Abstract
Muscle degeneration is one the main factors that lead to the high rate of retear after a successful repair of rotator cuff (RC) tears. The current surgical practices have failed to treat patients with chronic massive rotator cuff tears (RCTs). Therefore, regenerative engineering approaches are being studied to address the challenges. Recent studies showed the promising outcomes of electroactive materials (EAMs) on the regeneration of electrically excitable tissues such as skeletal muscle. Here, we review the most important biological mechanism of RC muscle degeneration. Further, the review covers the recent studies on EAMs for muscle regeneration including RC muscle. Finally, we will discuss the future direction toward the application of EAMs for the augmentation of RCTs.
Keywords: Skeletal muscle, Rotator Cuff, Electroconductivity, Regenerative engineering, scaffolds
Graphical abstract

Introduction
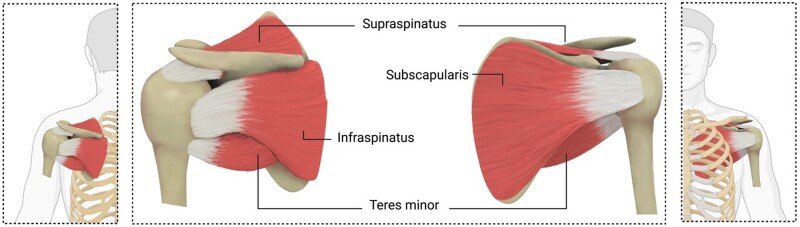
The rotator cuff (RC) is a cuff of soft tissue responsible for the shoulder movement (Figure 1). Rotator cuff tears (RCTs) with more than 200 000 annual repair procedures in the United States are a significant cause of disability in adults [1–13]. Tendon detachment can cause tendon retraction, muscle degeneration, and shoulder instability [1, 14, 15]. One of the challenges with RC repair is the high retear rate after surgery. Patient age, tear size, repair strategies, and the degeneration of RC muscle influence the rate of retear [2, 16, 17].
Figure 1.
RC anatomy and the involved tendons including supraspinatus, infraspinatus, teres minor, and subscapularis.
RC muscle degeneration is characterized by atrophy of muscle fibers, fibrosis, and fat accumulation within and around the muscles [14, 18, 19]. Especially, fat accumulation of the muscle has a significant influence on the high rate of RC retear after surgery.
Clinically, the delay of surgical repair after RC injury is one of the most commonly observed challenges. This duration between injury and repair is described by category, ‘acute’, ‘subacute’ and ‘chronic’. An acute RC injury involves the immediate repair of the injury, while subacute and chronic refer to a prolonged duration. Subacute and chronic RCTs are often associated with a set of degenerative changes (Figure 2) [20, 21]. The tendon retraction and degenerative changes of the musculotendinous unit over weeks and months lead to muscle atrophy, fibrosis formation, and fat accumulation [19, 22–25].
Figure 2.
Muscle atrophy and fat accumulation of RC muscles in a Sub-acute and chronic injury (ROI, region of interest).
The current surgical and nonsurgical strategies have failed to treat RCTs satisfactorily and tissue regeneration strategies are promising approaches to address the challenges [2, 26, 27]. Regenerative engineering stated as the convergence of disparate fields including stem cells science, advanced materials, physics, developmental biology, and clinical translation for the regeneration of various complex tissues, including musculoskeletal regeneration [1, 14, 19, 28].
Over the past two decades, significant progress has been made in the area of tissue engineering, which reveals a promising future for engineering complex tissues and organ systems [1, 14, 19, 29–34]. ‘Matrix’ or ‘Scaffold’ is a main component that plays an essential role in tissue regeneration, as it provides an important niche for cell attachment, proliferation, and differentiation.
For successful RC regeneration, materials need to be biodegradable and have appropriate physiological properties similar to the native tissue to provide a biomimetic substrate for cell activation [35, 36]. The materials and architecture that scaffolds can be fabricated from are determined based on the application of the scaffold in the body. Natural and synthetic polymers are a wide group of biomaterials that are used with different geometries for various kinds of applications [19, 29, 31, 37–43]. We have previously reported a comprehensive review of the use of natural and synthetic polymeric scaffolds for the regeneration of the RC [1].
Several strategies have been applied to stimulate cells and enhanced cell growth and activity for tissue regeneration [19, 29, 30, 33, 44–46]. One of the strategies for local stimulation are electroactive materials (EAMs). In the 1960s, Bassett et al. reported the efficiency of electrical stimulation (ES) on cells and tissues [47]. It has been reported that ES can rapidly affect cell migration and subsequently cell behavior [47]. Afterwards, several studies investigated the functionality of EAMs for tissue regenerative engineering. Electroactive-based materials have been widely used for the regeneration of many electrically excitable tissue types such as skeletal muscle [33], cardiac muscle [48], nerve [49], and bone [50] to enable the conduction of the natural electrical current along the bioactive graft after implantation in vivo [51]. ES has also been used in conjunction with such EAMs to recapitulate the in vivo electrical current passing through the native tissues to promote cell alignment, migration, proliferation, and differentiation to maintain the engineered tissue functionality [52, 53]. In this review, we first discuss the RC muscle degeneration and then review the applications of EAMs for the regeneration of skeletal muscle. In addition, we touch upon the utilization of ES with such EAMs.
RC muscle degeneration
RC muscle degeneration is characterized by muscle atrophy, fibrosis formation, and the fat accumulation in the muscle. Clinically, these degenerative changes in a chronic RC injury are considered permanent changes that cannot be retreated. In this section, we review the current understanding of these changes and underlying mechanism for their appearance.
RC muscle atrophy
Skeletal muscle forms almost half of the total body mass and comprises bundles of aligned muscle fibers [54, 55]. The growth and maintenance of muscle are supported by satellite cells which participate during the myogenesis process by their differentiation to myoblasts and subsequent fusion into myofibers [54–56]. The minor injuries of skeletal muscle are repaired by the intrinsic regeneration potential of satellite cells. However, this repair mechanism cannot support muscles in chronic RC injuries to inhibit or reduce fat accumulation and atrophy [14, 19]. Severe damage to muscle tissues prevents satellite cell recruitment and activation and limits regeneration [55].
The muscle atrophy is one of the critical factors that significantly affect the RC healing. It has been reported that muscle atrophy increases with aging even in healthy RC [57]. The number of satellite cells are specifically reduced in type II but not in type I skeletal muscle fibers in aged population [58]. However, the overall number of satellite cells did not change significantly with tear size [59], suggesting satellite cell niche is influenced by RCT. For example, muscle size and function are controlled by the balance of biogenesis/biosynthesis versus removal/destruction [60]. Insulin and insulin-like growth factor 1 are known to promote muscle growth, while TGF-β/myostatin/activin is known to prevent muscle growth [60]. In the RC, TGF-β signaling was upregulated followed by the RCT [61] and TGF-β expression was increased with age [62]. Another study showed the pro-myogenic gene expressions were suppressed in full-thickness tear and massive tear patients [63]. The ECM such as collagen type IV and laminin is another important regulator of skeletal muscle cell function and stem cell niche [64]. The remodeling of ECM followed by RCT may affect muscle regeneration through altered ECM environment [65]. The neuropathy of suprascapular nerve also influences to the progress of both muscle atrophy and fat accumulation [66].
Studies showed that the severe muscle atrophy makes the RC tendon irreparable [57, 67, 68]. RC muscle atrophy has been evaluated in several studies [19, 25, 67–70]. The common criteria to evaluate the muscle atrophy is muscle mass reduction, muscle fiber cross-sectional area, and muscle atrophy grading [19, 67–72]. The most common parameters for grading the muscle atrophy are the decreased muscle fiber size, a round shape of muscle fibers rather than an angular shape, and the decreased distance between myonuclei [68, 71, 72]. Several studies evaluated the different time-points of chronic injuries in different species, replicating the same muscle atrophy often seen clinically. Abdou et al. reported mild to moderate muscle atrophy from 2 to 6 weeks and severe atrophy at 8 and 12 weeks after injury in the supraspinatus muscle [68]. In a model developed by Kim et al. of chronic RCTs in rats, the extent of muscle degeneration after 16 weeks was found to be greater than that observed after 2 and 8 weeks [71]. Tang et al. [19], also reported muscle atrophy at 6 weeks following the RCT in a rat model.
RC fibrosis
Fibrosis, also known as fibrotic scarring, is characterized by excessive synthesis, remodeling, and contraction of extracellular matrix [73]. In general, wound healing process contains four overlapping phases: hemostasis, inflammation, proliferation, and tissue remodeling [74, 75]. Chronic inflammation or inappropriate regulation of inflammation result in continuous ECM production that prevent tissue repair [76]. Several signal pathways, such as TGF-β, bone morphogenic protein, and Wingless/Int signaling, have been linked to the pathophysiology of fibrosis [73, 77, 78]. Myofibroblasts differentiated from fibroblasts in accordance with macrophage stimulation are characterized by the expression of collagen type I and α-smooth muscle actin (α-SMA). Various cytokines and growth factors including TGF-β are released from inflammatory cells, which regulate myofibroblasts growth and ECM synthesis [79, 80].
Tendons are composed of a highly organized ECM bundle such as predominantly collagen type I and subsequently collagen type III [81], which is responsible for transmitting generated forces from muscle to bone. Tenocytes which express tendon marker genes such as scleraxis (Scx) and tenomodulin (Tnmd) are predominant cell type in tendon and located between collagen fibrils. Tendon possesses less cellularity and vascularity compared to other tissues [82, 83], and very little turnover of ECM after maturation [84]. Upon tendon injury, various cell types including intrinsic and extrinsic cell sources are involved in its wound healing process [85]. However, adult tendons lack the inherent regeneration ability and result in fibrotic scar formation. Lineage tracing analysis of transgenic mice showed the contribution of Scx-lineage cells in neonatal healing whereas no recruitment into the defect area in adult [86]. Another lineage tracing study suggested αSMA progenitors contribute to newly formed tenocytes [87]. Without Scx-lineage cells, extrinsic cells seem to be recruited to the wound site and differentiate into myofibroblasts which is contributed to fibrosis formation. Even though tendon stem/progenitor cells in tendon have been identified [88], little is known about their contribution during tendon healing. The niche of TSCPs might be affected by complex process of wound healing or aging.
Clinically, tendon disease are classified from acute inflammation stage, chronic tendinopathy, and tendon rupture, causing shoulder pain [89]. Followed by RCT, torn tendon tissue increased cellularity and disorganized ECM content which leads fibrosis formation. In addition, intramuscular fibrosis formation plays a significant role in RC pathology [90]. After the RCT, synthesized intramuscular collagen content seemed to cause increased passive stiffness that is associated with degenerative changes [91]. In a clinical setting, immobilization of joint is important for tendon healing, but disuse of joint and muscular weakness can lead to shoulder stiffness [92, 93]. Although collagen is the central component of tendon healing, establishment of aligned bundle is essential to appropriate repair otherwise fibrosis formation losing original function.
RC fat accumulation
In general, three types of adipose tissue have been identified by their anatomical location and metabolic features termed white adipose tissue, brown adipose tissue, and brite/beige adipose tissue (Figure 3) [94]. The white adipose tissue is the main energy-storing tissue and morphologically has one single big lipid droplet. On the other hand, brown adipose tissue’s responsibility is to dissipate energy as heat and is formed by many small lipid droplets. Despite white adipose tissue, the origin of brown adipose tissue is more closely related to muscle [95]. It derives from precursors that express Myf5 which is also expressed in myogenic lineages [96]. The expression of uncoupling protein 1 (UCP1) is a brown adipose tissue-specific marker. It has been reported that under specific stimulation, white adipocytes undergo a browning process which alters white adipose tissue into a brown adipose tissue phenotype, called beige adipocytes [94, 95, 97]. Brite/beige adipocytes form in white adipose tissue and show similarity to brown adipose tissue as they have multilocular lipid droplets and express UCP1, which distinguish them from white adipose tissue [96].
Figure 3.
The schematic images of the morphology of different types of adipocytes include brown, brite/beige, and white adipocytes. The H&E histology images were published by [96].
Fat accumulation is a more frequent challenge in RCTs compared with other musculotendinous injuries. Besides evaluating the effects of RCT, the effect of aging has also been reported on fat accumulation [57]. Many different cell types contribute to regenerate an efficient skeletal muscle. These cells can be either resident or not resident including but not limited to pericytes, fibro adipogenic progenitors (FAPs), muscle cells, and immune cells [98].
FAPs are muscle resident progenitor cells and were first described by Uezumi et al. [99]. These mesenchymal progenitors are positive for platelet-derived growth factor receptor alpha (PDGFRα). These cells are located in the muscle interstitium. The lack of paired box protein 7 (PAX7) expression distinguishes FAPs from muscle stem cells (satellite cells). It has been reported that these progenitors are the main source of fat accumulation and fibrosis in skeletal muscle [25, 65, 67, 99–102]. Lee et al. [101] demonstrated the intrinsic differences in the distribution and differentiation of FAPs in RC muscle compared with muscles from other anatomical locations in mice [101]. Supraspinatus and infraspinatus muscles showed the highest percentage of progenitors (17.5% and 15.7%, respectively) compared with tibialis anterior muscle (5.3%) based on fluorescence-activated cell sorting [101]. The highest concentration and adipogenic potential of FAPs in RC muscles can be the most important contributing factors in fat accumulation after RCTs. Clinically it has been reported that supraspinatus in a chronic RCT has a great number of FAPs and fibrosis compared with healthy RC [103]. Also, it has been shown that increasing the RC tear size and thickness leads to an increase in the number of FAPs [59].
Initially, after muscle injury, FAPs start to proliferate and provide redifferentiation signal for myogenesis which is beneficial for muscle regeneration [65]. After the initial expansion, a large number of progenitors undergo apoptosis. However, some of them persist and form the main source of fat accumulation and fibrosis [65, 98]. Although the exact underlying mechanism by which FAPs undergo adipogenesis and fibrogenesis is not identified yet [65].
The proliferation and differentiation of satellite cells depend on the composition of the niche and ECM signaling. The fat accumulation and fibrosis formation by FAPs can change the ECM composition which subsequently alters satellite cells pathways and can negatively affect their regenerative efficacy [65]. It has been reported that FAPs may also express anti-myogenic signals such as TGF-β [65].
Several studies reported an increase in the severity of fat accumulation by time in chronic RCT models [71]. The severity of fat accumulation is commonly reported by grading or fat area calculation [67, 68, 71,72, 104–106]. Goutallier et al. [107, 108], described a standard system to classify the severity of fat accumulation after RCTs. Clinically, stage 3 and stage 4 of Goutallier grading of the torn RC is considered irreparable while stage 1 or stage 2 may be reversed by an ideal treatment of fat accumulation [68, 107–109]. The developed strategies to stop, reduce, or reverse the fat accumulation following the RCTs will be discussed further in this article.
Electroactivity as a novel strategy to reverse the muscle degeneration after RCTs
Several strategies to treat RCT have been utilized and evaluated. These include various suture-based techniques such as a single row, and double row [110, 111], the use of tissue transplants (allograft, xenograft, autograft, decellularized structures), and tissue regeneration strategies [2]. The current standard treatment for muscle regeneration is to engraft healthy autologous tissue from an uninjured site [56]. Although successful, almost 10% of autograft transplantation fails due to infection and necrosis [112, 113]. Moreover, autografts present limitations such as donor morbidity and limited availability [114]. These limitations have driven efforts to develop alternative strategies to support muscle regeneration. Clinically, current surgical practices, including suture techniques and tissue transplants, have failed to treat RCTs successfully. It has been reported that the current strategies cannot reverse muscle degeneration after RC repair [19, 115–117]. There is still a need to reduce fat accumulation and muscle atrophy after RCT to improve RC regeneration. One of the most novel strategies is the applications of EAMs for reducing the muscle degeneration.
The human body is known for its ability to generates endogenous electrical potential, referred to as bio-electricity, that plays a key role in the homeostasis of various tissue types, including tissue development and wound healing [118]. When an injury occurs, an injury potential is initiated as a steady direct current (DC) electric field (EF) that localizes at the injury site. This localized EF guides cells to migrate and sprout directly toward the wound edge to aid in the healing process. Without EF, the wound healing is inhibited [119]. During cell migration to the wound edge, a voltage gradient of action potentials is initiated across the cell membrane as a result of the EF, causing the cell membrane to depolarize. This triggers cells to transmit biomolecular signals and upregulate certain genes and hormones that are essential during the healing process [119].
In some cases, some parts of the body such as tissues or organ systems lose their ability to endogenously generate an action potential EF or generates very weak action potential that cannot initiate a steady EF to perform the natural bioelectrical operations. However, an action potential can now be exogenously elicited by inducing an electrical charge to the cells, mimicking those of the endogenously produced through a process termed ‘electrical stimulation’ [120].
Electroconductive biomaterials can serve as a critical cue to promote muscle regeneration by improving the interactions between materials and cells [27, 121]. Since skeletal muscle is an electrically excitable tissue [27, 32, 121], the implantation of EAMs can support myoblast growth and maturation [27, 36, 121, 122]. Several studies indicated that EAMs could significantly promote myoblasts’ viability and differentiation, even with no external ES [19, 27, 32, 121, 123]. The efficacy of EAMs such as graphene [27, 124], and conductive polymers including poly(3,4-ethylene dioxythiophene) (PEDOT) [19, 32], polypyrrole (PPy) [125], and polyaniline (PANi) [121] has been reported in vitro and in vivo for skeletal muscle regeneration [121, 126–128].
External electrical stimulation
ES is a process used to provide an exogenous action potential to the cells, in the absence of the endogenous action potential [120]. In 1942, ES was proposed as a useful technique to preserve muscle tissue functions by substituting the nervous stimulation in denervated skeletal muscles [129]. The mass and contractility of denervated muscle tissues were maintained and improved by this technique [129]. Since then, ES has extensively been applied in both, clinical settings and tissue engineering as an alternate to treat various muscular and neuronal diseases, improve blood circulation at diseased tissues, promote wound healing, and maintain the functionality of the engineered tissues in culture [130]. The ability of various biological systems to respond to exogenous action potential suggests that ES may serve as an efficient tool to control and adjust cellular and tissue homeostasis [131, 132]. The responses of different types of cells to ES have been studied, including mesenchymal stem cells (MSCs), fibroblasts, osteoblasts, myoblasts, dorsal root ganglia, and neural crest cells [133–138]. These studies have shown that ES affects important cellular behaviors such as adhesion, proliferation, differentiation, directional migration, as well as cell division, all of which are essential elements during the tissue development and wound healing process. The variation in tissue composition, such as tissue type and density, cell membrane permeability, and electrolyte leads to the variation in the electrical resistivity of biological tissues. Therefore, the method by which the ES is applied must be optimized as a function of cell type and tissue composition to achieve the desired therapeutic effects [139].
Different methods of ES
ES is a feasible technique for both in vivo and in vitro two-dimensional (2D) and three-dimensional (3D) cultured cells [140]. Although methods for ES vary, no specific procedure/methodology/setup has been standardized for every cell or tissue type. Based on the literatures, ES methods can be characterized into: (i) DC/alternating current EF (AC EF), (ii) capacitive coupling EF (CC EF), and (iii) inductive coupling EF (IC EF) [139]. Various cell types have been studied and found to be responsive to these ES methods under different experimental settings [133–138].
Direct current/alternating current EF
ES was considered as a new therapeutic substitute and studied using both DC and AC EF following the finding of ossification enhancement by DC EF [141]. In medical literatures, DC and AC EFs refer to either electrical potential gradient or electrical current between two points of different electrical potentials generated by a power source [139]. DC/AC EF can be generated between an anode (−) and a cathode (+) immersed in electrolytes. In biological application, this electrolyte can be culture medium or tissue fluid and the current carriers are the ions in it. The current passing between the electrodes can cause depolarization of the membrane. Depending on certain parameters such as the EF frequency, current, and voltage, different degrees of depolarization can be obtained to achieve different experimental outcomes [142]. Electrodes can be metallic such as stainless steel, tungsten, platinum, platinum iridium alloys, iridium oxide, and titanium nitride; carbon-based such as carbon nanotubes (CNT); or made from conductive polymers such as PPy [139]. These electrodes can be simply altered into different forms and sizes, connected with a power source, and immersed directly into culture medium or tissue [139]. This procedure to apply ES to the cells is relatively simple; however, it has some disadvantages. The conversion of ionic conductivity into electron conductivity in these electrodes generates electrochemical reactions on the electrode surface which forms chemical species (faradic products) [139]. The faradic products in most cases lead to lowering of the media calcium levels, gas formation, and significantly increase the pH levels in the culture medium [143]. In addition, the direct exposure of the electrodes into the culture medium can cause disruption for the electrolyte composition when the electrical potential is applied and the current passes. Moreover, toxicity in the culture medium can arise, due to the insufficient biocompatibility of some electrodes, causing reduced levels of molecular oxygen which further hinder the use of this method [144, 145].
Capacitive coupling EF
Compared to the DC/AC EF method, CC EF offers a safer way for delivering the electrical stimulus to the cultured cells. In CC EF, a homogenous electromagnetic field is created between two parallel layers of metallic electrodes (e.g., stainless steel, gold) or carbon-based electrodes (i.e., a plate capacitor) that are placed above and below, the cell culture plate with a small (0.5–2 mm) gap between them [146–157]. CC EF has the advantage of generating a homogenous EF which leads to the equal amount of stimulation for every cell regardless of their position in the culture vessel. The main disadvantage of this method is the high voltage (e.g. 100 V) that is required between the electrodes [139].
Inductive coupling EF
In the IC EF, controlled electromagnetic fields are generated by coils placed around the cell culture plate. Compared to the CC EF method, IC EF has the advantage of generating small order of magnitude of currents and potentials near the targeted cells, rather than delivering the stimulation through electrodes [149, 152, 158–161]. Here, the coils are used in pairs, placed in a Helmholtz configuration, with a distance between the coils equal to their radius [162–164]. This method generates a homogenous magnetic field across the cell culture plate with uniform electromagnetic field properties. One of the subcategories of this method is pulsed electromagnetic field (PEMF) stimulation. In PEMF, the stimulus is delivered in pulses rather than being static or continuously harmonic [162, 165].
Electroconductive materials
The methodologies discussed in the previous section have been extensively used for exogenously delivering the electrical stimulus to the cultured cells, as well as to study the cell response to the ES. However, they can be problematic when applied for tissue regeneration applications, especially when a porous (3D) scaffold is used in culture. Since most fabricated scaffolds/matrices are not electrically conductive (dielectric), cells inside the porous scaffold or on its surface are not exposed to the same degree of EF and current. Therefore, this can generate a heterogeneous cell population of electrically stimulated and non-stimulated cells, which can lead to unmet experimental outcomes [139, 166].
Significant effort has been made in the past two decades for developing innovative strategies for the fabrication of biomimetic scaffolds for tissue regeneration [167, 168]. These efforts enabled the fabrication of scaffolds in a verity of physical forms such as fibers, meshes, membranes, gels or solids [167]. Of these, fibrous-based scaffolds have gained much attention and are particularly suitable for tissue regeneration applications due to their structural and topographical resemblance to the natural ECM [1, 14, 29, 32, 33, 169]. Fibers can be fabricated in the nanoscale/microscale by applying different fabrication techniques such as electrospinning, self-assembly, phase separation, and template melt-extrusion [32, 167, 170–173]. Moreover, fibers can be processed into complex fibrous structures using various textile techniques, such as knitting, weaving, or braiding, to create 3D structures with improved structural/mechanical properties [169]. It is nevertheless still a challenge to develop bioactive fibrous-based biomaterials that can enhance cell proliferation and guide their differentiation. Although, fibers can be modified either chemically or physically to show appropriate physiochemical and biological properties similar to natural niche environment to enhance cell adhesion, proliferation, and differentiation and ultimately improve tissue regeneration [51]. EAMs such as conductive polymers, electrets, piezoelectric and photovoltaic materials have been widely investigated for tissue regeneration applications due to their intrinsic chemical and physical properties, as well as high electrical conductivity that can modulate cellular response [174–181]. Due to nonbiodegradability and poor processability of such materials, they are often used in conjunction with other synthetic or natural biomaterials as composites, surface coatings, or functionalized on the surface of other materials via in situ polymerization technique [51, 182]. The electrical activity of these materials can be measured using conductivity measuring devices [183] and also other methods have been used to detect the composition and the dopant, functional groups, or coatings added to them such as UV-vis, Fourier transform infrared spectroscopy, elemental microanalysis, X-ray photoelectron spectroscopy, differential scanning calorimeter, and thermogravimetric analysis [184] in some studies researchers have measured the electrical resistivity of conductive polymers by four-point probe method [184–187]. Also, the characterization of PANI using dielectric relaxation spectroscopy and electron paramagnetic resonance has been done to study the electrical properties of polystyrene/PANI powder which is then compressed to form pellets [188]. The versatility of fibrous-based structures allows for combining such EAMs in their structure to form electro-active-based scaffolds [51].
EAMs include carbon-based composite, and conductive polymers can provide high similarity with the physiological properties of native tissues. EAMs intrinsic properties of simultaneously displaying the physical and chemical properties of polymers and the electrical characteristics of metals, all of which are key properties for regulating cellular functions, including differentiation, adhesion, proliferation, and migration [189]. Not only EAMs would allow for homogenous delivery of exogenous ES to the cultured cells in vitro but would also allow for homogenous delivery of endogenous ES along the bioactive scaffold after implantation in vivo. Studies have shown the potential of EAMs to enhance cellular activities, including cell adhesion, migration, proliferation, differentiation, and protein secretion with or without ES [125, 190–195]. This however, suggests the strong potential for such bioactive materials for use as suitable platforms for the regeneration of varies tissue types, especially of electrically excitable tissues such as skeletal muscles, cardiac muscles, nerves, connective tissues and bones [196, 197].
Conductive polymer
Among all types of organic and inorganic conductive biomaterials, PPy, PANi, and PEDOT are the most popular EAMs for tissue regeneration [36, 198]. Here, we briefly review the general properties of these conductive polymers.
Polypyrrole
Conjugated PPy is one of the most investigated conductive polymers because of its high electrical conductivity and good chemical stability in air and water and most importantly, good biocompatibility [199–201]. The polymerized PPy has a rigid conjugated backbone caused its insolubility in solvents and therefore limited processability. However, the monomer pyrrole is soluble in various solvents. To overcome this limitation, researchers incorporate the monomer pyrrole before polymerization into the matrices. It is difficult to fabricate pure PPy matrix due to its rigidness, brittleness, and nonbiodegradability [126, 194]. PPy can be polymerized in aqueous solution by various oxidizing agents such as FeCl3, (NH4)2S2O8, CuCl2. (NH4)2S2O8 is used for higher speed reactions (a few minutes) while FeCl3 is for lower speeds (e.g. 6 h) [202].Also, solvent-free oxidative polymerization methods have been used to mechanically mix the monomer and solid oxidant [183]. Due to the nonbiodegradability of PPy, it is important to use the lowest concentrations of PPy to ensure the biocompatibility of the PPy-matrix upon degradation [203, 204]. It has been reported that a small amount of PPy in the polymeric matrices such as poly(dl-lactic acid), poly(l-lactic acid) (PLLA), and polycaprolactone (PCL), has no adverse effects on their degradation behavior [205].
Polyaniline
PANi can be polymerized by different chemical and electrochemical oxidative methods. There are various structures of PANi based on the oxidation states; pernigraniline base (the fully oxidized structure), emeraldine base (the half-oxidized structure) and leucoemeraldine (the fully reduced structure) [201, 206, 207]. Besides the advantages of PANi including good conductivity, low cost, and good stability [208, 209], there are some challenges which limit its biological application. Similar to polymerized Ppy, PANi has poor processability, nonbiodegradability, and more importantly, it has been reported that it can cause chronic inflammation after implantation [210–213].
Poly(3,4-ethyl-enedioxythiophene)
PEDOT is another conductive polymer that is being investigated for tissue regeneration and is one of the polythiophene derivatives. PEDOT is a conjugated polymer which shows desirable chemical, electrical, and environmental stability and also better thermal stability and conductivity compared to PPy [214, 215].
Poor solubility is one of the critical challenges in using PEDOT which leads to poor processability. However, PEDOT with polystyrene sulfonate (PSS) composition shows improved solubility [216]. PEDOT could also be a promising conductor for biomedical applications. The good biocompatibility and low cytotoxicity of PEDOT have been reported in several in vitro and in vivo studies. Similar to other discussed conductive polymers, high stiffness and nonbiodegradability of PEDOT make some limitations which need to be addressed by keeping its concentration as low as possible [217].
Carbon-based materials
Carbon-based composites such as graphene, diamond, CNT, and nanowires have been investigated in different applications include electronic devices, drug delivery and tissue regenerative engineering [175, 218].
Graphene has been investigated for several biomedical and medicine applications owing to its unique features including the high electrical conductivity, high mechanical strength, stability in aqueous environments, ease of functionalization, flat structure with a large surface to volume ratio, heat conduction, and optical transparency [124, 219, 220].
A single atomic layer of sp2-hybridized carbon atoms (two single bonds and one double bond) organized in a hexagonal lattice is the base structure of graphene materials [219, 221]. The three covalent sigma (σ) bonds (the strongest type of covalent chemical bonds) among the carbon atoms form the sp2 configuration. This means a free valence electron is left which participates in a π bond that emerges from the lattice in the z-direction. The delocalized electrons in the π orbital give graphene its unique electronic and physical properties. The graphene’s inherent electrical conductivity can create electrical coupling with cells which may change the electrical activity of the cells [219, 221]. The formation of noncovalent interactions such as π–π, hydrogen bonding, and electrostatic between graphene nanomaterials and biomolecules can be considered as a contributing factor affecting cell differentiation.
Several studies reported the ability of various forms of graphene to support cell growth and induce differentiation in different cell lineages such as bone, neural, cardiac, and skeletal muscle [124, 219, 220, 222].
Mechanism of electrical field
Several studies demonstrated that applying ES on conductive polymers improve the cell activities and growth. Indeed, the conductive polymers with both electrical and ionic charges by inducing new conducting pathways/channels, help the cells communication together [36]. The mechanisms by which ES enhance the cells differentiation are still unknown. Wang et al. [223] reported a higher concentration of calcium ions in cardiac myoblasts on an EAMs-based scaffold. Under the condition with no external ES, the calcium ions concentration was higher on the conductive scaffold compared to the control group [223].
Calcium is one of the important intracellular messengers in myocytes. It plays an important role in muscle excitation–contraction and modulation of gene expression during myogenesis [224]. During the contraction, a voltage-gated L-type calcium channel is opened which allows the transport of small quantities of calcium ions into the cells. These calcium ions trigger the sarcoplasmic reticulum to release more calcium ions [224]. A large amount of intracellular calcium ions activates the myosin cross-bridge cycling which leads to muscle contraction [224]. Besides this direct activation, calcium ions can also indirectly induce the contraction through calcium/calmodulin-dependent protein kinase II and other enzymes and proteins involved in muscle contraction [224]. The concentration of calcium ions in the mitochondrial and nuclear matrices is similar to the calcium ion levels in the cytoplasm [225]. The increase in the cytoplasmic calcium ion levels can be attributed to various stimulations and result from either the release of calcium ions from internal stores or the influx of extracellular calcium ions by the calcium ion channels [225]. It has been reported that intracellular calcium ions alter the muscle gene’s expression and subsequently myogenesis. Porter et al. [224] showed that the presence of L-type calcium channel blockers inhibited the differentiation of C2C12 myoblasts to form myotubes. The study also reported that lowering the intracellular calcium ions through Dantrolene and Thapsigargin inhibited myotube formation and maturation (Dantrolene blocks calcium ions release from the sarcoplasmic reticulum and Thapsigargin inhibits the entry of calcium ions into the sarcoplasmic reticulum) [224].
Zhang et al. investigated the efficacy and the mechanisms of ES on PPy-based scaffold seeded with adipose-derived mesenchymal stem cells (AD-MSCs) [44]. Incorporation of PPy with the content of 9.6% increased the mechanical properties, alignment of cells, and hydrophilicity and subsequently the penetration of cells to the deeper/inner region. A 200 μA of direct current for 4 h per day for 3 weeks were applied on the cells. Based on the results, incorporation of EAMs with ES remarkably improved AD-MSCs migration and penetration. The significant enhancement of ALP activity, calcium deposition, and gene expression of osteogenic transcription factors confirmed the high potential of this strategy for bone tissue engineering. Further, different voltage-gated ion channels’ (VGIC) blockers (Ca2+, Na+, K+, and Cl− VGICs) were added to the AD-MSCs-seeded scaffolds. The data showed the key role of all VGICs and particularly Ca2+ VGIC on AD-MSCs migration and osteogenic differentiation [44]. The study indicated that applying ES to AD-MSCs seeded on conductive scaffold can change the membrane potential and activate the Ca2+, K+, Na+, and Cl− VGICs and subsequently alters the relevant ions flow among these VGICs to improve cell functions [44].
Applications of electroconductivity skeletal muscle regeneration
One of the most abundant tissues in our body is skeletal muscle [226]. Upon injury, skeletal muscle can regenerate the lost tissue but to a certain threshold [227]. However, if the injury is severe, the muscle tissue is unable to entirely regenerate its function [114, 228, 229]. Regenerative engineering approaches may be able to regenerate or restore the function of the impaired muscle tissue using biomaterial scaffolds, which attempt to mimic the ECM of the native muscle tissue. Due to the structural and topographical resemblance of fibrous scaffolds to the natural ECM, they have been tested as possible regenerative potential for muscle cells.
The aligned conductive fibers promote the myoblasts’ differentiation into myocytes and support the fusion of myocytes together to form the myotubes. The maturation of these myotubes can lead to the regeneration of skeletal muscle [1, 230].
Jun et al. developed an electroactive randomly aligned composite fibers made from poly(l-lactide-co-epsilon-caprolactone) (PLCL)/PANi using an electrospinning method and investigated the effect of the composite on myoblasts viability, proliferation, and differentiation. The authors showed that the PLCL/PANi fibers group was biocompatible and an enhanced number of cells were positive for sarcomeric myosin compared to the PLCL fibers group. In addition, PLCL/PANi fibers also improved myogenin expression when compared to the PLCL fibers group. The gene expression of troponin T and myosin heavy chain were also enhanced by the conductive PLCL/PANi fibers, indicating that electroactive fibrous structure has synergetic effects on modulating myoblasts differentiation and fusion into myotubes even without the addition of ES [128].
The aligned anisotropic structure of elongated myofibers in skeletal muscle suggests that providing aligned components to the engineered scaffold may serve as a guidance to myoblasts alignment and formation into myotubes. It was found that the aligned underlying orientation of electrospun scaffolds could serve as topographical cues that enhanced the alignment and elongation of skeletal muscle cells [127]. Based on these early findings, Chen et al. developed an electroactive PCL/PANi aligned and randomly aligned electrospun nanofiber scaffolds to study the effect of the fibers’ alignment on myoblasts response. Immunofluorescence staining data revealed that the PCL/PANi nanofibers with highly aligned structure could guide myoblast orientation and promote myotube formation better than random fibers. In addition, the electroactive and aligned PCL/PANi nanofibers showed further enhanced myotube maturation when compared to all the other groups (electroactive randomly aligned PCL/PANi, nonelectroactive aligned, and randomly aligned PCL alone), demonstrating the synergetic effect of the topographical and electroactive cues together on the differentiation of myoblasts [121].
Beside the underlying orientation of the fibrous scaffold, the concentration as well as the strategy of which EAMs are introduced to fibrous scaffolds can also affect the response of the cultured myoblasts. It has been reported that increasing conductivity via increasing the concentration of the EAMs, can improve the myoblasts attachment, proliferation, and differentiation [231]. However, increasing the concentration of the EAMs usually increases scaffold stiffness, which may be detrimental to myoblast development [231]. For example, Tang et al. developed aligned electrospun PCL/PEDOT: PSS electroactive scaffolds coated with different PEDOT: PSS concentrations. In this study, myoblasts viability, proliferation, and differentiation cultured on the electroactive scaffolds with the different PEDOT: PSS concentrations (from 1% to 100%) were assessed. Although all electroactive scaffolds were shown to be biocompatible, it was found that low and medium coating concentrations (1% and 10%) showed stimulatory effect on myoblasts growth and differentiation, as increasing the concentration of the EAMs was shown to suppress the growth and differentiation of the cultured myoblasts. Despite the improved outcomes from culturing myoblasts on the low and medium PEDOT: PSS-containing scaffolds, scaffolds with higher amounts of PEDOT: PSS had the highest electrical conductivity [32]. However, higher concentrations of PEDOT: PSS may have also increased the scaffold stiffness, which caused suppression in myoblasts bioactivities.
Browe et al. developed an aligned electrospun PPy/PCL scaffold with different PPy concentrations for muscle regeneration. In this study, both PPy and PCL were synthesized together into a copolymer (PPy/PCL), which was designed to increase scaffolds conductivity without significantly influencing their stiffness. The scaffolds were characterized in vitro and assessed for their suitability for myoblast proliferation and differentiation. In vitro data revealed that the 40% PPy/PCL group had the highest electrical conductivity when compared to all the other groups (pure PCL, 10% PPy/PCL, 20% PPy/PCL), and that increasing the PPy concentration had no significant effect on the stiffness of the scaffolds. The results of fusion index and number of nuclei per myotube showed promoted myoblast differentiation on PPy/PCL than PCL. It indicates that the method of which the EAM is introduced to the polymeric scaffold may play a key role in altering the stiffness and modulating myoblasts response [231]. As indicated here, different EAMs have different mechanical and conductive properties. Thus, these studies suggest that the strategy in which the EAMs are introduced to the fibrous scaffolds must be optimized as a function of a conductive material type to achieve the best possible scaffold stiffness and conductivity without suppressing the potential for myoblasts proliferation, differentiation and ultimately muscle regeneration.
ES is another factor contributing to the differentiation of myoblasts to myotubes. It has been shown that myoblasts are able to differentiate into myotube when cultured on electroactive fibrous-based scaffolds, even in the absence of ES [32, 121, 231]. However, the presence of ES further enhances myotube formation and muscle contractibility [127]. Several works have utilized ES along on electroactive fibrous-based scaffold for further enhancing the functionality of the formed myotubes in culture [232, 233]. For electrically stimulating muscle myoblasts, the ES parameters are often optimized as a function of scaffold material, as myoblasts cultured on different EAMs response differently to the applied ES due to the variation of the electrical conductivity of these materials.
Quigley et al. developed an aligned fibrous-based scaffold made from CNT and PPy as a potential myogenic scaffold and investigated the ES effects on myoblasts proliferation and differentiation. In this study, the cultured myoblasts were electrically stimulated with (0.125 mA cm−2) at a frequency of (10 Hz) for 8 h per 24 h period for 3 days. It was found that myoblasts orientation, length, and size were significantly enhanced when they were electrically stimulated on the electroactive fibrous scaffold when compared to the non-electrically stimulated controls. In addition, ES significantly increased myoblasts proliferation and differentiation into myotubes [232].
Sirivisoot et al. further investigated the effects of ES on myoblasts response by fabricating a multiwalled CNT/PU electrospun scaffold. In this study, the cultured myoblasts were electrically stimulated with (22 V/cm) at a frequency of (20 Hz) twice a day on the last 2 days of culture (4 days total). Immunofluorescence staining data revealed that after ES, the number of multinucleated myotubes on the electrospun CNT/PU scaffolds was significantly larger than that on nonelectrically stimulated CNT/PU scaffolds. In addition, myoblasts were able to differentiate into myotubes on both scaffolds groups, but the expression of Myf5 and myosin heavy chains was significantly greater on the electrically stimulated CNT/PU group [233]. These studies demonstrate that electroactive platforms can be utilized to influence muscle cell behaviors through fibrous structures and ES. In addition, the data clearly indicate that ES parameters are dependent on the type of the EAM used in the scaffold, as the ES parameters in these studies varied when different EAMs with different conductivities were used. Thus, optimizing the ES parameters for myoblasts cultured on electroactive fibrous-based scaffolds is crucial for integrative guidance to the cultured cells.
To the best of our knowledge, few studies have examined the efficacy of EAMs for muscle regeneration in vivo.
Zhao et al. [234], showed the potential of a conductive cryogel for skeletal muscle tissue regeneration. The cryogel was fabricated by cryopolymerization of gelatin (GT) and poly(tannic acid) reduced graphene oxide (GT/rGO@PTA). The in vitro study using C2C12 myoblasts demonstrated the enhanced cell proliferation and myogenic differentiation with cryogel. Thereafter, the cryogel was evaluated in a rat tibialis anterior volumetric muscle loss model. The samples were harvested at 2 and 5 weeks of implantation. At 2 weeks, the histology of muscles showed new tissue formation in cryogel implanted groups compared with the control group. After 5 weeks, the muscle formation was detected in the cryogel-implanted animals with the highest centronucleated myofibers and capillaries compared with control groups. Moreover, the trichrome staining showed that the blank group had the most fibrosis formation than GT/rGO@PTA group. These results demonstrated that the EAMs-based cryogel promoted the regeneration of skeletal muscle [234]. The potential of GT and polydopamine-coated carbon nanotubes (PCNTs) cryogel was also investigated by Hu et al. [235]. Both in vitro and in vivo results showed the efficacy of EAMs-based implantation for muscle regeneration. The fabricated cryogel promoted the myogenic differentiation of C2C12 cells and new muscle formation in a rat tibialis anterior muscle defect model [235].
Du et al. [27] developed poly (citric acid-octanediol-polyethylene glycol) (PCE)-graphene (PCEG) nanocomposites. The in vitro assays showed enhanced C2C12 myoblasts proliferation and myogenic differentiation on PCEG nanocomposite. The efficacy of the PCEG nanocomposite was further evaluated in a rat tibialis anterior muscle injury. The histological results showed the formation of new muscle tissue at 1 week and the new tissue significantly increased at 4 weeks in the nanocomposite implanted group. Besides they reported high numbers of centronucleated myofibers and capillaries in PCEG group compared with the control groups after 1 and 4 weeks [27].
Tang et al. reported the efficacy of EAMs-based aligned fibers to retain muscle quality and enhance muscle regeneration after RCT in rat acute and subacute models [19, 126]. The scaffold was fabricated by coating PEDOT: PSS on PCL nanofibers. The regenerative potential of the scaffold was evaluated using two repair models of RCTs include acute and subacute [19, 126]. For the acute model, the scaffold was implanted on the supraspinatus muscle immediately after the injury. The repair surgery for the sub-acute model was conducted 6 weeks after injury.
The results demonstrated the ability of aligned electroconductive fibrous scaffolds to reduce muscle atrophy 2 and 6 weeks after RC injury [19, 126]. Figure 4 shows H&E and trichrome staining of the rat supraspinatus muscle after 6 weeks of repair. The treated groups (A-RM and SA-RM/6w) showed better muscle organization 6 weeks after successful surgical repair [19, 126].
Figure 4.
(A) Diagram of experimental groups and schematic illustration of the RC surgery model. For subacute groups, 4 and 6 rats were sacrificed 2 and 6 weeks after repair respectively. (B) Histology images of the supraspinatus muscle for both acute and subacute groups, (A) H&E, and (B) Masson trichrome staining [19, 126].
The results demonstrated the ability of electroconductive scaffolds to guide and stimulate muscle regeneration and treat muscle atrophy in RC injury. The control group showed muscle degeneration and atrophy 6 weeks after RCT. However, the repair groups with scaffold showed significant improvement compared with control groups. This study demonstrated the efficacy of an electroconductive fibrous scaffold in healing the RCT by improving the muscle atrophy in acute and sub-acute models [19, 126].
Recently, our group reported the regenerative potential of graphene-based matrices to reverse muscle atrophy and reduce fat accumulation after chronic RCTs in a rat model [33]. We developed an EAM-based matrix by incorporating graphene nanoplatelets (GnPs) into aligned PLLA nanofibers. The GnP matrix significantly increased myotube formation of C2C12 myoblasts (Figure 5), which can be attributed to enhanced intracellular calcium ions in myoblasts as reported in the study [33]. The results also showed the efficiency of GnP matrices on the inhibition of fat formation in vitro.
Figure 5.
(A) Schematic illustration of muscle regeneration on graphene matrix, (B) immunofluorescent images of differentiated myotubes after 7 days on aligned matrices immune-stained for MHC and nucleus, (C) quantification of myotube length, fusion index, maturation index of differentiated myotubes after 7 days on aligned matrices [33].
Following the promising outcomes of the in vitro study, the matrices were implanted on the supraspinatus and infraspinatus muscles of chronic massive full-thickness RCTs in a rat model. The implantation of the matrices on the degenerated muscles demonstrated the ability of EAMs to reverse muscle degenerative changes in vivo. The histological evaluation exhibited the effects of the graphene-based matrix to reverse muscle atrophy, fat accumulation, and fibrosis in both supraspinatus and infraspinatus muscles at 24 and 32 weeks after the chronic massive full-thickness RCTs of the rat shoulder (Figure 6). The long-term biocompatibility of the matrix was also confirmed by pathological evaluation of internal organs. Furthermore, the study showed that reversing muscle degenerative changes improved the morphology and tensile properties of the tendon compared with current surgical techniques [33].
Figure 6.
(A) The histological images of infraspinatus muscle at 32 weeks. The evaluation of (B) fibrosis formation, (C) fat accumulation, and (D) muscle atrophy at 32 weeks. (E) The histological images of supraspinatus muscle at 32 weeks. The evaluation of (F) fibrosis formation, (G) fat accumulation, and (H) muscle atrophy at 32 weeks. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 [33].
The ability of the graphene-based matrix to reverse muscle degeneration and the long-term biocompatibility of the matrices in an animal model provide convincing evidence for using the EAMs in future studies.
Altering biologic factors
Several studies investigated different strategies to reduce muscle degeneration following the RCTs. Sevivas et al. reported the efficacy of the secretome of MSCs to prevent muscle degeneration in a rat chronic RCT model [236]. It was shown that the either systemic or local injection of MSCs secretome can reduce fat formation in a rat model of massive RCT [236]. Moreover, Wang et al. [237] demonstrated the preventive effects of local injection of adipose stem cell-derived exosomes on fat accumulation. The repair procedure was conducted in a rabbit RCT 6 weeks after the injury. The histological and biomechanical results showed a higher score of regeneration and strength compared with the control groups [237].
The efficacy of brown adipose tissue to promote muscle regeneration has been shown by Meyer et al. [238]. The results showed that transplantation of brown adipose tissue between the trapezius and supraspinatus muscles in a mouse model in conjunction with cardiotoxin injection to the supraspinatus muscle, significantly increased fiber cross-sectional area, muscle mass, and muscle function compared with the sham group [238]. These results showed that brown/beige adipocytes can be considered as a promising cell source for healing RCT. As mentioned earlier, many studies reported that FAPs are the main source of fat accumulation and atrophy after RCT. Gorski et al. [239], demonstrated the ability of FAPs to differentiate into UCP1-expressing brite adipocytes. The efficacy of local transplantation of these brite/beige adipocytes to reduce RC muscle degeneration in a mouse model has been demonstrated by Lee et al. [67]. The supraspinatus tendon and suprascapular nerve of mice were transected and repaired 2 or 6 weeks after the injury [67]. The repair procedures included: no treatment, PBS injection, or beige adipose FAPs injection. The samples were harvested 6 weeks after the repair surgery [67]. Consistent with other studies they reported fibrosis and fat accumulation after suture repair, confirming that current surgical repair cannot reverse muscle degeneration [67]. However, their results showed a significant reduction in fibrosis and fat accumulation in the beige adipose FAPs injection group [67]. The exact underlying mechanism is not clear however these promising results can be attributed to cytokine or other secreted factors by beige adipose FAPs, or the death and trans-differentiation of fibroblasts and adipocytes [67]. The group also reported the regenerative potential of beige FAPs in a massive RCT model [102]. The mice underwent supraspinatus, infraspinatus, and suprascapular nerve transection [102]. The repair procedure was performed 2 weeks after the first surgery. The treatment groups were no treatment, PBS injection, and beige FAPs injection into the supraspinatus muscles. The results showed a statistically significant reduction in fibrosis, muscle atrophy, and fat accumulation in the cell-treated group compared with control groups 6 weeks after the repair surgery [102].
Taken together, the fat stores in torn RC can become a novel therapeutic approach to improve muscle conditions after RCT, as shown in Figure 7 [95]. Kong et al. reported the potential of brown adipose tissue to control skeletal muscle function by the secretion of significant quantities of myostatin into the blood [97]. The EAMs-based scaffolds can act as the vehicle to deliver the beige/brown adipocyte to the degenerated RC muscles. The studies showed the ability of EAMs to regenerate skeletal muscle and reverse RC fat accumulation and muscle atrophy. The combination of EAMs with beige/brown adipocytes can boost the RCT treatment and speed up the healing process.
Figure 7.
The schematic illustration of FAPs behavior. The underlying mechanism of fat accumulation and fibrosis formation is attributed to FAPs. However, the ability of FAPs to differentiate into brown/beige adipocytes can make it a novel therapeutic approach to reverse muscle degenerative changes after RCTs.
Moreover, EAMs-based scaffold can be further developed by adding biological factors or small molecules such as signaling inhibitors. It has been shown that FGF-8b can induce myogenesis and inhibit adipogenesis [30]. Another study showed applying small molecule inhibitor of TGF-β signaling reduced RC muscle fibrosis and fatty infiltration in a mice RCT model [240]. Applying those molecules into EAMs can be a robust strategy to reverse the muscle degenerative changes following the chronic RCTs. The combination of an effective biological factor with EAMs can accelerate the treatment and have superior effects on the tendon-bone insertion regeneration and tensile properties (Figure 8).
Figure 8.
The schematic illustration showing the efficacy of EAMs either alone or in combination with ES and/or biological factors to reverse the muscle degeneration following RCTs.
Limitations and challenges
Implementing electroconductivity in regenerative engineering and more precisely in RCT regeneration has its own challenges and limitations. External ES is beneficial for muscle tissue activity hence promoting healing, however, delivering this to the cells is a challenge. When using scaffolds in tissue engineering, cells will migrate to the porous structure as it will support and promote their proliferation and function, but if this substrate is not electrically conductive, the external stimuli will not affect where it is most needed, cells within the scaffold. Thus, there is a need for using electroconductive materials to facilitate this connection and pass on the external or the internal ESs. There have been a wide variety of electroconductive materials used in industry, however, one most important shortcoming of these is nonbiodegradability and poor processability. As a result, most of these EAMs are used alongside other biomaterials as coating, functionalized on the surface, or composites. This gives researchers an opportunity for tuning, altering, and manipulating the fabricated material to gain the desired properties such as biodegradability along electroconductivity. However, there is still a gap that needs to be filled in this regard and more studies focused on the mechanism of action, incorporation the best material in an optimized manner is needed. Moreover, heterogenous in vivo and clinical studies are essential for this field to unveil the long-term effect and mechanism of clearance of these EAMs in the body. Due to these limitations, and unknown reactions to electrically conductive materials in the body, this studies have not been able to reach to clinical studies on RC injuries.
Conclusions
In this review, we discussed the biological mechanisms of RC muscle degeneration, then reviewed the applications of EAMs for skeletal muscle regeneration including RCT treatment. Various tissues are affected as a result of RCT injury which leads to a high retear rate after surgery. The current clinical strategies have failed to revert muscle degeneration and regenerative engineering is a promising approach to address the challenges. The endogenous electrical potential has a key role during the wound-healing process. By adding external ES, it has been shown to enhance the cellular activity of various types of cells such as MSCs, fibroblasts, and myoblasts. Therefore, researchers have developed and investigated EAMs to enhance local ES for the regeneration of electrically excitable tissue types such as skeletal muscle. Both in vitro and in vivo studies suggest the strong potential for the use of EAMs for the regeneration of skeletal muscles, although there is still room for improvement, including scaffold stiffness and conductivity without suppressing the potential for muscle regeneration. There are few studies that have examined the efficacy of EAMs for RCT treatment. However, our recent study demonstrated that EAMs application reverted muscle degeneration, not only muscle atrophy but also fibrosis formation and fat accumulation. Thus, it is beneficial to fabricate a scaffold or an augmentation that can be electrically stimulated. Then by implanting it in the injured RC, especially in contact with the muscle, it can act as a signaling facilitator and help repair the injury by improving muscle contractibility, reduce fat accumulation and fibrosis and improve regeneration in the area of effect. In addition, the use of biological factors such as secretome, brown/beige adipocytes, and growth factors showed promising results for the RCT treatment. EAMs can serve as a desirable carrier of those biological factors. In future studies, it is critical to focus on the efficient and controlled delivery by providing optimized electrical field to boost the regeneration of complex RC tissues, which will be a novel clinical strategy for the treatment of RCT.
Contributor Information
Nikoo Saveh-Shemshaki, The Cato T. Laurencin Institute for Regenerative Engineering, University of Connecticut, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA.
Mohammed A Barajaa, Department of Biomedical Engineering, Imam Abdulrahman Bin Faisal University, Dammam 31451, Saudi Arabia.
Takayoshi Otsuka, The Cato T. Laurencin Institute for Regenerative Engineering, University of Connecticut, Farmington, CT 06030, USA.
Elnaz S Mirdamadi, The Cato T. Laurencin Institute for Regenerative Engineering, University of Connecticut, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA.
Lakshmi S Nair, The Cato T. Laurencin Institute for Regenerative Engineering, University of Connecticut, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Orthopedic Surgery, University of Connecticut Health Center, Farmington, CT 06030, USA; Department of Materials Science and Engineering, University of Connecticut, Storrs, CT 06269, USA.
Cato T Laurencin, The Cato T. Laurencin Institute for Regenerative Engineering, University of Connecticut, Farmington, CT 06030, USA; Department of Biomedical Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Orthopedic Surgery, University of Connecticut Health Center, Farmington, CT 06030, USA; Department of Materials Science and Engineering, University of Connecticut, Storrs, CT 06269, USA; Department of Chemical & Biomolecular Engineering, University of Connecticut, Storrs, CT 06269, USA.
Funding
This work was funded by DHHS/NIH/NIAMS entitled, Regenerative Engineering of Complex Musculoskeletal Tissue and Joints (Grant No.: DP1AR068147 and NSF/EFRI Grant No: 1332329). M.A.B. was funded by Imam Abdulrahman Bin Faisal University, Dammam 34212, Saudi Arabia.
Conflicts of interest statement. None declared.
References
- 1. Saveh-Shemshaki NS, Nair L, Laurencin CT.. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater 2019;94:64–81. [DOI] [PubMed] [Google Scholar]
- 2. Zhao S, Su W, Shah V, Hobson D, Yildirimer L, Yeung KW, Zhao J, Cui W, Zhao X.. Biomaterials based strategies for rotator cuff repair. Colloids Surf B Biointerfaces 2017;157:407–16. [DOI] [PubMed] [Google Scholar]
- 3. Cronin KJ, Mair SD, Hawk GS, Thompson KL, Hettrich CM, Jacobs CA.. Increased health care costs and opioid use in patients with anxiety and depression undergoing rotator cuff repair. Arthroscopy 2020;36:2655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marigi EM, Kennon JC, Dholakia R, Visscher SL, Borah BJ, Sanchez-Sotelo J, Sperling JW.. Cost analysis and complication rate comparing open, mini-open, and all arthroscopic rotator cuff repair. JSES Rev Rep Tech 2021;1:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu S, Wang Y, Streubel PN, Duan B.. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater 2017;62:102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu JE, Horneff JG, Gee AO.. Immobilization after rotator cuff repair: what evidence do we have now? Orthop Clin North Am 2016;47:169–77. [DOI] [PubMed] [Google Scholar]
- 7. Novakova SS, Mahalingam VD, Florida SE, Mendias CL, Allen A, Arruda EM, Bedi A, Larkin LM.. Tissue‐engineered tendon constructs for rotator cuff repair in sheep. J Orthop Res 2018;36:289–99. [DOI] [PubMed] [Google Scholar]
- 8. Smietana MJ, Moncada-Larrotiz P, Arruda EM, Bedi A, Larkin LM.. Tissue-engineered tendon for enthesis regeneration in a rat rotator cuff model. Biores Open Access 2017;6:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN.. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet Disord 2014;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly BJ, Field LD.. Arthroscopic repair of medial transtendinous rotator cuff tears. Arthrosc Tech 2017;6:e2217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel S, Gualtieri AP, Lu HH, Levine WN.. Advances in biologic augmentation for rotator cuff repair. Ann N Y Acad Sci 2016;1383:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacevic D, Rodeo SA.. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res 2008;466:622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothrauff BB, Pauyo T, Debski RE, Rodosky MW, Tuan RS, Musahl V.. The rotator cuff organ: integrating developmental biology, tissue engineering, and surgical considerations to treat chronic massive rotator cuff tears. Tissue Eng Part B Rev 2017;23:318–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Washington KS, Shemshaki NS, Laurencin CT.. The role of nanomaterials and biological agents on rotator cuff regeneration. Regen Eng Transl Med 2021;7:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumbar SG, James R, Nukavarapu SP, Laurencin CT.. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater 2008;3:034002. [DOI] [PubMed] [Google Scholar]
- 16. Voos JE, Barnthouse CD, Scott AR.. Arthroscopic rotator cuff repair: techniques in 2012. Clin Sports Med 2012;31:633–44. [DOI] [PubMed] [Google Scholar]
- 17. Henry P, Wasserstein D, Park S, Dwyer T, Chahal J, Slobogean G, Schemitsch E.. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy 2015;31:2472–80. [DOI] [PubMed] [Google Scholar]
- 18. Osti L, Buda M, Del Buono A.. Fatty infiltration of the shoulder: diagnosis and reversibility. Muscles Ligaments Tendons J 2013;3:351–4. [PMC free article] [PubMed] [Google Scholar]
- 19. Tang X, Shemshaki NS, Vernekar VN, Prabhath A, Kuyinu E, Kan H-M, Barajaa M, Khan Y, Laurencin CT.. The treatment of muscle atrophy after rotator cuff tears using electroconductive nanofibrous matrices. Regen Eng Transl Med 2021;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thangarajah T, Henshaw F, Sanghani-Kerai A, Lambert SM, Pendegrass CJ, Blunn GW.. Supraspinatus detachment causes musculotendinous degeneration and a reduction in bone mineral density at the enthesis in a rat model of chronic rotator cuff degeneration. Shoulder Elbow 2017;9:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ.. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech 2004;37:739–49. [DOI] [PubMed] [Google Scholar]
- 22. Mierzejewski B, Archacka K, Grabowska I, Florkowska A, Ciemerych MA, Brzoska E.. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin Cell DevBiol 2020;104:93–104. [DOI] [PubMed] [Google Scholar]
- 23. Collao N, Farup J, De Lisio M.. Role of metabolic stress and exercise in regulating fibro/adipogenic progenitors. Front Cell Dev Biol 2020;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, Fuchs B.. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res 2009;27:504–9. [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, Feeley BT.. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscle Ligam Tendons J 2019;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg 2014;23:1508–13. [DOI] [PubMed] [Google Scholar]
- 27. Du Y, Ge J, Li Y, Ma PX, Lei B.. Biomimetic elastomeric, conductive and biodegradable polycitrate-based nanocomposites for guiding myogenic differentiation and skeletal muscle regeneration. Biomaterials 2018;157:40–50. [DOI] [PubMed] [Google Scholar]
- 28. Sun H, Zhou X, Zhang Y, Zhang L, Yu X, Ye Z, Laurencin CT. Bone implants (bone regeneration and bone cancer treatments). In: Cui W, Zhao X, Liu S (eds). Biofabrication for Orthopedics Germany: WILEY-VCH, 2022, pp. 265–321.
- 29. Shemshaki NS, Laurencin C. Graphene-based nanofibers for skeletal muscle tissue regeneration. US20210402054A1, 2021.
- 30. Otsuka T, Mengsteab PY, Laurencin CT.. Control of mesenchymal cell fate via application of FGF-8b in vitro. Stem Cell Res 2021;51:102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mengsteab PY, Otsuka T, McClinton A, Shemshaki NS, Shah S, Kan H-M, Obopilwe E, Vella AT, Nair LS, Laurencin CT.. Mechanically superior matrices promote osteointegration and regeneration of anterior cruciate ligament tissue in rabbits. Proc Natl Acad Sci USA 2020;117:28655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang X, Saveh-Shemshaki N, Kan H-M, Khan Y, Laurencin CT.. Biomimetic electroconductive nanofibrous matrices for skeletal muscle regenerative engineering. Regen Eng Transl Med 2020;6:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saveh Shemshaki N, Kan H-M, Barajaa M, Otsuka T, Lebaschi A, Mishra N, Nair LS, Laurencin CT.. Muscle degeneration in chronic massive rotator cuff tears of the shoulder: addressing the real problem using a graphene matrix. Proc Natl Acad Sci USA 2022;119:e2208106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mirdamadi ES, Kalhori D, Zakeri N, Azarpira N, Solati-Hashjin M. Liver tissue engineering as an emerging alternative for liver disease treatment. Tissue Engineering Part B: Reviews2020;26:145–63. [DOI] [PubMed] [Google Scholar]
- 35. Moffat KL, Kwei AS-P, Spalazzi JP, Doty SB, Levine WN, Lu HH.. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A 2009;15:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gajendiran M, Choi J, Kim S-J, Kim K, Shin H, Koo H-J, Kim K.. Conductive biomaterials for tissue engineering applications. J Ind Eng Chem 2017;51:12–26. [Google Scholar]
- 37. Naderi A, Palardy G.. Comparison of microstructure and tensile behavior of hydroxyapatite-coated PEEK meshes and cellulose-based fabrics and mats. J Ind Textiles 2022;51(1_suppl):1392S-1421S. doi: 10.1177/15280837211070021. [DOI] [Google Scholar]
- 38. Naderi A, Zhang B, Belgodere JA, Sunder K, Palardy G.. Improved biocompatible, flexible mesh composites for implant applications via hydroxyapatite coating with potential for 3-dimensional extracellular matrix network and bone regeneration. ACS Appl Mater Interfaces 2021;13:26824–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barajaa MA, Nair LS, Laurencin CT.. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen Eng Transl Med 2020;6:451–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nelson C, Khan Y, Laurencin CT.. Nanofiber/microsphere hybrid matrices in vivo for bone regenerative engineering: a preliminary report. Regen Eng Transl Med 2018;4:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ogueri KS, Ivirico JLE, Nair LS, Allcock HR, Laurencin CT.. Biodegradable polyphosphazene-based blends for regenerative engineering. Regen Eng Transl Med 2017;3:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naderi A, Dolati A, Afshar A, Palardy G.. A simple route to synthesize zirconia antistatic thin films on glass substrates and their application to polymer-based composites. Mater Chem Phy 2020;244:122696. [Google Scholar]
- 43. Mirdamadi ES, Khosrowpour Z, Jafari D, Gholipourmalekabadi M, Solati-Hashjin M.. 3D-printed PLA/gel hybrid in liver tissue engineering: effects of architecture on biological functions. Biotechnol Bioeng 2023;120:836–51. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Li M, Kang E-T, Neoh KG.. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater 2016;32:46–56. [DOI] [PubMed] [Google Scholar]
- 45. Sevivas N, Teixeira FG, Portugal R, Direito-Santos B, Espregueira-Mendes J, Oliveira FJ, Silva RF, Sousa N, Sow WT, Nguyen LTH, Ng KW, Salgado AJ.. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med 2018;46:449–59. [DOI] [PubMed] [Google Scholar]
- 46. Awale G, Kan H-M, Laurencin CT, Lo KWH.. Molecular mechanisms underlying the short-term intervention of forskolin-Mediated bone regeneration. Regen Eng Transl Med 2023;9:375–83. [Google Scholar]
- 47. Bassett CAL. Biologic significance of piezoelectricity. Calcif Tissue Res 1968;1:252–72. [DOI] [PubMed] [Google Scholar]
- 48. Alamdari SG, Alibakhshi A, de la Guardia M, Baradaran B, Mohammadzadeh R, Amini M, Kesharwani P, Mokhtarzadeh A, Oroojalian F, Sahebkar A.. Conductive and semiconductive nanocomposite‐based hydrogels for cardiac tissue engineering. Adv Healthc Mater 2022;11:e2200526. [DOI] [PubMed] [Google Scholar]
- 49. Ma H, Yu K, Wang H, Liu J, Cheng YY, Kang Y, Wang H, Zhang J, Song K.. Fabrication and detection of a novel hybrid conductive scaffold based on alginate/gelatin/carboxylated carbon nanotubes (Alg/gel/mMWCNTs) for neural tissue engineering. Tissue Cell 2023;80:101995. [DOI] [PubMed] [Google Scholar]
- 50. Peidavosi N, Azami M, Beheshtizadeh N, Ramazani Saadatabadi A.. Piezoelectric conductive electrospun nanocomposite PCL/polyaniline/barium titanate scaffold for tissue engineering applications. Sci Rep 2022;12:20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo B, Ma PX.. Conducting polymers for tissue engineering. Biomacromolecules 2018;19:1764–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S.. Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface 2010;7:S559–S579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahadian S, Ostrovidov S, Hosseini V, Kaji H, Ramalingam M, Bae H, Khademhosseini A.. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis 2013;9:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grasman JM, Zayas MJ, Page RL, Pins GD.. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater 2015;25:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matthias N, Hunt SD, Wu J, Lo J, Callahan LAS, Li Y, Huard J, Darabi R.. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res 2018;27:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang X, Saveh-Shemshaki N, Kan H-M, Khan Y, Laurencin CT.. Skeletal muscle regenerative engineering. Regen Eng Transl Med 2020;6:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lebaschi A, Deng XH, Zong J, Cong GT, Carballo CB, Album ZM, Camp C, Rodeo SA.. Animal models for rotator cuff repair. Annals N Y Acad Sci 2016;1383:43–57. [DOI] [PubMed] [Google Scholar]
- 58. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC.. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 2007;292:E151–7. [DOI] [PubMed] [Google Scholar]
- 59. Feeley BT, Liu M, Ma CB, Agha O, Aung M, Lee C, Liu X.. Human rotator cuff tears have an endogenous, inducible stem cell source capable of improving muscle quality and function after rotator cuff repair. Am J Sports Med 2020;48:2660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sartori R, Romanello V, Sandri M.. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 2021;12:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu X, Joshi SK, Ravishankar B, Laron D, Kim HT, Feeley BT.. Upregulation of transforming growth factor-β signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg 2014;23:1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tazawa R, Uchida K, Kenmoku T, Nakawaki M, Muneshige K, Ishii D, Inoue G, Takaso M.. Increasing transforming growth factor-beta concentrations with age decrease apelin in the rat rotator cuff. J Orthop Surg Res 2021;16:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choo A, McCarthy M, Pichika R, Sato EJ, Lieber RL, Schenk S, Lane JG, Ward SR.. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am 2014;96:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang W, Liu Y, Zhang H.. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci 2021;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Agha O, Diaz A, Davies M, Kim HT, Liu X, Feeley BT.. Rotator cuff tear degeneration and the role of fibro‐adipogenic progenitors. Annals N Y Acad Sci 2021;1490:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ek ET, Neukom L, Catanzaro S, Gerber C.. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199–208. [DOI] [PubMed] [Google Scholar]
- 67. Lee C, Liu M, Agha O, Kim HT, Liu X, Feeley BT.. Beige fibro-adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg 2020;29:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abdou MA, Kim G-E, Kim J, Kim B-H, Kim Y-K, Jeong S-E, Kim T-J, Park H-K, Kim M-S.. How long should we wait to create the Goutallier stage 2 fatty infiltrations in the rabbit shoulder for repairable rotator cuff tear model? Biomed Res Int 2019;2019:7387131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gerber C, Meyer DC, Frey E, von Rechenberg B, Hoppeler H, Frigg R, Jost B, Zumstein MA.. Neer Award 2007: reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg 2009;18:163–71. [DOI] [PubMed] [Google Scholar]
- 70. Wang Z, Liu X, Davies MR, Horne D, Kim H, Feeley BT.. A mouse model of delayed rotator cuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med 2018;46:2981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S.. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg 2012;21:847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sevivas N, Serra SC, Portugal R, Teixeira F, Carvalho M, Silva N, Espregueira-Mendes J, Sousa N, Salgado A.. Animal model for chronic massive rotator cuff tear: behavioural and histologic analysis. Knee Surg Sports Traumatol Arthrosc 2015;23:608–18. [DOI] [PubMed] [Google Scholar]
- 73. Piersma B, Bank RA, Boersema M.. Signaling in fibrosis: TGF-β, WNT, and Yap/TAZ converge. Front Med (Lausanne) 2015;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gurtner GC, Werner S, Barrandon Y, Longaker MT.. Wound repair and regeneration. Nature 2008;453:314–21. [DOI] [PubMed] [Google Scholar]
- 75. Voleti PB, Buckley MR, Soslowsky LJ.. Tendon healing: repair and regeneration. Annu Rev Biomed Eng 2012;14:47–71. [DOI] [PubMed] [Google Scholar]
- 76. Ariel A, Timor O.. Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol 2013;229:250–63. [DOI] [PubMed] [Google Scholar]
- 77. Dituri F, Cossu C, Mancarella S, Giannelli G.. The interactivity between TGFβ and BMP signaling in organogenesis, fibrosis, and cancer. Cells 2019;8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frangogiannis NG. Transforming growth factor–β in tissue fibrosis. J Exp Med 2020;217:e20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Werner S, Grose R.. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 80. Zakeri N, Mirdamadi ES, Kalhori D, Solati-Hashjin M.. Signaling molecules orchestrating liver regenerative medicine. J Tissue Eng Regen Med 2020;14:1715–37. [DOI] [PubMed] [Google Scholar]
- 81. Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports 2000;10:312–20. [DOI] [PubMed] [Google Scholar]
- 82. Tozer S, Duprez D.. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today 2005;75:226–36. [DOI] [PubMed] [Google Scholar]
- 83. Tempfer H, Traweger A.. Tendon vasculature in health and disease. Front Physiol 2015;6:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M.. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. Faseb J 2013;27:2074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nichols AE, Best KT, Loiselle AE.. The cellular basis of fibrotic tendon healing: challenges and opportunities. Transl Res 2019;209:156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, Huang AH.. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep 2017;7:45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW.. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One 2014;9:e96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo B-M, Zhang L, Shi S, Young MF.. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 2007;13:1219–27. [DOI] [PubMed] [Google Scholar]
- 89. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 1972;54:41–50. [PubMed] [Google Scholar]
- 90. Jensen AR, Kelley BV, Mosich GM, Ariniello A, Eliasberg CD, Vu B, Shah P, Devana SK, Murray IR, Péault B, Dar A, Petrigliano FA.. Neer Award 2018: platelet-derived growth factor receptor α co-expression typifies a subset of platelet-derived growth factor receptor β-positive progenitor cells that contribute to fatty degeneration and fibrosis of the murine rotator cuff. J Shoulder Elbow Surg 2018;27:1149–61. [DOI] [PubMed] [Google Scholar]
- 91. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B.. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am 2004;86:1973–82. [DOI] [PubMed] [Google Scholar]
- 92. Park H-S, Choi K-H, Lee H-J, Kim Y-S.. Rotator cuff tear with joint stiffness: a review of current treatment and rehabilitation. Clin Shoulder Elb 2020;23:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weber SC, Abrams JS, Nottage WM.. Complications associated with arthroscopic shoulder surgery. Arthroscopy 2002;18:88–95. [DOI] [PubMed] [Google Scholar]
- 94. Townsend LK, Wright DC.. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflugers Arch 2019;471:455–65. [DOI] [PubMed] [Google Scholar]
- 95. Meyer GA, Gibbons MC, Sato E, Lane JG, Ward SR, Engler AJ.. Epimuscular fat in the human rotator cuff is a novel beige depot. Stem Cells Transl Med 2015;4:764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Keipert S, Jastroch M.. Brite/beige fat and UCP1—is it thermogenesis? Biochim Biophys Acta 2014;1837:1075–82. [DOI] [PubMed] [Google Scholar]
- 97. Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, Dawes BA, Tsai L, Kahn BB, Spiegelman BM, Liu T, Rosen ED.. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 2018;28:631–43.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Theret M, Rossi F, Contreras O.. Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol 2021;12:673404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Uezumi A, Fukada S-i, Yamamoto N, Takeda S, Tsuchida K.. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143–52. [DOI] [PubMed] [Google Scholar]
- 100. Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S-I. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 2011;124:3654–64. [DOI] [PubMed] [Google Scholar]
- 101. Lee C, Agha O, Liu M, Davies M, Bertoy L, Kim HT, Liu X, Feeley BT.. Rotator cuff fibro‐adipogenic progenitors demonstrate highest concentration, proliferative capacity, and adipogenic potential across muscle groups. J Orthop Res 2020;38:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lee C, Liu M, Agha O, Kim HT, Feeley BT, Liu X.. Beige FAPs transplantation improves muscle quality and shoulder function after massive rotator cuff tears. J Orthop Res 2020;38:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kang X, Yang M-Y, Shi Y-X, Xie M-M, Zhu M, Zheng X-L, Zhang C-K, Ge Z-L, Bian X-T, Lv J-T, Wang Y-J, Zhou B-H, Tang K-L.. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun Signal 2018;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang C, Song W, Chen B, Liu X, He Y.. Exosomes isolated from adipose-derived stem cells: a new cell-free approach to prevent the muscle degeneration associated with torn rotator cuffs. Am J Sports Med 2019;47:3247–55. [DOI] [PubMed] [Google Scholar]
- 105. Davies MR, Lee L, Feeley BT, Kim HT, Liu X.. Lysophosphatidic acid‐induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J Orthop Res 2017;35:1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Künzler M, Nuss K, Karol A, Schär M, Hottiger M, von Rechenberg B, Raniga S, Zumstein MA.. Reduced muscle degeneration and decreased fatty infiltration after rotator cuff tear in a PARP-1 knock-out mouse model. J Shoulder Elbow Surg 2016;25:e187. [DOI] [PubMed] [Google Scholar]
- 107. Goutallier D, Postel J-M, Bernageau J, Lavau L, Voisin M-C.. Fatty muscle degeneration in cuff ruptures. Pre-and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994;1;304:78–83. [PubMed] [Google Scholar]
- 108. Goutallier D, Postel J-M, Gleyze P, Leguilloux P, Van Driessche S.. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg 2003;12:550–4. [DOI] [PubMed] [Google Scholar]
- 109. Thomazeau H, Rolland Y, Lucas C, Duval J-M, Langlais F.. Atrophy of the supraspinatus belly assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 1996;67:264–8. [DOI] [PubMed] [Google Scholar]
- 110. Rawson S, Cartmell S, Wong J.. Suture techniques for tendon repair; a comparative review. Muscle Ligaments Tendons J 2019;03:220. [PMC free article] [PubMed] [Google Scholar]
- 111. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K.. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004;86:219–24. [DOI] [PubMed] [Google Scholar]
- 112. Bianchi B, Copelli C, Ferrari S, Ferri A, Sesenna E.. Free flaps: outcomes and complications in head and neck reconstructions. J Craniomaxillofac Surg 2009;37:438–42. [DOI] [PubMed] [Google Scholar]
- 113. Hosseini FS, Abedini AA, Chen F, Whitfield T, Ude CC, Laurencin CT.. Oxygen-Generating biomaterials for translational bone regenerative engineering. ACS Appl Mater Interfaces 2023;15:50721–41. [DOI] [PubMed] [Google Scholar]
- 114. Grogan BF, Hsu JR, Consortium STR; Skeletal Trauma Research Consortium Volumetric muscle loss. J Am Acad Orthopaedic Surg 2011;19:S35–7. [DOI] [PubMed] [Google Scholar]
- 115. Gladstone J, Bishop J, Lo I, Flatow E.. American journal of sports fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and Am J Sports Med 2007;35:719–28. [DOI] [PubMed] [Google Scholar]
- 116. Shen P-H, Lien S-B, Shen H-C, Lee C-H, Wu S-S, Lin L-C.. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg 2008;17:1S–7S. [DOI] [PubMed] [Google Scholar]
- 117. Beeler S, Ek ET, Gerber C.. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg 2013;22:1537–46. [DOI] [PubMed] [Google Scholar]
- 118. Foulds IS, Barker AT.. Human skin battery potentials and their possible role in wound healing. Br J Dermatol 1983;109:515–22. [DOI] [PubMed] [Google Scholar]
- 119. McCaig CD, Rajnicek AM, Song B, Zhao M.. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005;85:943–78. [DOI] [PubMed] [Google Scholar]
- 120. Mayor D. Book review: electrotherapy: evidence-based practice (12th edition). Acupunct Med 2009;27:135–6. [Google Scholar]
- 121. Chen M-C, Sun Y-C, Chen Y-H.. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater 2013;9:5562–72. [DOI] [PubMed] [Google Scholar]
- 122. Zhang Z, Klausen LH, Chen M, Dong M.. Electroactive scaffolds for neurogenesis and myogenesis: graphene‐based nanomaterials. Small 2018;14:e1801983. [DOI] [PubMed] [Google Scholar]
- 123. Yang HS, Lee B, Tsui JH, Macadangdang J, Jang SY, Im SG, Kim DH.. Electroconductive nanopatterned substrates for enhanced myogenic differentiation and maturation. Adv Healthc Mater 2016;5:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mahmoudifard M, Soleimani M, Hatamie S, Zamanlui S, Ranjbarvan P, Vossoughi M, Hosseinzadeh S.. The different fate of satellite cells on conductive composite electrospun nanofibers with graphene and graphene oxide nanosheets. Biomed Mater 2016;11:025006. [DOI] [PubMed] [Google Scholar]
- 125. Björninen M, Gilmore K, Pelto J, Seppänen-Kaijansinkko R, Kellomäki M, Miettinen S, Wallace G, Grijpma D, Haimi S.. Electrically stimulated adipose stem cells on polypyrrole-coated scaffolds for smooth muscle tissue engineering. Ann Biomed Eng 2017;45:1015–26. [DOI] [PubMed] [Google Scholar]
- 126. Tang X. Electroconductive nanofiber matrices for muscle regeneration. Materials Science and Engineering. Doctoral Dissertation. University of Connecticut 2018.
- 127. Broda CR, Lee JY, Sirivisoot S, Schmidt CE, Harrison BS.. A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J Biomed Mater Res A 2011;98:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jun I, Jeong S, Shin H.. The stimulation of myoblast differentiation by electrically conductive Sub-micron fibers. Biomaterials 2009;30:2038–47. [DOI] [PubMed] [Google Scholar]
- 129. Gutmann E, Guttmann L.. Effect of electrotherapy on denervated muscles in rabbits. The Lancet 1942;239:169–70. [Google Scholar]
- 130. Shi G, Zhang Z, Rouabhia M.. The regulation of cell functions electrically using biodegradable polypyrrole–polylactide conductors. Biomaterials 2008;29:3792–8. [DOI] [PubMed] [Google Scholar]
- 131. Mehedintu M, Berg H.. Proliferation response of yeast saccharomyces cerevisiae on electromagnetic field parameters. Bioelectrochem Bioenergetics 1997;43:67–70. [Google Scholar]
- 132. Smith SD, McLeod BR, Liboff AR, Cooksey K.. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 1987;8:215–27. [DOI] [PubMed] [Google Scholar]
- 133. Mobini S, Leppik L, Thottakkattumana Parameswaran V, Barker JH.. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017;5:e2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rouabhia M, Park H, Meng S, Derbali H, Zhang Z.. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 2013;8:e71660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schemitsch E, Kuzyk P.. The science of electrical stimulation therapy for fracture healing. Indian J Orthop 2009;43:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Park H, Bhalla R, Saigal R, Radisic M, Watson N, Langer R, Vunjak-Novakovic G.. Effects of electrical stimulation in C2C12 muscle constructs. J Tissue Eng Regen Med 2008;2:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ye H, Steiger A.. Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J Neuroeng Rehabil 2015;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lee R, Rich J, Kelley K, Weiman D, Mathews M.. A comparison of in vitro cellular responses to mechanical and electrical stimulation. Am Surg 1982;48:567–74. [PubMed] [Google Scholar]
- 139. Meng S, Rouabhia M, Zhang Z, De DD, Laval U.. Electrical stimulation in tissue regeneration. In Applied Biomedical Engineering, InTech, Croatia 2011;37–62. [Google Scholar]
- 140. Sun S, Titushkin I, Cho M.. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006;69:133–41. [DOI] [PubMed] [Google Scholar]
- 141. Berg H, Zhang L.. Electrostimulation in cell biology by low-frequency electromagnetic fields. Electro- Magnetobiol 1993;12:147–63. [Google Scholar]
- 142. Schmid EW. Extracellular stimulation of nerve cells with electric current spikes induced by voltage steps. arXiv preprint, arXiv:1603.02062 2016.
- 143. Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR.. Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun 1999;264:657–61. [DOI] [PubMed] [Google Scholar]
- 144. Merrill DR, Bikson M, Jefferys JGR.. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 2005;141:171–98. [DOI] [PubMed] [Google Scholar]
- 145. Spadaro JA, Becker RO.. Function of implanted cathodes in electrode-induced bone growth. Med Biol Eng Comput 1979;17:769–75. [DOI] [PubMed] [Google Scholar]
- 146. Au HTH, Cheng I, Chowdhury MF, Radisic M.. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials 2007;28:4277–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim IS, Song JK, Song YM, Cho TH, Lee TH, Lim SS, Kim SJ, Hwang SJ.. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng Part A 2009;15:2411–22. [DOI] [PubMed] [Google Scholar]
- 148. Sauer H, Bekhite MM, Hescheler J, Wartenberg M.. Redox control of angiogenic factors and CD31-positive vessel-like structures in mouse embryonic stem cells after direct current electrical field stimulation. Exp Cell Res 2005;304:380–90. [DOI] [PubMed] [Google Scholar]
- 149. Ercan B, Webster TJ.. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials 2010;31:3684–93. [DOI] [PubMed] [Google Scholar]
- 150. Kim IS, Song JK, Zhang YL, Lee TH, Cho TH, Song YM, Kim DK, Kim SJ, Hwang SJ.. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochim Biophys Acta 2006;1763:907–16. [DOI] [PubMed] [Google Scholar]
- 151. Hartig M, Joos U, Wiesmann H-P.. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J 2000;29:499–506. [DOI] [PubMed] [Google Scholar]
- 152. Huang CP, Chen XM, Chen ZQ.. Osteocyte: the impresario in the electrical stimulation for bone fracture healing. Medical Hypotheses 2008;70:287–90. [DOI] [PubMed] [Google Scholar]
- 153. Xu J, Wang W, Clark CC, Brighton CT.. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis Cartilage 2009;17:397–405. [DOI] [PubMed] [Google Scholar]
- 154. Antov Y, Barbul A, Mantsur H, Korenstein R.. Electroendocytosis: exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys J 2005;88:2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhuang H, Wang W, Seldes RM, Tahernia AD, Fan H, Brighton CT.. Electrical stimulation induces the level of TGF-β1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun 1997;237:225–9. [DOI] [PubMed] [Google Scholar]
- 156. Abeed RI, Naseer M, Abel EW.. Capacitively coupled electrical stimulation treatment: results from patients with failed long bone fracture unions. J Orthop Trauma 1998;12:510–3. [DOI] [PubMed] [Google Scholar]
- 157. Zamora-Navas P, Borras AV, Antelo RL, Saras JA, Peña MR.. Electrical stimulation of bone nonunion with the presence of a gap. Acta Orthop Belg 1995;61:169–76. [PubMed] [Google Scholar]
- 158. Titushkin I, Cho M.. Regulation of cell cytoskeleton and membrane mechanics by electric field: role of linker proteins. Biophys J 2009;96:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mcleod KJ, Donahue HJ, Levin PE, Fontaine M-A, Rubin CT.. Electric fields modulate bone cell function in a density-dependent manner. J Bone Miner Res 1993;8:977–84. [DOI] [PubMed] [Google Scholar]
- 160. Yonemori K, Matsunaga S, Ishidou Y, Maeda S, Yoshida H.. Early effects of electrical stimulation on osteogenesis. Bone 1996;19:173–80. [DOI] [PubMed] [Google Scholar]
- 161. Thamsborg G, Florescu A, Oturai P, Fallentin E, Tritsaris K, Dissing S.. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2005;13:575–81. [DOI] [PubMed] [Google Scholar]
- 162. McLeod KJ, Rubin CT.. The effect of low-frequency electrical fields on osteogenesis. J Bone Joint Surg Am 1992;74:920–9. [PubMed] [Google Scholar]
- 163. Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ, Boyan BD.. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res 2003;21:326–34. [DOI] [PubMed] [Google Scholar]
- 164. Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, Abbott J, Blake DR, Stevens CR.. Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Commun 1998;250:458–61. [DOI] [PubMed] [Google Scholar]
- 165. Fu Y-C, Lin C-C, Chang J-K, Chen C-H, Tai I-C, Wang G-J, Ho M-L.. A novel single pulsed electromagnetic field stimulates osteogenesis of bone marrow mesenchymal stem cells and bone repair. PLoS One 2014;9:e91581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yu X, Tang X, Gohil SV, Laurencin CT.. Biomaterials for bone regenerative engineering. Adv Healthc Mater 2015;4:1268–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Shi Y, Wang Y, Zhang P, Liu W.. Fibrous scaffolds for tissue engineering. Inflam Regenerat 2014;34:23–32. [Google Scholar]
- 168. Laurencin CT, Ko FK. Hybrid nanofibril matrices for use as tissue engineering devices. Google Patents, US6689166B2, 2004.
- 169. Li W-J, Cooper JA.. Fibrous Scaffolds for Tissue Engineering. In: Burdick JA, Mauck RL (eds). Biomaterials for Tissue Engineering Applications. Vienna: Springer Vienna, 2011, 47–73. [Google Scholar]
- 170. Bagherzadeh R, Gorji M, Bafgi MS, Saveh-Shemshaki N.. Electrospun conductive nanofibers for electronics. In: Afshari M (ed.), Electrospun Nanofibers, Woodhead Publishing Series in Textiles. Cambridge, UK: Elsevier, 2017; Volume 186, pp. 467–519. [Google Scholar]
- 171. Saveh‐Shemshaki N, Latifi M, Bagherzadeh R, Malekshahi Byranvand M, Naseri N, Dabirian A.. Synthesis of mesoporous functional hematite nanofibrous photoanodes by electrospinning. Polym Adv Technol 2016;27:358–65. [Google Scholar]
- 172. Saveh-Shemshaki N, Bagherzadeh R, Latifi M.. Electrospun metal oxide nanofibrous mat as a transparent conductive layer. Organic Elect 2019;70:131–9. [Google Scholar]
- 173. Bagherzadeh R, Shemshaki NS.. Metal oxide nanofibers for flexible organic electronics and sensors. In: Metal Oxide-Based Nanofibers and Their Applications. Amsterdam, The Netherlands: Elsevier, 2022, pp. 159–171. [Google Scholar]
- 174. Hopley EL, Salmasi S, Kalaskar DM, Seifalian AM.. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol Adv 2014;32:1000–14. [DOI] [PubMed] [Google Scholar]
- 175. Goenka S, Sant V, Sant S.. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 2014;173:75–88. [DOI] [PubMed] [Google Scholar]
- 176. Fan Z, Wang J, Wang Z, Ran H, Li Y, Niu L, Gong P, Liu B, Yang S.. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014;66:407–16. [Google Scholar]
- 177. Abarrategi A, Gutiérrez MC, Moreno-Vicente C, Hortigüela MJ, Ramos V, López-Lacomba JL, Ferrer ML, del Monte F.. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008;29:94–102. [DOI] [PubMed] [Google Scholar]
- 178. Harrison BS, Atala A.. Carbon nanotube applications for tissue engineering. Biomaterials 2007;28:344–53. [DOI] [PubMed] [Google Scholar]
- 179. Nair RS, Ameer JM, Alison MR, Anilkumar TV.. A gold nanoparticle coated porcine cholecyst-derived bioscaffold for cardiac tissue engineering. Colloids Surf B Biointerfaces 2017;157:130–7. [DOI] [PubMed] [Google Scholar]
- 180. Shevach M, Fleischer S, Shapira A, Dvir T.. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett 2014;14:5792–6. [DOI] [PubMed] [Google Scholar]
- 181. Shevach M, Maoz BM, Feiner R, Shapira A, Dvir T.. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J Mater Chem B 2013;1:5210. [DOI] [PubMed] [Google Scholar]
- 182. Nezakati T, Seifalian A, Tan A, Seifalian AM.. Conductive polymers: opportunities and challenges in biomedical applications. Chem Rev 2018;118:6766–843. [DOI] [PubMed] [Google Scholar]
- 183. Seike M, Uda M, Suzuki T, Minami H, Higashimoto S, Hirai T, Nakamura Y, Fujii S.. Synthesis of polypyrrole and its derivatives as a liquid marble stabilizer via a solvent-free chemical oxidative polymerization protocol. ACS Omega 2022;7:13010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Poddar AK, Patel SS, Patel HD.. Synthesis, characterization and applications of conductive polymers: a brief review. Polym Adv Techs 2021;32:4616–41. [Google Scholar]
- 185. Yussuf A, Al-Saleh M, Al-Enezi S, Abraham G.. Synthesis and characterization of conductive polypyrrole: the influence of the oxidants and monomer on the electrical, thermal, and morphological properties. Int J Polymer Sci 2018;2018:1–8. [Google Scholar]
- 186. Tahir ZM, Alocilja EC, Grooms DL.. Polyaniline synthesis and its biosensor application. Biosens Bioelectron 2005;20:1690–5. [DOI] [PubMed] [Google Scholar]
- 187. Karim MR, Lee CJ, Lee MS.. Synthesis and characterization of conducting polythiophene/carbon nanotubes composites. J Polym Sci A Polym Chem 2006;44:5283–90. [Google Scholar]
- 188. Bourib FZ, Mezdour D, Tabellout M.. Investigation of the electrical properties of conductive polymers by the DRS and RPE techniques. Appl Surface Sci 2019;495:143535. [Google Scholar]
- 189. Chronakis IS, Grapenson S, Jakob A.. Conductive polypyrrole nanofibers via electrospinning: electrical and morphological properties. Polymer 2006;47:1597–603. [Google Scholar]
- 190. Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z.. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res 2010;93A:164–74. [DOI] [PubMed] [Google Scholar]
- 191. Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, Li J, Dao LH.. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs 2007;31:13–22. [DOI] [PubMed] [Google Scholar]
- 192. Harris AR, Wallace GG.. Organic electrodes and communications with excitable cells. Adv Funct Mater 2018;28:1700587. [Google Scholar]
- 193. Mawad D, Stewart E, Officer DL, Romeo T, Wagner P, Wagner K, Wallace GG.. A single component conducting polymer hydrogel as a scaffold for tissue engineering. Adv Funct Mater 2012;22:2692–9. [Google Scholar]
- 194. Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P.. Electrically conductive polymers and composites for biomedical applications. RSC Adv 2015;5:37553–67. [Google Scholar]
- 195. Schmidt CE, Shastri VR, Vacanti JP, Langer R.. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci USA 1997;94:8948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Qazi TH, Rai R, Boccaccini AR.. Tissue engineering of electrically responsive tissues using polyaniline based polymers: a review. Biomaterials 2014;35:9068–86. [DOI] [PubMed] [Google Scholar]
- 197. Li M, Guo Y, Wei Y, Macdiarmid A, Lelkes P.. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006;27:2705–15. [DOI] [PubMed] [Google Scholar]
- 198. Wang L, Wu Y, Hu T, Guo B, Ma P.. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater 2017;59:68–81. [DOI] [PubMed] [Google Scholar]
- 199. Bousalem S, Mangeney C, Chehimi MM, Basinska T, Miksa B, Slomkowski S.. Synthesis, characterization and potential biomedical applications of N-succinimidyl ester functionalized, polypyrrole-coated polystyrene latex particles. Colloid Polym Sci 2004;282:1301–7. [Google Scholar]
- 200. Cetiner S, Kalaoglu F, Karakas H, Sarac AS.. Electrospun nanofibers of polypyrrole-poly (acrylonitrile-co-vinyl acetate). Textile Res J 2010;80:1784–92. [Google Scholar]
- 201. Balint R, Cassidy NJ, Cartmell S.. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 2014;10:2341–53. [DOI] [PubMed] [Google Scholar]
- 202. Mattila H. Intelligent Textiles and Clothing. 2nd ed.; Sawston, Cambridge, UK: Woodhead Publishing Limited, 2006. [Google Scholar]
- 203. Shi G, Rouabhia M, Wang Z, Dao LH, Zhang Z.. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004;25:2477–88. [DOI] [PubMed] [Google Scholar]
- 204. Boutry CM, Gerber‐Hörler I, Hierold C.. Electrically conducting biodegradable polymer composites (polylactide‐polypyrrole and polycaprolactone‐polypyrrole) for passive resonant circuits. Polymer Eng Sci 2013;53:1196–208. [Google Scholar]
- 205. Wang Z, Roberge C, Dao LH, Wan Y, Shi G, Rouabhia M, Guidoin R, Zhang Z.. In vivo evaluation of a novel electrically conductive polypyrrole/poly (d, l‐lactide) composite and polypyrrole‐coated poly (d, l‐lactide‐co‐glycolide) membranes. J Biomed Mater Res A 2004;70:28–38. [DOI] [PubMed] [Google Scholar]
- 206. Zhou DD, Cui XT, Hines A, Greenberg RJ.. Conducting polymers in neural stimulation applications. In: Implantable Neural Prostheses 2: Techniques and Engineering Approaches. New York, NY, USA: Springer, 2009, pp. 217–52. [Google Scholar]
- 207. Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S.. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med 2011;5:e17–35. [DOI] [PubMed] [Google Scholar]
- 208. Lin P, Yan F.. Organic thin‐film transistors for chemical and biological sensing. Adv Mater 2012;24:34–51. [DOI] [PubMed] [Google Scholar]
- 209. Zhang Y, Kim JJ, Chen D, Tuller HL, Rutledge GC.. Electrospun polyaniline fibers as highly sensitive room temperature chemiresistive sensors for ammonia and nitrogen dioxide gases. Adv Funct Mater 2014;24:4005–14. [Google Scholar]
- 210. Humpolicek P, Kasparkova V, Saha P, Stejskal J.. Biocompatibility of polyaniline. Synth Metals 2012;162:722–7. [Google Scholar]
- 211. Huang L, Zhuang X, Hu J, Lang L, Zhang P, Wang Y, Chen X, Wei Y, Jing X.. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules 2008;9:850–8. [DOI] [PubMed] [Google Scholar]
- 212. Borriello A, Guarino V, Schiavo L, Alvarez-Perez M, Ambrosio L.. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J Mater Sci Mater Med 2011;22:1053–62. [DOI] [PubMed] [Google Scholar]
- 213. Zhang Q-S, Yan Y-H, Li S-P, Feng T.. Synthesis of a novel biodegradable and electroactive polyphosphazene for biomedical application. Biomed Mater 2009;4:035008. [DOI] [PubMed] [Google Scholar]
- 214. Furlani F, Campodoni E, Sangiorgi N, Montesi M, Sanson A, Sandri M, Panseri S.. Electroconductive scaffolds based on gelatin and PEDOT: PSS for cardiac regeneration. Int J Biol Macromol 2023;224:266–80. [DOI] [PubMed] [Google Scholar]
- 215. Peramo A, Urbanchek MG, Spanninga SA, Povlich LK, Cederna P, Martin DC. In situ polymerization of a conductive polymer in acellular muscle tissue constructs. Tissue Engineering Part A. 2008;14:423–32. [DOI] [PubMed] [Google Scholar]
- 216. Tomasino DV, Wolf M, Farina H, Chiarello G, Feldhoff A, Ortenzi MA, Sabatini V.. Role of doping agent degree of sulfonation and casting solvent on the electrical conductivity and morphology of PEDOT: SPAES thin films. Polymers (Basel) 2021;13:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Xu C, Guan S, Wang S, Gong W, Liu T, Ma X, Sun C.. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater Sci Eng C Mater Biol Appl 2018;84:32–43. [DOI] [PubMed] [Google Scholar]
- 218. Shin SR, Li Y-C, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, Zhang YS, Tamayol A, Khademhosseini A.. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev 2016;105:255–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Luong-Van E, Madanagopal TT, Rosa V.. Mechanisms of graphene influence on cell differentiation. Mater Today Chem 2020;16:100250. [Google Scholar]
- 220. Lee WC, Lim CHY, Shi H, Tang LA, Wang Y, Lim CT, Loh KP.. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011;5:7334–41. [DOI] [PubMed] [Google Scholar]
- 221. Bianco A, Cheng H-M, Enoki T, Gogotsi Y, Hurt RH, Koratkar N, Kyotani T, Monthioux M, Park CR, Tascon JM. All in the graphene family: a recommended nomenclature for two-dimensional carbon materials. Carbon 2013;65:1–6. [Google Scholar]
- 222. Uehara TM, Paino IM, Santos FA, Scagion VP, Correa DS, Zucolotto V.. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym Adv Techs 2020;31:1437–43. [Google Scholar]
- 223. Wang Y, Zhang W, Huang L, Ito Y, Wang Z, Shi X, Wei Y, Jing X, Zhang P.. Intracellular calcium ions and morphological changes of cardiac myoblasts response to an intelligent biodegradable conducting copolymer. Mater Sci Eng C Mater Biol Appl 2018;90:168–79. [DOI] [PubMed] [Google Scholar]
- 224. Porter GA, Makuck RF, Rivkees SA.. Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem 2002;277:28942–7. [DOI] [PubMed] [Google Scholar]
- 225. Bagur R, Hajnóczky G.. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol Cell 2017;66:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kwee BJ, Mooney DJ.. Biomaterials for skeletal muscle tissue engineering. Curr Opin Biotechnol 2017;47:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G.. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 2010;120:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wu X, Corona BT, Chen X, Walters TJ.. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access 2012;1:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR.. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev 2015;52:785–792. [DOI] [PubMed] [Google Scholar]
- 230. Patel A, Mukundan S, Wang W, Karumuri A, Sant V, Mukhopadhyay SM, Sant S.. Carbon-based hierarchical scaffolds for myoblast differentiation: synergy between nano-functionalization and alignment. Acta Biomater 2016;32:77–88. [DOI] [PubMed] [Google Scholar]
- 231. Browe D, Freeman J.. Optimizing C2C12 myoblast differentiation using polycaprolactone–polypyrrole copolymer scaffolds. J Biomed Mater Res A 2019;107:220–31. [DOI] [PubMed] [Google Scholar]
- 232. Quigley AF, Razal JM, Kita M, Jalili R, Gelmi A, Penington A, Ovalle-Robles R, Baughman RH, Clark GM, Wallace GG, Kapsa RMI.. Electrical stimulation of myoblast proliferation and differentiation on aligned nanostructured conductive polymer platforms. Adv Healthc Mater 2012;1:801–8. [DOI] [PubMed] [Google Scholar]
- 233. Sirivisoot S, Harrison BS.. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int J Nanomed 2011;6:2483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Zhao X, Zhang Z, Luo J, Wu Z, Yang Z, Zhou S, Tu Y, Huang Y, Han Y, Guo B.. Biomimetic, highly elastic conductive and hemostatic gelatin/rGO-based nanocomposite cryogel to improve 3D myogenic differentiation and guide in vivo skeletal muscle regeneration. Appl Mater Today 2022;26:101365. [Google Scholar]
- 235. Hu T, Shi M, Zhao X, Liang Y, Bi L, Zhang Z, Liu S, Chen B, Duan X, Guo B.. Biomimetic 3D aligned conductive tubular cryogel scaffolds with mechanical anisotropy for 3D cell alignment, differentiation and in vivo skeletal muscle regeneration. Chem Eng J 2022;428:131017. [Google Scholar]
- 236. Sevivas N, Teixeira FG, Portugal R, Araújo L, Carriço LF, Ferreira N, Vieira da Silva M, Espregueira-Mendes J, Anjo S, Manadas B, Sousa N, Salgado AJ.. Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am J Sports Med 2017;45:179–88. [DOI] [PubMed] [Google Scholar]
- 237. Wang C, Hu Q, Song W, Yu W, He Y.. Adipose stem cell–derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med 2020;48:1456–64. [DOI] [PubMed] [Google Scholar]
- 238. Bryniarski AR, Meyer GA.. Brown fat promotes muscle growth during regeneration. J Orthop Res 2019;37:1817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Gorski T, Mathes S, Krützfeldt J.. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle 2018;9:384–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT, Feeley BT.. TGF-β small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One 2016;11:e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]