Introduction
Epilepsy is a common neurological disorder affecting approximately 1% of the global population.1 Approximately 40% of patients don’t respond to medical treatment alone. Patients with drug resistant epilepsy (DRE) can be treated surgically through resection, ablation, or neuromodulation and between 65–70% of patients can achieve seizure freedom.2,3 Surgical treatment of epilepsy is predicated on the successful localization of the area thought to be generating seizures, the seizure onset zone (SOZ).4,5 However, localization is a complex process that can differ across institutions and does not always result in a clear surgical plan.6 In fact, nearly 50% of those evaluated for epilepsy surgery are not candidates for surgery and the principle reason is the lack of a hypothesis for SOZ localization (Figure 1).7 Despite this difference, surgical cases have been stable. Therefore, it has been proposed that the complexity of epilepsy being evaluated for surgery has increased in recent years.7
Figure 1: Epilepsy surgery evaluations are increasing, but resection trends are stable, and the most cited reason is the lack of a hypothesis.
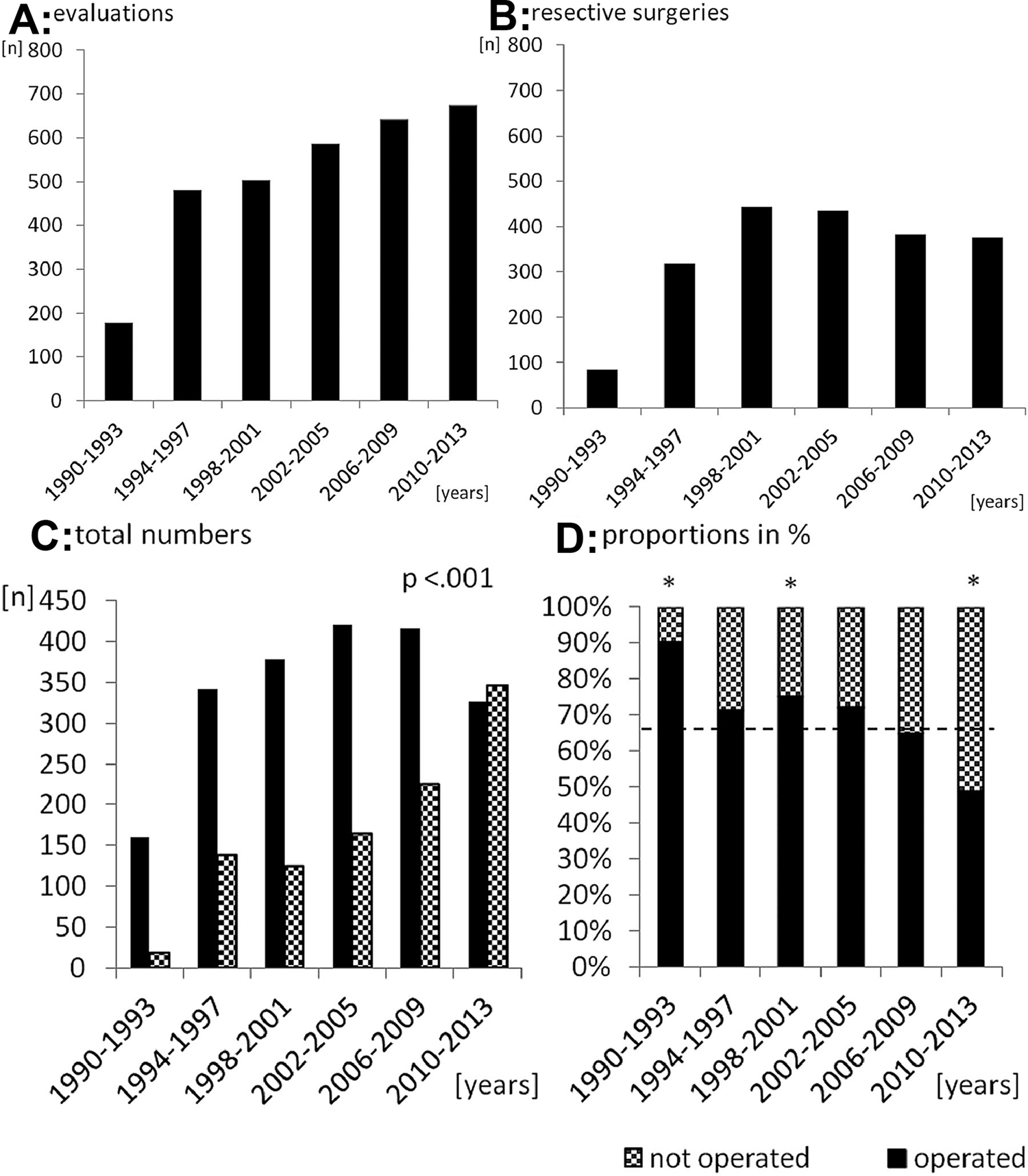
Panel A shows the number of presurgical evaluations across different timepoints. Panel B shows the number of resective surgeries during the same timepoints. Panel C shows the percentage of rejected cases by patient/parent or neurologist. Panel D shows the breakdown of reason for rejection, with the most common being risks and no hypothesis. Adapted from Cloppenborg et al7; with permission.
Shifts in patient complexity have occurred before. From the early to late 20th century, the surgical treatment of epilepsy vastly expanded as physicians were able to better localize the SOZ. Originally, epilepsy surgery was limited to only Jacksonian focal epilepsy syndromes. The clinical features combined with intraoperative stimulation to localize the “spasming center” were the only localizing methodologies available.8–10 The addition of electroencephalography (EEG) along with more advanced intraoperative stimulation spurred the expansion of epilepsy surgery to temporal lobectomies.11 Localization was improved further by intracranial EEG (iEEG) and neuroimaging such as magnetic resonance imaging (MRI).12–14 While challenging, the prior paradigm shift led to one of the most effective operations in the field of neurosurgery.9,10 Just as historical approaches to epilepsy surgery shifted their paradigm from the “spasming center” to the SOZ, the modern epilepsy surgery paradigm has shifted from the SOZ to the seizure network.
The “network revolution” in epilepsy has shifted the paradigm of epilepsy surgery towards localization strategies that quantify the network and treatment plans that disrupt the network (Figure 2A).15–18 The seizure network can be disrupted by resection, ablation, neuromodulation, or a combination of modalities (Figure 2B–D). However, the network must first be discovered, quantified, then treated. Representing the brain as a network relies on examining the connectivity, or relation of signals between areas of the brain. Structural connectivity (SC) represents white matter connections between regions. Functional connectivity (FC) represents neural communication between areas in the brain, with high FC representing a strong connection and low FC representing a weak connection. These measures are often derived from diffusion weighted imaging (DWI), functional magnetic resonance imaging (fMRI), and iEEG. Discovery of the seizure network may be completed noninvasively through neuroimaging techniques such as DWI and fMRI. DWI and fMRI have shown promise in the lateralization of the epileptic network as well as the prediction of seizure freedom after surgery. Localization of the network can be completed with iEEG. The addition of connectivity-based studies may be a missing link in identifying and treating the seizure network in patients previously not considered epilepsy surgery candidates.
Figure 2: Conceptual depiction of how various treatment modalities may disrupt the seizure network in epilepsy.
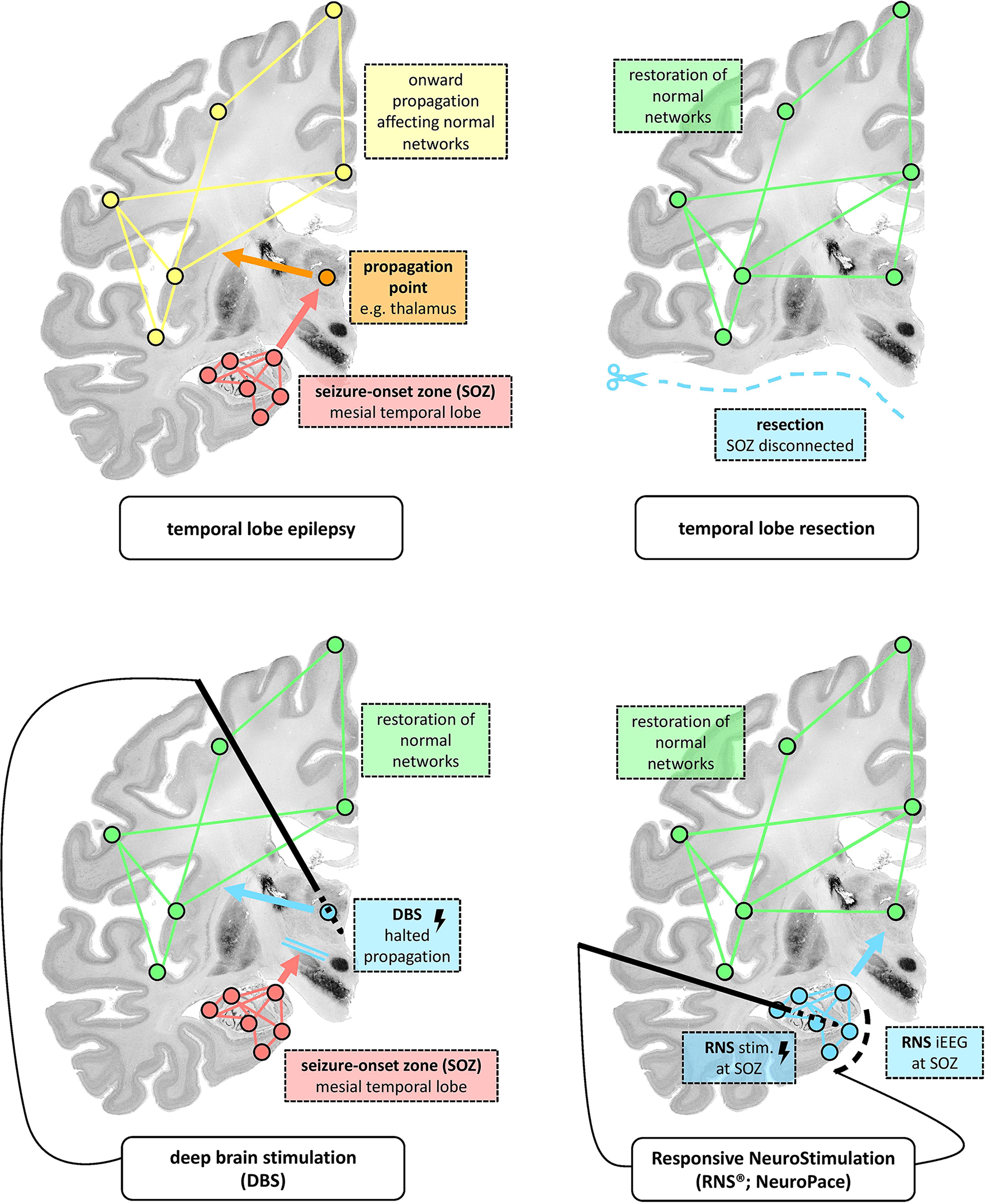
Panel A depicts normal networks, propagation networks, and the seizure network. Panel B demonstrates how resection is thought to disrupt the seizure network. Panel C demonstrates how DBS is thought to disrupt the seizure network. Panel D demonstrates how RNS is through to disrupt the seizure network. Adapted from Piper et al15; with permission.
Functional Magnetic Resonance Imaging (fMRI)
FMRI is a non-invasive imaging technique that is being increasingly utilized in the pre-surgical workup for epilepsy for lateralization and localization of language and memory.19,20 However, network studies with fMRI may benefit epilepsy surgery through lateralization of the seizure network and prediction of seizure freedom.
Lateralization
Lateralization of the epileptic network is one of the key predictors of seizure freedom.5,21,22 While conventional methods can lateralize seizure networks in most patients, more complex patients are difficult to lateralize and may benefit from fMRI augmented lateralization.23
FC abnormalities in mesial temporal networks have been relatively successful in lateralizing epilepsy.24 However, the exact connections vary between studies. Narasimhan et al used FC between mesial temporal structures and the default mode network to lateralize mesial temporal lobe epilepsy (mTLE) patients.25 Morgan et al sought to improve lateralization by splitting their cohort of temporal lobe epilepsy (TLE) patients into those who seizure free after surgery vs not seizure free after surgery. The authors found that FC from the hippocampi to the ventral lateral nucleus of the right thalamus was the best differentiator for left TLE vs right TLE.26
Given these slight disparities in lateralization hypotheses, machine learning methods have been utilized to improve epilepsy lateralization, but results have been mixed. A machine learning study by Gholipour et al used fMRI data from a multicenter cohort to lateralize TLE but achieved an accuracy below that of FC methods alone.27 Deep learning models may improve this accuracy, but they must remain interpretable. Only recently have studies began using these methods with Luckett et al publishing innovative work utilizing a convolutional neural network.28 A unique strength of this study was the interrogation of what anatomical areas the model used to classify left and right TLE, overcoming the “black box” nature of deep learning (Figure 3). Like prior FC studies, the authors found that resting state networks including the default mode network, medial temporal network, and dorsal attention network were all the strongest predictors of left vs right TLE (Figure 3). The overall accuracy was substantially greater than other methods, but the generalizability is unknown.
Figure 3: Deep learning approaches to lateralize TLE can be used both as tools for epilepsy surgery and hypothesis generation.
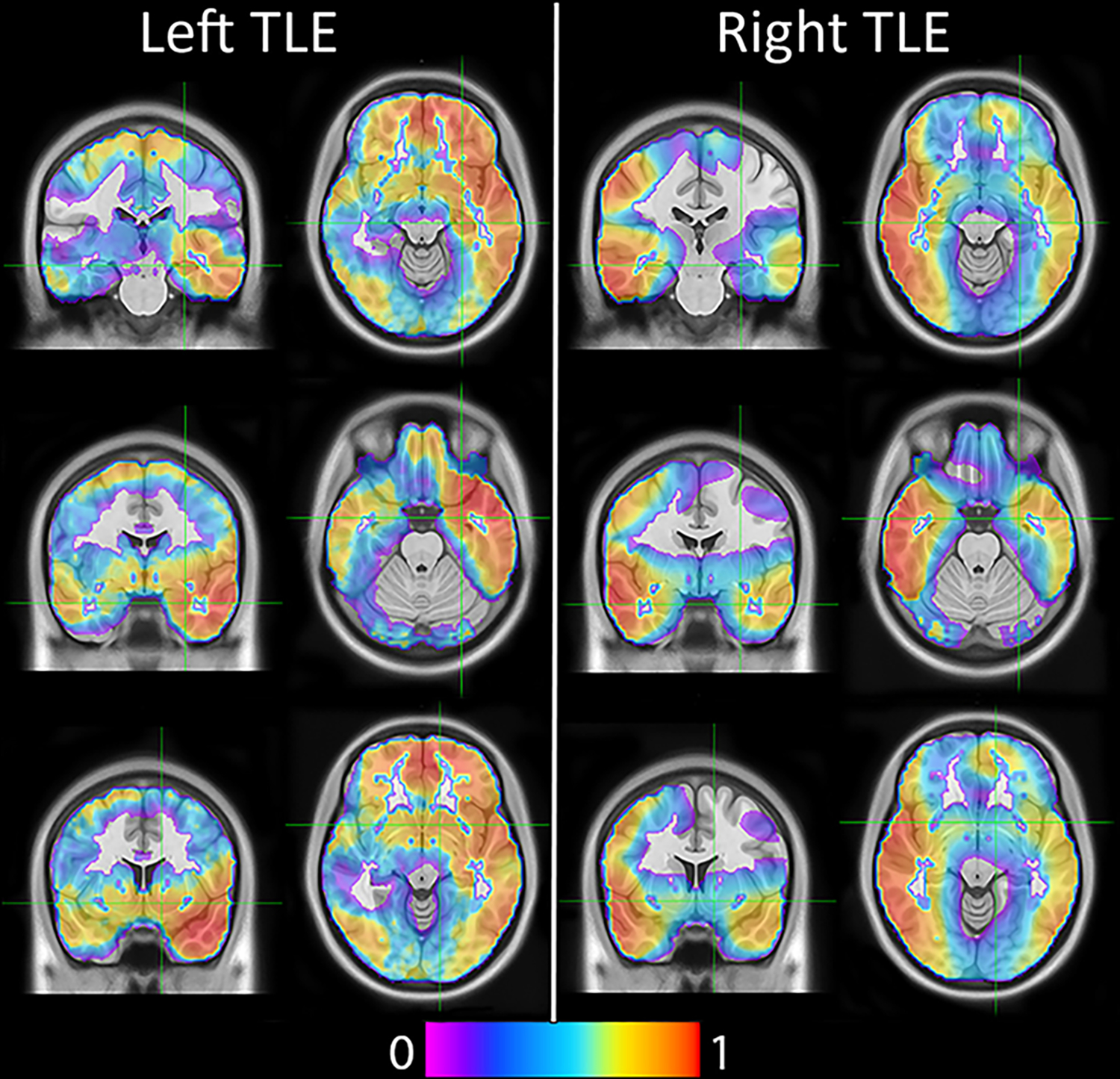
The displayed heatmap represents the spatial location of features used in a deep learning model. As can be seen, the right hemisphere is more important for classification of right TLE, and the left hemisphere is more important for classification of left TLE. From Luckett et al28; with permission
Outcome Prediction
Prediction of patients who will respond well to epilepsy surgery may aid in surgical decision making and provide insights into why some patients have less favorable outcomes. Although clinical variables have been used previously, FC may allow for a more accurate outcome prediction model. He et al demonstrated that hubness of the thalamus was increased in patients who weren’t seizure free after surgery, perhaps representing more widespread epilepsy.29 Guo et al used dynamic FC and found that the states that seizure free and not seizure free patients spent most of their time in were different.30 Additionally, Negishi et al found that patients who were seizure free had connectivity abnormalities localized to one lobe than those who were not seizure free, which may represent a more localized epilepsy.31
DWI
DWI is a method by which white matter tracts can be estimated. Tractography is typically performed in the pre-operative assessment for epilepsy surgery and has historically been used for stratifying risk.32 The risk of a visual field deficit, memory deficits, and naming deficits can all be predicted though localization of well-known white matter tracts.33–38 Prior work has demonstrated that structural connectivity is different in patients with epilepsy vs healthy controls and represents an opportunity to lateralize the seizure network and predict outcome in epilepsy.39,40
Lateralization
Lateralization of the seizure network with DWI is most helpful in ambiguous, non-lesional cases. It is possible to use common white matter tracts for this purpose, but these approaches are most accurate with lesional cases of TLE.41 The addition of machine learning and deep learning techniques on diffusion measures has improved lateralization.42 The combination of the structural connectome and machine learning was shown to lateralize TLE accurately, agnostic to lesional and non-lesional cases.43 Furthermore, the connections used by the network to lateralize were consistent with other studies of left vs right TLE, increasing interpretability of the network.
Outcome Prediction
It can be useful for surgical decision making to know the chances of achieving seizure freedom after epilepsy surgery. Prediction of seizure freedom can aid in the decision process to proceed with epilepsy surgery, modify the surgical plan, or avoid surgery for those that are not candidates. Several scoring systems have been proposed based on clinical variables, however, a connectome-based approach may achieve more accurate outcome predictions.44,45 Structural connectivity has been particularly accurate in the prediction of seizure freedom after surgery. Increased ipsilateral structural connectivity abnormalities, especially those related to the ipsilateral mesial structures, are most often used.46–48 One study examined seizure recurrence and found that more widespread SC abnormalities were associated with seizure recurrence (Figure 4).47 However, these studies were limited to anterior temporal lobectomies and were limited to TLE.
Figure 4: More widespread structural abnormalities were present in patients with a poor outcome after epilepsy surgery.
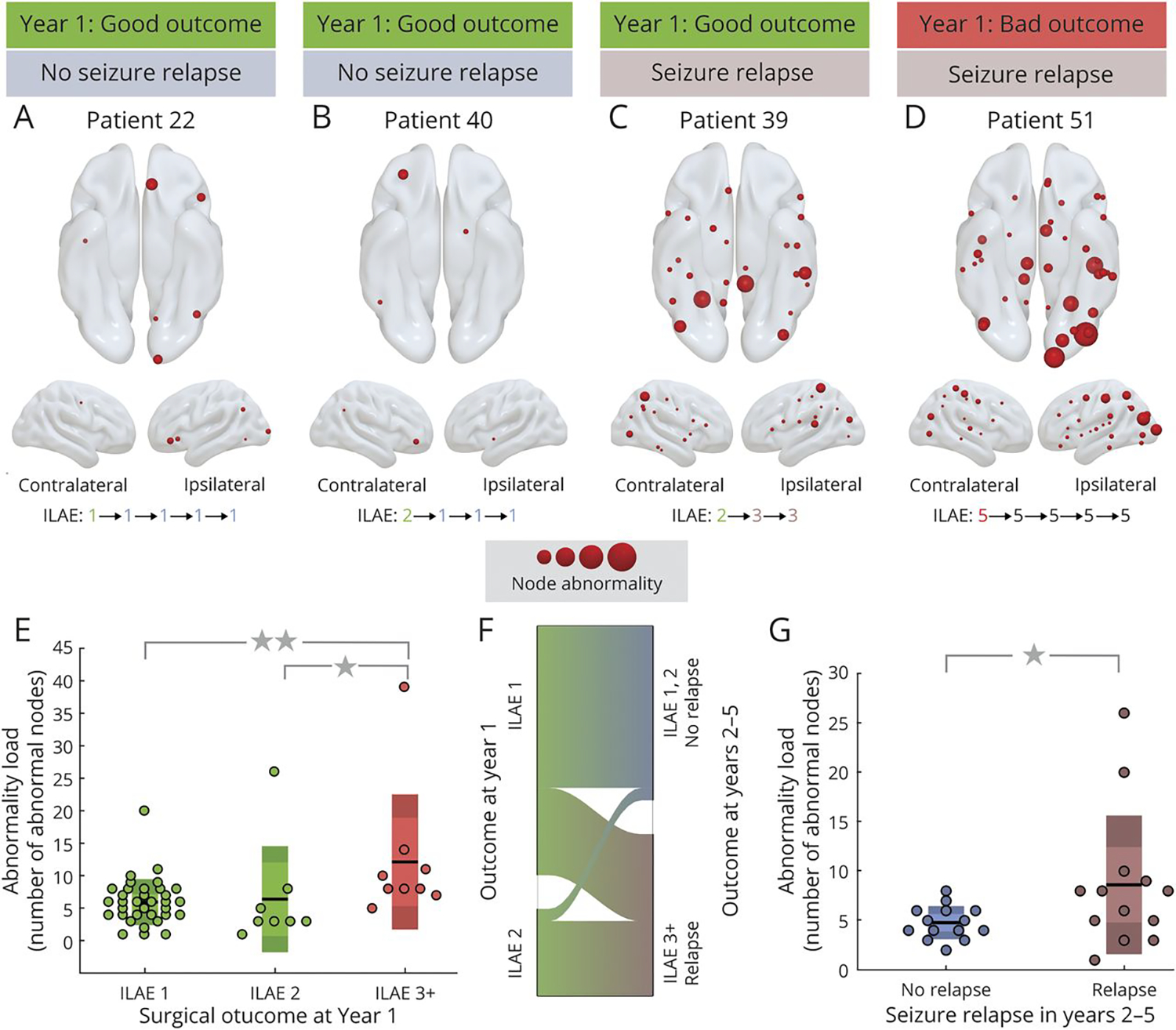
An example of these widespread abnormalities can be seen in panel D. Examples of more localized abnormalities can be seen in panels A and B. From Sinha et al47; with permission.
Machine learning approaches have also been used for seizure outcome prediction.49,50 However, it can be difficult to interpret what machine learning approaches use to stratify patients. One such example of this is a study by Johnson et al where the authors reduced the features down to the connections that best stratified patients who were and were not seizure free.50
Intracranial EEG
Neurostimulation
One of the first techniques for identifying the SOZ for resection in epilepsy was through intraoperative stimulation.10 Stimulation has also been employed for localization of eloquent cortex, both intraoperatively and pre-operatively.51,52 In the early 21st century, single pulse electrical stimulation (SPES) has been increasingly used after implantation of iEEG for the identification of epileptogenic tissue.53 A prospective study by Valentin et al demonstrated the effectiveness of SPES to identify epileptogenic tissue. They reported that when abnormal responses (delays and repetitive spiking) were present in the resected area, 96% of patients achieved an Engel I or II favorable seizure outcome.54,55 The results of this study were promising for patients who had abnormal responses to SPES, however, nearly 40% of patients had no abnormal responses. Can we make use of this technique in more patients using a network-based approach?
Network stimulation studies with SPES allow for directional information to be inferred, as a stimulus is delivered and responses are computed from all other contacts.56,57 Two major thoughts have predominated in this field: (1) SOZs influence the entire network more so than other regions and (2) SOZs are more highly connected to itself.
The idea of SOZs influencing the network more so than other regions has been proposed and studied by several in the field, with SPES studies providing evidence.58,59 Furthermore, these findings become even stronger when only contacts that were labeled as an SOZ and resected in patients with an Engel I outcome are analyzed.60 In addition to influencing the network, SOZs have been shown to demonstrate increased within SOZ connections.60,61 The finding of “hypercoupling” of SOZs may represent the seizure network and is most present in patients who were seizure free after surgery.62
Although the connectivity metrics used by prior studies have performed relatively well, many were originally developed for use in iEEG grids. This allowed for the assumption of directionality of neurons. This can represent a limitation as more centers are using stereotactic EEG (SEEG) which have varying directionality of pyramidal neurons. In order to overcome this limitation, one study used a deep learning model to analyze SPES responses, resulting in improvements in the accuracy of SOZ localization.43
Resting state networks
While ictal data acquired from iEEG is the gold standard for localization of the seizure network, interictal data has the potential to improve localization, especially in cases in which ictal localization fails. Interictal network approaches to iEEG have been utilized since the early 2000s. Initially, several studies reported that SOZs had increased connectivity to other SOZs.63–68 With the addition of directional connectivity measures, Narasimhan et al noted that SOZs had higher inward connectivity from all other regions in the brain in 2020.69 This finding was also observed by Jiang et al.70 More recent studies have noted that in addition to the increase in inward connectivity, there is a decreased outward connectivity from seizure networks, leading to the idea that seizure networks are suppressed at baseline.71,72 One such study proposed the Interictal Suppression Hypothesis (ISH), which postulated that SOZs had higher inward connectivity and lower outward connectivity during the resting state, suggesting that SOZs are suppressed at rest to prevent seizures (Figure 5).72 Other group have described a similar “Sources and Sinks” phenomenon, suggesting that inward inhibition of the SOZ may aid presurgical localization.71 While these network-based approaches appear valuable, they have yet to be prospectively evaluated.
Figure 5: The Interictal Suppression Hypothesis (ISH) is a novel conceptualization that may localize the seizure network.
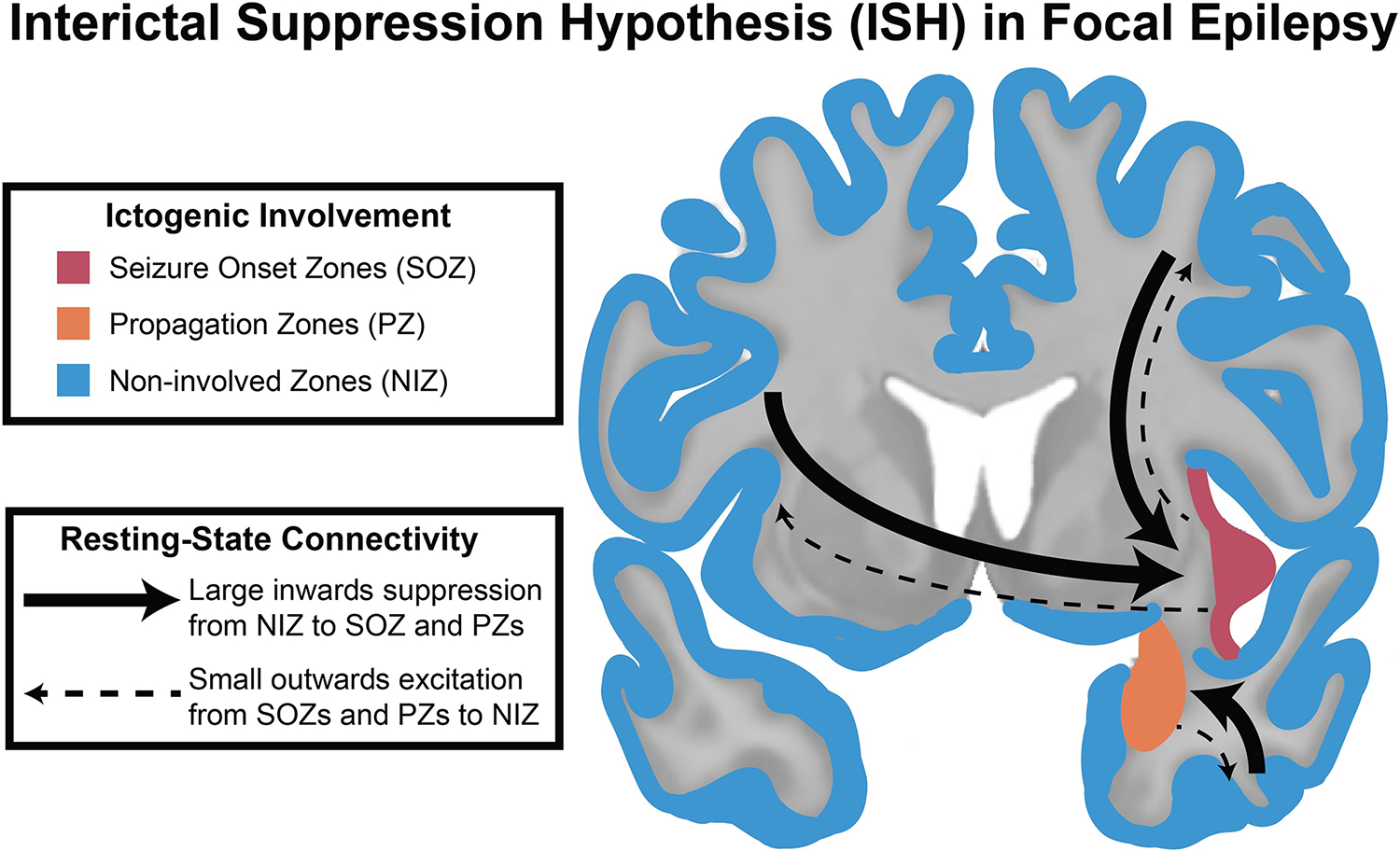
The ISH proposes that at baseline, the seizure network is suppressed by regions not involved in the seizure network. The non-involved zones can be seen in blue, the propagation zones can be seen in orange, and the seizure onset zones can be seen in red. From Johnson et al72; with permission.
Multimodal
The combination of structural and functional connectivity has been shown to improve the accuracy of outcome prediction.72–74 One such approach is to combine SC and FC to develop a connectivity profile of seizure free patients. Such a fingerprint can then be compared to a patient’s connectome to predict seizure freedom with an accuracy of 100% in one study (Figure 6).73,74
Figure 6: Structural and functional connectivity profiles can be used to accurately predict outcomes from epilepsy surgery.
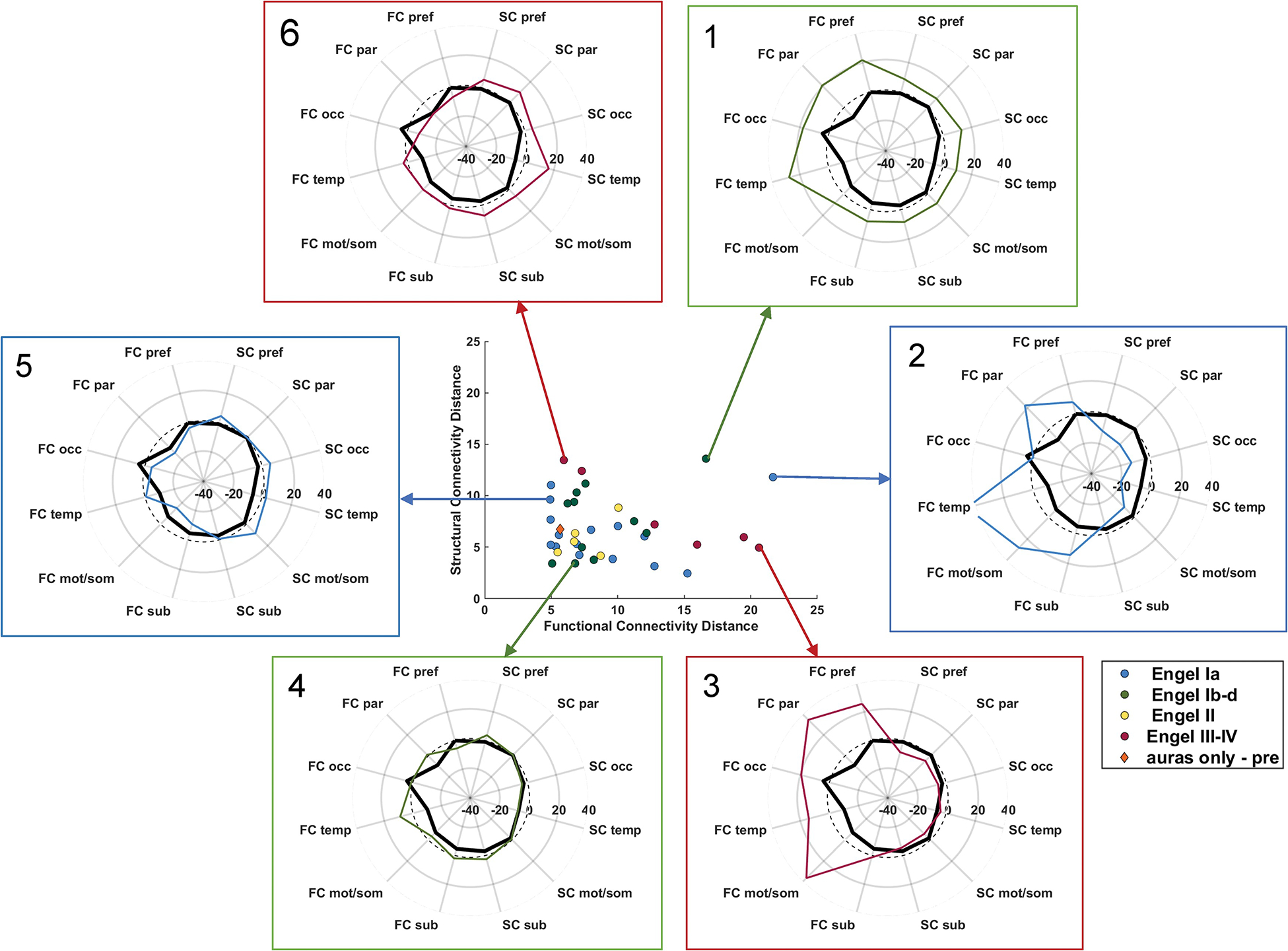
Both functional and structural connectivity were used to develop a “network fingerprint” of patients who were seizure free. Connectivity profiles of 6 example patients with their corresponding outcome data are depicted. Adapted from Morgan et al74; with permission. FC = functional connectome distance; SC = structural connectome distance; pref = prefrontal lobe; par = parietal lobe; occ = occipital lobe; temp = temporal lobe; mot/som = motor and sensory/motor lobe; sub = subcortical structures (all ipsilateral to seizure focus); dashed line = zero denoting age-matched control.
Others have had success expanding the combination of structural and functional connectivity to combine iEEG and DWI. Due to the difference in sampling between iEEG and neuroimaging methods, this can be a technical challenge. To overcome this, Johnson et al developed an algorithm for subsampling whole-brain tractography with iEEG near-field dynamic localization (SWiNDL), thus allowing for the computation of structural connectivity in the same areas sampled by iEEG.72 By combining the Interictal Suppression Hypothesis findings of increased inward FC and decreased outward FC along with SC, the authors found that they were able to better differentiate SOZs, PZs, and NIZs (Figure 7).
Figure 7: The combination of the Interictal Suppression Hypothesis functional connectivity and structural connectivity can more accurately differentiate the seizure network than functional or structural connectivity alone.
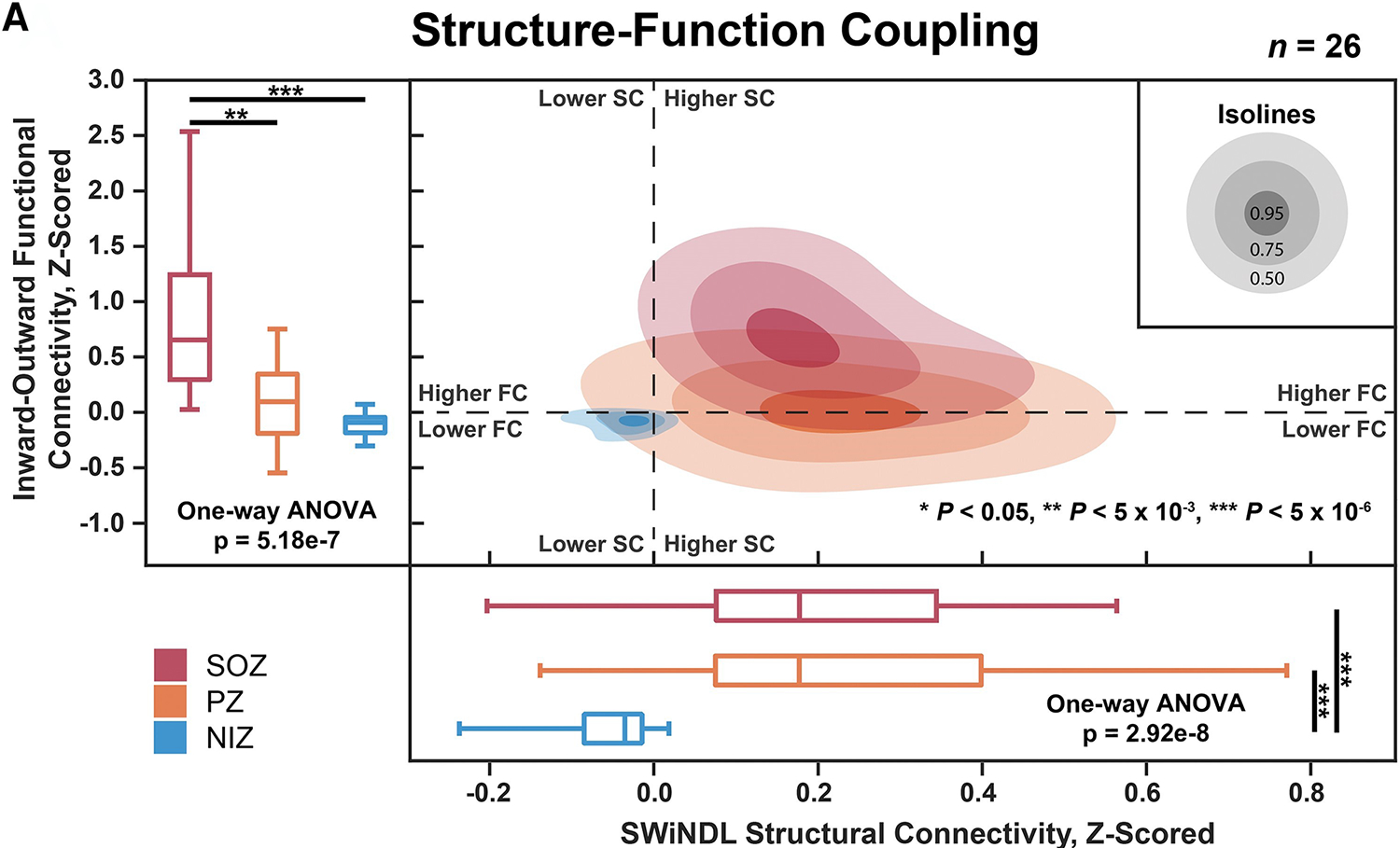
SOZs are depicted in red, PZs are depicted in orange, and NIZs are depicted in blue. Structural connectivity is on the x-axis and inward-outward connectivity is on the y-axis. Adapted from Johnson et al72; with permission.
Discussion
Despite an increase in surgical evaluations, there has not been an increase in surgical treatments.7 The reason most cited for patients not being surgical candidates is lack of a localization hypothesis. Why are we increasingly unable to understand the seizure network in patients when we have access to more technology than before?4,6 Some suggest increasingly complex patients are being evaluated for epilepsy surgery.7 Roadblocks such as these have been present in epilepsy surgery before and they were overcame with a paradigm shift, moving from “the spasming point” to the SOZ that if removed would result in seizure freedom. Now, the paradigm has shifted from a SOZ to a seizure network that can be treated with resection, ablation, neuromodulation, or a combination.5,15,75–77
There are limitations to network evaluations of epilepsy, and it is paramount to remember that network studies are a tool to augment clinical decision making. DWI and fMRI network studies are useful for lateralization and outcome prediction, but accurate localization of the seizure network with MRI connectivity alone has been more challenging. Network studies with iEEG have demonstrated promise in localizing and identifying the seizure network. However, a strong hypothesis must be developed due to the sparse sampling intrinsic to iEEG. A further limitation is the variability of processing methods between groups, creating challenges in generalizability. Thus, even with published methods and similar data acquisition between centers, connectomics can be difficult to implement. A further challenge is the paucity of prospective studies evaluating network measures for epilepsy surgery. However, the few studies published changed decision making in 58% of cases and increased epilepsy surgery candidacy by 26%.78
Currently the largest gap in epilepsy surgery is the lack of translation from the lab to the clinic. Despite the need for new ways to treat more complex epilepsy patients, the promising results from network studies, and several proposed implementation paths such as the one depicted in Figure 8, few have utilized network approaches to augment presurgical evaluations.77 Therefore, the next steps in this process require prospective evaluation of localization methodologies along with academic-commercial partnerships to bring the research from theoretical to practical. One could envision a network evaluation software package that is simple to use, repeatable, and clinically useful. Such an approach may allow for localization of a patient’s seizure network and identification of key areas for multimodal treatment.
Figure 8: How the addition of network studies could impact the presurgical evaluation for epilepsy surgery.
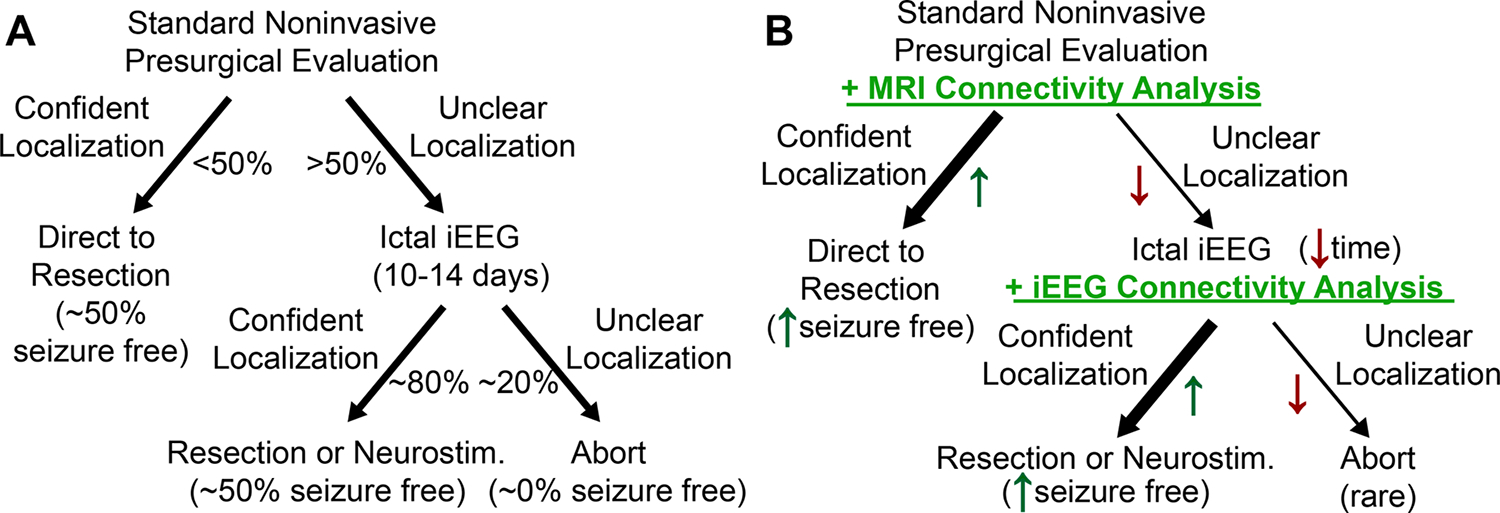
The typical presurgical evaluation is depicted in panel A. The proposed network augmented evaluation is depicted in panel B. From Johnson et al77; with permission.
Summary
The paradigm of epilepsy surgery has shifted to that of a network disorder.15–18,75–77 This shift in the paradigm of epilepsy surgery may allow for the localization of seizure networks that were previously unable to be localized and the treatment of epilepsies which were previously thought not to be surgical candidates. Connectivity can be evaluated with fMRI, DWI, and iEEG. FMRI and DWI connectivity studies have demonstrated the most success in lateralization of the seizure network and outcome prediction, whereas iEEG connectivity patterns have shown value in localizing the seizure network. Multimodal connectivity analyses, such as those combining structural and functional connectivity, have demonstrated the most success. However, translating connectomics from the lab to the clinic has been challenging. There is a need for improvement in localization and treatment of epilepsy. The field has taken a half measure in discussion and research of these methods, but there is a need for action in the form of prospective evaluations of connectomics and partnerships between industry and academia to bring connectomics to all centers.
Clinical Care Points
Epilepsy should be conceptualized as a network disorder with the goal of treatment being to disrupt the seizure network.
Connectomics can aid in localizing and quantifying the seizure network and these measures can be computed using existing data from the presurgical evaluation pipeline.
Key Points:
Despite improvements in technology, many patients evaluated for epilepsy surgery are not deemed to be candidates, often due to an incomplete hypothesis.
Connectomics can be implemented into epilepsy surgery evaluations.
Connectomics may help expand surgical options for patients who were previously not candidates.
Prospective evaluations of connectomics methods and partnerships between academia and industry are necessary to bring connectomics to the clinic.
Synopsis:
Epilepsy surgery is a potentially curative treatment for drug resistant epilepsy that has remained underutilized both due to inadequate referrals and incomplete localization hypotheses. The complexity of patients evaluated for epilepsy surgery has increased, thus new approaches are necessary to treat these patients. The paradigm of epilepsy surgery has evolved to match this challenge, now considering the entire seizure network with the goal of disrupting it through resection, ablation, neuromodulation, or a combination. The network paradigm has the potential to aid in identification of the seizure network as well as treatment selection.
Footnotes
Disclosure statement:
The authors declare no financial or commercial conflict of interest. This work was supported by the National Institutes of Health (grant nos. T32GM007347, T32EB021937, F31NS120401, and R01NS112252).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beghi E The Epidemiology of Epilepsy. Neuroepidemiology. 2020;54(2):185–191. doi: 10.1159/000503831 [DOI] [PubMed] [Google Scholar]
- 2.Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. The Lancet Neurology. 2014/11/01/ 2014;13(11):1114–1126. doi: 10.1016/S1474-4422(14)70156-5 [DOI] [PubMed] [Google Scholar]
- 3.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(5):1188–1198. doi: 10.1093/brain/awh449 [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner C, Koren JP, Britto-Arias M, Zoche L, Pirker S. Presurgical epilepsy evaluation and epilepsy surgery. F1000Res. 2019;8doi: 10.12688/f1000research.17714.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vakharia VN, Duncan JS, Witt JA, Elger CE, Staba R, Engel J Jr. Getting the best outcomes from epilepsy surgery. Ann Neurol. Apr 2018;83(4):676–690. doi: 10.1002/ana.25205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englot DJ. A modern epilepsy surgery treatment algorithm: Incorporating traditional and emerging technologies. Epilepsy & Behavior. 2018/03/01/ 2018;80:68–74. doi: 10.1016/j.yebeh.2017.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloppenborg T, May TW, Blümcke I, et al. Trends in epilepsy surgery: stable surgical numbers despite increasing presurgical volumes. J Neurol Neurosurg Psychiatry. Dec 2016;87(12):1322–1329. doi: 10.1136/jnnp-2016-313831 [DOI] [PubMed] [Google Scholar]
- 8.Krause F Surgery of the brain and spinal cord: based on personal experiences. vol 2. Rebman; 1912. [Google Scholar]
- 9.Meador KJ, Loring DW, Flanigin HF. History of epilepsy surgery. Journal of Epilepsy. 1989;2(1):21–25. [Google Scholar]
- 10.Feindel W, Leblanc R, De Almeida AN. Epilepsy Surgery: Historical Highlights 1909–2009. 10.1111/j.1528-1167.2009.02043.x. Epilepsia. 2009/03/01 2009;50(s3):131–151. doi: 10.1111/j.1528-1167.2009.02043.x [DOI] [PubMed] [Google Scholar]
- 11.GIBBS FA, LENNOX WG, Gibbs EL. The electro-encephalogram in diagnosis and in localization of epileptic seizures. Archives of Neurology & Psychiatry. 1936;36(6):1225–1235. [Google Scholar]
- 12.Talairach J, David M, Tournoux P. L’exploration chirurgicale stéréotaxique du lobe temporal dans l’épilepsie temporale: repérage anatomique stéréotaxique et technique chirurgicale. Masson & Cie, Editeurs; 1958. [Google Scholar]
- 13.Kuzniecky R, de la Sayette V, Ethier R, et al. Magnetic resonance imaging in temporal lobe epilepsy: pathological correlations. Ann Neurol. Sep 1987;22(3):341–7. doi: 10.1002/ana.410220310 [DOI] [PubMed] [Google Scholar]
- 14.Berkovic SF, Andermann F, Olivier A, et al. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1991;29(2):175–182. [DOI] [PubMed] [Google Scholar]
- 15.Piper RJ, Richardson RM, Worrell G, et al. Towards network-guided neuromodulation for epilepsy. Brain. Oct 21 2022;145(10):3347–3362. doi: 10.1093/brain/awac234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha N, Johnson GW, Davis KA, Englot DJ. Integrating Network Neuroscience Into Epilepsy Care: Progress, Barriers, and Next Steps. Epilepsy Curr. Sep-Oct 2022;22(5):272–278. doi: 10.1177/15357597221101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott RC, Menendez de la Prida L, Mahoney JM, Kobow K, Sankar R, de Curtis M. WONOEP APPRAISAL: The many facets of epilepsy networks. Epilepsia. Aug 2018;59(8):1475–1483. doi: 10.1111/epi.14503 [DOI] [PubMed] [Google Scholar]
- 18.Davis KA, Jirsa VK, Schevon CA. Wheels Within Wheels: Theory and Practice of Epileptic Networks. Epilepsy Curr. May 14 2021;21(4):15357597211015663. doi: 10.1177/15357597211015663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidhu MK, Duncan JS, Sander JW. Neuroimaging in epilepsy. Curr Opin Neurol. Aug 2018;31(4):371–378. doi: 10.1097/wco.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 20.Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. Jan 24 2017;88(4):395–402. doi: 10.1212/wnl.0000000000003532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. May 2010;89(2–3):310–8. doi: 10.1016/j.eplepsyres.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Krucoff MO, Chan AY, Harward SC, et al. Rates and predictors of success and failure in repeat epilepsy surgery: A meta-analysis and systematic review. Epilepsia. 2017;58(12):2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. Dec 2009;66(12):1491–9. doi: 10.1001/archneurol.2009.283 [DOI] [PubMed] [Google Scholar]
- 24.Bettus G, Bartolomei F, Confort-Gouny S, et al. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. Oct 2010;81(10):1147–54. doi: 10.1136/jnnp.2009.191460 [DOI] [PubMed] [Google Scholar]
- 25.Narasimhan S, González HFJ, Johnson GW, et al. Functional connectivity between mesial temporal and default mode structures may help lateralize surgical temporal lobe epilepsy. Journal of Neurosurgery. 01 Dec. 2022 2022;137(6):1571–1581. doi: 10.3171/2022.1.JNS212031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan VL, Sonmezturk HH, Gore JC, Abou-Khalil B. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia. Sep 2012;53(9):1628–35. doi: 10.1111/j.1528-1167.2012.03590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gholipour T, You X, Stufflebeam SM, et al. Common functional connectivity alterations in focal epilepsies identified by machine learning. Epilepsia. Mar 2022;63(3):629–640. doi: 10.1111/epi.17160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luckett PH, Maccotta L, Lee JJ, et al. Deep learning resting state functional magnetic resonance imaging lateralization of temporal lobe epilepsy. 10.1111/epi.17233. Epilepsia. 2022/06/01 2022;63(6):1542–1552. doi: 10.1111/epi.17233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI. Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology. Jun 13 2017;88(24):2285–2293. doi: 10.1212/wnl.0000000000004035 [DOI] [PubMed] [Google Scholar]
- 30.Guo D, Feng L, Yang Z, et al. Altered Temporal Variations of Functional Connectivity Associated With Surgical Outcomes in Drug-Resistant Temporal Lobe Epilepsy. Front Neurosci. 2022;16:840481. doi: 10.3389/fnins.2022.840481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52(9):1733–1740. doi: 10.1111/j.1528-1167.2011.03191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan JS, Winston GP, Koepp MJ, Ourselin S. Brain imaging in the assessment for epilepsy surgery. The Lancet Neurology. 2016/04/01/ 2016;15(4):420–433. doi: 10.1016/S1474-4422(15)00383-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper RJ, Yoong MM, Kandasamy J, Chin RF. Application of diffusion tensor imaging and tractography of the optic radiation in anterior temporal lobe resection for epilepsy: a systematic review. Clinical neurology and neurosurgery. 2014;124:59–65. [DOI] [PubMed] [Google Scholar]
- 34.Yogarajah M, Focke NK, Bonelli S, et al. Defining Meyer’s loop–temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132(6):1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper RJ, Yoong MM, Kandasamy J, Chin RF. Application of diffusion tensor imaging and tractography of the optic radiation in anterior temporal lobe resection for epilepsy: a systematic review. Clin Neurol Neurosurg. Sep 2014;124:59–65. doi: 10.1016/j.clineuro.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 36.Papagno C, Casarotti A, Comi A, et al. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Struct Funct. Jan 2016;221(1):687–94. doi: 10.1007/s00429-014-0920-8 [DOI] [PubMed] [Google Scholar]
- 37.McDonald CR, Leyden KM, Hagler DJ, et al. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex. Sep 2014;58:139–50. doi: 10.1016/j.cortex.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middlebrooks EH, Yagmurlu K, Szaflarski JP, Rahman M, Bozkurt B. A contemporary framework of language processing in the human brain in the context of preoperative and intraoperative language mapping. Neuroradiology. Jan 2017;59(1):69–87. doi: 10.1007/s00234-016-1772-0 [DOI] [PubMed] [Google Scholar]
- 39.Bernhardt BC, Fadaie F, Liu M, et al. Temporal lobe epilepsy. Hippocampal pathology modulates connectome topology and controllability. 2019;92(19):e2209–e2220. doi: 10.1212/wnl.0000000000007447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Chu L, Liu C, Huang M, Wang H. Alterations of white matter network in patients with left and right non-lesional temporal lobe epilepsy. Eur Radiol. Dec 2019;29(12):6750–6761. doi: 10.1007/s00330-019-06295-5 [DOI] [PubMed] [Google Scholar]
- 41.García-Pallero MA, Hodaie M, Zhong J, et al. Prediction of Laterality in Temporal Lobe Epilepsy Using White Matter Diffusion Metrics. World Neurosurg. Aug 2019;128:e700–e708. doi: 10.1016/j.wneu.2019.04.238 [DOI] [PubMed] [Google Scholar]
- 42.Gleichgerrcht E, Munsell BC, Alhusaini S, et al. Artificial intelligence for classification of temporal lobe epilepsy with ROI-level MRI data: A worldwide ENIGMA-Epilepsy study. Neuroimage Clin. 2021;31:102765. doi: 10.1016/j.nicl.2021.102765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson GW, Cai LY, Doss DJ, et al. Localizing seizure onset zones in surgical epilepsy with neurostimulation deep learning. Journal of Neurosurgery. 01 Apr. 2023. 2023;138(4):1002–1007. doi: 10.3171/2022.8.JNS221321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. Mar 2015;14(3):283–90. doi: 10.1016/s1474-4422(14)70325-4 [DOI] [PubMed] [Google Scholar]
- 45.Dugan P, Carlson C, Jetté N, et al. Derivation and initial validation of a surgical grading scale for the preliminary evaluation of adult patients with drug-resistant focal epilepsy. Epilepsia. May 2017;58(5):792–800. doi: 10.1111/epi.13730 [DOI] [PubMed] [Google Scholar]
- 46.Bonilha L, Jensen JH, Baker N, et al. The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology. May 5 2015;84(18):1846–53. doi: 10.1212/wnl.0000000000001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha N, Wang Y, Moreira da Silva N, et al. Structural Brain Network Abnormalities and the Probability of Seizure Recurrence After Epilepsy Surgery. Neurology. Feb 2 2021;96(5):e758–e771. doi: 10.1212/wnl.0000000000011315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Wang Y, Kopetzky SJ, Butz-Ostendorf M, Kaiser M. Connectivity within regions characterizes epilepsy duration and treatment outcome. Hum Brain Mapp. Aug 15 2021;42(12):3777–3791. doi: 10.1002/hbm.25464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gleichgerrcht E, Munsell B, Bhatia S, et al. Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia. Sep 2018;59(9):1643–1654. doi: 10.1111/epi.14528 [DOI] [PubMed] [Google Scholar]
- 50.Johnson GW, Cai LY, Narasimhan S, et al. Temporal lobe epilepsy lateralisation and surgical outcome prediction using diffusion imaging. J Neurol Neurosurg Psychiatry. Jun 2022;93(6):599–608. doi: 10.1136/jnnp-2021-328185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuanYu N, GuoJun Z, Liang Q, LiXin C, Tao Y, YongJie L. Surgery for perirolandic epilepsy: Epileptogenic cortex resection guided by chronic intracranial electroencephalography and electric cortical stimulation mapping. Clinical neurology and neurosurgery. 2010;112(2):110–117. [DOI] [PubMed] [Google Scholar]
- 52.Alarcon G, Nawoor L, Valentin A. Electrical Cortical Stimulation: Mapping for Function and Seizures. Neurosurg Clin N Am. Jul 2020;31(3):435–448. doi: 10.1016/j.nec.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 53.Trébuchon A, Chauvel P. Electrical Stimulation for Seizure Induction and Functional Mapping in Stereoelectroencephalography. J Clin Neurophysiol. Dec 2016;33(6):511–521. doi: 10.1097/wnp.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 54.Valentín A, Alarcón G, Honavar M, et al. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: a prospective study. The Lancet Neurology. 2005/11/01/ 2005;4(11):718–726. doi: 10.1016/S1474-4422(05)70200-3 [DOI] [PubMed] [Google Scholar]
- 55.Engel J Surgical treatment of the epilepsies. Raven Press; 1987. [Google Scholar]
- 56.Matsumoto R, Kunieda T, Nair D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure. 2017/01/01/ 2017;44:27–36. doi: 10.1016/j.seizure.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prime D, Rowlands D, O’Keefe S, Dionisio S. Considerations in performing and analyzing the responses of cortico-cortical evoked potentials in stereo-EEG. 10.1111/epi.13939. Epilepsia. 2018/01/01 2018;59(1):16–26. doi: 10.1111/epi.13939 [DOI] [PubMed] [Google Scholar]
- 58.Hays MA, Coogan C, Crone NE, Kang JY. Graph theoretical analysis of evoked potentials shows network influence of epileptogenic mesial temporal region. 10.1002/hbm.25418. Human Brain Mapping. 2021/09/01 2021;42(13):4173–4186. doi: 10.1002/hbm.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao C, Liang Y, Li C, et al. Localization of Epileptogenic Zone Based on Cortico-Cortical Evoked Potential (CCEP): A Feature Extraction and Graph Theory Approach. Front Neuroinform. 2019;13:31. doi: 10.3389/fninf.2019.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Blooijs D, Leijten FSS, van Rijen PC, Meijer HGE, Huiskamp GJM. Evoked directional network characteristics of epileptogenic tissue derived from single pulse electrical stimulation. Hum Brain Mapp. Nov 2018;39(11):4611–4622. doi: 10.1002/hbm.24309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boido D, Kapetis D, Gnatkovsky V, et al. Stimulus-evoked potentials contribute to map the epileptogenic zone during stereo-EEG presurgical monitoring. Hum Brain Mapp. Sep 2014;35(9):4267–81. doi: 10.1002/hbm.22516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Z-h, Zhao B-t, Toprani S, et al. Epileptogenic network of focal epilepsies mapped with cortico-cortical evoked potentials. Clinical Neurophysiology. 2020/11/01/ 2020;131(11):2657–2666. doi: 10.1016/j.clinph.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 63.Mormann F, Lehnertz K, David P, E. Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena. 2000/10/01/ 2000;144(3):358–369. doi: 10.1016/S0167-2789(00)00087-7 [DOI] [Google Scholar]
- 64.Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One. 2011;6(5):e20071. doi: 10.1371/journal.pone.0020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartolomei F, Bettus G, Stam CJ, Guye M. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin Neurophysiol. Dec 2013;124(12):2345–53. doi: 10.1016/j.clinph.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Lagarde S, Roehri N, Lambert I, et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain. Oct 1 2018;141(10):2966–2980. doi: 10.1093/brain/awy214 [DOI] [PubMed] [Google Scholar]
- 67.Paulo DL, Wills KE, Johnson GW, et al. SEEG Functional Connectivity Measures to Identify Epileptogenic Zones: Stability, Medication Influence, and Recording Condition. Neurology. May 17 2022;98(20):e2060–e2072. doi: 10.1212/wnl.0000000000200386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodale SE, González HFJ, Johnson GW, et al. Resting-State SEEG May Help Localize Epileptogenic Brain Regions. Neurosurgery. Jun 1 2020;86(6):792–801. doi: 10.1093/neuros/nyz351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narasimhan S, Kundassery KB, Gupta K, et al. Seizure-onset regions demonstrate high inward directed connectivity during resting-state: An SEEG study in focal epilepsy. Epilepsia. Nov 2020;61(11):2534–2544. doi: 10.1111/epi.16686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang H, Kokkinos V, Ye S, et al. Interictal SEEG Resting-State Connectivity Localizes the Seizure Onset Zone and Predicts Seizure Outcome. Adv Sci (Weinh). Jun 2022;9(18):e2200887. doi: 10.1002/advs.202200887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunnarsdottir KM, Li A, Smith RJ, et al. Source-sink connectivity: a novel interictal EEG marker for seizure localization. Brain. 2022;145(11):3901–3915. doi: 10.1093/brain/awac300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson GW, Doss DJ, Morgan VL, et al. The Interictal Suppression Hypothesis in focal epilepsy: network-level supporting evidence. Brain. 2023:awad016. doi: 10.1093/brain/awad016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgan VL, Englot DJ, Rogers BP, et al. Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia. Jul 2017;58(7):1251–1260. doi: 10.1111/epi.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan VL, Sainburg LE, Johnson GW, et al. Presurgical temporal lobe epilepsy connectome fingerprint for seizure outcome prediction. Brain Commun. 2022;4(3):fcac128. doi: 10.1093/braincomms/fcac128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engel J Jr, Thompson PM, et al. Connectomics and epilepsy. Current Opinion in Neurology. 2013;26(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zijlmans M, Zweiphenning W, van Klink N. Changing concepts in presurgical assessment for epilepsy surgery. Nature Reviews Neurology. 2019/10/01 2019;15(10):594–606. doi: 10.1038/s41582-019-0224-y [DOI] [PubMed] [Google Scholar]
- 77.Johnson GW, Doss DJ, Englot DJ. Network dysfunction in pre and postsurgical epilepsy: connectomics as a tool and not a destination. Current Opinion in Neurology. 2022;35(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boerwinkle VL, Mirea L, Gaillard WD, et al. Resting-state functional MRI connectivity impact on epilepsy surgery plan and surgical candidacy: prospective clinical work. Journal of Neurosurgery: Pediatrics PED. 01 Jun. 2020. 2020;25(6):574–581. doi: 10.3171/2020.1.PEDS19695 [DOI] [PubMed] [Google Scholar]


