Abstract
EDUCATION GAP
Influenza is among the most common infectious causes of pediatric emergency department visits and hospitalizations. Clinicians should use evidence-based guidelines to learn how to identify, manage, prevent, and treat influenza cases. Disease caused by influenza virus can be mitigated with appropriate treatment and prevention efforts.
OBJECTIVES After completing this article, readers should be able to:
1. Describe the virology and epidemiology of influenza.
2. List the clinical features and complications of influenza infections.
3. List the benefits and limitations of testing modalities for the diagnosis of influenza.
4. Appropriately apply American Academy of Pediatrics, Infectious Diseases Society of America, and Centers for Disease Control and Prevention (CDC) treatment guidelines for influenza or suspected influenza.
5. Describe the importance of influenza vaccination.
INTRODUCTION
Influenza is responsible for significant morbidity and mortality, accounting for close to 1,000,000 global hospitalizations in children younger than 5 years each year. (1) Influenza season duration and severity varies year to year and by influenza strain, and it is challenging to predict the severity of each upcoming influenza season. In this article, we aim to provide a review of the virology, epidemiology, clinical characteristics, complications, transmission, diagnostic testing, treatment, and prevention of influenza in the pediatric population.
EPIDEMIOLOGY
In the United States, 8% to 10% of children experience symptomatic influenza each year, resulting in 140,000 to 710,000 hospitalizations and 12,000 to 52,000 deaths (Table 1). (2)(3) Attack rates vary seasonally and are higher in unvaccinated compared with vaccinated children (20% vs 10%). (4) During the winter, influenza accounts for nearly 10% of pediatric hospitalizations in the United States. (5) Influenza accounts for significant health-care expenditures each year. For example, in 2015, direct and indirect costs to the US health-care system and society totaled $6.3 to $25.3 billion. (6) Most morbidity and mortality associated with influenza occurs in young children (<5 years) and the elderly (>65 years). In the pediatric population, children younger than 5 years, especially those younger than 2 years, and children with underlying medical conditions are at highest risk for influenza-associated complications.
Table 1.
Severity, Incidence, and Type of Influenza Infection by Season (2)
| TYPE OF INFLUENZA INFECTION, % | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| INFLUENZA SEASON | INFLUENZA SEASON SEVERITY AMONG CHILDREN AGED 0–17 ya | ESTIMATED SYMPTOMATIC ILLNESSES, No.b | A (H1N1) | A (H3) | A (NO SUBTYPING) | B (YAMAGATA AND VICTORIA) |
|
| ||||||
| 2015–2016 | Low | 24 million | 54 | 15 | 1 | 30 |
| 2016–2017 | Moderate | 29 million | 2 | 74 | 1 | 23 |
| 2017–2018 | High | 41 million | 11 | 59 | 1 | 29 |
| 2018–2019 | Moderate | 29 million | 50 | 41 | 3 | 6 |
| 2019–2020 | Moderate | 36 million | 51 | 4 | 4 | 41 |
| 2020–2021c | — | — | — | — | — | — |
| 2021–2022 | Low | 9 million | 0.4 | 79 | 20 | 0.6 |
As classified by the Centers for Disease Control and Prevention.
Among all children and adults in the United States.
Not calculated due to low influenza activity.
VIROLOGY
Influenza viruses are enveloped negative-sense single-stranded ribonucleic acid viruses classified as orthomyxo-viruses. Influenza viruses contain a lipid envelope, RNA segments, the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA), matrix proteins, and membrane proteins, among others. Viral replication is fostered by HA to facilitate viral entry and by NA to facilitate viral release, making glycoproteins and membrane proteins common targets for antiviral medications and vaccinations. (7)
Clinically relevant influenza types in humans are A, B, and C. Influenza A is classified into subtypes based on surface glycoproteins, of which 25 different antigenic subtypes exist in humans. Strains are named by virus type, location, year, and subtype (eg, A/Tennessee/2022/[H3N2]). (8)(9) Dominant strains known to cause epidemics in recent years include H1N1 and H3N2. Influenza B is classified into 2 lineages (Yamagata and Victoria) based on HA type. (10) Influenza C contains an HA-esterase fusion glycoprotein rather than HA/NA and causes mild illness. (11) There are only rare instances of hospitalization secondary to influenza C in children. (12)
HISTORICAL BACKGROUND AND PANDEMIC RISK
The influenza virus adapts by altering its genetic make-up through reproduction. These mutations can involve minor changes in HA/NA (genetic “drift”) or cause more dramatic changes that result in an entirely novel strain (genetic “shift”). Genetic drift results in seasonal variation, whereas genetic shift can result in an influenza pandemic. Historically, influenza A, rather than influenza B, has led to global pandemics as the genetic properties of influenza B viruses shift more slowly and spread almost exclusively in humans rather than in animal hosts. In the past 100 years, 5 influenza pandemics have occurred. The infamous 1918–1919 H2N2 influenza pandemic infected one-third of the world’s population, with significant mortality among infants, young adults, and the elderly. (13) Most recently, the 2009–2010 H1N1 influenza pandemic resulted in 60 million cases in the United States and 300,000 deaths worldwide. (14) Similar to the 1918–1919 outbreak, most deaths occurred in people younger than 65 years. (2)(3)
RACIAL AND ETHNIC DISPARITIES IN INFLUENZA
There are significant disparities in influenza-associated health-care utilization and outcomes by race/ethnicity in the United States. Influenza-associated hospitalization rates are higher in Black, Hispanic, American Indian or Alaskan native, and Asian or Pacific Islander children compared with White children. (15)(16) Black, Hispanic, and American Indian/Alaskan native adolescents receive influenza vaccinations at lower rates than White adolescents. (17)(18) Black pregnant women have the lowest rate of influenza immunization in pregnancy and report a lower rate of offer of vaccine. (19)
Reasons for these disparities are multifactorial and may include inequitable access to health-care, vaccination opportunity, misinformation, and mistrust secondary to a long history of structural racism. (16) Ongoing study is needed to better understand disparate outcomes and immunization rates in underserved populations.
INFLUENZA AND THE COVID-19 PANDEMIC
During the 2020–2021 influenza season, influenza activity was the lowest ever recorded (0.5% positivity rate). (20)(21) This coincided with the COVID-19 pandemic and global implementation of measures known to interrupt transmission of influenza, such as masking, social distancing, school closures, and reduced travel. Influenza activity during the 2021–2022 season was similarly low; however, the 2022–2023 season saw a marked increase of influenza cases. From 2017 to 2021, influenza vaccination coverage in children ranged from 57.8% to 62.5%. (22) The COVID-19 pandemic likely resulted in vaccine fatigue and, along with other factors, led to a drop to 36% vaccination coverage during the 2021–2022 season. (23)(24) Thus, the influenza resurgence in the 2022–2023 season likely resulted from several factors: the widespread reduction of pandemic-related protective measures combined with decreased influenza vaccination, waning natural/vaccine-induced antibodies, antigenic drift, and more time spent at home with influenza contacts. After the COVID-19 pandemic, evidence suggests an increase in the transmissibility of influenza, with household transmission increasing from 20% in prepandemic times to 50% in 2021–2022. (25)
TRANSMISSION
Seasonal Variation
In temperate climates, the influenza season typically spans October to March (northern hemisphere) or April to September (southern hemisphere). (26) In the United States, there is wide geographic variation in the start and end of influenza season, but it generally peaks between January and March. (27) In tropical climates, influenza seasons may occur year-round. (28)
Modes of Transmission
Three modes of transmission occur: droplet, contact, and aerosol (Table 2). Large-particle droplets (>100 μm) produced during coughing, sneezing, or talking are the primary mode of influenza transmission. Contact transmission, either direct transmission via body-to-body surface contact, such as hand-to-hand contact followed by hand-to-respiratory mucosa contact, or indirect via contaminated surfaces/objects, can occur. (29) Influenza virus has been experimentally found on surfaces 24 to 48 hours after inoculation. (30) According to the Centers for Disease Control and Prevention (CDC), health-care professionals should use standard and droplet precautions when interacting with patients with confirmed or suspected influenza.
Table 2.
Transmission Takeaways
| •Peak influenza season is January to March in the northern hemisphere |
| •Influenza can be spread by droplet, contact, and aerosol |
| •The Centers for Disease Control and Prevention recommends standard and droplet precautions for health-care providers interacting with patients with suspected or confirmed influenza |
| •Household transmission of influenza can result in infections in up to 38% of household contacts |
Viral Incubation and Shedding
The incubation period of influenza virus is 1 to 4 days. (7) Influenza can be detected and viral shedding can occur several days before the onset of illness. Viral shedding peaks within 24 hours of symptoms, and the highest infectious period is during the first 3 days of symptoms. (31) Recent studies suggest that children may have longer viral shedding periods and higher viral loads than adults. (32) Increased disease severity is associated with a longer shedding and infectious period. (33)
Household and School Transmission
Household transmission of influenza virus varies each year. On average, if a household member is infected with influenza, there is an approximately 40% risk of infection to other household contacts. (34) The CDC recommends isolating at home until fever free for at least 24 hours or after symptoms improve. (35) As demonstrated with the COVID-19 pandemic and H1N1 influenza pandemic, school closure can be an efficacious infection control mitigation strategy. (36)(37) A meta-analysis of short-term school closure studies demonstrate a resulting 30% reduction in peak epidemic influenza incidence in the community. (37)
CLINICAL CHARACTERISTICS
Signs and Symptoms
The clinical manifestations of acute influenza vary based on the age and health of the child. The broad spectrum of influenza illness spans asymptomatic infections to severe illness and death. The most common signs and symptoms in all age groups include fever, cough, and rhinorrhea, which are present in 85% of confirmed symptomatic influenza cases. (38) For surveillance purposes, an influenza-like illness is defined as fever with cough or sore throat. (39) It is important to note that although fever is a common symptom, the absence of fever should not exclude influenza as a diagnosis in those with respiratory symptoms during influenza season. Children older than 15 years are more likely to have an asymptomatic infection (26%) compared with younger children (6.6%). (38) In addition, immunocompromised children may be incapable of mounting a febrile response. Alternatively, influenza can also result in prolonged fever and is an uncommon cause of fever of unknown origin. (40)
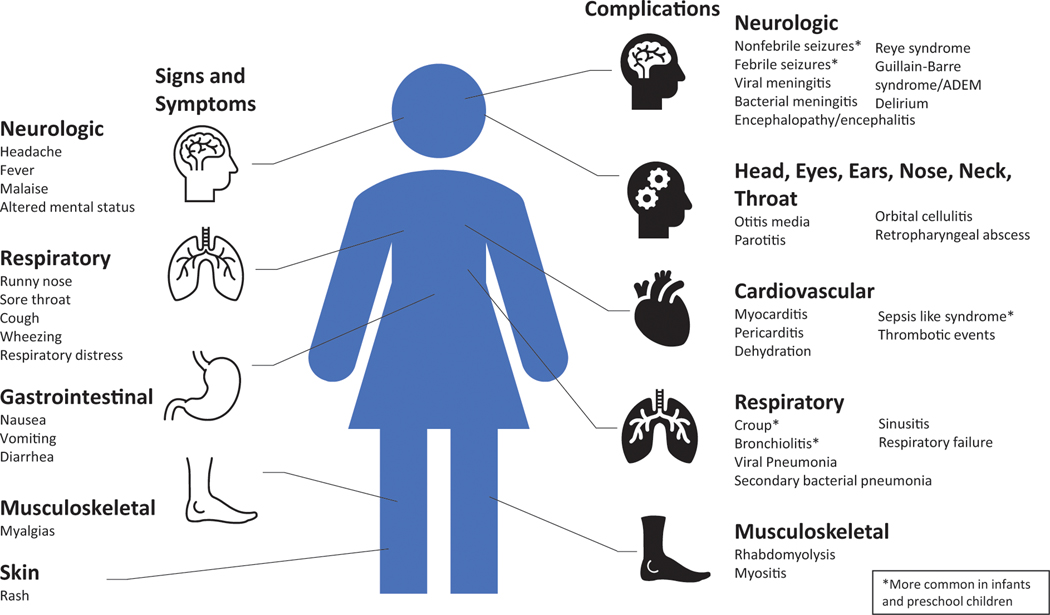
Gastrointestinal symptoms, including vomiting and diarrhea, are found in 40% of infants and children younger than 4 years. Older children are more likely to endorse headache compared with younger children. Other common symptoms include malaise, fatigue, and myalgias (Fig 1). Physical examination findings are generally nonspecific and can reveal pharyngeal erythema/ exudate and cervical lymphadenopathy, among other signs. Most healthy, immunocompetent children with influenza will have gradual improvement over 5 to 10 days.
Figure 1.
Signs, symptoms, and complications of influenza.
Adapted from Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258. (41)
Disease Severity
Disease severity may differ between influenza A and B, although multiple systematic reviews continue to generate conflicting evidence. (38)(42) Certain strains of influenza A (H3N2) in adults are associated with more severe disease, hospitalization, and death. (43) Ten years of surveillance data of children with influenza in Australia found that influenza A accounted for 68.6% of hospitalizations; however, influenza B was more often associated with acute renal failure, rhabdomyolysis, and cardiac complications. (44) There is also evidence of higher pediatric mortality from influenza B infections than from influenza A. (45) More data are needed to understand strain-specific mortality and morbidity and transmissibility in children.
COMPLICATIONS OF INFLUENZA
Although most influenza illness is self-limited, complications from influenza can be life-threatening. Certain pediatric groups are at high risk for severe primary influenza disease or complications secondary to influenza (Table 3). Children at high risk for complications of influenza are a particularly vulnerable population highlighted in national guidelines. (22)(46)(47) Strategies for testing, treatment, and chemoprophylaxis for influenza may differ based on high-risk status or exposure to a contact who is high risk, such as if the patient is high risk, if the patient is a health-care worker who cares for those who are high risk, or if there is a high-risk contact in the household.
Table 3.
Pediatric High-Risk Conditions for Influenza Complications
| CATEGORY | DESCRIPTION/EXAMPLES |
|---|---|
|
| |
| Demographics | Children <5 y of age, especially <2 y |
| Residential location | Residents of long-term care facilities or nursing homes |
| Extreme obesity | BMI threshold not well-defined in children |
| Certain long-term medications | Aspirin or salicylate-containing medications |
| Pregnancy | Pregnant and postpartum up to 2 wk after delivery |
| Chronic medical conditionsa | |
| Neurologic and neurodevelopmental conditions | Epilepsy, stroke, cerebral palsy, moderate to severe developmental delay, muscular dystrophy |
| Cardiovascular disease | Congenital heart disease |
| Endocrine disorders | Type 1 diabetes mellitus, type 2 diabetes mellitus |
| Pulmonary disease | Asthma, chronic lung disease, cystic fibrosis, chronic mechanical ventilation |
| Kidney disease | Chronic kidney disease, dialysis |
| Hematologic disease | Sickle cell disease and other hemoglobinopathies |
| Liver disorders | Chronic liver disease |
| Metabolic disorders | Inherited metabolic disorders and mitochondrial disorders |
| Immunosuppression | Congenital, acquired, or iatrogenic immune deficiency |
List of chronic medical conditions is not exhaustive.
Respiratory
Secondary bacterial infections of the respiratory tract are the most common complication of influenza. Among children hospitalized with influenza, 28% to 36% will have primary or secondary pneumonia. (48) When identified through respiratory samples, Streptococcus pneumoniae is the most common (67%) cause of secondary pneumonia, (49) and secondary necrotizing methicillin-resistant Staphylococcus aureus pneumonia is rare. (50) Critically ill children may experience acute respiratory failure or acute respiratory distress syndrome. Asthma exacerbations occur in up to 22% of children with asthma and influenza infection. (51) Other respiratory complications include laryngo-tracheobronchitis (croup) and bacterial tracheitis.
Neurologic
The top percentage of neurologic complications occurring in children hospitalized for influenza ranges from 7% to 10%. (52)(53) Seizures represent 66% to 75% of those complications, followed by encephalopathy. Less commonly, bacterial meningitis, encephalitis, acute demyelinating syndromes, stroke, Reye syndrome, and Guillain-Barré syndrome (GBS) may occur. (52)(54) Reye syndrome cases are rare after the Food and Drug Administration (FDA) issued its warning on aspirin use in children in 1980, (55) but cases can still occur in children with various illnesses who are exposed to aspirin therapy. (56)
Cardiac
Direct myocardial involvement by influenza may result in myocarditis and pericarditis. (57)(58) Cardiovascular complications are of particular significance in influenza-related mortality. In 1 case series of 47 influenza-associated pediatric deaths, 13% were attributed to cardiac involvement. (59)
Head, Ears, Eyes, Nose, Neck
Acute otitis media is common and can develop in 10% to 50% of patients, typically occurring 3 to 4 days after an influenza infection. (60) Rare complicated head and neck infections secondary to influenza include orbital cellulitis, parotitis, retropharyngeal abscesses, Pott puffy tumor, and Lemierre syndrome. (61)
Musculoskeletal
Myositis is uncommon but can be seen in both influenza A and B, although historically it has been associated more with influenza B. (62) Myositis presents as bilateral calf tenderness, often with refusal to walk in young children, with concurrent elevation in serum creatine phosphokinase level. (63) Influenza is also frequently implicated as a cause of viral-associated rhabdomyolysis. (64)
LABORATORY TESTING FOR INFLUENZA
Recommendations for Testing
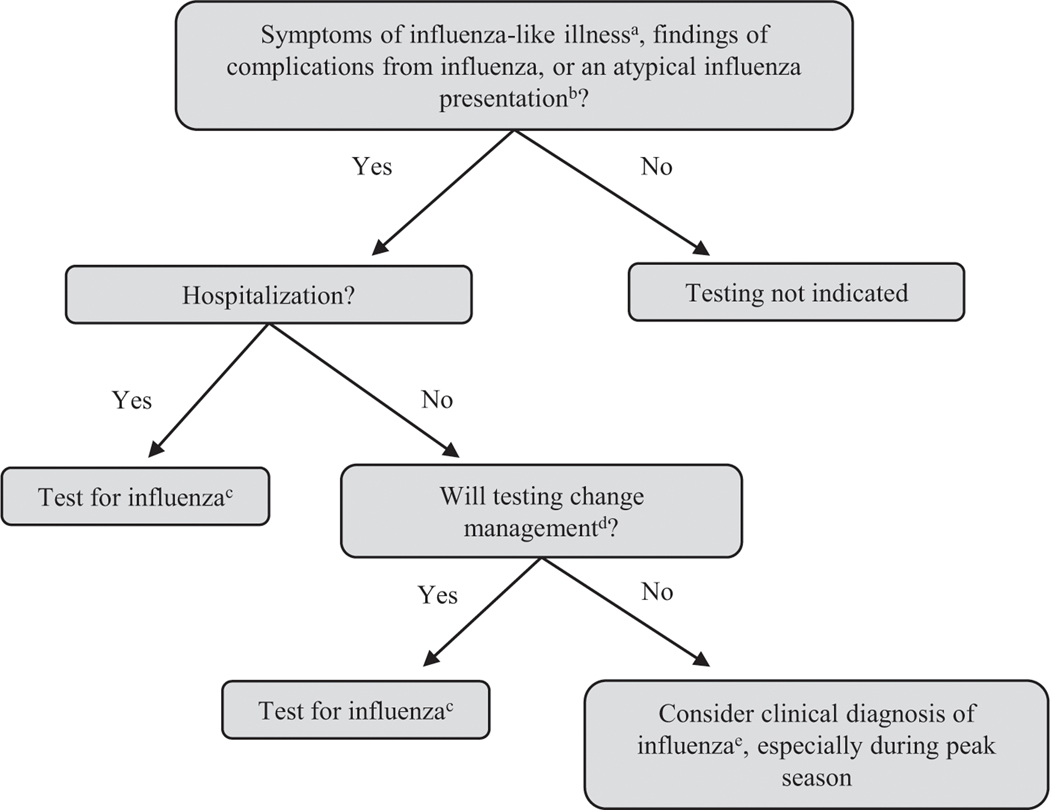
The American Academy of Pediatrics (AAP), the Infectious Diseases Society of America (IDSA), and the CDC provide guidance on influenza testing (Fig 2). A clinical diagnosis may be challenging in young children due to other atypical bacterial/viral infections (respiratory syncytial virus, human metapneumovirus, COVID, parainfluenza, adenovirus, rhinovirus/enterovirus, and Mycoplasma pneumoniae, among others) that present with similar symptoms during influenza season. According to national guidelines, laboratory confirmation is not necessary before initiating antiviral treatment in those with influenza-like illness during influenza season. (22)(46)(47) A history of vaccination does not exclude influenza, and vaccination status should not influence the decision to test for influenza. (23)
Figure 2.
Clinical flow diagram for influenza testing.
Adapted from the Centers for Disease Control and Prevention. Guide for considering influenza testing when influenza viruses are circulating in the community. Available at: https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm. (65)
aAn influenza-like illness is defined as a fever plus cough or sore throat.
bVaccination status should not influence testing algorithms.
cTesting for influenza should be interpreted based on the clinical picture and type of test used with corresponding sensitivity/specificity.
dManagement changes may include antiviral treatment, antibiotic treatment, further diagnostic tests, and decisions about infection prevention and control practices.
eDuring periods of high influenza activity, a clinical diagnosis of influenza can be made.
Based on AAP, CDC, and IDSA guidelines, outpatient clinicians should test for influenza in individuals with influenza-like illness if the result will change management (eg, antiviral treatment, need for further evaluation, infection control practices, chemoprophylaxis). (47) Clinicians should test all patients requiring hospitalization for influenza-like illness during the influenza season, especially children at high risk for influenza complications.
Types of Available Influenza Tests
Reverse transcriptase–polymerase chain reaction (RT-PCR) molecular assays provide highly sensitive and specific results. Specificity of all tests is relatively high (66); however, sensitivity can vary for rapid influenza diagnostic tests. (67) Influenza prevalence has a demonstrable effect on the positive predictive value of rapid antigen testing, with higher positive predictive values during high-prevalence weeks and lower positive predictive values during weeks of lower influenza prevalence. (68) The IDSA recommends the use of rapid influenza molecular assays in the outpatient setting and RT-PCR or other molecular assays for hospitalized patients (Table 4).
Table 4.
Common Diagnostic Testing Modalities for Influenza (67)
| TYPE OF TEST | METHOD | TIME TO RESULTS | PERFORMANCE | COMMENTS |
|---|---|---|---|---|
|
| ||||
| Rapid influenza diagnostic test | Antigen detection | 15–40 min | Sensitivity 50%–70% Specificity >90% |
CLIA allow most tests for point-of-care use |
| Rapid molecular assay | Nucleic acid amplification | 10–15 min | Sensitivity >95% Specificity >99% |
CLIA allow most tests for point-of-care use |
| Molecular assays (RT-PCR) |
Nucleic acid amplification | 1–6 h | Sensitivity >95% Specificity >99% |
Multiplex assays can distinguish among COVID, influenza A and B, and, in some cases, RSV Can also be part of a respiratory pathogen panel |
CLIA=Clinical Laboratory Improvement Amendments, RSV=respiratory syncytial virus, RT-PCR=reverse transcriptase–polymerase chain reaction.
Additional laboratory testing for influenza complications or alternative diagnoses may be performed depending on the clinical context. When obtained, laboratory findings such as the white blood cell count may be low, high, or normal, whereas elevated C-reactive protein or procalcitonin levels could point to a secondary bacterial infection. (69)(70)
ANTIVIRAL TREATMENT
Antiviral Medications
Four influenza antiviral medications are FDA approved for children and adults and are generally considered to have similar effectiveness (Table 5). NA inhibitors (oseltamivir, zanamivir, and peramivir) work by blocking NA to prevent virion release and are effective against both influenza A and B. Alternatively, the endonuclease inhibitor baloxavir interrupts the transcription of viral mRNA by targeting the virus polymerase complex.
Table 5.
Recommended Dosages for Treatment and Chemoprophylaxis for Influenza Antiviral Medications
| ANTIVIRAL | TREATMENT DOSING | CHEMOPROPHYLAXIS DOSING | ADVERSE EVENTS |
|---|---|---|---|
|
| |||
| Oseltamivir Adults Children >12 mo ≤15 kg >15–23 kg >23–40 kg >40 kg Infants 9–11 mo Term infant 0–8 mo Preterm infants <38 wk 38–40 weeks >40 weeks (Postmenstrual age) |
5-d oral course 75 mg, twice daily 30 mg, twice daily 45 mg, twice daily 60 mg, twice daily 75 mg, twice daily 3.5 mg/kg per dose, twice daily 3.0 mg/kg per dose, twice daily 1.0 mg/kg per dose, twice daily 2.0 mg/kg per dose, twice daily 3.0 mg/kg per dose, twice daily |
7-d oral course 75 mg, once daily 30 mg, once daily 45 mg, once daily 60 mg, once daily 75 mg, once daily 3.5 mg/kg per dose, once daily 3–8 mo: 3.0 mg/kg/dose, once daily 0–3 mo: Consult with an infectious disease specialist |
Nausea, vomiting, headache, diarrhea, rare transient neuropsychiatric events, severe cutaneous adverse reactions |
| Zanamivir Adults Children |
5-d inhaled course 10 mg (two 5-mg puffs, twice daily) ≥7 y 10 mg (two 5-mg puffs, twice daily) |
7-d inhaled course 10 mg (two 5-mg puffs, once daily) ≥5 y 10 mg (two 5-mg puffs, once daily) |
Risk of bronchospasm (avoid in people with chronic lung disease), dizziness, rare transient neuropsychiatric events, severe cutaneous adverse reactions |
| Peramivir 6 mo–12 y ≥13 y |
1 infusion 12 mg/kg dose (600 mg maximum) IV 600 mg dose IV |
Not recommended | Diarrhea, rare transient neuropsychiatric events, severe cutaneous adverse reactions |
| Baloxavir ≥5 y <20 kg 20 kg–<80 kg ≥80 kg |
1 oral dose 2 mg/kg once 40 mg once 80 mg once |
1 oral dose 2 mg/kg once 40 mg once 80 mg once |
Nausea, vomiting, diarrhea |
IV=intravenous.
Adapted from Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2022–2023. Pediatrics. 2022;150(4):e2022059275. (22)
NA Inhibitors.
Oseltamivir.
Oseltamivir is most commonly administered orally. The most frequent adverse effects include vomiting and epigastric discomfort, which occur in 10% to 15% of patients. (71) It is the only NA inhibitor to be approved for use in preterm and term infants and is also recommended in breastfeeding mothers with influenza. (72) In 2006, in response to case studies and subsequent reports (mainly out of Japan), the FDA added a warning label for rare neuropsychiatric adverse events, including suicidal ideation and psychosis. Subsequent studies have yielded mixed results, with some reporting a positive association, (73)(74) negative association, (75)(76) or no association (77)(78)(79) between oseltamivir and neuropsychiatric events. To date, the relationship between oseltamivir and neuropsychiatric events remains highly debated and is an area of much-needed research.
Zanamivir.
Zanamivir is an inhaled medication that is contraindicated in children with chronic lower respiratory disease, including asthma. Compared with outpatient treatment with oseltamivir, inhaled zanamivir is noninferior regarding reduction of symptom duration and hospitalizations. (80)
Peramivir.
Peramivir is administered intravenously and is approved for use in the ambulatory setting. In clinical practice, peramivir is typically used in hospitalized patients when other antiviral formulations are unable to be tolerated. (81)
Endonuclease Inhibitors.
Baloxavir.
Baloxavir, approved by the FDA in 2018, is the first polymerase acidic endonuclease inhibitor developed against influenza and works by inhibiting proteins involved in viral replication. (82) Baloxavir is administered as a 1-time oral agent and has similar efficacy to oseltamivir. At this time, it is not covered by all insurers and is more costly than other antiviral agents. (83)
Antiviral Resistance
The class of M2 protein inhibitors known as adamantane antiviral medications are no longer recommended for the treatment of influenza due to widespread viral resistance. (84) Similar concerns surrounding oseltamivir have not emerged in clinical studies. (85)(86)
Recommendations for Treatment
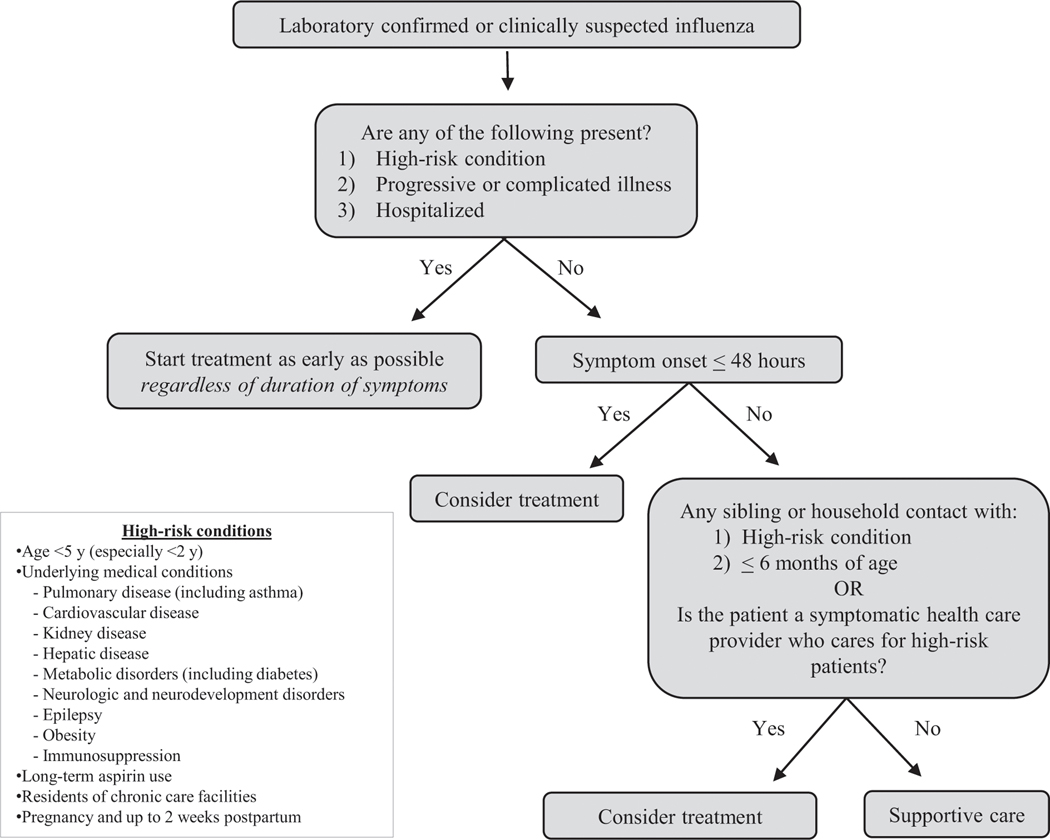
In general, most patients with influenza will have a self-limiting course regardless of treatment with antiviral medications. The AAP, CDC, and IDSA recommend that clinicians treat influenza in all cases, regardless of symptom duration, in individuals at high risk for influenza complications (Table 2), individuals with influenza complications, individuals with progressive illness, and individuals requiring hospitalization. Clinicians can consider treatment for non–high-risk patients if symptom onset is less than 48 hours, for non–high risk patients who have household contacts at high risk for influenza complications (including children <2 years of age), and for symptomatic health-care workers who care for patients at high risk for influenza complications (Fig 3).
Figure 3.
Clinical flow diagram for influenza antiviral treatment. (22)(46)(47)
EVIDENCE FOR IMPROVED OUTCOMES WITH ANTIVIRAL TREATMENT
Reduced Duration of Symptoms
In a meta-analysis of 5 randomized trials comparing oseltamivir and placebo, among those with less than 48 hours of symptoms, oseltamivir decreased duration of illness by 18 hours in all children and by 30 hours in children without asthma. (87) A randomized trial of baloxavir in children 1 to 12 years of age with less than 2 days of symptoms revealed similar time to symptom alleviation compared with oseltamivir. (83) In randomized trials of individuals older than 12 years, baloxavir reduced duration of illness by 27 hours in non–high-risk individuals and by 29 hours in high-risk individuals. (88)(89) Trials comparing zanamivir and peramivir found similar efficacy compared with oseltamivir. (90)
Decreased Influenza Complications
In a meta-analysis of 5 randomized trials in children, there was a 34% reduction in otitis media in those treated with oseltamivir compared with placebo. (87) In pediatric observational studies, oseltamivir was associated with decreased pneumonia, (74)(91) influenza-related hospitalization, (92) and influenza-related complications (including respiratory, cardiac, neurologic, and renal complications) regardless of duration of symptoms. (92) In children with chronic medical conditions, oseltamivir was associated with decreased risk of hospitalization and influenza-related complications, including pneumonia, nonpneumonia respiratory illness, and otitis media. (93)
Decreased Influenza Transmission
Antiviral treatment reduced the amount and volume of viral shedding, resulting in decreased household transmission of influenza. (33) Treatment with oseltamivir, zanamivir, or baloxavir was associated with a 23% to 48% reduction in transmission of influenza. (94)(95)(96)(97) There was no difference in reduction of transmission comparing antiviral agents with each other. (98)
Improved Hospital Outcomes
In a prospective cohort study of hospitalized children with laboratory-confirmed influenza, early oseltamivir use was associated with a decreased length of hospital stay (LOS) among children at high-risk for influenza complications and those admitted to the ICU. (99) Patients who received oseltamivir 3 days or more after symptom onset had no reduction in LOS compared with those who did not receive antiviral therapy. (99) Data on decreased LOS in this prospective cohort study support other retrospective studies of reduced LOS in children with high-risk conditions, (100) those admitted to the ICU, (101)(102)(103) and hospitalized children overall. (104) For example, a retrospective cohort study of hospitalized children demonstrated that early oseltamivir treatment was associated with a shorter LOS, reduced odds of readmission, reduced transfer to the ICU, and decreased composite outcome of death/extracorporeal membrane oxygenation use. (105) Findings were consistent among those with and without underlying conditions.
Limitations of this and other large retrospective studies of hospital antiviral use include missing information on potential confounders such as prehospitalization antiviral exposure, duration of illness, and seasonal influenza vaccination status, as well as lack of laboratory confirmation of influenza. An area of much-needed research is evaluation of antiviral effectiveness among hospitalized children and adolescents who are otherwise healthy and in those with more than 2 days of illness duration. Until further evidence is generated, we recommend following the AAP, CDC, and IDSA guidelines regarding treatment for all hospitalized children with influenza regardless of symptom duration.
PREVENTION
Influenza Vaccination
Influenza vaccination is paramount to providing protection against influenza and is recommended for all children older than 6 months. Although influenza vaccination may not avert all clinical illness, it has significant secondary benefit and reduces the risk of influenza illness by 40% to 60%. (106) Of children who become ill with influenza, immunization prevents influenza-associated hospitalization in 16% of those aged 5 to 17 years and 28% aged 6 months to 4 years. Immunization also reduces severity of disease and reduces the risk of influenza-associated death by 51% for high-risk children and 65% for non– high-risk children. (107) Nearly 80% of influenza-related pediatric deaths occur in unvaccinated children. (107)
Types of Available Influenza Vaccines
Each year, the CDC, World Health Organization, and FDA predict which strains are likely to cause illness in the upcoming influenza season and combine 2 influenza A strains and 2 influenza B strains into 1 quadrivalent vaccine. Several licensed vaccine products exist (for more information see the current AAP technical report “Recommendations for Prevention and Control of Influenza in Children, 2022–2023”). (22) Influenza vaccination can be given with either an inactivated influenza vaccine (IIV) or a live attenuated influenza vaccine (LAIV). In 2016–2018, the LAIV was not recommended by the AAP due to concerns about its effectiveness. After new virus strain replacement, studies demonstrated similar effectiveness to IIV, and the AAP updated their guidelines to recommend either LAIV or IIV in 2018. (108)(109)
IIVs are licensed for children older than 6 months and are administered via intramuscular injection. Four IIVs (Afluria ® Quadrivalent [Seqirus, Parkville, Victoria, Australia], Fluarix® Quadrivalent [GlaxoSmithKline Biologicals, Dresden, Germany], FluLaval® Quadrivalent [GlaxoSmithKline Biologicals], and Fluzone® Quadrivalent [Sanofi Pasteur, Swiftwater, PA]) are egg-based and 1 (Flucelvax® Quadrivalent [Seqirus]) is cell culture based. Dosing is age and vaccine specific. IIV is the agent of choice for immunocompromised children, those with high exposure to immunocompromised individuals, and pregnant women. The most common adverse effects are injection site pain (17%–67%), redness, and swelling. (22) Systemically, IIV may cause fatigue, irritability, muscle aches, and gastrointestinal symptoms. Rare adverse effects include GBS and anaphylaxis. (110)(111) Quadrivalent LAIV (FluMist® Quadrivalent, MedImmune, Gaithersburg, MD) is licensed for children 2 years and older and is administered intranasally. The most commonly reported reactions to LAIV are rhinorrhea (20%–75%), headache (2%−46%), fever (up to 26%), wheezing, and myalgias (up to 21%). (22)
Influenza Vaccination Precautions and Contraindications
Precautions for influenza vaccinations include a history of GBS within 6 weeks after influenza vaccine and anaphylaxis to a previous influenza vaccine. Children aged 2 to 4 years with wheezing in the past 12 months or asthma and any child with cochlear implants, asplenia, or cerebrospinal fluid leak should not receive LAIV. Mild illness (with or without fever) is not a contraindication, although influenza immunization may be deferred in children with active COVID-19 until they are no longer moderately to severely ill. Individuals with egg allergy no longer need special consideration and can have any influenza vaccination because they are at no greater risk for anaphylaxis than the general population. (112)
When to Vaccinate
Children should be vaccinated as soon as the seasonal vaccine becomes available. (22) Children younger than 9 years who have not yet had an influenza vaccine should receive 2 doses administered at least 4 weeks apart. For those yet to receive their annual influenza vaccine, vaccination should be offered and recommended through the duration of the influenza season. Although uncommon, children may be infected with a different strain in the same influenza season, and thus a previous influenza infection does not contraindicate vaccination. Both IIV and LAIV can be administered simultaneously with all available COVID-19 vaccines as well as other inactivated or live vaccines. (113)
Influenza Vaccination Advocacy
The AAP provides an influenza tool kit (https://www.aap.org/en/news-room/campaigns-and-toolkits/flu-campaign-toolkit/) containing evidence-based strategies for effective vaccine communication and tools to combat vaccine misinformation. The emergency department and hospital provide another opportunity for influenza education and/or vaccination of children before discharge. (114)
Chemoprophylaxis
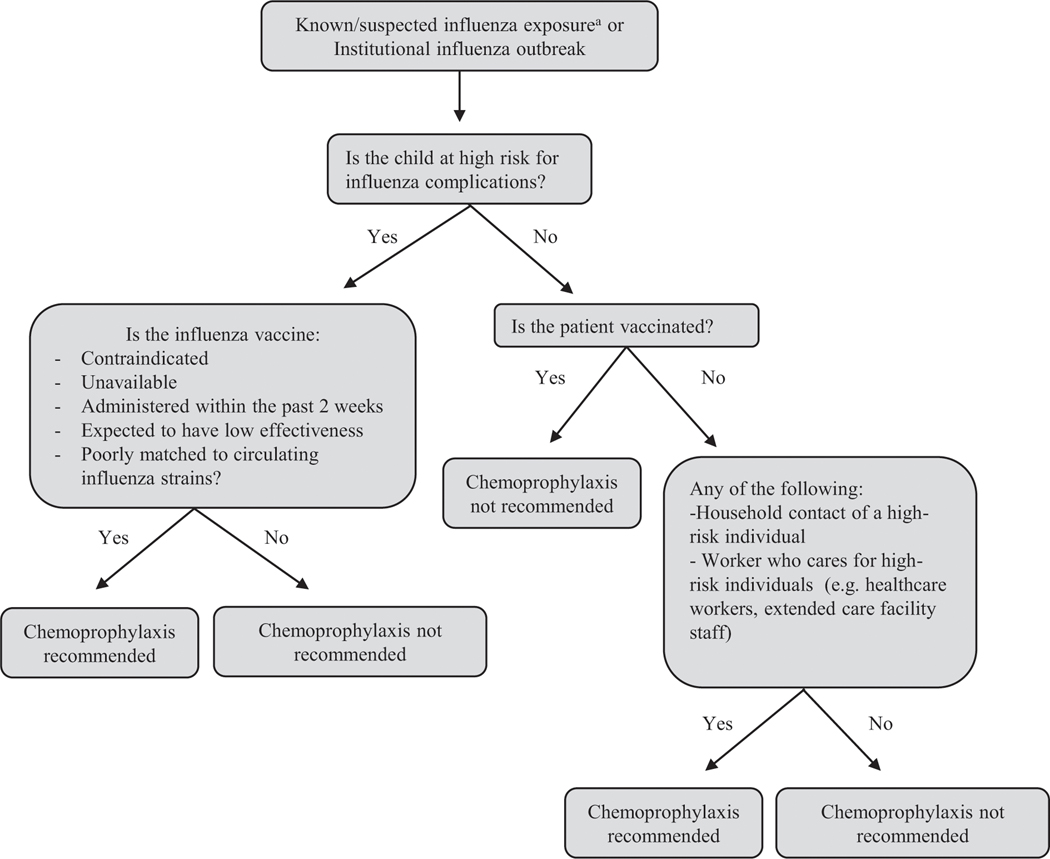
The IDSA and AAP recommend chemoprophylaxis after confirmed or suspected influenza exposure in high-risk children who have yet to receive a seasonal influenza vaccine, immunocompromised high-risk children, those with close contacts who are at high risk for influenza complications, and health-care workers (Fig 4). (22)(47) A randomized controlled trial demonstrated that chemoprophylaxis with oseltamivir had protective efficacy of 84% against household transmission of symptomatic influenza. (115) If a child receiving chemoprophylaxis develops symptoms of an influenza-like illness, clinicians should convert to treatment dosing. Table 5 provides chemoprophylaxis and treatment dosage recommendations. Provider discretion should be used in other situations where prophylaxis may be warranted, such as upcoming travel after exposure. For recommendations on chemoprophylaxis in select populations in the absence of influenza exposure, please reference pages 6 and 7 of the IDSA influenza guidelines. (47)
Figure 4.
Clinical flow diagram for influenza chemoprophylaxis. (22)(46)(47)
aThe Infectious Diseases Society of America defines exposure as living with/caring for a person with influenza or being in a setting with a high chance of contact with respiratory droplets from an infected person.
PREGNANCY AND BREASTFEEDING CONSIDERATIONS
Pregnancy is considered a high-risk condition for the development of influenza complications, and vaccination is highly recommended for pregnant women. (47) Maternal vaccination also has conferred benefits to newborns. Infants born to mothers who received influenza vaccines have a 72% decreased risk of influenza hospitalization in the first month of life. (116) Oseltamivir is the agent of choice for pregnant and breastfeeding women with influenza, and there is robust safety and effectiveness data in this population. (117)
QI project suggestion –
Recent data suggest that 25% of hospitalized children, 42% of high-risk outpatients, and 75% of children younger than 2 years with influenza do not receive evidence-and guideline-based antiviral medications. (118)(119) A quality improvement project could target guideline-concordant treatment of influenza infections in hospitalized children with an aim of increasing appropriate treatment.
Summary.
Influenza is responsible for significant morbidity, mortality, and health-care costs in children, especially high-risk children. (On the basis of strong epidemiologic evidence)
Testing for influenza can be considered for influenza-like illness and should be performed for all hospitalized children with influenza-like illness. (On the basis of consensus and expert opinion)
Antiviral treatment should be administered to eligible children. (On the basis of strong observational evidence and randomized controlled trials)
Influenza vaccination is effective at prevention of influenza and its complications. (On the basis of strong observational evidence and randomized controlled trials)
REQUIREMENTS:
Learners can take Pediatrics in Review quizzes and claim credit online only at: http://pedsinreview.org.
To successfully complete 2023 Pediatrics in Review articles for AMA PRA Category 1 Credit™, learners must demonstrate a minimum performance level of 60% or higher on this assessment. If you score less than 60% on the assessment, you will be given additional opportunities to answer questions until an overall 60% or greater score is achieved.
This journal-based CME activity is available through Dec. 31, 2025, however, credit will be recorded in the year in which the learner completes the quiz.
2023 Pediatrics in Review is approved for a total of 30 Maintenance of Certification (MOC) Part 2 credits by the American Board of Pediatrics (ABP) through the AAP MOC Portfolio Program. Pediatrics in Review subscribers can claim up to 30 ABP MOC Part 2 points upon passing 30 quizzes (and claiming full credit for each quiz) per year. Subscribers can start claiming MOC credits as early as November 2023. To learn how to claim MOC points, go to: https://publications.aap.org/journals/pages/moc-credit.
AUTHOR DISCLOSURE:
Dr Wolf has disclosed no financial relationships relevant to this article. Dr Antoon was supported in part by the National Institute for Allergy and Infectious Diseases of the National Institutes of Health under award number K23 AI168496. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- CDC
Centers for Disease Control and Prevention
- FDA
Food and Drug Administration
- GBS
Guillain-Barré syndrome
- HA
hemagglutinin
- IDSA
Infectious Diseases Society of America
- IIV
inactivated influenza vaccine
- LAIV
live attenuated influenza vaccine
- LOS
length of hospital stay
- NA
neuraminidase
- RT-PCR
reverse transcriptase–polymerase chain reaction
APPENDIX
PIR QUIZ
- A 7-year-old previously healthy boy is brought to the emergency department by his parents due to increased work of breathing and fever. He became febrile 4 days earlier, with malaise, fatigue, myalgias, and cough. Several children at his school have been diagnosed as having influenza. During the past day he has worsened, with an increased respiratory rate, decreased appetite, and elevated oral temperature up to 103.6°F (39.8°C). On physical examination he is illappearing. His temperature is 102.8°F (39.3°C), oxygen saturation is 88% on room air, respiratory rate is 34 breaths/min, and heart rate is 140 beats/min. Crackles are noted in the left lung base. Chest radiography shows a left lower lobe consolidation with a small to moderate parapneumonic effusion. A multiplex polymerase chain reaction nasopharyngeal swab is positive for influenza A. In addition to influenza A, which one of the following is the most likely cause of his consolidative pneumonia?
- Methicillin-susceptible Staphylococcus aureus.
- Methicillin-resistant S aureus.
- Mycoplasma pneumoniae.
- Streptococcus intermedius.
- Streptococcus pneumoniae.
- A 4-year-old fully vaccinated girl with moderate persistent asthma is admitted to the hospital in November. She has a 4-day history of fever, malaise, myalgias, and cough. She has not had vomiting and has been able to maintain hydration. Her 7-year-old brother had a similar illness starting 6 days ago and his symptoms have improved. The community is noted to have a high incidence of influenza A, a low incidence of influenza B, a low incidence of COVID-19, and a low incidence of respiratory syncytial virus. On admission, she is noted to have a respiratory rate of 35 breaths/min, mild subcostal retractions, and bilateral wheezing. Her oxygen saturation is 92% on room air. Which one of the following is most likely to confirm the etiology of her febrile illness?
- Chest radiography.
- Nasopharyngeal antigen.
- Nasopharyngeal polymerase chain reaction assay.
- Throat swab antigen assay.
- Throat swab viral culture.
- For the same patient in question 2, in addition to nebulized albuterol and corticosteroid for her asthma exacerbation, which one of the following is the most appropriate next step in treatment?
- No antiviral treatment is recommended.
- Oseltamivir.
- Ribavirin.
- Rimantadine.
- Zanamivir.
- A 5-year-old boy is seen in the office in March for a 36-hour history of fever, cough, myalgias, and fatigue. A close friend at school was diagnosed as having influenza A by a rapid point-of-care assay this past week, and there is noted to be a high incidence of influenza A in the community. He is not in respiratory distress. He received the influenza vaccine last October, and he has received appropriate influenza vaccination since infancy. The patient has a 5-week-old sister at home. The mother received influenza vaccine during pregnancy, and the father was also vaccinated. A rapid point-of-care assay for the boy is positive for influenza A. Both parents and the sister are currently asymptomatic. Which one of the following is the most appropriate next step in management?
- Antiviral therapy is not indicated.
- Antiviral therapy is not indicated, and the sister should receive the influenza vaccine.
- Begin oseltamivir.
- Begin oseltamivir and have him isolate at home for 14 days from the onset of symptoms.
- Begin peramivir.
- An 18-month-old girl is brought to the clinic by her parents for a health supervision visit in September. Her history is remarkable for 2 episodes of wheezing associated with viral upper respiratory infections during the past 8 months that resolved with outpatient management. Her mom states that there is a family history of egg allergy and that the only time she gave her eggs (a week ago) she vomited. She currently has some nasal congestion but no fever. She has not previously received the influenza vaccine. She is scheduled today to receive her second hepatitis A vaccine, and she is up-to-date with her other vaccinations. Which one of the following immunizations is recommended today?
- Hepatitis A only and defer influenza vaccine for 4 weeks.
- Hepatitis A and inactivated influenza vaccine (IIV) with a second dose of IIV in 4 weeks.
- IIV only with a second dose in 6 months and hepatitis A vaccine in 4 weeks.
- Live attenuated influenza vaccine and hepatitis A vaccine.
- Hepatitis A vaccine but no influenza vaccine until she is tested for egg allergy.
References
- 1.Lafond KE, Nair H, Rasooly MH, et al. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLOS Medicine. 2016;13(3):e1001977. doi: 10.1371/journal.pmed.1001977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tokars JI, Olsen SJ, Reed C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin Infect Dis. 2018;66(10):1511–1518. doi: 10.1093/cid/cix1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Burden of Influenza. Centers for Disease Control and Prevention. Published October 4, 2022. Accessed January 18, 2023. https://www.cdc.gov/flu/about/burden/index.html
- 4.Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207. doi: 10.1016/j.vaccine.2018.04.063 [DOI] [PubMed] [Google Scholar]
- 5.Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132–137. doi: 10.1111/irv.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putri WCWS Muscatello DJ, Stockwell MS Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–3966. doi: 10.1016/j.vaccine.2018.05.057 [DOI] [PubMed] [Google Scholar]
- 7.Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. doi: 10.1038/s41572-018-0002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assaad FA, Bres P, Chi-Ming C, Dowdle WR. A revision of the system of nomenclature for influenza viruses: a WHO Memorandum. Bull World Health Organ. 1980;58(4):585–591. [PMC free article] [PubMed] [Google Scholar]
- 9.Fox TG, Christenson JC. Influenza and Parainfluenza Viral Infections in Children. [DOI] [PubMed] [Google Scholar]
- 10.Bouvier NM, Palese P. THE BIOLOGY OF INFLUENZA VIRUSES. Vaccine. 2008;26(Suppl 4):D49–D53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sederdahl BK, Williams JV. Epidemiology and Clinical Characteristics of Influenza C Virus.Viruses. 2020;12(1):89. doi: 10.3390/v12010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njouom R, Monamele GC, Ermetal B, et al. Detection of Influenza C Virus Infection among Hospitalized Patients, Cameroon. Emerg Infect Dis. 2019;25(3):607–609. doi: 10.3201/eid2503.181213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taubenberger JK, Morens DM. 1918 Influenza: the Mother of All Pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet Infectious Diseases. 2012;12(9):687–695. doi: 10.1016/S1473-3099(12)70121-4 [DOI] [PubMed] [Google Scholar]
- 15.O’Bryant SC, Dongarwar D, Sailhu HM, Gillespie S. Racial and Ethnic Differences of Influenza-Associated Pediatric Hospitalizations and Deaths, 2008–2017. Pediatric Allergy, Immunology, and Pulmonology. 2022;35(2):49–109. [DOI] [PubMed] [Google Scholar]
- 16.O’Halloran AC, Holstein R, Cummings C, et al. Rates of Influenza-Associated Hospitalization, Intensive Care Unit Admission, and In-Hospital Death by Race and Ethnicity in the United States From 2009 to 2019. JAMA Netw Open. 2021;4(8):e2121880. doi: 10.1001/jamanetworkopen.2021.21880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb NS, Dowd-Arrow B, Taylor MG, Burdette AM. Racial/Ethnic Disparities in Influenza Vaccination Coverage Among US Adolescents, 2010–2016. Public Health Rep. 2018;133(6):667–676. doi: 10.1177/0033354918805720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Inequities in Flu Vaccine Uptake. Centers for Disease Control and Prevention. Published October 18, 2022. Accessed February 27, 2023. https://www.cdc.gov/vitalsigns/fluinequities/index.html
- 19.Ag C, Vh CC, J S, Ml P. Racial disparities in influenza immunization during pregnancy in the United States: A narrative review of the evidence for disparities and potential interventions. Vaccine. 2021;39(35). doi: 10.1016/j.vaccine.2021.07.028 [DOI] [PubMed] [Google Scholar]
- 20.Olsen SJ, Winn AK, Budd AP, et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic — United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1013–1019. doi: 10.15585/mmwr.mm7029a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoon JW, Williams DJ, Thurm C, et al. The COVID-19 Pandemic and Changes in Healthcare Utilization for Pediatric Respiratory and Nonrespiratory Illnesses in the United States. J Hosp Med. 2021;16(5):294–297. doi: 10.12788/jhm.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee on Infectious Diseases. Recommendations for Prevention and Control of Influenza in Children, 2022–2023. Pediatrics. 2022;150(4). doi: 10.1542/peds.2022-059275 [DOI] [PubMed] [Google Scholar]
- 23.Price AM, Flannery B, Talbot HK, et al. Influenza Vaccine Effectiveness Against Influenza A(H3N2)-Related Illness in the United States During the 2021–2022 Influenza Season. Clinical Infectious Diseases. Published online December 12, 2022:ciac941. doi: 10.1093/cid/ciac941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC Seasonal Flu Vaccine Effectiveness Studies | CDC. Published December 22, 2022. Accessed January 24, 2023. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
- 25.Rolfes MA, Talbot HK, McLean HQ, et al. Household Transmission of Influenza A Viruses in 2021–2022. JAMA. Published online January 26, 2023. doi: 10.1001/jama.2023.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azziz Baumgartner E, Dao CN, Nasreen S, et al. Seasonality, Timing, and Climate Drivers of Influenza Activity Worldwide. The Journal of Infectious Diseases. 2012;206(6):838–846. doi: 10.1093/infdis/jis467 [DOI] [PubMed] [Google Scholar]
- 27.Perez A, Lively JY, Curns A, et al. Respiratory Virus Surveillance Among Children with Acute Respiratory Illnesses - New Vaccine Surveillance Network, United States, 2016–2021. MMWR Morb Mortal Wkly Rep. 2022;71(40):1253–1259. doi: 10.15585/mmwr.mm7140a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moura FE. Influenza in the tropics. Current Opinion in Infectious Diseases. 2010;23(5):415. doi: 10.1097/QCO.0b013e32833cc955 [DOI] [PubMed] [Google Scholar]
- 29.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza Virus Aerosols in Human Exhaled Breath: Particle Size, Culturability, and Effect of Surgical Masks. PLoS Pathog. 2013;9(3):e1003205. doi: 10.1371/journal.ppat.1003205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein RA, Bridges CB, Kuehnert MJ, Hall CB. Transmission of Influenza: Implications for Control in Health Care Settings. Clinical Infectious Diseases. 2003;37(8):1094–1101. doi: 10.1086/378292 [DOI] [PubMed] [Google Scholar]
- 31.Lau LLH, Cowling BJ, Fang VJ, et al. Viral Shedding and Clinical Illness in Naturally Acquired Influenza Virus Infections. J Infect Dis. 2010;201(10):1509–1516. doi: 10.1086/652241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng S, Lopez R, Kuan G, et al. The Timeline of Influenza Virus Shedding in Children and Adults in a Household Transmission Study of Influenza in Managua, Nicaragua. Pediatr Infect Dis J. 2016;35(5):583–586. doi: 10.1097/INF.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding JE, Kelly HA, Mercer GN, Glass K. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza and Other Respiratory Viruses. 2014;8(2):142–150. doi: 10.1111/irv.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang TK, Lau LLH, Cauchemez S, Cowling BJ. Household Transmission of Influenza Virus. Trends Microbiol. 2016;24(2):123–133. doi: 10.1016/j.tim.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stay Home When You Are Sick | CDC. Published May 6, 2021. Accessed January 19, 2023. https://www.cdc.gov/flu/business/stay-home-when-sick.htm
- 36.Jackson C, Mangtani P, Hawker J, Olowokure B, Vynnycky E. The Effects of School Closures on Influenza Outbreaks and Pandemics: Systematic Review of Simulation Studies. PLoS One. 2014;9(5):e97297. doi: 10.1371/journal.pone.0097297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bin Nafisah S, Alamery AH, Al Nafesa A, Aleid B, Brazanji NA. School closure during novel influenza: A systematic review. Journal of Infection and Public Health. 2018;11(5):657–661. doi: 10.1016/j.jiph.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 38.Hoy G, Kuan G, López R, et al. The Spectrum of Influenza in Children. Clinical Infectious Diseases. Published online September 7, 2022:ciac734. doi: 10.1093/cid/ciac734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Influenza Surveillance: Purpose and Methods | CDC. Published October 19, 2022. Accessed January 20, 2023. https://www.cdc.gov/flu/weekly/overview.htm
- 40.Antoon JW, Potisek NM, Lohr JA. Pediatric Fever of Unknown Origin. Pediatr Rev 2015;36(9):380–390; quiz 391. doi: 10.1542/pir.36-9-380 [DOI] [PubMed] [Google Scholar]
- 41.Cohen JM, Silva ML, Caini S, et al. Striking Similarities in the Presentation and Duration of Illness of Influenza A and B in the Community: A Study Based on Sentinel Surveillance Networks in France and Turkey, 2010–2012. PLoS One. 2015;10(10):e0139431. doi: 10.1371/journal.pone.0139431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen JD, Ross TM. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum Vaccin Immunother. 2018;14(8):1840–1847. doi: 10.1080/21645515.2018.1462639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teutsch SM, Zurynski YA, Nunez C, et al. Ten Years of National Seasonal Surveillance for Severe Complications of Influenza in Australian Children. Pediatr Infect Dis J. 2021;40(3):191–198. doi: 10.1097/INF.0000000000002961 [DOI] [PubMed] [Google Scholar]
- 44.Tran D, Vaudry W, Moore D, et al. Hospitalization for Influenza A Versus B. Pediatrics. 2016;138(3):e20154643. doi: 10.1542/peds.2015-4643 [DOI] [PubMed] [Google Scholar]
- 45.CDC. Information for Health Professionals. Centers for Disease Control and Prevention. Published August 31, 2022. Accessed March 7, 2023. https://www.cdc.gov/flu/professionals/index.htm
- 46.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clinical Infectious Diseases. 2019;68(6):e1–e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawood FS, Fiore A, Kamimoto L, et al. Influenza-associated pneumonia in children hospitalized with laboratory-confirmed influenza, 2003–2008. Pediatr Infect Dis J. 2010;29(7):585–590. doi: 10.1097/inf.0b013e3181d411c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dananché C, Sánchez Picot V, Bénet T, et al. Burden of Influenza in Less Than 5-Year-Old Children Admitted to Hospital with Pneumonia in Developing and Emerging Countries: A Descriptive, Multicenter Study. Am J Trop Med Hyg. 2018;98(6):1805–1810. doi: 10.4269/ajtmh.17-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillet Y, Issartel B, Vanhems P, Fournet JC. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. The Lancet. 2002;359(9308). [DOI] [PubMed] [Google Scholar]
- 50.Dawood FS, Chaves SS, Pérez A, et al. Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003–2010. J Infect Dis. 2014;209(5):686–694. doi: 10.1093/infdis/jit473 [DOI] [PubMed] [Google Scholar]
- 51.Antoon JW, Hall M, Herndon A, et al. Prevalence, Risk Factors, and Outcomes of Influenza-Associated Neurologic Complications in Children. J Pediatr. 2021;239:32–38.e5. doi: 10.1016/j.jpeds.2021.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antoon JW, Williams, Derek J, Bruce Jean, et al. Population-Based Incidence of Influenza-Associated Serious Neuropsychiatric Events in Children. JAMA Pediatrics. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frankl S, Coffin SE, Harrison JB, Swami SK, McGuire JL. Influenza-Associated Neurologic Complications in Hospitalized Children. J Pediatr. 2021;239:24–31.e1. doi: 10.1016/j.jpeds.2021.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB. Reye’s Syndrome in the United States from 1981 through 1997. New England Journal of Medicine. 1999;340(18):1377–1382. doi: 10.1056/NEJM199905063401801 [DOI] [PubMed] [Google Scholar]
- 55.Wei CM, Chen HL, Lee PI, Chen CM, Ma CY, Hwu WL. Reye’s syndrome developing in an infant on treatment of Kawasaki syndrome. J Paediatr Child Health. 2005;41(5–6):303–304. doi: 10.1111/j.1440-1754.2005.00617.x [DOI] [PubMed] [Google Scholar]
- 56.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. International Journal of Cardiology. 2008;130. doi: 10.1016/j.ijcard.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 57.Aykac K, Ozsurekci Y, Kahyaoglu P, et al. Myocarditis associated with influenza infection in five children. Journal of Infection and Public Health. 2018;11(5):698–701. doi: 10.1016/j.jiph.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 58.Guarner J, Paddock CD, Shieh WJ, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43(2):132–140. doi: 10.1086/505122 [DOI] [PubMed] [Google Scholar]
- 59.Henderson FW, Collier AM, Sanyal MA, et al. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982;306(23):1377–1383. doi: 10.1056/NEJM198206103062301 [DOI] [PubMed] [Google Scholar]
- 60.Foster CE, Kaplan SL. Complicated Head and Neck Infections Following Influenza Virus Infection in Children. The Pediatric Infectious Disease Journal. 2019;38(9):e226. doi: 10.1097/INF.0000000000002294 [DOI] [PubMed] [Google Scholar]
- 61.Turan C, Yurtseven A, Cicek C, Keskin G, Saz EU. Benign acute childhood myositis associated with influenza A/B in the paediatric emergency department and the efficacy of early-onset oseltamivir. Journal of Paediatrics and Child Health. 2022;58(6):1022–1027. doi: 10.1111/jpc.15894 [DOI] [PubMed] [Google Scholar]
- 62.Hu JJ, Kao CL, Lee PI, et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect. 2004;37(2):95–98. [PubMed] [Google Scholar]
- 63.You J, Lee J, Park YS, Lee JH. Virus-associated Rhabdomyolysis in Children. Child Kidney Dis. 2017;21(2):89–93. doi: 10.3339/jkspn.2017.21.2.89 [DOI] [Google Scholar]
- 64.Merckx J, Wali R, Schiller I, et al. Diagnostic Accuracy of Novel and Traditional Rapid Tests for Influenza Infection Compared With Reverse Transcriptase Polymerase Chain Reaction. Ann Intern Med. 2017;167(6):394–409. doi: 10.7326/M17-0848 [DOI] [PubMed] [Google Scholar]
- 65.Overview of Influenza Testing Methods | CDC. Published May 6, 2021. Accessed January 23, 2023. https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm
- 66.Grijalva CG, Poehling KA, Edwards KM, et al. Accuracy and Interpretation of Rapid Influenza Tests in Children. Pediatrics. 2007;119(1):e6–e11. doi: 10.1542/peds.2006-1694 [DOI] [PubMed] [Google Scholar]
- 67.Canavaggio P, Boutolleau D, Goulet H, Riou B, Hausfater P. Procalcitonin for clinical decisions on influenza-like illness in emergency department during influenza a(H1N1)2009 pandemic. Biomarkers. 2018;23(1):10–13. doi: 10.1080/1354750X.2016.1276626 [DOI] [PubMed] [Google Scholar]
- 68.Caini S, Kroneman M, Wiegers T, El Guerche Séblain C, Paget J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: A systematic literature review. Influenza Other Respir Viruses. 2018;12(6):780–792. doi: 10.1111/irv.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalvi PS, Singh A, Trivedi HR, Mistry SD, Vyas BR. Adverse drug reaction profile of oseltamivir in children. J Pharmacol Pharmacother. 2011;2(2):100–103. doi: 10.4103/0976-500X.81901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CDC. Influenza (maternal and infant). Centers for Disease Control and Prevention. Published January 12, 2021. Accessed January 19, 2023. https://www.cdc.gov/breastfeeding/breastfeeding-specialcircumstances/maternal-or-infant-illnesses/influenza.html
- 71.Life-Threatening Abnormal Behavior Incidence in 10–19 Year Old Patients Administered Neuraminidase Inhibitors | PLOS ONE. Accessed February 22, 2023. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0129712 [DOI] [PMC free article] [PubMed]
- 72.Kang HR, Lee EK, Kim WJ, Shin JY. Risk of neuropsychiatric adverse events associated with the use of oseltamivir: a nationwide population-based case-crossover study. J Antimicrob Chemother. 2019;74(2):453–461. doi: 10.1093/jac/dky445 [DOI] [PubMed] [Google Scholar]
- 73.Kang HR, Jang SC, Shin JY. Association between oseltamivir use and neuropsychiatric adverse events in influenza patients: a nationwide population-based cohort study. Expert Opin Drug Saf. 2021;20(2):245–253. doi: 10.1080/14740338.2021.1850690 [DOI] [PubMed] [Google Scholar]
- 74.Huh K, Kang M, Shin DH, Hong J, Jung J. Oseltamivir and the Risk of Neuropsychiatric Events: A National, Population-based Study. Clin Infect Dis. 2020;71(9):e409–e414. doi: 10.1093/cid/ciaa055 [DOI] [PubMed] [Google Scholar]
- 75.Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. MedGenMed. 2007;9(4):23. [PMC free article] [PubMed] [Google Scholar]
- 76.Casscells SW, Granger E, Kress AM, Linton A. The association between oseltamivir use and adverse neuropsychiatric outcomes among TRICARE beneficiaries, ages 1 through 21 years diagnosed with influenza. Int J Adolesc Med Health. 2009;21(1):79–89. doi: 10.1515/ijamh.2009.21.1.79 [DOI] [PubMed] [Google Scholar]
- 77.Harrington R, Adimadhyam S, Lee TA, Schumock GT, Antoon JW. The Relationship Between Oseltamivir and Suicide in Pediatric Patients. Ann Fam Med. 2018;16(2):145–148. doi: 10.1370/afm.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su C ping Chan KA, Huang CT Fang CT. Inhaled Zanamivir vs Oral Oseltamivir to Prevent Influenza-related Hospitalization or Death: A Nationwide Population-based Quasi-experimental Study. Clinical Infectious Diseases. 2022;75(8):1273–1279. doi: 10.1093/cid/ciac217 [DOI] [PubMed] [Google Scholar]
- 79.Witcher R, Tracy J, Santos L, Chopra A. Outcomes and Adverse Effects With Peramivir for the Treatment of Influenza H1N1 in Critically Ill Pediatric Patients. The Journal of Pediatric Pharmacology and Therapeutics : JPPT. 2019;24(6):497. doi: 10.5863/1551-6776-24.6.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ng KE. Xofluza (Baloxavir Marboxil) for the Treatment Of Acute Uncomplicated Influenza. Pharmacy and Therapeutics. 2019;44(1):9. [PMC free article] [PubMed] [Google Scholar]
- 81.Baker J, Block SL, Matharu B, et al. Baloxavir Marboxil Single-dose Treatment in Influenza-infected Children. Pediatr Infect Dis J. 2020;39(8):700–705. doi: 10.1097/INF.0000000000002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention (CDC). Update: influenza activity - United States, 2011–12 season and composition of the 2012–13 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2012;61(22):414–420. [PubMed] [Google Scholar]
- 83.Hurt AC, Chotpitayasunondh T, Cox NJ, et al. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. The Lancet Infectious Diseases. 2012;12(3):240–248. doi: 10.1016/S1473-3099(11)70318-8 [DOI] [PubMed] [Google Scholar]
- 84.Holmes EC, Hurt AC, Dobbie Z, Clinch B, Oxford JS, Piedra PA. Understanding the Impact of Resistance to Influenza Antivirals. Clin Microbiol Rev. 2021;34(2):e00224–20. doi: 10.1128/CMR.00224-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clinical Infectious Diseases. 2018;66(10):1492–1500. doi: 10.1093/cid/cix1040 [DOI] [PubMed] [Google Scholar]
- 86.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197 [DOI] [PubMed] [Google Scholar]
- 87.Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–1214. doi: 10.1016/S1473-3099(20)30004-9 [DOI] [PubMed] [Google Scholar]
- 88.Liu JW, Lin SH, Wang LC, Chiu HY, Lee JA. Comparison of Antiviral Agents for Seasonal Influenza Outcomes in Healthy Adults and Children: A Systematic Review and Network Meta-analysis. JAMA Netw Open. 2021;4(8):e2119151. doi: 10.1001/jamanetworkopen.2021.19151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai Z, Zhang L, Yu Q, Liu L, Yang M, Fan K. Early Administration of Oseltamivir Within 48 Hours After Onset of Flulike Symptoms Can Reduce the Risk of Influenza B Virus-Associated Pneumonia in Hospitalized Pediatric Patients with Influenza B Virus Infection. The Pediatric Infectious Disease Journal. 2020;39(2):e20. doi: 10.1097/INF.0000000000002528 [DOI] [PubMed] [Google Scholar]
- 90.Huh K, Kang M, Shin DH, Hong J, Jung J. Oseltamivir and the Risk of Neuropsychiatric Events: A National, Population-based Study. Clinical Infectious Diseases. 2020;71(9):e406–e414. doi: 10.1093/cid/ciaa055 [DOI] [PubMed] [Google Scholar]
- 91.Lee JJ, Smith M, Bankhead C, et al. Oseltamivir and influenza-related complications in children: a retrospective cohort in primary care. Eur Respir J. 2020;56(5):1902246. doi: 10.1183/13993003.02246-2019 [DOI] [PubMed] [Google Scholar]
- 92.Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124(1):170–178. doi: 10.1542/peds.2008-0977 [DOI] [PubMed] [Google Scholar]
- 93.Fielding JE, Kelly HA, Mercer GN, Glass K. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza Other Respir Viruses. 2014;8(2):142–150. doi: 10.1111/irv.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goldstein E, Cowling BJ, O’Hagan JJ, et al. Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect Dis. 2010;10:211. doi: 10.1186/1471-2334-10-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirotsu N, Wada K, Oshitani H. Risk factors of household transmission of pandemic (H1N1) 2009 among patients treated with antivirals: a prospective study at a primary clinic in Japan. PLoS One. 2012;7(2):e31519. doi: 10.1371/journal.pone.0031519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakano T, Shiosakai K. Spread of viral infection to family members from influenza patients treated with a neuraminidase inhibitor. J Infect Chemother. 2014;20(7):401–406. doi: 10.1016/j.jiac.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 97.Fry AM, Goswami D, Nahar K, et al. Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial. Lancet Infect Dis. 2015;15(6):654–662. doi: 10.1016/S14733099(15)70041-1 [DOI] [PubMed] [Google Scholar]
- 98.Hayden FG, Asher J, Cowling BJ, et al. Reducing Influenza Virus Transmission: The Potential Value of Antiviral Treatment. Clin Infect Dis. 2022;74(3):532–540. doi: 10.1093/cid/ciab625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campbell AP, Tokars JI, Reynolds S, et al. Influenza Antiviral Treatment and Length of Stay. Pediatrics. 2021;148(4):e2021050417. doi: 10.1542/peds.2021-050417 [DOI] [PubMed] [Google Scholar]
- 100.Miyakawa R, Barreto NB, Kato RM, Neely MN, Russell CJ. Early Use of Anti-influenza Medications in Hospitalized Children With Tracheostomy. Pediatrics. 2019;143(3):e20182608. doi: 10.1542/peds.2018-2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Louie J, Yang S, Samuel M, Uyeki T. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics. Published online November 2013. doi: 10.1542/peds.2013-2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farias JA, Fernández A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 2010;36(6):1015–1022. doi: 10.1007/s00134-010-1853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30(11):962–966. doi: 10.1097/INF.0b013e318232ede9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katzen J, Kohn R, Houk JL, Ison MG. Early Oseltamivir After Hospital Admission Is Associated With Shortened Hospitalization: A 5-Year Analysis of Oseltamivir Timing and Clinical Outcomes. Clin Infect Dis. 2019;69(1):52–58. doi: 10.1093/cid/ciy860 [DOI] [PubMed] [Google Scholar]
- 105.Walsh PS, Schnadower D, Zhang Y, Ramgopal S, Shah SS, Wilson PM. Association of Early Oseltamivir With Improved Outcomes in Hospitalized Children With Influenza, 2007–2020. JAMA Pediatr. Published online September 19, 2022. doi: 10.1001/jamapediatrics.2022.3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vaccine Effectiveness: How Well Do Flu Vaccines Work? | CDC. Published August 25, 2022. Accessed January 27, 2023. https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
- 107.Flannery B, Reynolds SB, Blanton L, et al. Influenza Vaccine Effectiveness Against Pediatric Deaths: 2010–2014. Pediatrics. 2017;139(5):e20164244. doi: 10.1542/peds.2016-4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.COMMITTEE ON INFECTIOUS DISEASES. Recommendations for Prevention and Control of Influenza in Children, 2021–2022. Pediatrics. 2021;148(4):e2021–053745. doi: 10.1542/peds.2021053745 [DOI] [PubMed] [Google Scholar]
- 109.Buchan SA, Booth S, Scott AN, et al. Effectiveness of Live Attenuated vs Inactivated Influenza Vaccines in Children During the 2012–2013 Through 2015–2016 Influenza Seasons in Alberta, Canada: A Canadian Immunization Research Network (CIRN) Study. JAMA Pediatrics. 2018;172(9):e181514. doi: 10.1001/jamapediatrics.2018.1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.GBS (Guillain-Barré Syndrome) and Vaccines | Vaccine Safety | CDC. Published February 6, 2023. Accessed March 7, 2023. https://www.cdc.gov/vaccinesafety/concerns/guillain-barre-syndrome.html
- 111.McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelso JM, Greenhawt MJ, Li JT, Joint Task Force on Practice Parameters (JTFPP). Update on influenza vaccination of egg allergic patients. Ann Allergy Asthma Immunol. 2013;111(4):301–302. doi: 10.1016/j.anai.2013.07.030 [DOI] [PubMed] [Google Scholar]
- 113.Lazarus R, Baos S, Cappel-Porter H, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. The Lancet. 2021;398(10318):2277–2287. doi: 10.1016/S0140-6736(21)02329-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45(3):191–195. doi: 10.1016/j.jcv.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of Oseltamivir in Preventing Influenza in Household ContactsA Randomized Controlled Trial. JAMA. 2001;285(6):748–754. doi: 10.1001/jama.285.6.748 [DOI] [PubMed] [Google Scholar]
- 116.Omer SB, Clark DR, Aqil AR, et al. Maternal Influenza Immunization and Prevention of Severe Clinical Pneumonia in Young Infants: Analysis of Randomized Controlled Trials Conducted in Nepal, Mali and South Africa. Pediatr Infect Dis J. 2018;37(5):436–440. doi: 10.1097/INF.0000000000001914 [DOI] [PubMed] [Google Scholar]
- 117.Beigi RH, Venkataramanan R, Caritis SN. Oseltamivir for influenza in pregnancy. Semin Perinatol. 2014;38(8):503–507. doi: 10.1053/j.semperi.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Antoon JW, Hall M, Feinstein JA, et al. Guideline-Concordant Antiviral Treatment in Children at High Risk for Influenza Complications. Clinical Infectious Diseases. Published online July 22, 2022:ciac606. doi: 10.1093/cid/ciac606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walsh PS, Schnadower D, Zhang Y, Ramgopal S, Shah SS, Wilson PM. Assessment of Temporal Patterns and Patient Factors Associated With Oseltamivir Administration in Children Hospitalized With Influenza, 2007–2020. JAMA Network Open. 2022;5(9):e2233027. doi: 10.1001/jamanetworkopen.2022.33027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. Published online December 7, 2016:i6258. doi: 10.1136/bmj.i6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guide for considering influenza testing when influenza viruses are circulating in the community | CDC. Published May 6, 2021. Accessed January 24, 2023. https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm