Abstract
Introduction:
Despite improvements in survival of patients with high-grade osteosarcoma after the implementation of perioperative chemotherapy, osteosarcoma remains among the most lethal cancers. Prescription of all chemotherapy courses before the surgery may provide this opportunity to eliminate micrometastases more efficiently, increase the chances of pathologic complete response and organ preserving surgery. This study aimed to compare the outcomes of total neoadjuvant chemotherapy vs. standard perioperative chemotherapy with cisplatin/doxorubicin regimen in patients with extremities osteosarcoma.
Methods:
In this retrospective cohort, all patients with high-grade osteosarcoma admitted to oncologic centers affiliated to Iran University of Medical Sciences in Tehran, Iran from 2015 to 2021 were included. Organ preserving rates, pathologic responses, and survival of patients who received all six courses of cisplatin/doxorubicin regimen preoperatively were compared to those who received the regimen perioperatively.
Results:
Sixty-three patients were enrolled (total neoadjuvant chemotherapy: 32 patients and perioperative chemotherapy: 31 patients). In total neoadjuvant chemotherapy and perioperative chemotherapy groups, favorable pathology responses (necrosis>90%) were reported in 80.6% and 15.6% of patients, respectively (p<0.001). With a median follow-up of 24 months, mean overall survival of total neoadjuvant chemotherapy and perioperative chemotherapy groups were 21.29 months (95% CI; 19.3-23.27) and 23.46 months (95% CI; 22.7-24.1), respectively (p=0.2). The mean disease-free survival of patients in total neoadjuvant chemotherapy and perioperative chemotherapy groups were 19.54 months (95% CI; 17.0-22.0) and 21.37 months (95% CI; 19.4-23.2), respectively (p=0.2).
Conclusion:
Our results showed that prescription of all courses of doxorubicin/cisplatin chemotherapy prior to surgery can increase favorable pathologic response rates, although this improvement is not translated into overall and disease-free survival benefits.
Key Words: Osteosarcoma, Perioperative chemotherapy, total neoadjuvant therapy, Doxorubicin, Cisplatin
Introduction
High grade osteosarcoma, as the most prevalent primary tumor of bone, is considered as one of the most lethal malignancies although various chemotherapy regimens and approaches have been adopted over the past decades (Allison et al., 2012). Distant metastases developed during the course of disease are the main prognostic factors affecting non-metastatic patients survival rate, highlighting the substantial role of chemotherapy in eliminating micrometastases (Yasin et al., 2020). Rosen et al. developed the concept of neoadjuvant chemotherapy in the 1970s as a means to delay surgery while patients were waiting for the preparation of endoprostheses to proceed organ preserving surgery (Rosen et al., 1979; Rosen et al., 1976). Consequently, the standard of care in the management of osteosarcoma is perioperative chemotherapy using methotrexate, doxorubicin, and cisplatin with and without ifosfamide (Bielack et al., 2015; Marina et al., 2016). Prescription of all chemotherapy courses before the surgery may provide this opportunity to eliminate micrometastases more efficiently and increase the chances of pathologic complete response and organ preserving surgery. While the concept of total neoadjuvant therapy has been successfully tested in other malignancies such as breast cancer and rectal cancer (Cercek et al., 2018; Lee et al., 2020), this hypothesis has not been scrutinized in osteosarcoma patients. Contrarily, the coronavirus pandemic (COVID-19) imposed excessive pressure on all health care sections (including the oncology departments) and contributed to delayed elective surgeries (Javadinia et al., 2021; Soroosh and Javadinia, 2020; Uimonen et al., 2021). Accordingly, the number of patients with osteosarcoma receiving all cycles of chemotherapy significantly increased in the present study setting (i.e. Department of Radiation Oncology at Firoozgar Hospital, as one of the leading oncologic centers in Iran). Therefore, we aimed to compare the outcomes of total neoadjuvant chemotherapy vs. standard perioperative chemotherapy with cisplatin/doxorubicin regimen in patients with extremities osteosarcoma.
Materials and Methods
In this retrospective cohort, all patients with high-grade osteosarcoma admitted to oncologic centers affiliated to Iran University of Medical Sciences in Tehran, Iran from 2015 to 2021 were included. Patients with a histologic-confirmed diagnosis of high-grade osteosarcoma who aged less than 40 years old and had an ECOG performance score of 0-3, ejection fraction above 50%, normal kidney and liver function tests, and preserved bone marrow function at the baseline were evaluated to determine if there was no distant metastasis, no pathologic fracture, no uncontrolled underlying diseases and no previous history of chemotherapy and radiotherapy (except basal cell carcinoma of skin).
Primary staging was done through pathologic assessment of core needle biopsy of skeletal lesion. Moreover, comprehensive local and distal extension were performed using whole body bone scan, chest computed topography (CT) scan and magnetic resonance imaging (MRI) of the limb. After three courses of chemotherapy, the distant work-ups were repeated.
Patients were included if they received six cycles of doxorubicin (25 mg/m2 D1-3)/cisplatin (100 mg/m2 D1) regimen either perioperatively or preoperatively and the doses were not adjusted more than 25% due to side effects. The protocol of the study was approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1400.159); a written informed consent form was also obtained from the patients or their legal guardian.
Data on the type of surgery and pathologic responses were extracted from their medical records; all patients were followed up for the survival data. Patients who perioperatively received all of the six cycles of doxorubicin/cisplatin protocol were considered as the total neoadjuvant therapy (TNT) group. The standard group consisted of patients who received three cycles of chemotherapy before and after the surgery. A favorable pathologic response was defined as the presence of necrosis more than 90% upon post-operative pathologic evaluation. Disease-free and overall survival rates were defined as the time interval between the diagnosis and the first relapse and death, respectively. Organ preserving rates, pathologic responses, and survival of patients who received all courses of cisplatin/doxorubicin regimen were compared to those who received the regimen perioperatively.
For statistical analyses, SPSS 21 was used to analyze the data. After assessing their normality by Shapiro-Wilk test, the independent t-test was used to compare differences between the two groups; the repeated measures analysis was also used for assessing the within-group changes. If the normality was not retained, Mann-Whitney U test was used to compare between-group differences; and Friedman’s test was used for measuring within-group changes. To compare the qualitative variables, Chi-square test or Fisher’s exact test were used. In addition, Log-rank test and Kaplan-Meier curves were used for survival analysis. P value less than 0.05 was considered significant.
Results
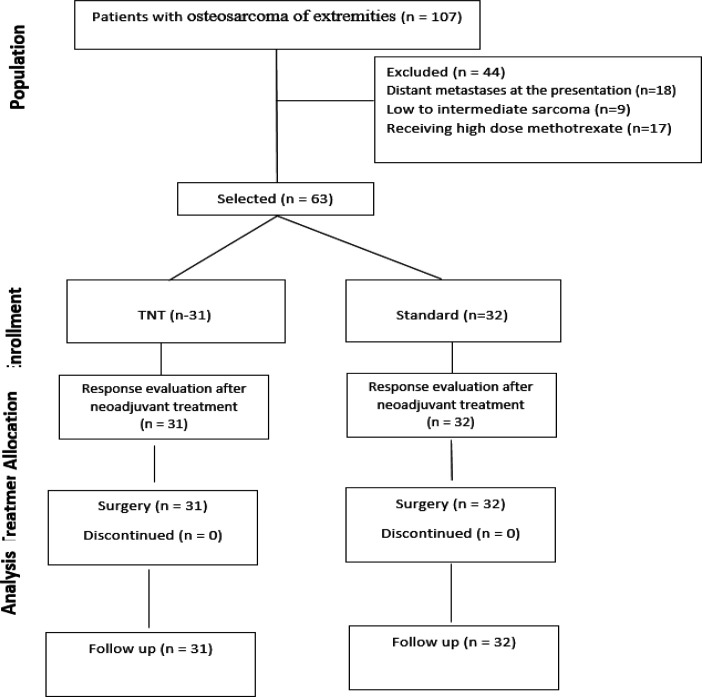
One hundred and seven patients with osteosarcoma were assessed. Eighteen patients due to distant metastasis, nine patients due to low-to-intermediate sarcoma pathology and seventeen patients due to receiving high doses of methotrexate were excluded; finally, 63 patients were enrolled (TNT group: 31 patients and standard group: 32 patients) (Figure 1). The baseline characteristics of the two groups were similar except the disease stage which was significantly higher in the TNT group (p=0.01).Organ preserving surgery was performed in all patients. In the total neoadjuvant chemotherapy and perioperative chemotherapy groups, favorable pathology responses (necrosis>90%) were reported in 80.6% and 15.6% of patients, respectively (p<0.001) (Table 2).
Figure 1.
Flow Diagram of Patients Enrolled in the Current Study
Table 2.
Oncologic Outcomes of the Patients
| Standard group (n=32) | TNT group (n=31) | P value | |
|---|---|---|---|
| Surgery [n (%)] | - | ||
| Organ preserving surgery | 32 (100) | 31 (100) | |
| amputation | 0 | 0 | |
| Pathologic response [n (%)] | <0.001 | ||
| Favorable | 5 (15.6) | 25 (80.6) | |
| Unfavorable | 27 (84.4) | 6 (19.4) | |
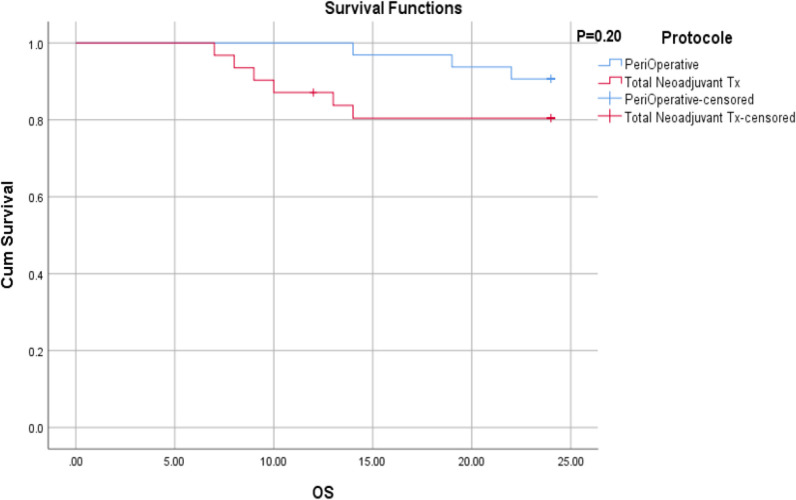
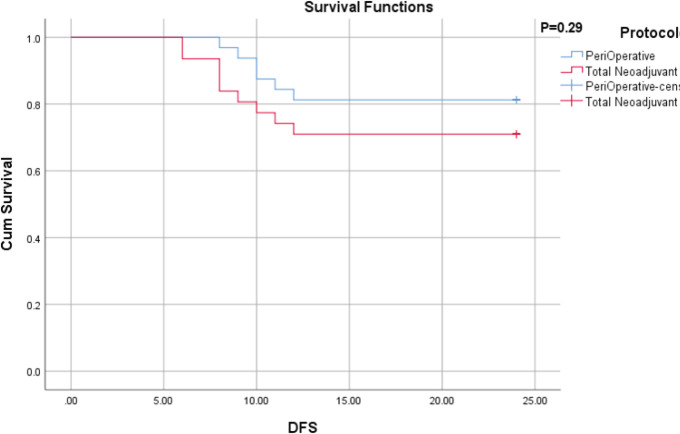
With a median follow-up of 24 months, there was no local recurrence in the two groups. All relapses were reported as lung metastases. Moreover, nine patients died due to active cancer. The mean overall survival of total neoadjuvant chemotherapy and perioperative chemotherapy groups were 21.29 months (95% CI; 19.3-23.27) and 23.46 months (95% CI; 22.7-24.1), respectively (p=0.20) (Figure 1). In the standard group, all deaths (3 cases) were reported within the first year after the diagnosis and the 1-year and 2-year survival rates were both 90.6%. In the TNT group, four deaths were reported in the first year, and other two deaths were reported in the second year; the 1-year and 2-year survival rates were 87.7% and 80.4%, respectively. The mean disease-free survival of patients in total neoadjuvant chemotherapy and perioperative chemotherapy groups were 19.54 months (95% CI; 17.0-22.0) and 21.37 months (95% CI; 19.4-23.2), respectively (p=0.29) (Figure 2).
Figure 2.
Sarcoma-Specific Survival by Groups
All lung metastases in our patients were disseminated ruling out the role of curative surgery. Therefore, all patients underwent palliative chemotherapy with agents that were different from the primary chemotherapy protocols.
Table 1.
The Baseline Characteristics
| Standard group (n=32) | TNT group (n=31) | P value | |
|---|---|---|---|
| Age (mean±SD) | 17.8 ± 3.1 | 18.2 ± 3.2 | 0.60 |
| Gender [n (%)] | 0.38 | ||
| Male | 11 (34.4) | 14 (45.2) | |
| Female | 21 (65.6) | 17 (54.8) | |
| ECOG performance score [n (%)] | - | ||
| 0 | 32 (100) | 31 (100) | |
| 1≤ | 0 | 0 | |
| Location [n (%)] | 0.80 | ||
| Lower extremities | 32 (100) | 29 (93.5) | |
| Upper extremities | 0 | 2 (6.5) | |
| Size of lesion (cm) | 5.7 ± 1.1 | 6.2 ± 1.6 | 0.15 |
| AJCC TNM stage group [n (%)] | 0.01 | ||
| II | 32 (100) | 26 (83.9) | |
| IIIA | 0 | 5 (16.1) | |
Figure 3.
Disease-Free Survival by Groups
Discussion
Previous studies have confirmed that several chemotherapeutic agents including cisplatin, doxorubicin, methotrexate, and ifosfamide play a considerable role in the management of osteosarcoma of the extremities (Bielack et al., 2002; Ferrari et al., 2005; Laitinen et al., 2015; Whelan et al., 2015), resulting in a significant improvement in the survival of patients (Coventry and Dahlin, 1957; Rosen, 1985). Currently, the standard approach involves partial chemotherapy before the surgery to facilitate the organ preserving surgery, to assess the efficacy of chemotherapeutic agents, to prevent pathologic fracture, and to eliminate the micrometastases (Benjamin et al., 1984; Benjamin, 1989; Benjamin et al., 1993; Benjamin and Patel, 2010; Cade, 1955; Jaffe et al., 1974; Jaffe et al., 1978; Jaffe et al., 1983; Jaffe et al., 1985; Kamalakar et al., 1977; Lee and Mackenzie, 1964; Link et al., 1986; Marcove et al., 1970; Marina et al., 2016; Mavligit et al., 1981; Wagner et al., 2016). Despite this practice changing evidence, the survival of osteosarcoma patients remains steady after tremendous improvements in the 1970’s, emphasizing the importance of new treatment strategies and new effective medications (Allison et al., 2012).
Since distant metastases are still the main factor affecting the survival of patients even after introducing the current standard surgical interventions (i.e. organ preserving surgery), (Bacci et al., 2001), we hypothesized that prescription of all courses of chemotherapy prior to the definitive surgery may enhance the survival of patients through elimination of the potential micrometastases. However, changing the post-operative chemotherapy protocol failed to improve survival (Marina et al., 2016). Furthermore, considering the new concept of total neoadjuvant therapy in the management of breast cancer and rectal cancer (Cercek et al., 2018; Lee et al., 2020) and delayed elective surgeries due to the COVID-19 pandemic (Javadinia et al., 2021; Soroosh and Javadinia, 2020; Uimonen et al., 2021), we were encouraged to assess the efficacy of total neoadjuvant therapy in the osteosarcoma.
In this retrospective cohort of 63 sarcoma patients, pathologic responses, disease-free survival and overall survival of patients receiving all courses of doxorubicin/cisplatin chemotherapy prior to surgery (total neoadjuvant chemotherapy) were compared to patients receiving standard perioperative chemotherapy. Our results confirmed that total neoadjuvant chemotherapy in patients suffering from osteosarcoma of the extremities was associated with higher rates of favorable pathologic responses. Surprisingly, however, this improvement did not yield a higher survival rate.
Tumor histopathological responses to neoadjuvant chemotherapy are among the strongest prognostic factors in patients with osteosarcoma (Bacci et al., 2006). In the present study, the favorable pathologic response, defined as 90% or more tumor necrosis in the post-operative pathologic evaluation (Picci et al., 1985), was observed in 15.6% of the standard and 80.6% of the TNT groups. It is, however, uncertain if the observed improved favorable pathologic rates are either related to the improved efficacy due to increased number of chemotherapy course or just a plain consequence of longer preoperative time intervals. On the other hand, our patients did not reach their median survival showing that the follow-up period was not long enough to report the differences in the overall and disease-free states of both groups despite the observed significant differences in the pathologic responses. In short, the current study employed a retrospective approach for data collection. Therefore, the accuracy of regular postoperative re-examination to detect the local or distant recurrence was debatable.
In conclusion, our results showed that prescription of all courses of doxorubicin/cisplatin chemotherapy prior to surgery was likely to increase the favorable pathologic response rates, although this improvement is not translated into overall and disease-free survival benefits.
Author Contribution Statement
Study concept and design: A.N. and A.M.A.; acquisition of data: A.F.; analysis and interpretation of data: M.S.; drafting of the manuscript: S.A.J.; critical revision of the manuscript for important intellectual content: S.H.M.; statistical analysis: F.A.
Acknowledgements
This research was funded by Iran University of Medical Sciences (grant number 20796 to A.N.). Authors would like to thank all patients who participated in the project, and sincerely thank Vasei Clinical Research Development Office at Sabzevar University of Medical Sciences, Iran, for technical advice and counselling in conducting this research.
Ethical approval statement
Ethical approval statement
The protocol of the study was approved by the Ethics Committee of Iran University of Medical Sciences, Iran (IR.IUMS.FMD.REC.1400.159); a written informed consent form was also obtained from the patients or their legal guardian.
Clinical trial registration number
Not applicable.
Data availability statement
All data generated and analyzed during this study can be accessed through direct communication with the corresponding author and the agreement of all research team members.
Conflict of interest statement
The authors report no conflicts of interest.
References
- Allison DC, Carney SC, Ahlmann ER, et al. A Meta-Analysis of Osteosarcoma Outcomes in the Modern Medical Era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci G, Ferrari S, Longhi A, et al. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–8. doi: 10.1016/s0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–61. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- Benjamin RS. Regional chemotherapy for osteosarcoma. Semin Oncol. 1989;16:323–7. [PubMed] [Google Scholar]
- Benjamin RS, Legha SS, Patel SR, Nicaise C. Single-agent ifosfamide studies in sarcomas of soft tissue and bone: the M D Anderson experience. Cancer Chemother Pharmacol. 1993;31:174–9. [PubMed] [Google Scholar]
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J Clin Oncol. 2015;33:2279–87. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade S. Osteogenic sarcoma; a study based on 133 patients. J R Coll Surg Edinb. 1955;1:79–111. [PubMed] [Google Scholar]
- Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. doi: 10.1001/jamaoncol.2018.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry MB, Dahlin DC. Osteogenic sarcoma: a critical analysis of 430 cases. J Bone Jt Surg. 1957;39:741–58. [PubMed] [Google Scholar]
- Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- Jaffe N, Frei E, Traggis D, Bishop Y. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med. 1974;291:994–7. doi: 10.1056/NEJM197411072911902. [DOI] [PubMed] [Google Scholar]
- Jaffe N, Frei E, Watts H, Traggis D. High-dose methotrexate in osteogenic sarcoma: a 5-year experience. Cancer Treat Rep. 1978;62:259–64. [PubMed] [Google Scholar]
- Jaffe N, Knapp J, Chuang VP, et al. Osteosarcoma: intra-arterial treatment of the primary tumor with cis-diammine-dichloroplatinum II (CDP) Angiographic, pathologic, and pharmacologic studies. Cancer. 1983;51:402–7. doi: 10.1002/1097-0142(19830201)51:3<402::aid-cncr2820510308>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Jaffe N, Robertson R, Ayala A, et al. Comparison of intra-arterial cis-diamminedichloroplatinum II with high-dose methotrexate and citrovorum factor rescue in the treatment of primary osteosarcoma. J Clin Oncol. 1985;3:1101–4. doi: 10.1200/JCO.1985.3.8.1101. [DOI] [PubMed] [Google Scholar]
- Javadinia SA, Welsh JS, Mosavi Jarrahi A. COVID-19 Vaccination and Cancer, the Need for more Data. Asian Pac J Cancer Prev. 2021;22:3053–4. doi: 10.31557/APJCP.2021.22.10.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalakar P, Freeman AI, Higby DJ, Wallace HJ, Sinks LF. Clinical response and toxicity with cis-dichlorodiammineplatinum(II) in children. Cancer Treat Rep. 1977;61:835–9. [PubMed] [Google Scholar]
- Laitinen M, Parry M, Albergo JI, et al. The prognostic and therapeutic factors which influence the oncological outcome of parosteal osteosarcoma. Bone Joint J. 2015;97-b:1698–703. doi: 10.1302/0301-620X.97B12.35749. [DOI] [PubMed] [Google Scholar]
- Lee ES, Mackenzie DH. Osteosarcoma A Study Of The Value Of Preoperative Megavoltage Radiotherapy. Br J Surg. 1964;51:252–74. doi: 10.1002/bjs.1800510405. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yost SE, Yuan Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers. 2020;12:1404. doi: 10.3390/cancers12061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- Marcove RC, Miké V, Hajek JV, Levin AG, Hutter RV. Osteogenic sarcoma under the age of twenty-one A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970;52:411–23. [PubMed] [Google Scholar]
- Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17:1396–408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavligit GM, Benjamin R, Patt YZ, et al. Intraarterial cis-platinum for patients with inoperable skeletal tumors. Cancer. 1981;48:1–4. doi: 10.1002/1097-0142(19810701)48:1<1::aid-cncr2820480102>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Picci P, Bacci G, Campanacci M, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy Regional mapping of viable and nonviable tumor. Cancer. 1985;56:1515–21. doi: 10.1002/1097-0142(19851001)56:7<1515::aid-cncr2820560707>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rosen G, Marcove RC, Caparros B, et al. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–77. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37:1–11. doi: 10.1002/1097-0142(197601)37:1<1::aid-cncr2820370102>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Soroosh D, Javadinia SA. The COVID-19 outbreak and oncology centers in Iran. Int J Cancer Manage. 2020:13. [Google Scholar]
- Uimonen M, Kuitunen I, Paloneva J, et al. The impact of the COVID-19 pandemic on waiting times for elective surgery patients: A multicenter study. PLoS One. 2021;16:e0253875. doi: 10.1371/journal.pone.0253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Livingston JA, Patel SR, Benjamin RS. Chemotherapy for Bone Sarcoma in Adults. J Oncol Pract. 2016;12:208–16. doi: 10.1200/JOP.2015.009944. [DOI] [PubMed] [Google Scholar]
- Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–14. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin NF, Abdul Rashid ML, Ajit Singh V. Survival analysis of osteosarcoma patients: A 15-year experience. J Orthop Surg. 2020;28:2309499019896662. doi: 10.1177/2309499019896662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study can be accessed through direct communication with the corresponding author and the agreement of all research team members.