Abstract
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by aberrant myeloid lineage hematopoiesis with excessive red blood cell and pro-inflammatory cytokine production. Patients with PV present with a range of thrombotic and hemorrhagic symptoms that affect quality of life and reduce overall survival expectancy. Thrombotic events, transformation into acute myeloid leukemia, and myelofibrosis are largely responsible for the observed mortality. Treatment of PV is thus primarily focused on symptom control and survival extension through the prevention of thrombosis and leukemic transformation. Patients with PV frequently experience thrombotic events and have elevated cardiovascular risk, including hypertension, dyslipidemias, obesity, and smoking, all of which negatively affect survival. To reduce the risk of thrombotic complications, PV therapy should aim to normalize hemoglobin, hematocrit, and leukocytosis and, in addition, identify and modify cardiovascular risk factors. Herein, we review what is currently known about the associated cardiovascular risk and propose strategies for diagnosing and managing patients with PV.
Keywords: cardiovascular risk, myeloproliferative neoplasms, polycythemia vera, thrombosis
Plain Language Summary
Patients with the myeloproliferative neoplasm (MPN) polycythemia vera (PV) are at increased risk of cardiovascular (CV) events, including stroke, heart attacks, and peripheral arterial disease. High blood pressure, smoking, and dyslipidemia are common in MPN and contribute to the increased cardiovascular risk. Effectively controlling cardiovascular risk factors in PV, along with appropriate hematological therapy such as direct-acting oral anticoagulants alone or in combination with aspirin, may improve the outcomes of patients with PV, but further research is needed.
Introduction
Polycythemia vera (PV) is a hematological disorder classified under myeloproliferative neoplasms (MPN) by the World Health Organization (WHO). Although there are seven subcategories under the MPN category, the term “MPN” is commonly reserved for the three pathological entities that lack the Philadelphia chromosome (Ph-) but carry mutations in the Janus kinase 2 (JAK2), calreticulin (CALR) or proto-oncogene, thrombopoietin receptor (MPL) genes. In addition to PV, these include essential thrombocythemia (ET), and primary myelofibrosis (PMF), and represent clonal proliferations arising in stem cells. The most frequent JAK2 mutation associated with Ph-MPN is in exon 14 and is JAK2V617F.1,2
Clinical symptoms of PV include fatigue and itching, microvascular symptoms (eg headaches, visual disturbances, lightheadedness, paresthesia, and atypical chest discomfort), and features such as splenomegaly, hyperviscosity, leukocytosis, thrombocytosis, thrombotic and bleeding complications. PV may progress into acute myeloid leukemia or secondary myelofibrosis.3
Patients with Ph-MPN frequently experience thrombotic events. A pooled prevalence of 20% for thrombotic events (either arterial 16.2% or venous 6.2%) at diagnosis of Ph-MPN (PV – 28.6%) was identified by a recent meta-analysis.4 This included stroke (7.4%), transient ischemic attack (3.5%), acute ischemic heart disease (6.1%), acute peripheral ischemia (3.3%), deep vein thrombosis (3.4%) and pulmonary embolism (0.9%). Atypical anatomical site thromboses are also characteristic of Ph-MPN. They include splanchnic vein thrombosis or cerebral sinus thrombosis.5
In the European Collaboration on Low-dose Aspirin (ECLAP) study,6 the most extensive PV epidemiological study conducted to date (N = 1638), thrombotic events were responsible for 41% of mortality (1.5 deaths/100 patients/year). They consisted mainly (70%) of arterial thrombosis (acute coronary syndromes, ischemic stroke, and peripheral arterial thrombosis). In this study, 587 patients (36%) had evidence of a thrombotic event at diagnosis, whereas 169 patients (10%) experienced a thrombosis during follow-up.
Heart or vascular disease and stroke are also observed in the general population and are correlated with cardiovascular (CV) risk factors, including weight control behaviors and smoking, and health factors such as blood pressure, cholesterol level, and glucose control. In the yearly update of heart disease, stroke, and CV risk factors that contribute to CV health, the American Heart Association (AHA) recently reported that the prevalence of CV risk factors observed in the general population was 13.3%, 41.4%, 32.8%, 46%, and 10.6% for smoking, obesity, total cholesterol ≥200 mg/dL, hypertension, and diabetes, respectively.7
It is not easy to critically appraise the relationship between PV and CV risks. The results obtained from studies of MPN patient cohorts have the advantage of analyzing real-world evidence and therefore produce reliable conclusions. However, in the case of thrombosis, which arises due to the interaction of many factors, it is not easy to understand which factor is primarily responsible for the thrombotic phenotype. Studies comparing PV/MPN patients with and without thrombosis could provide more precise information. However, the few such studies do not always allow comparisons between patients with similar characteristics, as MPNs are heterogeneous diseases characterized by different mutations and a diverse hematological picture. Retrospective analyses using data from electronic patient records often do not detail information on parameters such as blood pressure, weight, diabetes, smoking, mutation status, and therapy. Furthermore, there is some debate regarding whether elevated CV risk factors in patients with PV has a negative impact on clinical outcomes independent of factors such as advanced age, history of thrombosis, or leukocytosis. Cardiovascular risk factors are not incorporated into risk prognostication systems such as those of the European LeukemiaNet (ELN), who do not consider the evidence sufficiently robust to differentiate the risk in patients with PV versus that in the general population.8,9
Another risk of bias can be represented by the fact that patients with MPNs are subjected to more stringent check-ups than healthy individuals and it is, therefore, feasible that problems that would otherwise remain undiagnosed in the normal population are detected in the MPN population. For this reason, a higher number of subjects with CV conditions (stroke, heart attack, and peripheral arterial diseases) and a higher number of CV risk factors (obesity, hypertension, and diabetes) are observed in MPN patients than in age-matched controls.
Therefore, a multidisciplinary approach that considers not only the hematological features of the disease but also internist and cardiological ones should be implemented when managing patients with PV. Similarly to the paper recently published for essential thrombocythemia,10 this review aims to assist in the multidisciplinary management of the PV patient.
Cardiovascular Risk Factors and Risk of Thrombosis in Polycythemia Vera
Evidence shows that conventional CV risk factors are frequent in patients with Ph-MPN (Table 1): arterial hypertension is reported in 39–70% of patients with PV,6,11–13 diabetes mellitus in 7–16%,6,12,13 dyslipidemia in 15–38%,6,12,13 obesity in 7.5%,13 and a smoking habit in 10–15%.6,12,13 About three-quarters of patients with PV possess at least one CV risk factor and 37.7% have more than one CV risk factor.12,13 The most dangerous complication of CV disease (CVD) is thrombosis14 which is also a serious complication of PV.3 However, how classical CV risk factors contribute to the incidence of thrombotic events in patients with Ph-MPN is not fully understood. In the ECLAP study, smoking, but not arterial hypertension or diabetes mellitus, was associated with a greater risk of arterial events during follow-up.6 Other studies have shown that arterial hypertension represents an independent risk factor in patients with low-risk PV.11 In a Japanese real-world study, the presence of CV conditions (diabetes, hypertension, and hyperlipidemia) significantly increased the risk of thromboembolic events in both univariate and multivariate analyzes.15 These results have also been confirmed in a Brazilian study.16 Importantly, there is a significant worsening of survival as the number of CV risk factors in PV increases.13
Table 1.
Cardiovascular Risk Factors in Polycythemia Vera
| Risk Factor | Notes | Reference |
|---|---|---|
| Polycythemia vera-specific: | ||
| Clonal hematopoiesis | Epidemiological and experimental evidence supports the hypothesis that immune cell dysfunction mediated by clonal hematopoiesis is a risk factor for heart failure and CVD. Individuals with clonal hematopoiesis supported by the DNMT3A, TET2, ASXL1, or JAK2 (JAK2V617F) mutation are at increased risk of developing coronary heart disease after adjusting for classic CV risk factors. | [17,18] |
| Generic: | ||
| Smoking | Smoking facilitates the phenomenon of atherosclerosis and thrombotic phenomena. The risk of CV events doubles in smokers. In individuals under age 50, smoking increases the risk of CV events by 4–5 times. Passive smoking increases CV risk by 30%. | [19] |
| Blood pressure | Arterial hypertension is the main risk factor for ischemic heart disease, heart failure, cerebrovascular diseases, atrial fibrillation, and chronic renal failure. | [19] |
| Cholesterol | High levels of LDL-C produce atherosclerosis and are correlated with high CV risk. | [20] |
| Body weight | Being overweight and obesity are both associated with an increased risk of CVD because of hypertension, dyslipidemia, insulin resistance, systemic inflammation and prothrombotic status which increase the incidence of CVD and secondary events. | [21] |
| Diabetes mellitus | Diabetes mellitus is a major risk factor for CVD, while CVD is the major cause of death in patients with diabetes mellitus. | [22] |
| Diet | Alimentary habits impact CV risk and the risk of other chronic diseases such as cancer. | [21] |
| Physical activity | Sedentary (<0.5 h/week of physical activity) lifestyle is recognized as one of the major CV risk factors. | [21] |
Abbreviations: ASXL1, ASXL transcriptional regulator 1; CV, cardiovascular; CVD, cardiovascular disease; DNMT3A, DNA methyltransferase 3 alpha; JAK2, Janus kinase 2; LDL-C, low-density lipoprotein cholesterol; TET2, tet methylcytosine dioxygenase 2.
Recently, studies have shown that hematopoietic and immune cells from the bone marrow play key roles in the onset and progression of CVD. Clonal expansion of bone marrow hematopoietic stem and progenitor cells carrying somatic gene mutations, described as clonal hematopoiesis, has been shown by genetic analysis to be common in healthy individuals not otherwise showing any hematologic disorders.17,18,23 The finding is present in up to 10% of the general population and increases with age, and it is becoming apparent that clonal hematopoiesis is a significant risk factor for CVD as distinct from a cumulative incident risk of blood cancers. JAK2V617F and Tet methylcytosine dioxygenase 2 (TET2) are the most important mutations in clonal hematopoiesis. Hematopoietic cell clones that harbor JAK2V617F or TET2 are associated with the pathogenesis of CVD (Table 1).17,18,23
The risk of arterial thrombotic events in PV is higher than that for venous events.24 The accepted risk factors for thrombosis are “age >60” and “past thrombotic events”.25 In fact, the two factors allow for the classification of patients into low-risk (in the absence of both factors) or high-risk (in their presence), guiding therapeutic choices. In a study conducted by the International Working Group on Myeloproliferative Neoplasms Research and Treatment, prior arterial events, hyperlipidemia, and hypertension were predictive of future arterial events. By contrast, prior venous events, leukocytosis ≥11x109/L, and a major hemorrhagic event predicted future venous events. Arterial thrombosis was associated with age ≥60 years, hypertension, diabetes, hyperlipidemia, and a normal karyotype, whereas venous thrombosis was associated with age ≤60 years, palpable splenomegaly, female sex, and a history of major hemorrhage.26 In some studies, the allele burden of the JAK2V617F variant has been identified as a potential risk factor for thrombosis in MPN patients.27
In the absence of further evidence, it seems intuitive to conclude that patients with Ph-MPN need to be assessed to identify classical modifiable CV risk factors (smoking, hypercholesterolemia, arterial hypertension), with the scope of optimizing their baseline risk profile, to which the excess risk caused by the hematological disease is added.
Cardiovascular Risk Assessment
The 2021 guidelines issued by the European Association of Preventive Cardiology/European Society of Cardiology (EAPC/ESC) recommend systematic global CVD risk assessment in persons with any major vascular risk factor (such as family history of premature CVD or familial hypercholesterolemia, smoking, arterial hypertension, diabetes mellitus, raised lipid levels, obesity, or in those with comorbidities increasing CVD risk; recommendation Class I, evidence level A).21 Given that PV patients have an excess of 1.7% thrombotic events per patient/year at follow-up, even when treated according to the best standard of antithrombotic therapy (phlebotomy and acetylsalicylic acid; ASA),28 PV should be considered a comorbidity that increases CV risk, comparable to other disorders increasing CV risk, such as diabetes mellitus and chronic kidney disease (CKD).
Therefore, we propose to stratify the CV risk in patients with PV, similarly to what was done in other hematological disorders (eg chronic myelogenous leukemia)29 according to the EAPC/ESC guidelines.21
An initial evaluation of CV risk is done on the basis of anamnesis (Table 2). In particular, data on the presence of CVD and major risk factors (history of a CV event, diabetes mellitus, peripheral arteriopathy, CKD, resistant arterial hypertension, atrial fibrillation and left ventricle hypertrophy) must be collected. Also, the factors that modify CV risk must be identified. These include a family history of CVD, obesity, physical inactivity, psychiatric disease, autoimmune or inflammatory disorders, the use of antivirals, and obstructive sleep apnea syndrome. Risk assessment is further performed with the use of laboratory and instrumental examinations (Table 3). For patients with no CVD, CKD or diabetes mellitus, the Systematic COronary Risk Estimation 2 (SCORE2) algorithm estimates the 10-year risk of fatal/non-fatal CVD events in apparently healthy individuals aged 40–69. For individuals over 69, SCORE2-Older Persons (SCORE2-OP) is used.30
Table 2.
Cardiovascular Risk Determination Based on Anamnesis with Indications of Who Should Treat the patient21
| Anamnesis | Yes? | Risk level | Treater |
|---|---|---|---|
| Past myocardial infarction | □ | Very high risk | Referral to a specialist cardiology/hypertension center |
| Coronary or other arterial revascularization procedures | □ | ||
| ACS and other arterial atherosclerotic occlusions | □ | ||
| Past stroke or transient ischemic attack | □ | ||
| Aorta aneurism | □ | ||
| Peripheral artery disease | □ | ||
| Diabetes mellitus: | □ | ||
|
□ | ||
|
□ | ||
| Severe CKD (GFR<30 mL/min/1.73 m2) | □ | ||
| Age <50 years and SCORE2 ≥7.5% | □ | ||
| Age 50–69 years and SCORE2 ≥10.0% | □ | ||
| Age ≥70 years and SCORE2-OP ≥15.0% | □ | ||
Markedly elevated single risk factor:
|
□ | High risk | Consider a referral to a specialist cardiology/hypertension center |
| Diabetes mellitus: | □ | ||
|
□ | ||
| Moderate CKD (GFR 30–59 mL/min/1.73 m2) | □ | ||
| Age <50 years and SCORE2 2.5–7.49% | □ | ||
| Age 50–69 years and SCORE2 5–9.99% | □ | ||
| Age ≥70 years and SCORE2-OP 7.5–14.99% | □ | ||
| TT echocardiogram: | |||
|
□ | ||
|
□ | ||
| Resistant hypertension (not controlled by 3 drugs including a diuretic at full dose) | □ | ||
Diabetes mellitus:
|
□ | Moderate risk | GP |
| Age <50 years and SCORE2 <2.5% | □ | Low risk | GP |
| Age 50–69 years and SCORE2 <5.0% | □ | ||
| Age ≥70 years and SCORE2-OP <7.5% | □ |
Notes:  Very High Risk
Very High Risk  High Risk
High Risk  Moderate Risk
Moderate Risk  Low Risk.
Low Risk.
Abbreviations: ACS, acute coronary syndrome; CKD, chronic kidney disease; EF, ejection fraction; F, female; GFR, glomerular filtration rate; GLS, global longitudinal strain; GP, general practitioner; LVMi, left ventricular mass index; M, male; SCORE2, Systematic COronary Risk Evaluation 2; SCORE2-OP, Systematic COronary Risk Evaluation 2 in Older Persons; TT, transthoracic.
Table 3.
Laboratory and Instrumental Examinations to Assess Cardiovascular Risk
| First-line hemato-chemical examinations |
|---|
| Creatinine, Na and K |
| Creatinine clearance |
| Total cholesterol |
| HDL-C |
| Triglycerides |
| Glycemia |
| HbA1c (in patients with diabetes mellitus) |
| Albuminuria/creatininuria (to be carefully evaluated in patients with plasma cell dyscrasias) |
| Uricemia, iron levels |
| Instrumental examinations |
| Electrocardiogram |
| Echocardiogram with the assessment of systolic function and of the left ventricle mass |
| Home blood pressure monitoring ABPM in patients with arterial hypertension |
Abbreviations: ABPM, ambulatory 24-hour blood pressure monitoring; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; K, potassium; Na, sodium.
A comprehensive assessment of CV risk factors is at the basis of patient management, aiming at diminishing risk. CV risk assessment should be performed annually.31
Measures to Modify Cardiovascular Risk and Risk of Thrombosis
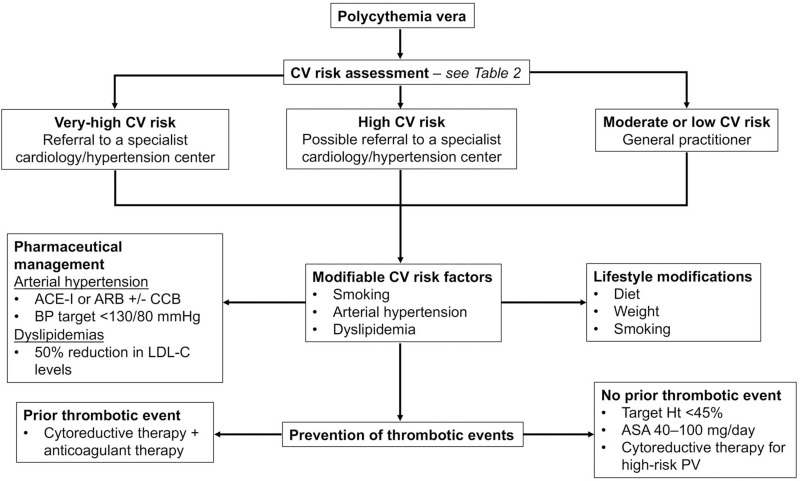
Modifying the CV risk profile in PV patients includes lifestyle modifications and pharmaceutical management of arterial hypertension and dyslipidemias (Figure 1).
Figure 1.
Flow diagram for the diagnosis and management of the cardiovascular risk profile and prevention of thrombotic events in patients with PV.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; BP, blood pressure; CCB, calcium channel blockers; CV, cardiovascular; Ht, hematocrit; LDL-C, low density lipoprotein cholesterol; PV, polycythemia vera.
Lifestyle Modifications
Stopping smoking is the most important of all CV risk-reducing measures. Smoking has been shown to reduce treatment response and overall survival in MPN,32 and the EAPC/ESC 2021 guidelines recommend that all smokers should be convinced to quit smoking, with similar benefits seen when passive smoking ceases. Patients should also be instructed on a correct diet (reduced saturated fat, salt, and sugar, increased dietary fiber) and alcohol consumption limitations. Moreover, patients must be encouraged to engage in regular physical exercise and control their weight.21
Arterial Hypertension
While no specific guidelines exist for treating hypertension in PV patients, lifestyle modifications are recommended for all patients. The ESC/European Society of Hypertension (ESH) 2018 guidelines recommend that patients with PV and arterial hypertension should be treated with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor antagonists (ARB) with or without calcium channel blockers to obtain a blood pressure reduction to the target of <130 (or less if tolerated)/80 mmHg. The therapeutic goal for arterial hypertension may vary according to the patient’s age with comorbidities (Table 4). Data analysis of hypertensive PV patients in the ECLAP study showed that patients treated with an ACE-I had a significantly lower need for cytoreductive therapy than those using other antihypertensive agents. The authors suggested a possible role of ACE-I is controlling the marrow renin-angiotensin system, which has a role in the mechanisms of erythropoiesis and is activated in clonal forms.33,34 This link with renin-angiotensin system expression in bone marrow may also partly explain reductions in thrombotic complications observed in PV patients with arterial hypertensive treated with ACE-I or ARB.35 ACE inhibitors have also been shown to improve kidney function in PV patients, as evidenced by improvements in estimated glomerular filtration rate, suggesting renoprotective properties.36
Table 4.
Therapeutic Goals for Modifiable Cardiovascular Risk Factors
| Risk Factor | Target | Notes |
|---|---|---|
| Systolic blood pressure | ||
| 18–65 years | 130 mmHg | Standard target |
| >65 years | 130–139 mmHg | If tolerated |
| Diastolic blood pressure | 70–79 mmHg | |
| LDL-C targeta | ||
| <100 mg/dL | Patients with moderate CV risk or young patients with diabetes mellitus duration <10 years without other risk factors | |
| <70 mg/dL | Patients with high CV risk or diabetes mellitus without target organ damage | |
| <55 mg/dL | Patients with very high CV risk or diabetes mellitus with target organ damage or severe CKD or established atherosclerotic CVD | |
| Triglycerides | <150 mg/dL | |
| Fasting glycemia | <110 mg/dL | |
| HbA1c | <6.5% |
Notes: aIn patients with high and very high CV risk, at least a 50% reduction is required.
Abbreviations: CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; LDL-C, low-density lipoprotein cholesterol.
The use of ACE-I is to be preferred in patients with PV and arterial hypertension if no contraindications exist. A beta-blocker can be added (or maintained, as in the case of coexisting ischemic heart disease) in order to obtain the blood pressure target.37 In the presence of diabetes mellitus, microalbuminuria, or frank proteinuria, antihypertensive agents that act on the renin-angiotensin system are preferred.37 There are no data on the safety of diuretics in patients with PV; however, it may be prudent to limit their use in patients with elevated hematocrit (Ht) values. In the case of resistant hypertension, after evaluating compliance and excluding other secondary causes, further therapeutic possibilities are represented by introducing mineralocorticoid receptor antagonists (spironolactone/canrenone) or alpha-1 blockers.
Dyslipidemias
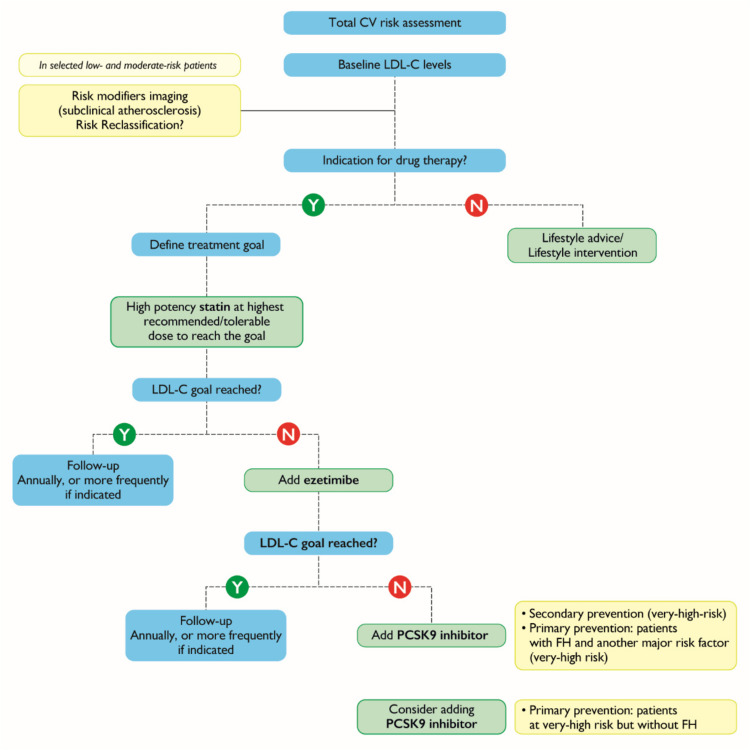
Similar to the lack of specific guidelines for treating arterial hypertension in patients with PV, no such guidelines exist for the treatment of dyslipidemias. ESC/European Atherosclerosis Society (EAS) established therapeutic goals for the treatment of dyslipidemias according to the level of low-density lipoprotein cholesterol (LDL-C) (Table 4).38 Given that patients with PV have a higher risk of CV events than patients without PV, we believe they should be treated more aggressively than currently recommended for patients in the same risk class without PV. Indeed, there is evidence that LDL-C levels <70 mg/dL (1.8 mmol/L) may be an appropriate therapeutic goal in moderate CV risk PV patients, rather than just in those at high CV risk or with diabetes mellitus.39 Regardless, lifestyle modifications must be introduced as a first-line measure in the case of dyslipidemias. If needed, according to the ESC/EAS guidelines, the initial therapeutic approach is based on the use of highly potent statins. High-potency statins are expected to provide a 50% reduction in LDL-C levels. If targets are not reached, ezetimibe is added, and if this fails, inhibitors for proprotein convertase subtilisin/kexin type 9 may be used according to the risk of the patient (Figure 2).
Figure 2.
The algorithm for the sequential introduction of cholesterol lowering therapy.
Notes: Adapted with permission from Oxford University Press. Mach F, Baigent C, Catapano AL et al 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. The European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines, permission conveyed through Copyright Clearance Center, Inc.38
Abbreviations: CV, cardiovascular; FH, familial hypercholesterolemia; LDL-C, low density lipoprotein cholesterol; N, no; PCSK9, proprotein convertase subtilisin/kexin type 9; Y, yes.
There is substantial variability in the response to lipid-modifying treatment. The use of predefined therapeutic targets may help patient-doctor communication and increase treatment adherence.38
Thrombosis Prophylaxis Specific to Polycythemia Vera
Primary prevention in PV is focused on the interventions necessary to reduce the risk of developing thrombosis in patients who have never experienced a thrombotic event (Table 5). A randomized clinical trial showed the antithrombotic value of maintaining Ht below 45%.40 Therefore, phlebotomy (or erythropheresis) is recommended in patients with PV, with the aim of maintaining Ht <45%. In patients who are intolerant to phlebotomy (ie individuals who experienced 2 episodes of post-phlebotomy syncope despite appropriate management or those who have blood or needle phobia leading to treatment avoidance) or in those with inadequate Ht control with phlebotomies (ie a need for ≥6 phlebotomies per year for at least 2 years in the maintenance phase after reaching Ht concentrations <45% in the induction phase), cytoreductive therapy should be considered.9,41,42 A lower Ht target level may be preferred in specific clinical situations (eg pregnancy or past history of splanchnic vein thrombosis).43 Primary prophylaxis with low-dose ASA 40–100 mg/day should be introduced in all low-risk PV patients in addition to phlebotomy.2 ASA twice daily may be considered in patients with inadequate control of microvascular symptoms who present CV risk factors, especially hypertension, or those with leukocytosis.2 However, further controlled studies are necessary to confirm the superiority of twice-daily versus once-daily dosing of ASA.
Table 5.
Primary and Secondary Prevention of Thrombotic Events in Polycythemia Vera
| Clinical Feature | Intervention |
|---|---|
| Primary prophylaxis | |
| All PV patients | ASA |
| Unless | |
| vWF activity <30% | Consider holding ASA |
| Platelet >1 million | Consider holding ASA |
| Ht ≥45% | Phlebotomy/cytoreduction to target Ht <45% |
| High-risk PV (age >60 years/past thrombosis) | Cytoreduction |
| Secondary prophylaxis | |
| All patients | Cytoreduction |
| Typical VTE | Consider indefinite VKA for most patients, ASA if not on VKA |
| Atypical VTE | Indefinite VKA and DOAC |
| Arterial thrombosis | ASA |
Notes: Adapted with permission from Springer Nature. Martin K. Risk factors for and management of MPN-associated bleeding and thrombosis. Curr Hematol Malig Rep. 2017;12(5):389–396.44
Abbreviations: ASA, acetylsalicylic acid; DOAC, direct oral anticoagulant; Ht, hematocrit; PV, polycythemia vera; VKA, vitamin K antagonists; VTE, venous thromboembolism; vWf, von Willebrand factor.
Moreover, based on ELN 2021 recommendations, cytoreductive therapy should also be considered to reduce the risk of thrombosis in progressive (at least 100% increase if baseline count is <10 × 10⁹ cells/L or at least 50% increase if baseline count is >10 × 10⁹ cells/L) and persistent (leukocyte count >15 × 10⁹ cells/L confirmed at 3 months) leukocytosis or in extreme thrombocytosis (>1500 × 10⁹ platelets/L), disease-related bleeding manifestations irrespective of the platelet count, or both situations.9
Secondary Thrombosis Prevention
Secondary prevention is a set of measures introduced to individuals who have already had a CV event (eg myocardial infarction, angina, myocardial revascularization, stroke, transient ischemic attack, peripheral arterial disease of lower limbs or carotids, or revascularization procedures) in order to reduce the risk of recurrent events, improve survival and quality of life.21,45 All patients are strongly encouraged to participate in a cardiologic rehabilitation program.
In MPN patients with a history of arterial and/or venous thrombosis, it is crucial to act on modifiable CV risk factors (as described above) and to estimate the individual risk of thrombosis and hemorrhage.3,46 In patients with high CV risk and previous thrombosis, cytoreductive therapy in addition to ASA should also be considered (Table 5).37,47 The acute treatment of organ-specific arterial thrombosis (stroke, acute myocardial infarction, peripheral artery disease) is no different in patients with an MPN from the general population except for the addition of cytoreductive therapy and phlebotomy (Table 5). Antiplatelet agents together with cytoreduction are typically used in patients with a history of an arterial thrombotic event, while in PV patients who have experienced a venous episode, anticoagulants are administered.48 Secondary prophylaxis with ASA is usually done at 100 mg/day, although 200 mg/day (100 mg twice daily), otherwise recommended in high-risk essential thrombocythemia, may be administered to some patients.3 There is no specific anticoagulant recommended for MPN patients by the existing guidelines.49 Results from small retrospective studies show that the overall incidence of new thrombotic and hemorrhagic events in patients treated with vitamin K antagonists (VKAs) and direct-acting oral anticoagulants (DOACs) were comparable (reviewed in).48 Currently, their use must be decided case-by-case based on individual characteristics.50 Analysis of data from 494 MPN patients with ET (52%) or PV (48%) in the Italian Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) registry revealed that VKA treatment reduced the recurrence of venous thromboembolic events (VTE), with a hazard ratio of 0.32 and a VTE-related bleeding risk of 0.9%).51
A 2018 study showed the merits of associating antithrombotic drugs with cytoreductive therapy in secondary prevention. In this study, MPN patients, who had experienced an ischemic stroke or transient ischemic attack and were subsequently treated with ASA or oral anticoagulants and cytoreduction.52 The composite incidence of recurrent ischemic stroke, transient ischemic attack, myocardial infarction and CV death was lower in these patients than that reported in the general population without an increased risk of major bleeding.52
However, evidence for the use of DOAC in MPNs predominantly comes from small retrospective studies. Of interest, the prospective AIRPORT-MPN study is comparing thrombosis prophylaxis with low-dose ASA with or without cytoreductive therapy with the DOAC apixaban, with or without cytoreductive therapy, in patients with MNP (ClinicalTrials.gov Identifier: NCT04243122).
A recently-published retrospective study reported data from MPN patients treated with DOAC or VKA. Patients had venous (87.3%) and arterial (12.7%) thromboses; 45 of 71 patients were treated with a VKA, and 26 received a DOAC.53 There were no significant differences in bleeding episodes between the two groups, although the prevalence of recurrent thromboses were more frequent in patients treated with VKA (35%) than DOAC (0%, p = 0.0003).
Recently, Barbui et al conducted an international, multicenter, real-world observational study in 442 MPN patients with atrial fibrillation and VTE receiving treatment with a DOAC.54 After estimating the incidence and risk factors for thrombotic and bleeding complications, they concluded that DOACs and VKAs had a largely similar risk-benefit profile for preventing VTE in patients with MPN. However, the concomitant use of hydroxyurea may be a compounding factor in the apparent favorable efficacy of DOAC in the prevention of ischemic cerebrovascular events in atrial fibrillation, as hydroxyurea is an independent protective factor against recurrence in MPN. However, a significant bleeding tendency is associated with dabigatran in primary myelofibrosis.54
Nevertheless, given the ease of administration and improved patient convenience, DOACs could be considered an alternative to VKAs as antithrombotic prophylaxis.
In patients affected by venous thromboembolism and thrombocytopenia, anticoagulant therapy using low molecular weight heparin must be modified in individuals with a platelet count <50,000 platelets/mm3; half of the recommended dose should be prescribed if platelet count ranges from 25,000–50,000/mm3, and no anticoagulation therapy should be used in patients with a platelet count <25,000/mm3.55,56 The duration of necessary anticoagulation in PV is still under study.48
Thrombophilia screening (eg testing for mutations in genes coding for factor II, factor V, factor VIII, antithrombin, protein C, protein S, activated protein C resistance, lupus anticoagulant, anticardiolipin antibodies or anti-B2 glycoprotein 1 antibodies, and homocysteine) does not change the management of the patient and should only be considered in selected patients.57 If suspected, a search for lupus anticoagulant, anticardiolipin or anti-beta-2 glycoprotein 1 antibodies should be performed. For patients with a confirmed diagnosis of antiphospholipid antibody syndrome (APS) requiring long-term anticoagulation to prevent recurrent thrombosis, the 2019 ESC and American Society of Hematology (ASH) guidelines do not recommend the use of DOACs. However, evidence-based guidelines developed by the European League Against Rheumatism (EULAR), the British Society for Haematology (BSH), and the International Society on Thrombosis and Haemostasis (ISTH) recommend warfarin as the first-choice treatment for this indication.58–60 They also propose that DOACs may be considered in certain situations, specifically in patients already stably anticoagulated with a DOAC, those on low-quality anticoagulation while on warfarin, those unwilling or unable to undergo standard international normalized ratio monitoring, and patients with contraindications to or having experienced serious adverse events while on warfarin. Warfarin is recommended for patients with arterial APS or triple positivity, although DOACs may be considered for those with venous APS and single or double positivity.58–60
Management of Specific Pathologies
Acute Ischemic Heart Disease
Lifestyle modifications and close blood pressure monitoring should be done in all ischemic heart disease patients. Pharmacological therapy should include an ACE-I combined with a beta-blocker in patients with heart failure or left ventricular ejection fraction ≤40% (Class I recommendation, evidence level A) and statins to achieve target LDL-C levels. Table 6 includes details of antiplatelet treatment modalities in patients who had an ST-elevation myocardial infarction, non-ST-elevation myocardial infarction and/or an aortocoronary bypass.61
Table 6.
Antiplatelet Therapy in Patients with Acute Ischemic Heart Disease Who Had an ST-Elevation Myocardial Infarction, Non-ST-Elevation Myocardial Infarction or Aortocoronary Bypass
| Clinical Situation | Intervention |
|---|---|
| All patients: |
|
| PCI to place a stent: |
|
| PCI to place a stent with atrial fibrillation or flutter: |
|
| PCI to place a stent and intraventricular thrombosis: |
|
Abbreviations: ACS, acute coronary syndrome; ASA, acetylsalicylic acid; BID, twice daily; DAPT, double antiplatelet therapy; DOAC, direct oral anticoagulant; PCI, percutaneous coronary intervention; VKA, vitamin K antagonists.
Ischemic Stroke or Transient Ischemic Attack
In patients who have had ischemic strokes or a transient ischemic attack, antiplatelet therapy with ASA at a dose of 100 mg/day is recommended to prevent secondary events.62
Deep Vein Thrombosis
Anticoagulation therapy effectively prevents the recurrence of deep vein thrombosis and reduces the risk of thromboembolic events by up to two times compared with untreated patients. The duration of anticoagulant therapy must be established based on the patient’s characteristics, the risk of thrombosis recurrence, MPN activity, ongoing treatments, and on the individual’s risk of bleeding. Experts recommend indefinite anticoagulant therapy in the context of atypical thrombosis, including splanchnic vein thrombosis and cerebral venous sinuses thrombosis.5,44,63
Conclusion
Ph-MPN are associated with an increased CV risk independent of the presence of conventional CV risk factors. High blood pressure, smoking, and dyslipidemia are common in MPN and contribute to an increased risk of CV events. Identifying patients at very high risk of fatal CV events is necessary to introduce early co-management by hematologists, cardiologists, and metabolic disease specialists. The definition of CV risk class (% fatal events at 10 years) based on the appropriate ESC scores is necessary to define the thresholds and intensity of intervention on pharmacologically modifiable risk factors, such as hypercholesterolemia, glucose metabolism, and blood pressure, and on risk factors modifiable with lifestyle, such as weight, diet, and smoking. Strict control of CV risk factors, in association with appropriate hematological therapy, may improve outcomes of patients with Ph-MPN. Further studies will clarify if the combination of ASA and DOAC could improve the management of patients with MPN at high risk of relapse. However, CVD characteristics in MPN may differ from those in the general population, and optimal thresholds for metabolic in these diseases have not been fully elucidated.64 MPN-specific treatment goals may be necessary to optimize risk factor management in these patients.
Acknowledgments
The authors would like to thank Dr Alicja M. Gruszka, MD, PhD, for providing medical writing assistance on behalf of Springer Healthcare Communications, Arneak Kooner of Springer Healthcare Communications and Ray Hill and Melanie Gatt on behalf of Springer Healthcare Communications for providing styling assistance prior to submission. This medical writing and editorial assistance was funded by Novartis Farma SpA.
Funding Statement
The authors have not received fees or financial compensation for their participation in this article.
Abbreviations
ABPM, ambulatory 24-hour blood pressure monitoring; ACE-I, angiotensin converting enzyme inhibitors; ACS, acute coronary syndrome; APS, antiphospholipid antibody syndrome; ARB, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; ASH, American Society of Hematology; ASXL1, ASXL transcriptional regulator 1; BID, twice daily; BSH, British Society for Haematology; CALR, calreticulin; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; DAPT, double antiplatelet therapy; DNMT3A, DNA methyltransferase 3 alpha; DOAC, direct oral anticoagulant; EAPC, European Association of Preventive Cardiology; EAS, European Atherosclerosis Society; ECLAP, European Collaboration on Low-dose Aspirin Study; EF, ejection fraction; ELN, European LeukemiaNet; ESC, European Society of Cardiology; ESH, European Society of Hypertension; ET, essential thrombocythemia; EULAR, European League Against Rheumatism; F, female; FH, familial hypercholesterolemia; GFR, glomerular filtration rate; GLS, global longitudinal strain; GP, general practitioner; HbA1c, glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; Ht, hematocrit; ISTH, International Society on Thrombosis and Haemostasis; JAK2, janus kinase 2; K, potassium; LDL-C, low-density lipoprotein cholesterol; LVMi, left ventricular mass index; M, male; MPL, proto-oncogene thrombopoietin receptor; MPN, myeloproliferative neoplasms; Na, sodium; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9; Ph-, Philadelphia chromosome; PMF, primary myelofibrosis; PV, polycythemia vera; SCORE2, systematic coronary risk estimation 2; SCORE2-OP, systematic coronary risk estimation-older persons; TET2, tet methylcytosine dioxygenase 2; TT, transthoracic; VKA, vitamin K antagonists; VTE, venous thromboembolism; vWF, von Willebrand factor; WHO, World Health Organization.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
G.B. has been on the advisory board, and been a speaker for Novartis, Janssen, BMS-Celgene M.M. has received consultancy fees from Gilead srl. E.B. has been a speaker for Novartis, Alexion and Incyte. A.M. has taken part in an advisory board for Amgen. D.R. has been advisor for Blueprint Medicines and has received travel grants from Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Benevolo G, Vassallo F, Urbino I, Giai V. Polycythemia vera (PV): update on emerging treatment options. Ther Clin Risk Manag. 2021;17:209–221. doi: 10.2147/TCRM.S213020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer J. 2018;8(1):3. doi: 10.1038/s41408-017-0042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(12):1599–1613. doi: 10.1002/ajh.26008 [DOI] [PubMed] [Google Scholar]
- 4.Rungjirajittranon T, Owattanapanich W, Ungprasert P, Siritanaratkul N, Ruchutrakool T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer. 2019;19(1):184. doi: 10.1186/s12885-019-5387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finazzi G, De Stefano V, Barbui T. Splanchnic vein thrombosis in myeloproliferative neoplasms: treatment algorithm 2018. Blood Cancer J. 2018;8(7):64. doi: 10.1038/s41408-018-0100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446–2452. doi: 10.1182/blood-2006-08-042515 [DOI] [PubMed] [Google Scholar]
- 7.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the american heart association. Circulation. 2023;147(8):e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krečak I, Morić Perić M, Zekanović I, et al. No impact of the increased number of cardiovascular risk factors on thrombosis and survival in polycythemia vera. Oncol Res Treat. 2021;44(4):201–203. doi: 10.1159/000514347 [DOI] [PubMed] [Google Scholar]
- 9.Marchetti M, Vannucchi AM, Griesshammer M, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: european LeukemiaNet 2021 recommendations. Lancet Haematol. 2022;9(4):e301–e311. doi: 10.1016/S2352-3026(22)00046-1 [DOI] [PubMed] [Google Scholar]
- 10.Kuipers RS, Kok L, Virmani R, Tefferi A. Essential thrombocytosis: diagnosis, differential diagnosis, complications and treatment considerations of relevance for a cardiologist. Neth Heart J. 2023;31(10):371–378. doi: 10.1007/s12471-12023-01757-12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbui T, Vannucchi AM, Carobbio A, et al. The effect of arterial hypertension on thrombosis in low-risk polycythemia vera. Am J Hematol. 2017;92(1):E5–E6. doi: 10.1002/ajh.24583 [DOI] [PubMed] [Google Scholar]
- 12.Horvat I, Boban A, Zadro R, et al. Influence of blood count, cardiovascular risks, inherited thrombophilia, and JAK2 V617F burden allele on type of thrombosis in patients with Philadelphia chromosome negative myeloproliferative neoplasms. Clin Lymphoma Myeloma Leuk. 2019;19(1):53–63. doi: 10.1016/j.clml.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 13.Mancuso S, Santoro M, Accurso V, et al. Cardiovascular risk in polycythemia vera: thrombotic risk and survival: can cytoreductive therapy be useful in patients with low-risk polycythemia vera with cardiovascular risk factors? Oncol Res Treat. 2020;43(10):526–530. doi: 10.1159/000509376 [DOI] [PubMed] [Google Scholar]
- 14.Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–682. doi: 10.1038/s41569-021-00552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komatsu N, Jun G, Yonezu T, Ohashi Y. Real-world, retrospective study evaluating thromboembolic events, associated risk factors, and health-care resource utilization in Japanese patients with polycythemia vera. Int J Hematol. 2020;112(2):176–184. doi: 10.1007/s12185-020-02887-w [DOI] [PubMed] [Google Scholar]
- 16.Seguro FS, Teixeira LLC, da Rosa LI, et al. Risk factors and incidence of thrombosis in a Brazilian cohort of patients with Philadelphia-negative myeloproliferative neoplasms. J Thromb Thrombolysis. 2020;49(4):667–672. doi: 10.1007/s11239-019-02029-y [DOI] [PubMed] [Google Scholar]
- 17.Min KD, Kour A, Sano S, Walsh K. The role of clonal haematopoiesis in cardiovascular diseases: epidemiology and experimental studies. J Intern Med. 2020;288(5):507–517. doi: 10.1111/joim.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yura Y, Sano S, Walsh K. Clonal hematopoiesis: a new step linking inflammation to heart failure. JACC Basic Transl Sci. 2020;5(2):196–207. doi: 10.1016/j.jacbts.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. Evidence from genetic, epidemiologic, and clinical studies: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 22.Sarwar N, Gao P; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misaka T, Kimishima Y, Yokokawa T, Ikeda K, Takeishi Y. Clonal hematopoiesis and cardiovascular diseases: role of JAK2V617F. J Cardiol. 2023;81(1):3–9. doi: 10.1016/j.jjcc.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 24.Arachchillage DR, Laffan M. Pathogenesis and management of thrombotic disease in myeloproliferative neoplasms. Semin Thromb Hemost. 2019;45(6):604–611. doi: 10.1055/s-0039-1693477 [DOI] [PubMed] [Google Scholar]
- 25.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–2232. doi: 10.1200/JCO.2005.07.062 [DOI] [PubMed] [Google Scholar]
- 26.Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi: 10.1038/s41408-017-0035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guglielmelli P, Loscocco GG, Mannarelli C, et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021;11(12):199. doi: 10.1038/s41408-021-00581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wille K, Sadjadian P, Becker T, et al. High risk of recurrent venous thromboembolism in BCR-ABL-negative myeloproliferative neoplasms after termination of anticoagulation. Ann Hematol. 2019;98(1):93–100. doi: 10.1007/s00277-018-3483-6 [DOI] [PubMed] [Google Scholar]
- 29.Seguro FS, Cmpdc S, Moura CMB, et al. Recommendations for the management of cardiovascular risk in patients with chronic myeloid leukemia on tyrosine kinase inhibitors: risk assessment, stratification, treatment and monitoring. Hematol Transfus Cell Ther. 2021;43(2):191–200. doi: 10.1016/j.htct.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooney MT, Selmer R, Lindman A, et al. Cardiovascular risk estimation in older persons: SCORE O.P. Eur J Prev Cardiol. 2016;23(10):1093–1103. doi: 10.1177/2047487315588390 [DOI] [PubMed] [Google Scholar]
- 31.McMullin MFF, Mead AJ, Ali S, et al. A guideline for the management of specific situations in polycythaemia vera and secondary erythrocytosis: a British Society for Haematology guideline. Br J Haematol. 2019;184(2):161–175. doi: 10.1111/bjh.15647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen AL, Knudsen TA, Skov V, et al. Smoking impairs molecular response, and reduces overall survival in patients with chronic myeloproliferative neoplasms: a retrospective cohort study. Br J Haematol. 2021;193(1):83–92. doi: 10.1111/bjh.17130 [DOI] [PubMed] [Google Scholar]
- 33.Barbui T, Masciulli A, Ghirardi A, Carobbio A. ACE inhibitors and cytoreductive therapy in polycythemia vera. Blood. 2017;129(9):1226–1227. doi: 10.1182/blood-2016-11-752600 [DOI] [PubMed] [Google Scholar]
- 34.Vrsalovic MM, Pejsa V, Veic TS, et al. Bone marrow renin-angiotensin system expression in polycythemia vera and essential thrombocythemia depends on JAK2 mutational status. Cancer Biol Ther. 2007;6(9):1434–1436. doi: 10.4161/cbt.6.9.4568 [DOI] [PubMed] [Google Scholar]
- 35.Mulas O, Mola B, Costa A, et al. Renin-angiotensin inhibitors reduce thrombotic complications in essential thrombocythemia and polycythemia vera patients with arterial hypertension. Ann Hematol. 2023;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krečak I, Morić Perić M, Zekanović I, et al. Beneficial effect of ACE inhibitors on kidney function in polycythemia vera. Wien Klin Wochenschr. 2021;1:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 38.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 39.Krečak I, Holik H, Coha B, et al. Low-density lipoprotein (LDL) and the risk of thrombotic events in essential thrombocythemia and polycythemia vera. Ann Hematol. 2021;100(5):1335–1336. doi: 10.1007/s00277-021-04431-0 [DOI] [PubMed] [Google Scholar]
- 40.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500 [DOI] [PubMed] [Google Scholar]
- 41.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. doi: 10.1200/JCO.2010.31.8436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbui T, Passamonti F, Accorsi P, et al. Evidence- and consensus-based recommendations for phlebotomy in polycythemia vera. Leukemia. 2018;32(9):2077–2081. doi: 10.1038/s41375-018-0199-5 [DOI] [PubMed] [Google Scholar]
- 43.Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia. 2021;35(12):3339–3351. doi: 10.1038/s41375-021-01401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin K. Risk factors for and management of MPN-associated bleeding and thrombosis. Curr Hematol Malig Rep. 2017;12(5):389–396. doi: 10.1007/s11899-017-0400-3 [DOI] [PubMed] [Google Scholar]
- 45.Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d [DOI] [PubMed] [Google Scholar]
- 46.Breccia M, Arboscello E, Bellodi A, et al. Proposal for a tailored stratification at baseline and monitoring of cardiovascular effects during follow-up in chronic phase chronic myeloid leukemia patients treated with nilotinib frontline. Crit Rev Oncol Hematol. 2016;107:190–198. doi: 10.1016/j.critrevonc.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 47.De Stefano V, Finazzi G, Barbui T. Antithrombotic therapy for venous thromboembolism in myeloproliferative neoplasms. Blood Cancer J. 2018;8(7):65. doi: 10.1038/s41408-018-0101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koschmieder S. The approach to thrombosis prevention across the spectrum of Philadelphia-negative classic myeloproliferative neoplasms. Hemato. 2021;2(3):392–402. doi: 10.3390/hemato2030025 [DOI] [Google Scholar]
- 49.Vannucchi AM, Barbui T, Cervantes F, et al. Philadelphia chromosome-negative chronic myeloproliferative neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v85–99. doi: 10.1093/annonc/mdv203 [DOI] [PubMed] [Google Scholar]
- 50.Ianotto JC, Couturier MA, Galinat H, et al. Administration of direct oral anticoagulants in patients with myeloproliferative neoplasms. Int J Hematol. 2017;106(4):517–521. doi: 10.1007/s12185-017-2282-5 [DOI] [PubMed] [Google Scholar]
- 51.De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93(3):372–380. doi: 10.3324/haematol.12053 [DOI] [PubMed] [Google Scholar]
- 52.De Stefano V, Carobbio A, Di Lazzaro V, et al. Benefit-risk profile of cytoreductive drugs along with antiplatelet and antithrombotic therapy after transient ischemic attack or ischemic stroke in myeloproliferative neoplasms. Blood Cancer J. 2018;8(3):25. doi: 10.1038/s41408-018-0048-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huenerbein K, Sadjadian P, Becker T, et al. Direct oral anticoagulants (DOAC) for prevention of recurrent arterial or venous thromboembolic events (ATE/VTE) in myeloproliferative neoplasms. Ann Hematol. 2021;100(8):2015–2022. doi: 10.1007/s00277-020-04350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbui T, De Stefano V, Carobbio A, et al. Direct oral anticoagulants for myeloproliferative neoplasms: results from an international study on 442 patients. Leukemia. 2021;35(10):2989–2993. doi: 10.1038/s41375-021-01279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napolitano M, Saccullo G, Marietta M, et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: an expert consensus. Blood Transfus. 2019;17(3):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of non-Vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–1676. doi: 10.1093/europace/euab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connors JM, Longo DL. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377(12):1177–1187. doi: 10.1056/NEJMra1700365 [DOI] [PubMed] [Google Scholar]
- 58.Arachchillage DRJ, Gomez K, Alikhan R, et al. Addendum to British Society for haematology guidelines on investigation and management of antiphospholipid syndrome, 2012 (Br. J. Haematol. 2012; 157: 47–58): use of direct acting oral anticoagulants. Br J Haematol. 2020;189(2):212–215. doi: 10.1111/bjh.16308 [DOI] [PubMed] [Google Scholar]
- 59.Koval N, Alves M, Placido R, et al. Direct oral anticoagulants versus vitamin K antagonists in patients with antiphospholipid syndrome: systematic review and meta-analysis. RMD Open. 2021;7(2):e001678. doi: 10.1136/rmdopen-2021-001678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pastori D, Menichelli D, Cammisotto V, Pignatelli P. Use of direct oral anticoagulants in patients with antiphospholipid syndrome: a systematic review and comparison of the international guidelines. Front Cardiovasc Med. 2021;8:715878. doi: 10.3389/fcvm.2021.715878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 62.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 63.Gulizia MM, Parrini I, Colivicchi F, et al. HCF-ANMCO/AICPR/GIEC/ITAHFA/SICOA/SICP/SIMG/SIT Cardiological Societies Council consensus document: anticoagulant therapy in venous thromboembolism and atrial fibrillation of the patient with cancer. Current knowledge and new evidence. G Ital Cardiologia. 2020;21(9):687–738. [DOI] [PubMed] [Google Scholar]
- 64.Krečak I, Verstovsek S, Lucijanic M. Optimization of cardiovascular risk factor management in patients with BCR:: ABL1 negative chronic myeloproliferative neoplasms, current knowledge, and perspectives. Ann Hematol. 2023;2023:1–11. [DOI] [PubMed] [Google Scholar]