy–driven stages such as adhesion, integrin activation, aggregation, degranulation, and clot retraction. Besides the conventional role of metabolic pathways in fulfilling the energy requirements of platelets in a quiescent state, increasing evidence now suggests their ability in regulating platelet activation as well. Studies performed in the last few years have established that metabolic pathways, principally, glycolysis, and mitochondrial oxidative phosphorylation (OXPHOS), become engaged to generate ATP to facilitate platelet activation.5–12 For example, glucose is converted to pyruvate in the cytoplasm through glycolysis, which involves the transfer of phosphates from glycolytic intermediates to ADP to generate ATP (Figure 1). Next, the pyruvate gets converted to acetyl coenzyme A, which is further oxidized via the Krebs cycle to produce ATP through OXPHOS (Figure 1). In addition, platelet activation characteristically exhibits aerobic glycolysis (conversion of glucose/pyruvate to lactate in the presence of oxygen, also known as the Warburg effect), a phenomenon that is unique to cancer cells and known to play a critical role in rapid ATP production (Figure 1).6,7,9,12,13 Besides these, platelets also demonstrate the ability to metabolize substrates, such as fatty acids and glutamine via β-oxidation and glutaminolysis, respectively, to replenish the Krebs cycle and fuel OXPHOS for ATP synthesis (Figure 1).14–17 These varied metabolic choices to fulfill the high energy demand of platelets suggest the existence of metabolic flexibility in platelets. The extent to which these metabolic pathways can compensate for one another, especially during platelet activation, remains unclear. Broadly, the choice of metabolic pathway upon platelet activation depends on three factors: (i) the nature and strength of platelet stimulation, (ii) the existing physiological environment such as oxygen tension, and (iii) the availability of metabolic substrates (glucose, glycogen, fatty acids, and glutamine).5,8 Besides this, it is highly likely that substrate flexibility not only benefits circulating platelets undergoing activation but also plays a pivotal role in supporting activated platelets present within the thrombus milieu, challenged with limited access to nutrients and oxygen. Active metabolic switching within the growing thrombus may allow platelets to adapt their metabolism based on the available nutrients to perform energy-intensive processes such as cytoskeletal reorganization to ensure thrombus stabilization.18
Figure 1.
Schematic representation of the principal metabolic pathways fueling bioenergetic needs of activated platelets. Glucose undergoes glycolysis to form two pyruvate molecules along with ATP (2) and NADH (2). Pyruvate then enters the Krebs cycle to produce ATP (2), NADH (6), and FADH2 (2). The reducing equivalents (NADH and FADH2) are used by the electron transport chain to generate ATP through OXPHOS. Through aerobic glycolysis, pyruvate gets converted to lactate along with the regeneration of NAD+ (2) and ATP (2) synthesis. Platelets also metabolize fatty acids and glutamine via β-oxidation and glutaminolysis, respectively, to replenish the Krebs cycle and fuel OXPHOS. ADP, adenosine diphosphate; ATP, adenosine triphosphate; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide (NAD) + hydrogen (H); FADH, flavin adenine dinucleotide; PDH, pyruvate dehydrogenase.
It is getting increasingly recognized that targeting metabolites, metabolic enzymes, or vital metabolic checkpoints in different metabolic pathways can elicit profound effects on platelet activation and thrombosis.5–10 This provides a platform to investigate platelet metabolism with a completely different perspective that can open a new paradigm for the therapeutic intervention of thrombosis. In this review, we have comprehensively discussed the role of different metabolic pathways and associated metabolic checkpoints that have been uncovered as potential therapeutic targets for combatting platelet activation and thrombosis.
2. Glycolysis
Resting platelets fuel their baseline bioenergetic requirements through both glycolysis and mitochondrial OXPHOS.5,8,19 In contrast and irrespective of the nature of stimulation (GPVI or GPCR mediated), the transition of platelets from a resting to an activated state is characterized by an increase in glycolysis and OXPHOS.5–9,11,12 It is getting increasingly clear that the rise in the extent of glycolysis is much higher in activated platelets compared to mitochondrial respiration.5–9 This categorically implies a metabolic switching towards aerobic glycolysis, relative to OXPHOS, in activated platelets. It may seem rather paradoxical that activated platelets prefer aerobic glycolysis for energy production, which is a less efficient pathway (yields only two ATP molecules per molecule of glucose) in contrast to OXPHOS (yields 36 ATP molecules per molecule of glucose). However, evolving evidence suggests the intervention from a dynamically regulated and self-correcting homeostatic system, involving a set of enzymes that respond appropriately to meet the specific requirements of the cell.20 Moreover, an inefficient pathway for ATP production can be a limiting factor in cases where metabolic substrates are scarce relative to the ATP requirements. Contrastingly, platelets have an abundant supply of glucose and other nutrients circulating in the blood that can facilitate an accelerated generation of ATP through aerobic glycolysis, which is estimated to be 100× faster compared to OXPHOS (Figure 2).21,22 Therefore, increased aerobic glycolysis upon agonist challenge may facilitate faster glucose metabolism to meet the short-term high-energy demand of activated platelets to form a blood clot and prevent blood loss in a short period. In addition to rapid ATP synthesis, increased glucose consumption also facilitates de novo synthesis of nucleotides, lipids, and proteins that enters different branches emanating from glycolysis to further favor platelet activation (Figure 2).23 For example, the pentose phosphate pathway (PPP) branching from glycolysis along with serine metabolism are an important source of NADPH synthesis.23 NADPH oxidase subsequently catalyze the synthesis of reactive oxygen species (ROS) using NADPH to promote platelet activation via multiple signaling processes.24 While the role of glycolysis intermediates as the precursors of anabolic reactions (biosynthesis) may appear less relevant during the rapid process of platelet activation, it certainly holds importance for activated platelets that are present within the thrombus. Confronted by a restricted supply of nutrients inside the thrombus, biosynthesis may suffice the energy requirements of activated platelets to perform processes such as cytoskeleton rearrangement and ROS-mediated signaling to ensure the stability of the thrombus. Furthermore, aerobic glycolysis is also an important source of NAD+ regeneration in the cytoplasm through the conversion of pyruvate to lactate by lactate dehydrogenase (LDH) (Figure 2). NAD+ is a vital cofactor that becomes reduced in glycolysis to form NADH and H+ at the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reaction to maintain a high glycolytic flux required for platelet activation.25 Consequently, a limited supply of NAD+ can significantly reduce glycolysis in the cytoplasm, which may further reduce the extent of platelet activation. Besides glycolysis, the progression of other major metabolic pathways such as the Krebs cycle, fatty acid oxidation, and OXPHOS (via the respiratory chain) requires a continuous supply of NAD+ for NAD-dependent redox reactions.26 Therefore, the NADH synthesized during glycolysis must be trafficked to the mitochondria. Given the impermeability of the inner mitochondrial membrane to NAD+ and NADH, the malate-aspartate shuttle and the glycerol-3-phosphate shuttle pathways transfer the reducing equivalents into the mitochondria.27 These shuttles also regenerate cytosolic NAD+ necessary to further support glycolytic flux.28 It is also important to note that unlike cytoplasmic NAD, the mitochondrial NAD pool is sufficiently stable to preserve OXPHOS in times of cellular stress.25 During cytoplasmic NAD+ depletion, mitochondrial NAD+ levels can still be maintained for up to 24 h, and up to 3 days.27 This further suggests the importance of cytoplasmic NAD+ regeneration for the advancement of glycolysis. The increased NAD+/NADH ratio can also have profound implications on molecular signaling in platelets (Figure 2). For instance, an increase in NAD+/NADH ratio can trigger the de novo synthesis of diacylglycerol (DAG), which can activate protein kinase C (PKC), leading to the activation of integrin αIIbβ3 and platelet aggregation downstream of both GPVI- and GPCR-mediated platelet activation pathways.7,29
Figure 2.
The Warburg effect in activated platelets. The Warburg effect or aerobic glycolysis involves the conversion of glucose/pyruvate to lactate in the presence of oxygen. It plays a highly significant role in platelet activation by ensuring a rapid production of ATP, regeneration of NAD+, regulation of platelet signaling, and biosynthesis of precursors such as nucleotides, lipids, and proteins. LDH, lactate dehydrogenase; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide + hydrogen (H).
Given the rapid requirement of glucose to sustain aerobic glycolysis, the role of glucose transporters (GLUTs) on the platelet surface is highly fundamental. Platelet activation is characterized by a significant increase in the number of GLUTs on the platelet surface, which facilitates glucose entry into the cell to sustain a high rate of glycolysis.6,30 Genetic ablation of GLUT3 was observed to reduce platelet functions including degranulation and integrin activation, which were further associated with less susceptibility to experimental thrombosis in mice.30 Some of the early studies that identified the importance of glycolysis in regulating platelet activation are now getting validated using modern metabolic analysis such as the estimation of extracellular acidification rate (ECAR) and oxygen consumption rate (OCR).31–33 Cells with a glycolytic phenotype exhibit significantly higher rates of proton production (measured by ECAR) than cells using OXPHOS (measured by OCR).34 A previous study confirmed the contribution of glycolysis upon platelet activation by measuring ECAR.8 In resting platelets, 36% of the ECAR, and in thrombin-stimulated platelets 90% of the ECAR were inhibited by a hexokinase inhibitor 2-deoxy-D-glucose (2-DG). Moreover, treatment with 2-DG also inhibited thrombin-stimulated platelet aggregation by 50%.8 Consistent with this, another study observed a rapid increase in exogenous glucose uptake along with a dramatic shift toward a predominantly glycolytic phenotype during the transition of platelets from a quiescent to an activated state.5 Notably, an increase in ECAR was observed following thrombin stimulation even in the absence of externally supplied glucose. This not only indicates a switch to a glycolytic phenotype but provides evidence that glycogen has a similar metabolic fate as glucose as endogenous glycogen (in the absence of glucose) can fuel platelet activation.5 Furthermore, biosynthesis and consumption of glycogen have also been reported to play a critical role in facilitating platelet functions.35 Platelets in a resting state were observed to exhibit a steady turnover of glycogen with its active biosynthesis and consumption. This shifted towards a net degradation (via glycogen phosphorylase) and consumption of glycogen upon activation to support the high energy requirements of platelet function. Blocking glycogenolysis by inhibiting glycogen phosphorylase increased glycogen levels in resting and thrombin-activated platelets, suppressing platelet secretion and clot contraction, with minimal effects on aggregation.35
As these findings suggest a fundamental role of aerobic glycolysis in fueling the platelet activation process, it seems rational to speculate that targeting the same may have profound implications on platelet functions. Consequently, there has been a renewed interest in the past few years in revisiting aerobic glycolysis in platelets to identify vital metabolic targets and checkpoints that drive this process and investigate whether targeting these may modulate platelet activation and thrombosis. We will now discuss some of the recently identified metabolic targets and checkpoints that drive aerobic glycolysis in platelets.
2.1. Pyruvate dehydrogenase kinases
The mitochondrial pyruvate dehydrogenase (PDH) complex converts pyruvate (the end product of glycolysis) to acetyl coenzyme A, which enters the Krebs cycle and undergoes oxidation to produce ATP using the OXPHOS pathway. During platelet activation, the pyruvate dehydrogenase kinase (PDK) phosphorylates the E1α subunit of PDH to inhibit its activity.7,9 This diverts the pyruvate flux from the Krebs cycle to aerobic glycolysis, leading to lactic acid production (Figure 3).7,9 Therefore, the PDK/PDH metabolic checkpoint provides an essential metabolic switch that regulates pyruvate flux during platelet activation. Four isozymes of PDK (PDK1-4) have been identified in humans and rodents. Our lab identified that pharmacological targeting of PDKs in platelets using dichloroacetic acid (DCA), a small-molecule inhibitor of PDKs (Figure 3), significantly inhibited a variety of human and mouse platelet functions including aggregation and secretion.7 Furthermore, mice treated with DCA exhibited less susceptibility to arterial thrombosis without any effect on the tail-bleeding time, suggesting a normal hemostatic response. There was an increased glucose uptake by human and mouse platelets following agonist stimulation that was inhibited by DCA treatment. Moreover, under arterial shear rate, DCA-treated human blood perfused ex vivo over collagen in a microfluidic flow chamber formed significantly smaller thrombi.7 Bioenergetic studies identified an increase in ECAR upon stimulation with convulxin (a GPVI-specific platelet agonist) compared to resting platelets, suggesting an increase in aerobic glycolysis in activated platelets. The increase in ECAR in convulxin-stimulated platelets was significantly reduced following treatment with DCA.7 Interestingly, the extent of ECAR was comparable between untreated and DCA-treated platelets that were stimulated with thrombin or ADP. While informative, the ECAR estimated in all these studies5,7,8 provides only an indirect measure of the glycolytic rate as it encompasses acidification from both mitochondrial respiration-derived CO2 (which forms H2CO3 and then dissociates to HCO3− + H+) and extracellular protons generated through glycolysis (which forms lactate + H+).14,34 In earlier investigations, the contributions of CO2 to total extracellular acidification was thought to be negligible.34 However, besides mitochondrial respiration, other metabolic pathways such as those carried out by mitochondrial matrix dehydrogenases in the Krebs cycle also generate CO2 from glucose catabolism.36 Thus, the correct measurement of the glycolytic rate must involve the subtraction of acidification caused by mitochondrial CO2. The glycolytic proton efflux rate (glycoPER) is measured by subtracting the mitochondrial CO2-dependent acidification from the total acidification and is regarded as a direct estimate of aerobic glycolysis and extracellular lactate production.37
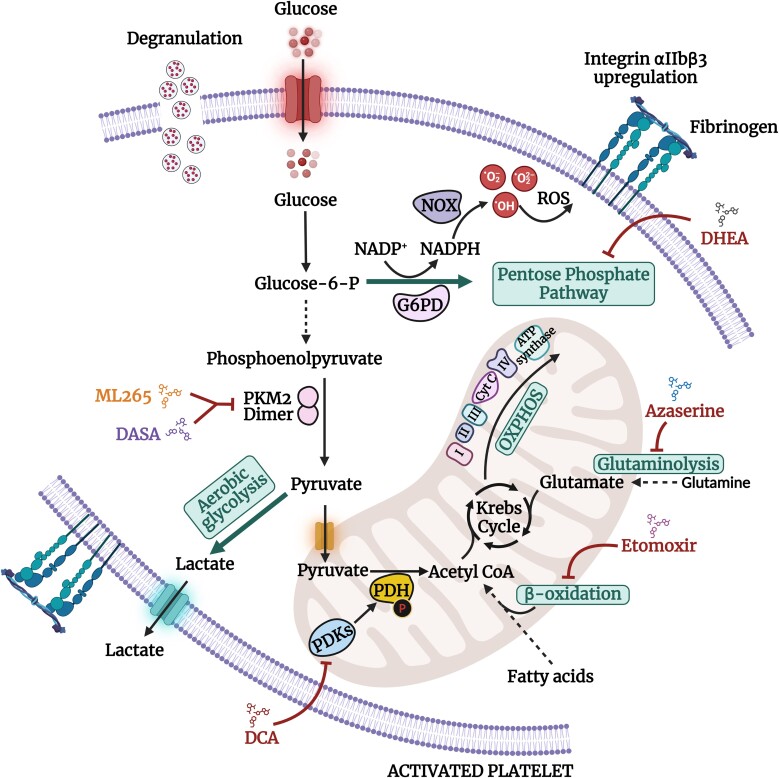
Figure 3.
A schematic demonstrating the metabolic pathways and checkpoints that have been shown to modulate platelet activation. Activation of platelets is characterized by an increased rate of aerobic glycolysis relative to OXPHOS. Upon agonist stimulation, PDKs phosphorylate the E1α subunit of PDH to inhibit its activity, thereby diverting the pyruvate flux from the Krebs cycle to aerobic glycolysis, leading to lactate production. Treatment with DCA (small-molecule inhibitor of PDKs) or genetic deletion of PDK2/4 has been shown to inhibit platelet activation. The PKM2 catalyzes the conversion of phosphoenolpyruvate pyruvate to pyruvate. The dimeric PKM2 exhibits low enzymatic activity and provides a metabolic switch to regulate aerobic glycolysis. Treatment with ML265 or DASA (small-molecule activator that limits the formation of PKM2 dimers) or platelet-specific deletion of PKM2 has been reported to inhibit platelet functions. Additionally, the dimeric PKM2 form also facilitates the production of glycolytic intermediates such as glucose-6-P that enter the pentose phosphate pathway (PPP). The increased glucose-6-P flux generates NADPH, which is catalyzed by NOX to promote ROS generation, leading to platelet activation. Treatment with DASA or DHEA significantly reduced ROS generation and subsequent platelet functions. Treatment of platelets with the inhibitors of etomoxir (inhibitor of β-oxidation of fatty acids) has been demonstrated to attenuate platelet functions. Additionally, platelets treated with azaserine (glutaminase inhibitor) attenuated thrombin-stimulated OCR. Glucose-6-P, glucose-6-phosphate; G6PD, glucose-6-phosphate-dehyrogenase; DASA, diaryl sulfonamide; DHEA, dehydroepiandrosterone sulphate; DCA: dichloroacetate; PDK, pyruvate dehydrogenase kinase; PDH, pyruvate dehydrogenase; PKM2: pyruvate kinase M2; NOX, NADPH oxidase; ROS, reactive oxygen species; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenine dinucleotide phosphate + hydrogen (H).
Others and our lab reported an increase in PDH phosphorylation in agonist-stimulated platelets.6,12 This provides further evidence that PDK-mediated phosphorylation of PDH in activated platelets inhibits PDH activity to increase pyruvate flux toward aerobic glycolysis (more lactate generation) (Figure 3). The enhanced PDH phosphorylation was abolished following treatment with DCA.6 In alignment with our findings, treatment with DCA attenuated multiple agonist-stimulated platelet functions and significantly delayed the time to occlusion of the mouse mesenteric arterioles following ferric chloride injury.6 Furthermore, utilizing mice devoid of both PDK2 and PDK4 (PDK2/4), we provided genetic evidence that suggested a regulatory role of the PDK/PDH axis in modulating platelet function.9 We showed that platelets from PDK2/4 double-knockout (KO) mice exhibit impaired platelet functions including, aggregation, integrin αIIbβ3 activation, degranulation, spreading, and clot retraction. PDK2/4-null mice exhibited reduced susceptibility to arterial thrombosis with no effect on tail bleeding time. As the PDK2/4−/− mice used in this study were global KOs, we also delineated the platelet-specific effects of PDK2/4 on regulating arterial thrombosis using the adoptive transfer of WT or PDK2/4−/− platelets in thrombocytopenic hIL-4Rα/GPIbα transgenic mice. Mechanistically, the inhibitory effects of PDK2/4 deletion on platelet functions were found to be associated with reduced PDH phosphorylation and glycoPER.9 In addition to the effects on aerobic glycolysis, treatment with DCA or genetic ablation of PDK2/4 was associated with the inhibition of GPVI-mediated signaling in activated platelets.7,9 Furthermore, using PDK2 or PDK4 single-KO mice, we identified a more prominent role of PDK4 compared to PDK2 in regulating platelet secretion and arterial thrombosis.9 While this study focused mainly on PDK2/4 due to their ubiquitous tissue distribution and prominent association with metabolic diseases,38–40 the role of PDK1 or PDK3 (or both) cannot be ruled out and requires further evaluation using mutant mouse models.
Interestingly, in addition to the antithrombotic effects, the antiatherosclerotic effects of targeting the PDK/PDH metabolic checkpoint were recently identified in the apolipoprotein-deficient (Apoe−/−) mice, a mouse model of atherosclerosis.41 Using DCA, the PDK/PDH axis was recognized as a major metabolic checkpoint that regulates immune cell polarization, plaque development, and fibrous cap formation in Apoe−/− mice.41 Collectively, the antithrombotic and antiatherosclerotic effects of targeting the PDK/PDH axis can potentially offer cardioprotective benefits in combating thrombotic disorders.
2.2. Pyruvate kinase M2
Four isomeric and tissue-specific forms of pyruvate kinase (PK) exist in mammals: PKL, PKR, PKM1, and PKM2. While PKR is highly expressed in red blood cells, PKL is predominantly expressed in the liver, kidney, and intestine. PKM1 and PKM2 are the spliced products of the single PKM gene. PKM1 is exclusively present in the tissues that require a high amount of energy for their functions such as the heart, brain, and skeletal muscle, whereas PKM2 expression is limited to tissues with anabolic functions such as proliferating cells, tumors, and embryonic tissues.42 The PKs play a vital role in glycolysis by catalyzing its final rate-limiting step that involves the transfer of the phosphate group from phosphoenolpyruvate (PEP) to ADP to form pyruvate along with ATP (Figure 3). While all the isoforms of PK function in tetrameric forms, PKM2 is found in both tetrameric and dimeric forms.43 The tetrameric PKM2 exhibits high enzymatic activity, which is associated with ATP synthesis through OXPHOS and catabolic metabolism in cells. In contrast, the low enzymatic activity of the dimeric PKM2 provides a metabolic switch to regulate aerobic glycolysis (Figure 3).44 Additionally, the dimeric PKM2 form also facilitates the production of glycolytic intermediates that enters different branches of glycolysis, such as the PPP and glycerol synthesis (Figure 3).45 Thus, given the ability of PKM2 in regulating aerobic glycolysis, its function as a potential metabolic target in preventing cardiovascular disorders such as thrombosis, atherosclerosis, and stroke has been investigated in multiple cell types including platelets, macrophages, and neutrophils.6,10,46–51
Our lab reported that human platelet activation is associated with an increase in the dimeric PKM2 expression (relative to its tetrameric form) along with a rise in glucose uptake and lactate production, implying an increase in aerobic glycolysis.10 The treatment with ML265 (a small-molecule activator that stabilizes PKM2 tetramers and limits the formation of PKM2 dimers) (Figure 3)52 prevented the formation of PKM2 dimers along with a significant reduction in glucose uptake and lactate production in agonist-stimulated platelets, suggesting a reduction in the extent of aerobic glycolysis.10 Furthermore, the phosphorylation of PKM2 has been reported to promote the dimeric state of PKM2 relative to its tetrameric form.53 In agreement with this, another study reported an increase in PKM2 phosphorylation in thrombin-stimulated platelets, providing further evidence that demonstrates the association of dimeric PKM2 with aerobic glycolysis in activated platelets.6 Our group also observed that reduced dimeric PKM2 formation following ML265 treatment attenuated multiple aspects of human and mouse platelet functions, including aggregation, integrin αIIbβ3 affinity upregulation, secretion, and clot retraction.10 Furthermore, the size of the thrombi was significantly reduced in ML265-treated human whole blood that was perfused over collagen ex vivo at an arterial shear rate in a microfluidic flow chamber.10 In agreement with this, treatment of platelets with diaryl sulfonamide (DASA), a small-molecule modulator of PKM2 (Figure 3), inhibited multiple agonist-induced platelet functions.6 Furthermore, treatment with ML265 or DASA also reduced the susceptibility to arterial thrombosis.6,10 Interestingly, while ML265 did not affect the tail-bleeding time, treatment with DASA was associated with prolonged bleeding time.6,10 We further validated these observations using platelet-specific PKM2-deficient mice (PKM2fl/flPF4Cre+). The platelet-specific deletion of PKM2 inhibited agonist-evoked lactate production and platelet functions.10 Furthermore, similar to ML265 treatment, platelet-specific PKM2 ablation significantly reduced the susceptibility to arterial thrombosis.10 In addition to the effects on platelet functions, pharmacological and genetic targeting of dimeric PKM2 reduced the PI3-kinase–mediated Akt/GSK3 signaling in convulxin or thrombin-stimulated platelets.10
Besides modulating aerobic glycolysis, the dimeric PKM2 also enhances the production of glycolytic intermediates such as glucose-6-phosphate (glucose-6-P), an important substrate for the PPP. The increased flux of glucose-6-P through PPP generates NADPH, which is further catalyzed by NADPH oxidase (NOX) to promote ROS generation, a stimulant that enhances platelet activation (Figure 3).54,55 Consistent with this, a significant increase in the NADPH to total NADP(H) ratio in platelets following thrombin stimulation was identified. This finding demonstrates NADPH enrichment, a direct estimation of the PPP flux, in activated platelets.6 Indeed, NADPH accumulation also caused a NOX-mediated rise in ROS generation in thrombin-stimulated platelets.6 The NADPH enrichment and ROS generation were substantially reduced following treatment with DASA or dehydroepiandrosterone sulphate (DHEA; an inhibitor of glucose-6-P-dehydrogenase, a rate-limiting enzyme of the PPP), indicating a reduction in the PPP.6 These findings uncover a fundamental role of targeting dimeric PKM2 in modulating platelet activation, potentially through the regulation of aerobic glycolysis and the PPP.
Our lab also identified the role of PKM2 in modulating the progression of atherosclerosis using myeloid cell–specific PKM2−/− mice on Ldlr (low-density lipoprotein receptor)–deficient background (PKM2mye−KOLdlr−/−).47 The PKM2mye−KOLdlr−/− mice on a high-fat diet exhibited significantly reduced atherosclerotic lesions compared to littermate PKM2WTLdlr−/− controls.47 Furthermore, the macrophage content in lesions of these mice was significantly reduced with impaired glycolytic rate. The underlying mechanism for reduced atherosclerosis in PKM2mye−KOLdlr−/− mice was attributed to reduced inflammation coupled with enhanced efferocytosis (a process of phagocytic clearance of apoptotic cells).47 Moreover, several studies have reported that PKM2 can also exhibit neuroprotective effects and improve ischemic stroke outcomes.48–51 Our group identified that the expression of PKM2 was upregulated in human and mouse neutrophils after the onset of ischemic stroke, whereas the myeloid cell–specific deletion of PKM2 improved stroke outcome by limiting post-ischemic cerebral thrombo-inflammation in peripheral neutrophils.48 Taken together, these findings provide sufficient evidence to demonstrate that PKM2 plays an important role in the progression of atherosclerosis, thrombosis, and cerebral ischemic events such as stroke.
3. Mitochondrial OXPHOS
Despite lacking a nucleus, platelets feature a functional mitochondrion that enables ATP generation through OXPHOS, to support platelet energy requirements.5,8,56 Several studies have identified a transient increase in oxygen consumption through OXPHOS during platelet activation.6,7,9,11 While the glycolytic phenotype of platelets upon agonist stimulation is well established, the relative importance of OXPHOS in sustaining platelet activation remain unclear and requires further investigation. Notably, platelets have been shown to demonstrate the highest basal OCR compared to other blood cells such as monocytes and lymphocytes.19,56 Some of the early studies, using the inhibitors of the mitochondrial respiratory chain reported that ATP generated via OXHPOS is essential for normal platelet activation.57–59 The predominant glycolytic phenotype observed during the transition of platelet from resting to an activated state was found to be coupled with an increase in the ATP-linked respiration or the mitochondrial OCR,5,7–9 whereas the oligomycin-mediated inhibition of mitochondrial ATP synthase exhibits a large increase in basal ECAR, suggesting a rise in compensatory glycolysis to meet energy requirements. Similarly, treatment with oligomycin was also reported to increase the level of glycolysis as a compensatory response to meet ATP requirements in thrombin-stimulated platelets, further validating that OXPHOS contributes to ATP requirements in platelets.5,7 Another study suggested that platelet aggregation was driven principally through glycolytic ATP with a minimal role of mitochondrial ATP.60 Interestingly, the role of mitochondrial ATP was observed to be more pertinent in sustaining platelet granule secretion, platelet–neutrophil interactions, and thrombus growth, especially during insufficient compensation from glycolytic ATP.60 In agreement with this, it has been reported that blocking mitochondrial function using OXPHOS inhibitors does not impair platelet aggregation due to an increase in the level of compensatory glycolysis.6,8,61 As expected, the combined inhibition of glycolysis and OXPHOS synergistically facilitates a robust attenuation of platelet aggregation.8,62,63 Another interesting study reported that there is a significant decline in the number of mitochondria, mitochondrial proteins, and mitochondrial activity as platelets age in circulation.64 This significantly reduced the spreading of old platelets on fibrinogen along with a reduction in secretion upon agonist stimulation. Moreover, old platelets in comparison to the young ones were proposed to have a blunt hemostatic response.64 This study further suggests the importance of a viable mitochondria in ensuring a normal platelet activation.
Mitochondria, in addition to glucose oxidation, can also oxidize fatty acids and glutamine in platelets to generate ATP under resting and activated states.5,8,17 The choice of fuel for mitochondrial oxidation is influenced by the extent of energy requirement and the environment in which platelets function such as the nutrient availability and oxygen tension within the growing thrombus. Following an abrupt increase in the bioenergetic demand, such as during agonist stimulation, the mitochondrial ‘reserve capacity’ (MRC) can provide a boost to sustain mitochondrial functions to meet ATP requirements and avoid ATP crisis.5,19,65 The MRC in platelets is regulated chiefly by the oxidation of glucose-derived pyruvate or fatty acids.5,66 In comparison to monocytes and lymphocytes, only a modest increment in platelet MRC under resting state was observed that reached only 20% of the maximal mitochondrial respiration, whereas the ATP-linked OCR was identified to be highest (57%) in resting platelets compared to monocytes and lymphocytes.19,56 In addition to these findings, thrombin-stimulated platelets were also reported to use their reserve mitochondrial capacity besides enhanced glycolysis to meet the increased necessity for ATP synthesis.5
3.1. β-Oxidation of fatty acids
Fatty acids not only preserve the structural integrity of the platelet plasma membrane but also play a fundamental role in regulating platelet activation signaling and sustaining mitochondrial oxidation for platelet energy metabolism, especially upon agonist stimulation.67 Mitochondrial β-oxidation of fatty acids is a catabolic pathway that involves the enzymatic conversion of fatty acids into acetyl-CoA, which is then oxidized through the Krebs cycle.68 The reduced electron carriers (NADH+ and FADH2), generated by both fatty acid β-oxidation and the Krebs cycle, undergo OXPHOS to produce ATP.69 The fatty acid β-oxidation has been reported to contribute toward basal and thrombin-stimulated OCR in platelets.8 Inhibition of endogenous fatty acid β-oxidation using etomoxir (small-molecule inhibitor of fatty acid oxidation) decreased basal and thrombin-stimulated OCR, indicating that platelets utilize fatty acids to produce ATP. Treatment with etomoxir enhanced basal and thrombin-stimulated ECAR potentially as a glycolytic compensatory mechanism in response to reduced mitochondrial ATP generation.8 Consequently, compensation provided from glycolysis prevented inhibition of thrombin-stimulated platelet aggregation upon blocking of fatty acid β-oxidation. Interestingly, supplementation with exogenous fatty acids increased basal OCR, indicating the ability of platelets to transport extracellular fatty acids to sustain respiration. However, endogenous free fatty acids were sufficient to support the metabolic requirements of activated platelets as the addition of exogenous fatty acids did not increase thrombin-stimulated respiration.8 In line with this, platelet mitochondria were observed to switch freely between glucose and fatty acid oxidation under resting and activated conditions.5 Moreover, blocking either glucose oxidation or fatty acid β-oxidation using UK5099 or etomoxir, respectively, could decrease the OCR. However, in contrast to the previous observations,8 independent downregulation of glucose or fatty acid oxidation did not elicit any effect on ECAR. Meanwhile, the combined suppression of glucose and fatty acid β-oxidation enhanced aerobic glycolysis as a compensatory feedback to meet ATP requirements.5
Recently, a comprehensive bioenergetics analysis performed on resting and thrombin-stimulated human platelets reported a reduction in OXPHOS following treatment with three pharmacologically distinct inhibitors of fatty acid oxidation inhibitors (etomoxir, trimetazidine, and oxfenicine).17 The impaired OXPHOS inhibited a variety of agonist-stimulated platelet functions and reduced the extent of in vitro thrombus formation in human washed platelets that were perused on immobilized collagen under arterial shear. Furthermore, treatment with fatty acid oxidation inhibitors protected mice from ferric chloride–induced mesenteric thrombosis without affecting the tail-bleeding time.17 Besides mitochondrial oxidation, the synthesis of fatty acids has also been shown to play a profound role in facilitating platelet activation and thrombosis.67 Acetyl-CoA carboxylase (ACC) exists in two isoforms (ACC1 and ACC2) that are well-known regulators of fatty acid synthesis. While ACC1 catalyzes the synthesis of malonyl-CoA for fatty acid production, ACC2 prevents the entry of fatty acids into the mitochondria to drive fatty acid oxidation.70 The activity of ACC is functionally regulated by AMP-activated protein kinase (AMPK)–mediated inhibitory phosphorylation of the serine residues.71 Incorporation of alanine double knock-in (DKI) mutation on the Ser79 and Ser212 residues of ACC1 and ACC2, respectively, reduced AMPK signaling to ACC, leading to an increased synthesis of arachidonic acid (AA)–containing phosphatidylethanolamine plasmalogen lipids. The AA facilitates thromboxane A2 (TxA2) generation via COX-1, which in turn stimulates platelet activation and secretion of ADP from dense granules. Consequently, impaired AMPK-ACC signaling amplified collagen-evoked TxA2 generation and dense granule secretion. Additionally, the suppression of ACC phosphorylation in DKI mice enhanced arterial thrombosis in vivo and thrombus growth ex vivo on collagen-coated surfaces under flow.67
In addition to long-chain fatty acids (LCFAs), plasma also contains short- and medium-chain fatty acids (SCFAs and MCFAs) that are readily imported by the platelets. Given the abundance of LCFAs, compared to the other two forms, the oxidation of fatty acids in platelets in general refers to the oxidation of LCFAs. There is a paucity of studies suggesting the contribution of SCFAs and MCFAs in fueling mitochondrial β-oxidation and requires investigation. Nonetheless, the findings discussed in this review provide evidence that suggests the vital role of mitochondrial oxidation of LCFAs in fulfilling ATP requirements of resting and agonist-stimulated platelets.
3.2. Glutamine oxidation
Glutaminolysis is a process characterized by the glutaminase-mediated conversion of glutamine into the Krebs cycle metabolites such as alpha-ketoglutarate (α-KG) to support mitochondrial OXPHOS.72 The basal and thrombin-stimulated OCR was found to be significantly downregulated in the assay performed using glutamine-depleted XF DMEM media. Additionally, the treatment of platelets with azaserine (Aza), a structural analog to glutamine that inactivate glutaminase, attenuated thrombin-stimulated OCR, suggesting the role of glutamine in supporting mitochondrial respiration.8 The inhibition of glutaminolysis alone or in combination with fatty acid β-oxidation did not prevent platelet aggregation, due to compensatory ATP synthesis from glycolysis. Notably, it is important to emphasize that the concentration of glutamine used in this study was 5- to 8-fold higher (4 mM) compared to the physiological plasma levels.8 On the flip side, Aibibula et al. reported minimal contribution of glutamine in supporting mitochondrial OCR compared to glucose and fatty acid oxidation.5 Moreover, blocking glutamine oxidation using BPTES (glutaminase inhibitor) did not affect the ECAR.5
4. Future directions
While these outcomes are promising, further studies in several key areas are required to conclusively delineate that metabolic targeting of platelets using small-molecule inhibitors can be an alternative antithrombotic strategy. In this section, we briefly describe some important avenues that require investigation to further dissect the effects of targeting metabolic checkpoints in platelets and further establish their role in combating thrombosis.
4.1. Metabolic targeting of platelets in the presence of comorbidities
The antithrombotic effects of targeting these metabolic checkpoints in the presence of comorbidities such as hypercholesterolemia, obesity, and diabetes require comprehensive examination using animal models and clinical studies. This is particularly important as most of the cardiovascular complications including atherothrombosis are generally associated with the presence of one or multiple comorbidities.73–75 As glucose is an essential energy substrate for platelets, prolonged exposure to high concentrations of glucose in cases of type 2 diabetes can significantly alter the functioning of platelet mitochondria.76,77 High levels of glucose can enhance mitochondrial function leading to increased oxygen consumption and mitochondrial membrane potential.78 Hyperglycemia induces mitochondrial membrane hyperpolarization in normal platelets causing excessive ROS production and intensified platelet aggregation.59,76 Therefore, preserving platelet mitochondrial function is also necessary to decrease the risk of thrombotic events in diabetic patients. It is plausible that inhibitors of OXHOS or fatty acid β-oxidation may offer protective benefits by preventing platelet activation in the context of diabetes.
4.2. Effects of metabolic targeting in other cell types known to contribute to thrombosis
Other cell types such as neutrophils and activated monocytes exhibit a high rate of aerobic glycolysis and are known to participate in the progression of arterial thrombosis.19,79–82 Thus, it would be worthwhile to investigate whether targeting metabolic checkpoints (such as PDKs and PKM2) in these cells using small-molecule inhibitors or cell-specific KO models affects the development of thrombosis.
4.3. Effects of metabolic targeting on deep vein thrombosis
The studies performed so far provide evidence that suggests the effects of targeting different metabolic pathways in platelets to prevent experimental arterial thrombosis. However, venous thrombosis is characterized by different pathophysiology and specific treatments, which is highly distinct in comparison to arterial thrombosis.83 Therefore, future studies are also warranted to identify the effects of metabolic targeting on the progression of deep vein thrombosis in mice, followed by clinical investigations.
4.4. The effects of metabolic small-molecule inhibitors on human arterial thrombosis
The studies discussed in this article exclusively focused on evaluating the in vitro effects of small-molecule inhibitors of metabolic enzymes in inhibiting human or mouse platelet functions and thrombosis in vivo in the arterial circulation of mouse models. These studies are indeed proof-of-concept findings; however, clinical investigations are needed to validate these findings in the context of human arterial thrombosis and to further evaluate their application in a broader context.
4.5. Potential off-target effects of metabolic small-molecule inhibitors
In contrast to conventional antiplatelet targets such as GPIb, GPVI, and GPIIb/IIIa receptors that are almost exclusively expressed on platelets,84–86 metabolic enzymes such as PDKs and PKM2 exhibit a broad expression profile across different cell types. This poses an increased risk of unwanted off-target effects of the small-molecule metabolic modulators, which needs to be scrutinized rigorously through clinical investigations.
5. Conclusions
The last few years have provided definitive insights into the role of various metabolic checkpoints that can be targeted to prevent platelet activation to prevent experimental thrombosis. For instance, the switch to a ‘Warburg’ or aerobic glycolytic phenotype over OXPHOS is a prerequisite to the process of platelet activation and has received considerable attention recently. Both genetic or pharmacological (using small-molecule inhibitors) targeting of vital metabolic checkpoints that fuel aerobic glycolysis (such as PDKs and dimeric PKM2) in platelets have demonstrated the apparent requirement of this process for normal platelet activation (Figure 3). Favoring this less efficient but rapid process of ATP production is a conserved metabolic feature that platelets share with different developmentally related lineages of blood cells including, dendritic cells, monocytes, T cells, and macrophages.87–93 Besides aerobic glycolysis, it has also been shown that fatty acids, and glutamine oxidation can also be therapeutically targeted for antiplatelet effects (Figure 3).
An ideal antiplatelet drug must provide global protection against multiple platelet activators without affecting hemostasis, an aim that has remained largely elusive for decades. Targeting vital metabolic checkpoints, especially those regulating aerobic glycolysis in platelets, may have potential therapeutic benefits in preventing thrombosis. As discussed previously, targeting metabolic pathways can provide broad protection against a variety of agonists to prevent pathological platelet activation. Additionally, the emerging antiatherosclerotic effects of targeting metabolic pathways coupled with their plausible ability to inhibit neutrophil activity (known to participate in this thrombus development) may provide additional benefits in reducing the development of thrombosis, though these speculations require examination.
In conclusion, the findings summarized herein suggest that targeting metabolic pathways and related checkpoints might offer cardioprotective effects by combating thrombosis and related disorders. However, as applicable to any novel antiplatelet therapy in development, it would be of paramount importance to establish that the anti-ischemic benefits of metabolic targeting in platelets are not offset by an increased risk of bleeding and off-target effects.
Authors’ contributions
G.D.F. did the literature research and wrote the article. M.K.N., M.G., and A.K.C. performed the critical revision. All the authors reviewed the manuscript and approved the submitted version.
Acknowledgements
The figures in this article were created using BioRender.com.
Contributor Information
Gagan D Flora, Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa, Iowa City, IA, USA.
Manasa K Nayak, Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa, Iowa City, IA, USA.
Madankumar Ghatge, Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa, Iowa City, IA, USA.
Anil K Chauhan, Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa, Iowa City, IA, USA.
Funding
The A.K.C laboratory is supported by grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute (R35HL139926), and the National Institute of Neurological Disorders and Stroke (R01NS109910 and U01NS130587). The American Heart Association postdoctoral award (916430) and career development award (942168) support G.D.F. and M.K.N., respectively.
Data availability
Data availability is not applicable to this review as no datasets were generated or analysed.
References
- 1. Tatsumi K, Mackman N. Tissue factor and atherothrombosis. J Atheroscler Thromb 2015;22:543–549. [DOI] [PubMed] [Google Scholar]
- 2. Asada Y, Yamashita A, Sato Y, Hatakeyama K. Pathophysiology of atherothrombosis: mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol Int 2020;70:309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Estevez B, Du X. New concepts and mechanisms of platelet activation signaling. Physiology (Bethesda) 2017;32:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Uden R, Houtenbos I, Griffioen-Keijzer A, Odekerken DAM, van den Bemt P, Becker ML. Guidelines for mono, double and triple antithrombotic therapy. Postgrad Med J 2021;97:730–737. [DOI] [PubMed] [Google Scholar]
- 5. Aibibula M, Naseem KM, Sturmey RG. Glucose metabolism and metabolic flexibility in blood platelets. J Thromb Haemost 2018;16:2300–2314. [DOI] [PubMed] [Google Scholar]
- 6. Kulkarni PP, Tiwari A, Singh N, Gautam D, Sonkar VK, Agarwal V, Dash D. Aerobic glycolysis fuels platelet activation: small-molecule modulators of platelet metabolism as anti-thrombotic agents. Haematologica 2019;104:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nayak MK, Dhanesha N, Doddapattar P, Rodriguez O, Sonkar VK, Dayal S, Chauhan AK. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood advances 2018;2:2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravi S, Chacko B, Sawada H, Kramer PA, Johnson MS, Benavides GA, O’Donnell V, Marques MB, Darley-Usmar VM. Metabolic plasticity in resting and thrombin activated platelets. PLoS One 2015;10:e0123597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flora GD, Nayak MK, Ghatge M, Kumskova M, Patel RB, Chauhan AK. Mitochondrial pyruvate dehydrogenase kinases contribute to platelet function and thrombosis in mice by regulating aerobic glycolysis. Blood Adv 2023;7(11):2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nayak MK, Ghatge M, Flora GD, Dhanesha N, Jain M, Markan KR, Potthoff MJ, Lentz SR, Chauhan AK. The metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood 2021;137:1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sowton AP, Millington-Burgess SL, Murray AJ, Harper MT. Rapid kinetics of changes in oxygen consumption rate in thrombin-stimulated platelets measured by high-resolution respirometry. Biochem Biophys Res Commun 2018;503:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghatge M, Nayak MK, Flora GD, Kumskova M, Jain A, Patel RB, Lin Z, Usachev YM, Chauhan AK. Mitochondrial calcium uniporter b deletion inhibits platelet function and reduces susceptibility to arterial thrombosis. J Thromb Haemost: JTH 2023;21(8):2163–2174. [DOI] [PubMed] [Google Scholar]
- 13. Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J Clin Invest 1967;46:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kholmukhamedov A, Jobe S. Platelet respiration. Blood Advances 2019;3:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vasta V, Meacci E, Farnararo M, Bruni P. Glutamine utilization in resting and stimulated platelets. J Biochem 1993;114:163–166. [DOI] [PubMed] [Google Scholar]
- 16. Rosenzweig A, Ways P. The oxidation of long-chain fatty acids by the formed elements of human blood. Blood 1966;27:57–64. [PubMed] [Google Scholar]
- 17. Kulkarni PP, Ekhlak M, Singh V, Kailashiya V, Singh N, Dash D. Fatty acid oxidation fuels agonist-induced platelet activation and thrombus formation: targeting β-oxidation of fatty acids as an effective anti-platelet strategy. Faseb j 2023;37:e22768. [DOI] [PubMed] [Google Scholar]
- 18. Bender M, Palankar R. Platelet shape changes during thrombus formation: role of actin-based protrusions. Hamostaseologie 2021;41:14–21. [DOI] [PubMed] [Google Scholar]
- 19. Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest 2013;93:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001;292:504–507. [DOI] [PubMed] [Google Scholar]
- 22. Zheng J. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (review). Oncol Lett 2012;4:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 2016;41:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol 2018;14:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y, Sauve AA. NAD(+) metabolism: bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta 2016;1864:1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S, Fu S, Wang G, Cao Y, Li L, Li X, Yang J, Li N, Shan Y, Cao Y, Ma Y, Dong M, Liu Q, Jiang H. Glycerol-3-phosphate biosynthesis regenerates cytosolic NAD(+) to alleviate mitochondrial disease. Cell Metab 2021;33:1974–1987.e1979. [DOI] [PubMed] [Google Scholar]
- 27. Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 2012;23:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kane DA. Lactate oxidation at the mitochondria: a lactate-malate-aspartate shuttle at work. Front Neurosci 2014;8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci 2012;13:10697–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fidler TP, Campbell RA, Funari T, Dunne N, Balderas Angeles E, Middleton EA, Chaudhuri D, Weyrich AS, Abel ED. Deletion of GLUT1 and GLUT3 reveals multiple roles for glucose metabolism in platelet and megakaryocyte function. Cell Rep 2017;21:1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akkerman JW, Holmsen H. Interrelationships among platelet responses: studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2 + secretion. Blood 1981;57:956–966. [PubMed] [Google Scholar]
- 32. Misselwitz F, Leytin VL, Repin VS. Effect of metabolic inhibitors on platelet attachment, spreading and aggregation on collagen-coated surfaces. Thromb Res 1987;46:233–240. [DOI] [PubMed] [Google Scholar]
- 33. Chaudhry AA, Sagone AL Jr, Metz EN, Balcerzak SP. Relationship of glucose oxidation to aggregation of human platelets. Blood 1973; 41:249–258. [PubMed] [Google Scholar]
- 34. Mookerjee SA, Brand MD. Measurement and analysis of extracellular acid production to determine glycolytic rate. J Vis Exp 2015;(106):e53464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prakhya KS, Vekaria H, Coenen DM, Omali L, Lykins J, Joshi S, Alfar HR, Wang QJ, Sullivan P, Whiteheart SW. Platelet glycogenolysis is important for energy production and function. Platelets 2023;34:2222184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mookerjee SA, Goncalves RL, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochimica et Biophysica Acta (BBA)-Bioenergetics 2015;1847:171–181. [DOI] [PubMed] [Google Scholar]
- 37. Montilla A, Ruiz A, Marquez M, Sierra A, Matute C, Domercq M. Role of mitochondrial dynamics in microglial activation and metabolic switch. ImmunoHorizons 2021;5:615–626. [DOI] [PubMed] [Google Scholar]
- 38. Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 2003;31:1143–1151. [DOI] [PubMed] [Google Scholar]
- 39. Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond) 2014;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Min BK, Park S, Kang HJ, Kim DW, Ham HJ, Ha CM, Choi BJ, Lee JY, Oh CJ, Yoo EK, Kim HE, Kim BG, Jeon JH, Hyeon DY, Hwang D, Kim YH, Lee CH, Lee T, Kim JW, Choi YK, Park KG, Chawla A, Lee J, Harris RA, Lee IK. Pyruvate dehydrogenase kinase is a metabolic checkpoint for polarization of macrophages to the M1 phenotype. Front Immunol 2019;10:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forteza MJ, Berg M, Edsfeldt A, Sun J, Baumgartner R, Kareinen I, Casagrande FB, Hedin U, Zhang S, Vuckovic I, Dzeja PP, Polyzos KA, Gisterå A, Trauelsen M, Schwartz TW, Dib L, Herrmann J, Monaco C, Matic L, Gonçalves I, Ketelhuth DFJ. Pyruvate dehydrogenase kinase regulates vascular inflammation in atherosclerosis and increases cardiovascular risk. Cardiovasc Res 2023;119(7):1524–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol 2020;10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 2011;43:969–980. [DOI] [PubMed] [Google Scholar]
- 44. Wong N, De Melo J, Tang D. PKM2, A central point of regulation in cancer metabolism. Int J Cell Biol 2013;2013:242513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F. PKM2 And cancer: the function of PKM2 beyond glycolysis. Oncol Lett 2016;11:1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li M, Lu W, Meng Y, Zhang W, Wang F, Sun L, Xu Y. Tetrahydroxy stilbene glucoside alleviates ischemic stroke by regulating conformation-dependent intracellular distribution of PKM2 for M2 macrophage polarization. J Agric Food Chem 2022;70:15449–15463. [DOI] [PubMed] [Google Scholar]
- 47. Doddapattar P, Dev R, Ghatge M, Patel RB, Jain M, Dhanesha N, Lentz SR, Chauhan AK. Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circ Res 2022;130:1289–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dhanesha N, Patel RB, Doddapattar P, Ghatge M, Flora GD, Jain M, Thedens D, Olalde H, Kumskova M, Leira EC, Chauhan AK. PKM2 Promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood 2022;139:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feng Y, Li X, Wang J, Huang X, Meng L, Huang J. Pyruvate kinase M2 (PKM2) improve symptoms of post-ischemic stroke depression by activating VEGF to mediate the MAPK/ERK pathway. Brain Behav 2022;12:e2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen D, Wei L, Liu ZR, Yang JJ, Gu X, Wei ZZ, Liu LP, Yu SP. Pyruvate kinase M2 increases angiogenesis, neurogenesis, and functional recovery mediated by upregulation of STAT3 and focal adhesion kinase activities after ischemic stroke in adult mice. Neurotherapeutics 2018;15:770–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin L, Wang X, Yu Z. Ischemia-reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem Pharmacol (Los Angel) 2016;5(4):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang JK, Walsh MJ, Brimacombe KR, Anastasiou D, Yu Y, Israelsen WJ, Hong BS, Tempel W, Dimov S, Veith H, Yang H, Kung C, Yen KE, Dang L, Salituro F, Auld DS, Park HW, Vander Heiden MG, Thomas CJ, Shen M, Boxer MB. ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. Probe reports from the NIH molecular libraries program. Bethesda (MD): National Center for Biotechnology Information (US); 2010. [PubMed] [Google Scholar]
- 53. Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2009;2:ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dayal S, Wilson KM, Motto DG, Miller F Jr, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013;127:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 2016;36:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2014;2:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barile CJ, Herrmann PC, Tyvoll DA, Collman JP, Decreau RA, Bull BS. Inhibiting platelet-stimulated blood coagulation by inhibition of mitochondrial respiration. Proc Natl Acad Sci U S A 2012;109:2539–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rusak T, Tomasiak M, Ciborowski M. Peroxynitrite can affect platelet responses by inhibiting energy production. Acta Biochim Pol 2006;53:769–776. [PubMed] [Google Scholar]
- 59. Yamagishi S-I, Edelstein D, Du X-L, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 2001;50:1491–1494. [DOI] [PubMed] [Google Scholar]
- 60. Kulkarni PP, Ekhlak M, Sonkar VK, Dash D. Mitochondrial ATP generation in stimulated platelets is essential for granule secretion but dispensable for aggregation and procoagulant activity. Haematologica 2022;107:1209–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaczara P, Sitek B, Przyborowski K, Kurpinska A, Kus K, Stojak M, Chlopicki S. Antiplatelet effect of carbon monoxide is mediated by NAD+ and ATP depletion. Arterioscler Thromb Vasc Biol 2020; 40:2376–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Holmsen H, Setkowsky CA, Day HJ. Effects of antimycin and 2-deoxyglucose on adenine nucleotides in human platelets. Role of metabolic adenosine triphosphate in primary aggregation, secondary aggregation and shape change of platelets. Biochem J 1974;144:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holmsen H, Robkin L, Day HJ. Effects of antimycin A and 2-deoxyglucose on secretion in human platelets. Differential inhibition of the secretion of acid hydrolases and adenine nucleotides. Biochem J 1979;182:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Allan HE, Hayman MA, Marcone S, Chan MV, Edin ML, Maffucci T, Joshi A, Menke L, Crescente M, Mayr M, Zeldin DC, Armstrong PC, Warner TD. Proteome and functional decline as platelets age in the circulation. J Thromb Haemost 2021;19:3095–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marchetti P, Fovez Q, Germain N, Khamari R, Kluza J. Mitochondrial spare respiratory capacity: mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J 2020;34:13106–13124. [DOI] [PubMed] [Google Scholar]
- 66. Nguyen QL, Corey C, White P, Watson A, Gladwin MT, Simon MA, Shiva S. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. Jci Insight 2017;2(5):e91415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lepropre S, Kautbally S, Octave M, Ginion A, Onselaer MB, Steinberg GR, Kemp BE, Hego A, Wéra O, Brouns S, Swieringa F, Giera M, Darley-Usmar VM, Ambroise J, Guigas B, Heemskerk J, Bertrand L, Oury C, Beauloye C, Horman S. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood 2018;132:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumari A. Chapter 4—Beta oxidation of fatty acids. In Kumari A (ed). Sweet Biochemistry: Academic Press, 2018. pp. 17–19. [Google Scholar]
- 69. Ma Y, Wang W, Devarakonda T, Zhou H, Wang X-Y, Salloum FN, Spiegel S, Fang X. Functional analysis of molecular and pharmacological modulators of mitochondrial fatty acid oxidation. Sci Rep 2020;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ito H, Nakamae I, Kato JY, Yoneda-Kato N. Stabilization of fatty acid synthesis enzyme acetyl-CoA carboxylase 1 suppresses acute myeloid leukemia development. J Clin Invest 2021;131(12):e141529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zordoky BN, Nagendran J, Pulinilkunnil T, Kienesberger PC, Masson G, Waller TJ, Kemp BE, Steinberg GR, Dyck JR. AMPK-dependent inhibitory phosphorylation of ACC is not essential for maintaining myocardial fatty acid oxidation. Circ Res 2014;115:518–524. [DOI] [PubMed] [Google Scholar]
- 72. Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med 2020;52:1496–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020;126:1477–1500. [DOI] [PubMed] [Google Scholar]
- 74. Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr 2019;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci 2020;21(5):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Siewiera K, Kassassir H, Talar M, Wieteska L, Watala C. Higher mitochondrial potential and elevated mitochondrial respiration are associated with excessive activation of blood platelets in diabetic rats. Life Sci 2016;148:293–304. [DOI] [PubMed] [Google Scholar]
- 77. Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets 2009;20:588–593. [DOI] [PubMed] [Google Scholar]
- 78. Siewiera K, Labieniec-Watala M, Kassassir H, Wolska N, Polak D, Watala C. Potential role of mitochondria as modulators of blood platelet activation and reactivity in diabetes and effect of metformin on blood platelet bioenergetics and platelet activation. Int J Mol Sci 2022;23(7):3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood J Am Soc Hematol 2016;128:753–762. [DOI] [PubMed] [Google Scholar]
- 80. Richer BC, Salei N, Laskay T, Seeger K. Changes in neutrophil metabolism upon activation and aging. Inflammation 2018;41:710–721. [DOI] [PubMed] [Google Scholar]
- 81. Tan C, Gu J, Chen H, Li T, Deng H, Liu K, Liu M, Tan S, Xiao Z, Zhang H, Xiao X. Inhibition of aerobic glycolysis promotes neutrophil to influx to the infectious site via CXCR2 in sepsis. Shock 2020;53:114–123. [DOI] [PubMed] [Google Scholar]
- 82. Kapoor S, Opneja A, Nayak L. The role of neutrophils in thrombosis. Thromb Res 2018;170:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Delluc A, Lacut K, Rodger MA. Arterial and venous thrombosis: what’s the link? A narrative review. Thromb Res 2020;191:97–102. [DOI] [PubMed] [Google Scholar]
- 84. Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost 2013;11:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Perrella G, Nagy M, Watson SP, Heemskerk JWM. Platelet GPVI (glycoprotein VI) and thrombotic complications in the venous system. Arterioscler Thromb Vasc Biol 2021;41:2681–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yakushkin VV, Zyuryaev IT, Khaspekova SG, Sirotkina OV, Ruda MY, Mazurov AV. Glycoprotein IIb-IIIa content and platelet aggregation in healthy volunteers and patients with acute coronary syndrome. Platelets 2011;22:243–251. [DOI] [PubMed] [Google Scholar]
- 87. Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010;115:4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013;38:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Palmer CS, Cherry CL, Sada-Ovalle I, Singh A, Crowe SM. Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis. EBioMedicine 2016;6:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 91. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O'Neill LA. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 2015;21:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. de-Brito NM, Duncan-Moretti J, da-Costa HC, Saldanha-Gama R, Paula-Neto HA, Dorighello GG, Simões RL, Barja-Fidalgo C. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta Mol Cell Res 2020;1867:118604. [DOI] [PubMed] [Google Scholar]
- 93. O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013;493:346–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this review as no datasets were generated or analysed.