Abstract
Migraine is a common neurological disease displaying an unusual dependence on age. For most patients, the peak intensity of migraine headaches occurs in 20s and lasts until 40s, but then headache attacks become less intense, occur less frequently and the disease is more responsive to therapy. This relationship is valid in both females and males, although the prevalence of migraine in the former is 2-4 times greater than the latter. Recent concepts present migraine not only as a pathological event, but rather as a part of evolutionary adaptive response to protect organism against consequences of stress-induced brain energy deficit. However, these concepts do not fully explain that unusual dependence of migraine prevalence on age. Many aspects of aging, both molecular/cellular and social/cognitive, are interwound in migraine pathogenesis, but they neither explain why only some persons are affected by migraine, nor suggest any causal relationship. In this narrative/hypothesis review we present information on associations of migraine with chronological aging, brain aging, cellular senescence, stem cell exhaustion as well as social, cognitive, epigenetic, and metabolic aging. We also underline the role of oxidative stress in these associations. We hypothesize that migraine affects only individuals who have inborn, genetic/epigenetic, or acquired (traumas, shocks or complexes) migraine predispositions. These predispositions weakly depend on age and affected individuals are more prone to migraine triggers than others. Although the triggers can be related to many aspects of aging, social aging may play a particularly important role as the prevalence of its associated stress has a similar age-dependence as the prevalence of migraine. Moreover, social aging was shown to be associated with oxidative stress, important in many aspects of aging. In perspective, molecular mechanisms underlying social aging should be further explored and related to migraine with a closer association with migraine predisposition and difference in prevalence by sex.
Keywords: migraine, chronological aging, biological aging, brain aging, social aging, cognitive aging, metabolic aging, molecular markers of aging, cellular senescence, stem cell exhaustion
1. Introduction
The prevalence of most human disorders increases with chronological age, but several diseases, including some kinds of cancer, peak in juvenile and adolescence [1, 2]. This supports the thesis that true aging rate is associated with biological rather than chronological aging [3]. Moreover, the occurrence of certain diseases at young age suggests that biological aging may not only differ from its chronological counterpart, but also different organs and tissues may age at different rates. These organ-specific rates of aging, along with other aspects of aging, contribute to complete aging-related characteristic of an individual called “ageotype” [4].
Migraine is a complex, chronic neurological disease with largely unknown mechanisms of pathogenesis. This gap in knowledge results from a restricted accessibility to the live human brain and limited value of animal models of human migraine [5]. Although targeting calcitonin gene related peptide (CGRP) and its receptor with antibodies and antagonists revolutionized migraine therapy, some questions on the long-term application of anti-CGRP treatment, especially in children, adolescent and the elderly are still open [6]. However, the basic question is why some patients do not response to anti-CGRP therapy. Equally important is the question why some people develop migraine and others do not.
Clinical picture of migraine dependence on age is unusual as the disease prevalence peaks between the ages of 20 and 40 years and the intensity of headache attacks decreases and responsiveness to therapy increases with age [7]. Migraine is the mean reason of disability in young women [8]. Therefore, migraine has the greatest effect when people are professionally and socially most active [9]. Therefore, it is important to understand how specific aspects of aging may influence migraine pathogenesis and vice versa - how migraine may influence the process of aging.
Aging leads to progressive loss of homeostasis, which is at the heart of age-related health outcomes [11]. Age-related loss of homeostasis is underlined by cellular and molecular processes also known as molecular hallmarks of aging [12]. They can be divided into three categories: primary hallmarks (genomic instability, telomere erosion, changes in the epigenetic profile, and loss of proteostasis), antagonistic hallmarks (deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence), and integrative hallmarks (stem cell depletion, and altered intercellular communication). However, this set of markers is not sufficient to explain all aspects of aging and its consequences [13]. In a 2022 research symposium “New Hallmarks of Ageing” held in Copenhagen, Denmark, these hallmarks were revised and supplemented by five new ones [10] (Fig. 1). They are compromised autophagy, microbiome disturbance, altered mechanical properties, splicing dysregulation and inflammation. It is suggested that these hallmarks should be considered in an integrative form, instead of being analyzed individually to help to assess biological aging. However, we are surprised that there is not a metabolic marker among these markers. We will address that problem in subsequent paragraphs.
Figure 1.
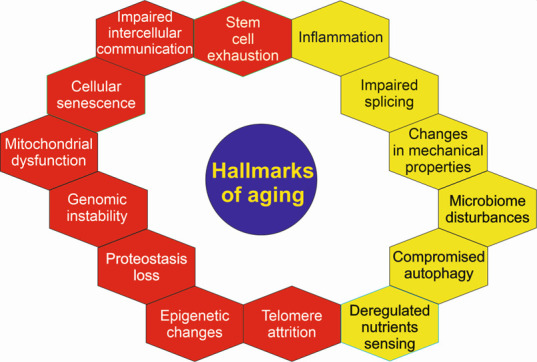
Original (red) and new (yellow) molecular hallmarks of aging. These hallmarks were adopted by international panels of experts and serve to identify targets in anti-aging strategies that provide precise mechanisms of aging [10]. This set of hallmarks is not complete and many of them overlap, e.g., genomic instability can be mechanistically linked to all remaining hallmarks, like epigenetic changes. Therefore, they may be considered as non-specific indicators of age as well as factors contributing to both physiological and premature aging.
Many reports address aspects of aging that are not directly linked with biological markers of aging. They are usually divided into psychological and social aging. The following social determinants (hallmarks) of aging are considered: low socioeconomic status, minority status, adverse life events, adverse psychological states, and adverse behaviors [14]. Like biological hallmarks, these determinants form a network of integrated and mutually dependent features that collectively characterize the process of social aging. The concept of cognitive aging has been developed to describe a process of gradual, longitudinal changes in cognitive functions that accompany the aging process [15]. The term metabolic aging may be understood as progressive decline in metabolic rate with age and integrates some aspects of biological hallmarks of aging, first deregulation of nutrients sensing, mitochondrial impairment and declined proteostasis. All molecular hallmarks of aging and above-mentioned aspects of aging depend on genetic constitution and epigenetic profile. However, the genome sequence changes slowly, if at all, in response to environmental changes and thus epigenetic aging and epigenetic clocks are considered [16-18].
In this narrative review/perspective we present some general aspects of migraine and its connections with brain aging, hallmarks of biological aging: cellular senescence, epigenetic alterations, and inflammation as well as cognitive, social, and metabolic aging. As oxidative stress is involved in almost all, if not all, of these phenomena, its role in migraine in the context of aging is also included. Some potential implications of these connections for migraine management, especially in the elderly, are also presented.
2. Migraine
Migraine is a neurological disorder featured by throbbing or pulsing headache attacks and such syndromes such as vomiting, nausea, hypersensitivity to noise, light and other environmental stimuli as well as mood changes from depression to euphoria [19].
Migraine affects over one billion individuals worldwide with the highest prevalence in young adults and is a serious burden for affected individuals and societies (reviewed in [5]) (Fig. 2). Annual loss of productivity due to migraine prevalence in the United States is estimated for more than USD 20 billion [20]. It is the second cause of disability worldwide [8].
Figure 2.
Years lived with disability (YLD) caused by headaches in 2019 according to WHO (both sexes, all ages; https://vizhub.healthdata.org/gbd-compare/). The numbers below the colored scale correspond to YLDs per 100,000; inset - illustrative migraine prevalence by age and sex.
Migraine prevalence in women is 2-3 times higher than in men and this difference has not been rationally explained yet, although some studies suggest that hormonal or X-linked components as well as some mitochondrial pathways can contribute to migraine pathogenesis (reviewed in [21]). Accordingly, migraine is the first cause of disability among young women [8]. It is arbitrary divided into two forms: episodic migraine when the number of days of headache attacks is less than 15 per month or chronic migraine when the attacks occur for 15 or more days per month [22]. Episodic migraine may transform into chronic migraine in the process of chronification [23]. There are more classifications of migraine, including its categorization into migraine with or without aura - sensory disturbances, such as flashes of light, blind spots, and other vision changes or tingling in hand or face [24]. The clinical pictures of migraine with and without aura are so diverse that some researchers suggest considering them as two different entities [25, 26].
The prevalence of migraine in the elderly (above 60 years old) is less than in younger individuals with both incidence and prevalence decreasing with age [27]. The clinical phenotype and symptoms of migraine in the elderly are different from that observed in younger patients, e.g., headaches are bilateral rather than hemispheric. Treatment options are complicated due to many comorbidities and polypharmacy in this group of migraine patients. In addition, there are much fewer publications on migraine in the elderly than in younger patients.
Migraine is a complex disease with both environmental/lifestyle and genetic/epigenetic factors involved in its pathogenesis. Genetic studies performed on rare monogenic migraine subtypes, including familial hemiplegic migraine, identified causal mutations in the calcium voltage-gated channel subunit alpha1 A (CACNA1A), ATPase Na+/K+ transporting subunit alpha 2 (ATP1A2) and sodium voltage-gated channel alpha subunit 1 (SCN1A) genes that are involved in transport of ions at synapses and glutamatergic transmission (reviewed in [21]). A recent study by the International Headache Genetics Consortium (IHGC) with over 100 000 migraine patients identified 123 loci that conferred risk for development of migraine [28]. These loci contain variants of genes implicated in vascular function, neurotransmission at glutamatergic synapses, synapse development and plasticity, nitric oxide signaling, and metalloproteinases activity [29]. In addition, variants of genes encoding effective therapeutic targets in migraine: CGRP and 5-hydroxytryptamine (serotonin) receptor 1F (5HT-1F), were associated with migraine. Environmental/lifestyle factors of migraine pathogenesis, which are considered as the disease triggers, are stress or subsequent relaxation, fasting or skipping a meal, too much or too little sleep, ovarian hormone changes, including menstruation and oral contraception, weather changes, physical exercise, alcohol, odors, including cigarette smoke, intense light and noise as well as many diet components [30, 31].
Although precise mechanisms of migraine pathogenesis are not known, it is accepted that the trigeminal nerve, the main sensory nerve innervating the head, plays a key role [32]. The sensitization of trigeminal ganglion (TG) neurons is involved in migraine and other primary headache disorders, but the type of cells and pattern of expression of genes implicated in migraine pathogenesis are largely unknown. Recently, an integrated single-cell transcriptomics and epigenomics analysis identified mouse and human neuronal and non-neuronal TG cell types that were transcriptionally active in migraine [33]. The non-neural cells were satellite glia, myelinating and non-myelinating Schwann cells, fibroblasts, immune cells, and vascular endothelial cells. Also, TG cell types that were implicated in migraine by human genetic variation were identified. The authors compared the atlases of transcriptionally active cells within TG from human and mice, indicating some important differences between them, including the expression of the calcitonin related polypeptide alpha (CALCA) gene encoding CGRP in pruriceptors in humans and lack of such expression in mice. Targeting CGRP and its receptor by antibodies and antagonists revolutionized migraine treatment [34]. This and other differences should be considered in the use of a mouse model of human migraine.
Migraine prevalence with age displays somehow unusual age dependence as it peaks between the ages of 20 and 40 years, both in women and men [35]. Therefore, it is rational to take a closer look at the dependence of migraine prevalence and onset on age.
3. Chronological aging
Age-specific clinical differences in migraineurs were shown in a large, population-based study [36]. A decrease with age was shown in such variables as stress as a migraine trigger, photophobia, phonophobia, dizziness, throbbing, pressure, stabbing, and being forced to sleep or rest with headache. On the other hand, an increase with age were seen in alcohol, smoke and neck pain triggers, neck pain location, and running of the nose/tearing of the eyes. Older adults reported less intense migraine symptoms.
Although migraine in the elderly is a major neurological disease, its prevalence tends to decrease with age (reviewed in [27]). The phenotype of migraine cases in the elderly is also different from that in younger patients - migraine occurs more frequently in the bilateral form in the former than in the latter. This provokes the question on the role of aging in migraine pathogenesis and possible influence of migraine on the process of biological aging. The answer to these questions is especially important in the context of the worldwide increase in life expectancy.
As mentioned, the average prevalence of migraine in women is 2-3 times greater than in men. However, the percentage of males with migraine decreased in the course from childhood to adulthood. Moreover, some migraine features in females, such as duration of headache and unilateral pain as well as aura-associated symptoms, such as noise and light sensitivity, increased with age [37]. Sensory sensitivities and nausea and vomiting were reported to reduce in older patients, while autonomic symptoms, such as facial flush and tachycardia were observed to increase in that group of patients [27]. These and other differences indicate the need of different therapeutic strategies for women, children, young adults, and the elderly. However, the question about the significance of true aging in migraine remains unanswered.
Late-life migraine accompaniments (LLMA) were for the first time described in older adults mimicking transient neurological episodes and other serious conditions [38]. LLMA are observed in migraine with aura but without headaches (silent migraine). Visual symptoms dominate in clinical presentation of LLMA along with sensory, aphasic, and motor indicators. This is another example illustrating a complex relationship between migraine and aging.
4. Brain aging
The brain is particularly sensitive to aging, but some elderly individuals show an exceptionally high intellectual ability, despite physical impairment typical for their chronological age. Therefore, the brain may be an example of not only differences between chronological and biological aging, but also organ-specific aging. The term “normal aging” or “healthy aging” is also applied to the brain, suggesting that it may age without any disease sign, but recent research suggests that the aged brain is a pathological state per se (reviewed in [39]). On average, ten percent of individuals at 65 years or older displayed age-related pathological changes that can be linked to Alzheimer disease (AD) [40]. Brain aging alone may impair inductive reasoning, spatial orientation, verbal memory and affect prognosis in stroke [41, 42].
Morphologically, brain aging is primarily characterized by brain volume loss, cortical thinning, white matter degradation, loss of gyrification, and ventricular enlargement. From the pathophysiological point of view, brain aging is linked with shrinking of neurons, dendritic degeneration, demyelination, small vessel disease, decreased metabolic rate, microglial activation, gray and white matter volume changes and white matter lesions (reviewed in [43]). On the cellular level, glial cells, astrocytes and microglia, play a major role in brain aging mechanisms. Astrocytes support nutrients intake, ion transport, exert an anti-inflammatory effect and assist integrity of the blood-brain barrier (BBB) [44]. However, these features of astrocytes are weakened and lost during aging and instead these cells release toxic factors killing neurons and oligodendrocytes [45]. Microglia are thought as a leader of white matter aging, acting as a modulator of diverse glial cells and phagocytosing white matter-derived myelin debris (reviewed in [39]). Single-cell RNA sequencing from white and gray matter allowed for the identification of white matter-associated microglia (WAMs), which partly bear disease-associated microglia (DAM) gene signature and are characterized by the activation of genes involved in phagocytic activity and lipid metabolism [46]. WAMs may be a kind of protective response needed to remove degenerated myelin accumulating during brain aging and disease. However, aging microglia show impaired phagocytic function causing accumulation of lipid metabolites and further degeneration of myelin sheath.
This is not the purpose of this work to explore general aspects of brain aging - this subject is presented in several excellent recent reviews, e.g., [39, 43, 47]. We try to relate some aspects of migraine pathogenesis to brain aging hallmarks. However, an association of migraine with an impairment in cognitive ability linked with aging brain, will be here considered in a broader context of cognitive and social aging in further sections.
As indicated, brain aging is associated with brain lesions, especially white matter damage [43]. For a long time, migraine has been considered not to have long-term consequences to the brain. However, several studies suggest that this may not be a universal case, but there are some conflicting results. An association between migraine occurrence and brain lesions risk was observed in a cross-sectional magnetic resonance imaging (MRI) study in an already existing population-based sample of adults with migraine and controls with not a headache history, the Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis (CAMERA) study [48]. Migraineurs with aura were reported to predominantly have such lesions as subclinical infarcts in the posterior circulation. Migraineurs had brainstem hyperintense lesions and female migraineurs were at increased risk of white matter lesions. However, the CAMERA-2 study found no association between deep WML load and change in cognitive features [49]. Furthermore, longitudinal studies showed no association between a history of migraine and an increased risk of dementia. Several subsequent works reported an association between migraine and brain lesions, but they neither showed nor suggested brain aging to mediate these changes [50]. Instead, several other reasons of brain lesions, especially in white matter, are suggested, including ischemia, neuroinflammation and endothelial dysfunction in the generation of spreading depolarization [51]. Therefore, it is difficult to assess how migraine is affected by brain aging and vice-versa and this is an important question which should be addressed in further research.
5. Social aging
Social aging is a term used to describe changes across the lifespan in social interactions and behavior in group-living organisms to cope with socio-environmental challenges [52]. The changes across the lifespan, although relevant to migraine, are difficult to analyze in migraine patients. Therefore, lifelong risk factors for accelerating aging are usually analyzed for their association with migraine. In humans, the social hallmarks of aging are low lifetime socioeconomic status, adversity in childhood and adulthood, being a member of a minority group, adverse health behaviors, and adverse psychological states [14]. Such hallmarks of aging were investigated as potential risk factors in migraine.
Low socioeconomic status (SES) was recognized as a risk factor in migraine and a factor aggravating its course. First of all, low SES can hinder and delay patient access to migraine treatment and result in poorer outcomes as shown in the Chronic Migraine Epidemiology and Outcomes Study [53]. An analysis of data from the American Migraine Prevalence and Prevention Study showed that both women and men with lower household (HH) income, reliably reported a higher symptom severity during acute migraine attacks, greater interictal symptom burden, and more serious functional impairment from their migraine symptoms [54]. The observed differences were not explained by race and other confounders. A higher migraine incidence rate at the lower HH income for both females and males were observed. An increase in prevalence with decreased HH income is typical not only for migraine. However, migraine remission rates did not depend on HH income. The obtained results agree with the social causation hypothesis [55]. Once initiated, migraine remission is independent of HH income. Therefore, onset and remission of migraine may have etiologically distinct causes. Although HH income not always positively correlates with SES, it can be considered as its good hallmark.
What has SES to do with aging? This is somehow puzzling as SES may be determined in an objective or a subjective way. In childhood and adolescence SES usually strongly depends on parents. It was shown that HH income did not have any significant effects on migraine prevalence in adolescents with family history of migraine [56]. The authors interpreted such results because of higher biological predisposition to migraine that might overwhelm any influence of HH income. This conception was confirmed by the results showing a positive correlation between migraine prevalence and HH income in adolescents without a family history of migraine. Altogether those results suggest social causation rather than social selection and indicate the need to study environmental risk factors related to HH income and migraine.
SES plays an important role in the migraine treatment and prevention, especially with anti-CGRP drugs that are still relatively expensive. However, it should be noted that migraine may influence SES, especially in education and employment. Also, migraine risk factors, such as smoking, or alcohol drinking may affect SES. On average, there is a dependence of SES on chronological aging, but more importantly some reports claim that it may influence the rate of biological aging [57, 58]. Again, on average, SES increases with age until retirement, but in many cases high professional activity and the highest income in gained around 40s. Therefore, time dependences of SES and migraine might have something in common.
If we assume that SES is timely correlated with the prevalence of migraine, the difference between sexes requires further explanations. An increased risk of migraine for low educated, retired, and unemployed individuals as well as smokers was shown in a large population twin-based Danish study [59]. Men displayed a higher migraine risk when they performed a heavy physical work, overused alcohol or were overweighted or obese (BMI > 25). A decreased risk of migraine for men compared to women was observed in individuals who were low educated, unemployed or studying. Using the data from the Korean Headache Survey, an influence of SES, especially living in rural areas, on migraine prevalence was shown in women, but not in men [60]. That study extended on Asia our knowledge on the role of SES on migraine prevalence, as majority of such studies were performed in Europe and the US. It also provided similar results for tension-type headache (TTH). It was observed that social determinants of health were associated with migraine prevalence both in men and women, but physical activity as an intervention factor in migraine seemed to be especially significant in women [61]. It was observed in a large cohort study, the Women Health Study, that women with low SES, expressed as a low HH income and a low measure of education, had an increased risk of all types of headaches, including migraine headache [62].
There are other aspects of social aging that are reported to be associated with migraine. It was hypothesized that insecure attachment was overexpressed in migraine patients and these patients had less social support as compared with general population [63]. This hypothesis was verified in a study with 101 consecutive patients evaluated with a battery of tests and questionnaires. An overrepresentation of insecure attachment styles was observed in migraine patients who had less social support than the general population, both in the fraction providing support and the level of satisfaction. However, aging was not considered as a confounding factor in that study, but the results confirm a possible role of social support in migraine pathogenesis. In a cross-sectional study with 37 migraine patients and 40 controls, it was shown that social support scores were negatively correlated with depression and anxiety scores. Multidimensional Scale of Perceived Social Support score was lower in migraine patients than controls. These studies suggest that social support may play a role in decreasing migraine attacks as it was found to affect the anxiety and depression scores of patients with migraine. In a similar study, assessing the role of work social support in migraine among 607 Chinese bank workers, it was shown that high social support in workplace was associated with a 74% reduction of chance to be affected by migraine [64]. Co-worker support had the greatest protective effect against migraine among six aspects of workplace support. Studies on work-associated and migraine performed in 94 consecutive subjects recruited in the outpatient clinic in Spain revealed that the MIDAS (Migraine Disability Assessment) score for both chronic and episodic migraine was inversely proportional to personal accomplishment at work [65]. Research on 900 nursing staffers in a tertiary medical center in Taiwan showed that 222 had migraine, 104 - tension headache, 37 - mixed migraine and tension headache, and 11 had other forms of headaches [66]. Headache sufferers had more stress at work than non-headache sufferers. The youngest and least experienced of the nursing staff, the unmarried, and those with a lower level of education had a higher level of stress, which illustrates an important role of social aging in migraine. Studies on 1076 workers from companies operating in different sectors showed that almost half of them suffered from headaches, which were associated with female gender, recent bereavement, intrusive leadership, and sleep problems [67]. A large cohort Brazilian study showed that migraine was associated with low job control, high job demands and low social support [68]. Job control was more strongly associated with migraine in women. In summary, stress at work is often reported to be associated with migraine, supporting the thesis that social aging may be an important contributor to migraine pathogenesis, but most of these works did not consider chronological aging as a confounding factor. Retirement is a period in life when an individual is relieved from stress work but may face other kinds of stress. Studies on the French GAZEL cohorts showed that statutory retirement was followed by improved sleep and lower rates of self-rated suboptimal health [69, 70]. An 11-13% reduction in headache prevalence was observed during pre- and postretirement in a study with nearly 16 thousand participants [70]. However, the maximal reduction in headache, 46%, was observed during the retirement transition. In absolute terms, such reduction was greater among persons with high work stress or persons with stress-prone personality than other participants [71]. Therefore, reduction of migraine prevalence with retirement supports thesis that social aging may be important in migraine pathogenesis and explains, at least in part, why migraine prevalence decreases with chronological aging.
In summary, social aging may influence onset and the course of migraine but it has not any effect on the disease remission. Different socioeconomic status of women and men as well as difference in the work and lifestyle, may contribute to differences in migraine prevalence between women and men. Biological susceptibility to migraine may overwhelm the effect of social aging in migraine pathogenesis.
6. Stem cell exhaustion and cellular senescence
Stem cells play an important role in organismal aging and stem cell exhaustion is a well-established molecular marker of aging [10]. Resident stem cells can be found in the CNS within specific niches in which they maintain their capacity to renew the populations of neurons, astrocytes and oligodendrocytes [72]. Endothelial progenitor cells (EPCs) are of interest to study in migraine due to its association with an increased risk of cardiovascular and cerebrovascular diseases [73, 74]. The number of circulating EPCs is a surrogate biological marker for endothelial function [75].
Cellular senescence was originally restricted to an irreversible inhibition of cellular division, but now it is seen as a process associated with numerous changes in the affected cell and its vicinity, including other cells. Besides replicative senescence, resulting from the replication end problem, senescence may be prematurely induced by a stress that may also affect stem cells, both during producing progenitors and in their resting state in a niche. The relationship between stem cell exhaustion and cellular senescence is complex, may be mediated by many factors and is not completely known (reviewed in [76]). Besides a simple implication that cellular senescence contributes to stem cell exhaustion, both phenomena likely interplay to drive the process of aging [77]. Senescent cells develop the senescence-associated secretory phenotype (SASP) featured by the overproduction of growth factors, proteases, pro-inflammatory cytokines and extracellular vesicles, which may act in autocrine or paracrine manner [78]. Cellular senescence can contribute to physiological aging, but its connection with pathological aging and diseases, although presented in many reports, is not fully known [16, 79]. The presence of a small fraction of senescent cells in early life is favorable for further development and homeostasis, but accumulation of senescent cells in advanced age may be detrimental [80].
The active products released by senescent cells may induce low-grade inflammation important in the pathogenesis of migraine [81, 82]. A decreased number and reduced functionality of circulating EPCs was observed in migraine patients with and without aura as compared with subjects with TTH and without headaches [83]. Regression analysis showed that migraine was a predictor for EPCs number. Functionally, EPCs from migraine patients displayed decreased migratory capacity and increased cellular senescence compared with EPCs from TTH or subjects without headaches. Therefore, migraine may be associated with a decrease in the stem cells population needed to replenish cells that lost their functionality. These results were generally confirmed and extended in a subsequent study in which a reduced number of EPCs was observed in episodic migraine patients both during headache attacks and in interictal periods [84]. That study also showed that the number of EPCs decreased as migraine progressed in time.
These studies also suggest therapeutic use of stem cells in migraine. ClinicalTrails.gov provides information on one clinical trial with autologous mesenchymal stem cell preparation for treatment of refractory migraine (https://clinicaltrials.gov/ct2/show/NCT04064879?term=stem+cell&cond=Migraine&draw=2&rank=1). The rationale for the use of mesenchymal stem cells to counteract reduced number of endothelial cells was that the former might differentiate into the latter and both kinds of cell might crosstalk in tissue regeneration [85, 86]. However, this phase I study started in August 2018, has been suspended. Prior to that study, it was shown that autologous adipose-derived stromal vascular fraction, rich in mesenchymal stromal cells, injected in patients with chronic refractory migraine improved their state [87]. Seven out of nine migraine patients showed an improvement in MIDAS score, but only in two of them such improvement was significant. The authors explained their results by the potential of mesenchymal stromal cells to target migraine-related neurogenic inflammation, but that speculation requires further research. That study was some kind of extension of an earlier study showing an improvement in a few women suffering from migraine and TTH after administration of stromal vascular fraction [88].
The involvement of CGRP, a key target in migraine therapy, in stem cell maintenance and functions was reported in many studies (e.g., [89-91]), but results of these studies cannot be directly related to migraine. In general, many reports show a positive role of CGRP in stem cell maintenance, first of all through the management of the stem cell niche [92-94]. This may be related to migraine as a protective mechanism against stress-related energy deficit in the brain and antioxidant properties of CGRP that will be discussed further [95].
SASP may induce signaling pathways that produce reactive oxygen and nitrogen species (RONS) [96]. DNA damage response (DDR), an evolutionary developed cellular reaction to DNA damage, induces cellular senescence to stop cell cycle with highly damaged DNA [97]. DNA damage may be considered as a prototype to induce cellular senescence. The efficacy of DDR decreases with age, which is important in the pathogenesis of many diseases, including cancer [98]. Recently we suggested that DDR may be compromised in migraine, but further studies including chronological age as a confounding factor are needed to address that issue [99].
In summary, vascular aspects of migraine may be associated with exhaustion of endothelial stem cells with aging and progenitors of mesenchymal stem cells may target neurogenic inflammation associated with migraine. A low-grade inflammation may be induced by senescence associated secretory phenotype along with oxidative stress and CGRP may be involved in stem cell maintenance by counteracting oxidative stress. Therefore, migraine may correlate with stem cell exhaustion and cellular senescence increasing with aging.
7. Epigenetic aging
Epigenetic regulation of gene expression finally decides about the phenotype of a cell or organism. It includes DNA methylation, posttranslational histone modifications and the action of non-coding RNAs. The epigenetic pattern of the genome (epigenome) is partly perpetuated from one generation to the next, but this process is not so precise and tightly regulated as DNA replication. The epigenetic profile or epigenome, understood as the complete set of its epigenetic modifications and associated non-coding RNAs, changes with age, but at present the precise nature and the course of these changes are not known [18]. A partial explanation of such change may be an observation that the activity of enzymes responsible for the maintenance of the profile declines with age, but such activity may depend on the expression of genes encoding these enzymes, which in turn, depends on their epigenetic profile.
Historically, epigenetic aging is a term that was used to correlate changes in DNA methylation with chronological or biological aging [100]. It was observed that the number of the CpG dinucleotides in CpG islands with methylated cytosine changed with age [100-102]. Consequently, the term “epigenetic clock” was introduced to predict the chronological age of an individual from the methylation status of CpG dinucleotides and accordingly the term “epigenetic aging” was also introduced. Currently, many CpG sites in various tissues are considered to correlate with biological rather than chronological aging (reviewed in [17]). It is not the purpose of this review to discuss aspects of epigenetic clocks and epigenetic aging, especially since we think that these terms are somehow overused as they are limited to DNA methylation, which is a single component of the epigenetic profile. Moreover, association of DNA methylation with brain age may not be so strong as it is reported when properly adjusted for confounding factors. Over four hundred thousand CpG sites were interrogated across the genome of postmortem brain from 740 individuals aged 66-108 years [103]. An association of DNA methylation with aging was observed in 8156 CpG sites, but it decreased to 4263 after adjustment for sex, six common age-related neuropathologies, and cell type mixing proportion. Moreover, the number of methylated sites in DNA may not be a measure of the importance of methylation in aging as it is not known whether aging is a mass effect of many small changes.
Migraine chronification was shown to accompany changes in DNA methylation profile in an 11-year retrospective, genome-wide association study (GWAS) [104]. The most pronounced changes occurred in the SH2 domain containing 5 (SH2D5) and neuronal pentraxin 2 (NPTX2) genes. These results were confirmed in a subsequent, case-control study [105]. The products of both genes are involved in the control of synaptic plasticity, a process that is important for migraine pathogenesis [106]. SH2D5 does so through its involvement in the inhibition of excitatory synapse formation through the control of Rac (rac family small GTPase)-GTP level and NPTX2 - trough binding and clustering of the glutamatergic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor [107, 108]. SH2D5 was not evidenced to be directly involved in aging, but there are reports on the involvement of reduced NPTX2 in cognitive dysfunctions, especially in AD [109]. The connection of cognitive function with aging is presented in the subsequent section.
Medication overuse headache (MOH) is a syndrome that in many cases is secondary to chronic migraine and in fact, there are not reliable markers to distinguish these two diseases [110]. It was observed that the catechol-O-methyltransferase (COMT) gene was hypermethylated in MOH patients [111]. Variability of the COMT gene was associated with changes in processes that can be related to aging, including decline in cognitive functions [112-114]. However, the relationship between genetic and epigenetic profile of a specific gene, if any, is not known, so these studies require validation by further research to be related to aging in migraine.
No differences were observed in epigenetic aging, measured by five different epigenetic clocks between episodic migraine and MOH patients as compared with individuals without headache [115]. Many parameters of epigenetic clock were investigated, but the study suffered from a relatively small number of subjects. Another problem was with chronological aging as a confounding factor, because a commonly used method for calculation introduces a bias in the final results. The authors used two methods of analysis to include chronological aging in their considerations.
Sixty-three differentially methylated regions (DMRs) rich in regulatory elements were identified close to some genes in another GWAS project in migraine [116]. These genes included members of the solute carrier family: solute carrier family 2 member 9 (SLC2A9), SLC38A4 and SLC6A5. These genes were associated with aging in pathological conditions [117, 118]. Another gene, encoding diacylglycerol kinase gamma (DGKG) was associated with aging and it was dysregulated in colorectal cancer through DNA hypermethylation [119]. Remaining genes differentially methylated in migraine were kinesin family member 26A (KIF26A), dedicator of cytokinesis 6 (DOCK6), and complement factor D (CFD) that were reported to associate with various aspects of the aging process [120-122].
A higher migraine risk was reported in females with a low level of DNA methylation in the gene encoding receptor activity modifying protein 1 (RAMP1), which is a key receptor unit of CGRP [123]. The expression of receptor activity proteins in specific tissues was associated with rat aging [124].
Many studies explored epigenetic connections of CGRP in migraine (reviewed in [125]). Modifications of the epigenetic profile of the CALCA gene in migraine include DNA methylation, as the promoter of the gene has two CpG islands with two CpG sites that are hypomethylated in migraine patients [126]. Changes in histone modification pattern and effects of micro RNAs (miRNAs), circular RNAs, and long-coding RNAs (lncRNAs) on the CALCA gene were also related to migraine [127-130]. Conversely, CGRP can change the epigenetic profile of neuronal and glial cells as shown for its involvement in epigenetic regulation of neural cytoarchitecture changes related to migraine chronification mediated by histone deacetylase 6 (HDAC6) [131]. The fundamental role of CGRP in the transmission of neurogenic pain may be epigenetically regulated [132]. In line with this research, it was observed that reduced CGRP signaling led to lifespan extension in mice and roundworms (C. elegans) [133]. The longest-lived C57BL/6 male mouse reached an age of nearly 41 months. The longevity was at least in part due to decreased incidence of cancer. Therefore, reducing the growth factor signaling from CGRP may play the key role in longevity, but these results cannot be directly translated into humans, in which cardiovascular disease, not cancer, is the main cause of mortality, as CGRP may protect the endothelium. However, that study showed an improved energy expenditure and circadian rhythm when CGRP signaling was disrupted.
In summary, migraine may be associated with changes in the cellular epigenetic profile. These changes may affect genes that can be related to aging. However, at present it is difficult to decide whether these changes can be classified as “epigenetic aging”, especially since they are not limited to DNA methylation. Moreover, at present there are too few studies to specify a causal relationship between epigenetic aging and migraine.
8. Cognitive aging
Changes in cognitive functions are typical to an aging brain [134]. It is still an open question whether migraine may modulate such changes in cognitive functions or/and vice versa - whether impairment in cognitive functions may contribute to migraine pathogenesis. It seems especially important in the context of the association between migraine, all-causes dementia and AD [135-137].
Cognitive aging is a term introduced to underline mental changes occurring during chronological aging. It may be understood as a process of gradual, longitudinal changes in cognitive functions that are associated with aging [138]. Several studies suggest that strong headache attacks may impair cognitive functions (e.g., [139] for review). Furthermore, neuropsychological impairments in executive functions, memory and learning were shown to resume after headache attacks. Many studies point at an impairment in cognitive functions during migraine attacks, but the data on the interictal period are conflicting [140]. The specific cognitive domains that may be impaired in migraine are information processing speed, basic attention, executive functions, verbal and non-verbal memory and verbal skills.
Some studies, including the Rotterdam Study, suggest an improvement of cognitive functions in migraine patients [141]. It was shown that older (between 5th and 7th decades of life) individuals with migraine and non-migraine headaches were not at an increased risk of cognitive decline, cognitive impairment or dementia [142]. That study used a comprehensive neuropsychological battery of tests of memory, language and executive functions, repeated 5 years apart. It was observed that individuals with migraine presented more subjective cognitive complaints and depressive symptoms than non-headache controls. In the context of aging, it is interesting whether such a state is maintained for longer time. In general, it requires further research to determine whether cognitive aging contributes to migraine or migraine modulates cognitive aging. Also, the Epidemiology of Vascular Aging (EVA) study, provided negative results on the association between migraine and decline in cognitive functions [143].
Many more recent studies suggest a positive association between migraine and impairment in cognitive functions/dementia (reviewed in [137]). Not only all-cause dementia but also AD, were observed at a greater ratio in 65+ years old migraineurs than age-matched controls [144]. Similar results were obtained for women diagnosed in general practices in the United Kingdom [145]. A Danish national register-based follow-up study revealed that migraine was a midlife risk factor for dementia in later life [146]. A higher ratio of dementia was observed in individuals with migraine with aura than without aura. That study suggests that migraine may affect cognitive aging. A randomized, cross-sectional, within subject approach study showed that not only migraine occurrence, but also its duration and frequency of headache attacks were positively correlated with an impairment in cognitive functions [147].
Studies on the association between migraine and cognitive functions in children and adolescents provided some contradictory results, but certain studies suggest that children and adolescents affected by migraine may display specific cognitive deficits, such as impaired short and long-term verbal memories, speed processing information, and selective/divided attention [148, 149].
An association between chronological aging and a decline in headache days in the absence of any confounding cognitive pathology was observed [150]. Moreover, aging can be a weak predictive indicator of headache days across the cognitive spectrum. Further research may explain whether results of these observations represent a reporting bias due to dementia or are biologically relevant.
In summary, studies on the association between migraine and impairment in cognitive functions did not yield consisted results in general, but many recent data report an association between them. Certainly, migraine impairs cognitive functions, but several studies show that this may be a transient state, which resumes after cessation of the headache attacks and so it cannot be attributed to cognitive aging. However, the state of the brain was not investigated in those studies. Further studies might determine a possible causative relationship between migraine and cognitive aging.
9. Metabolic aging and oxidative stress
The rate of metabolism and the amount and composition of its by-products change with age and were considered as drivers of the aging process (reviewed in [151]). The term “metabolic aging” relates the rate of metabolism to chronological aging. Aging may be metabolically characterized by insulin resistance, changes in body composition and expression of genes involved in growth control. Regulation of metabolic processes in aging organisms may be linked with extension of longevity through nutritional intervention and lifestyle modifications, both of potential significance in migraine pathogenesis. Not only lifespan, but also healthspan can be modified by metabolic intervention [152]. Metabolic stress may be linked with inflammation, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, changes in the unfolded protein response, impairment in proteostasis and other effects that may modulate aging [153].
It was hypothesized that migraine may be an element of a defense mechanism to protect the organism against a metabolic challenge [154]. An immediate question is why some people experience migraine and the others do not, despite the same metabolic challenge, but the answer is still the same - some people may have a biological predisposition to migraine increasing their sensitivity to other biological factors and environmental/lifestyle influences.
Metabolic pathways involved in energy production in the brain are natural candidates to associate with migraine pathogenesis. Synthesis of ATP from glucose is the main pathway of brain energy production, which occurs in three key stages: glycolysis; the citric acid cycle also known as the Krebs cycle or tricarboxylic acid (TCA) cycle and oxidative phosphorylation [155]. Deficit in brain energy metabolism in migraineurs was reported in MRI studies showing a lower phosphocreatine to creatine ratio and an enhanced concentration of ADP as compared to non-migraineurs (reviewed in [156]). Energy deficit positively correlated with severity of headache attacks [157]. Mechanistically, brain energy deficit may be a source of oxidative stress, which may contribute to migraine pathogenesis [95].
It was suggested that insulin resistance was an adaptive response to migraine to increase energy supply to ameliorate its deficit in the migraineurs brain [158]. Conversely, the “neuroenergetic hypothesis” suggests that postprandial hypoglycemia may play a major role in the pathogenesis of episodic migraine and that brain insulin resistance, which is a key risk factor for Alzheimer disease, may significantly contribute to migraine chronification [159, 160]. Reduced glucose metabolism, volume and energy metabolism in migraine are reported in several areas of brain, including insular cortex, parietal cortex, left prefrontal cortex and orbitofrontal cortex (reviewed in [159]).
The main reasons for the brain energy deficit in migraine are increased energy demand by hyperexcitable brain and lowered energy supply by impaired mitochondria [95]. The extent of energy production determines the amount of reduced nicotinamide adenine dinucleotide phosphate (NADPH), which, in turn, determines the activity of enzymes involved in antioxidant defense of brain neurons [161]. In addition, the expression of these enzymes is regulated by epigenetic modifications resulting from the expression and activity of proteins involved in the maintenance of the cellular epigenetic pattern. Mitochondrial NADPH is synthesized by enzymes involved in energy generation, including isocitrate dehydrogenase of the TCA cycle and nicotinamide nucleotide transhydrogenase (NNT), that depend on the Krebs cycle and oxidative phosphorylation [162]. However, when energy generation is impaired, these enzymes may consume antioxidants. Cortical spreading depolarization, an effect associated with migraine aura, induces a decrease in NADH (reduced nicotinamide adenine dinucleotide) level resulting in impairing antioxidant defense by NNT and overproduction of RONS [95]. The cellular antioxidant system consists of antioxidant enzymes, DNA repair proteins and small molecular weight antioxidants. All these elements are reported to progressively decline with aging [163].
An acute oxidative stress in the brain may induce a migraine attack as a defense reaction against potentially harmful stress-related changes [164]. However, a causative relationship between oxidative stress and migraine has not been experimentally documented, but several pathways of association were suggested (reviewed in [95]). Hydrogen peroxide, and likely other RONS, may be released by neurons and astrocytes, trapped by transient receptor potential cation channel subfamily A member 1 (TRPA1) and transduced into a neural signal of oxidative stress through the activation of NADPH oxidase 1/2 (NOX1/2) in trigeminal ganglion neurons [165, 166] (Fig. 3). This may sensitize meningeal nociceptors and second-order trigeminal neurons, important in migraine-like headaches [167]. Activated TRPA1 induces CGRP release from TGN neurons and dura matter [168]. Hydrogen peroxide also stimulates CGRP release, supporting the role of oxidative stress in migraine pathogenesis [169]. Furthermore, oxidative stress results in enhanced levels of lipid peroxidation products that may stimulate TRPA1 [170]. Consequently, the activation of TRPA1 may result in pain signaling and neuroinflammation, typical for migraine attacks [167].
Figure 3.
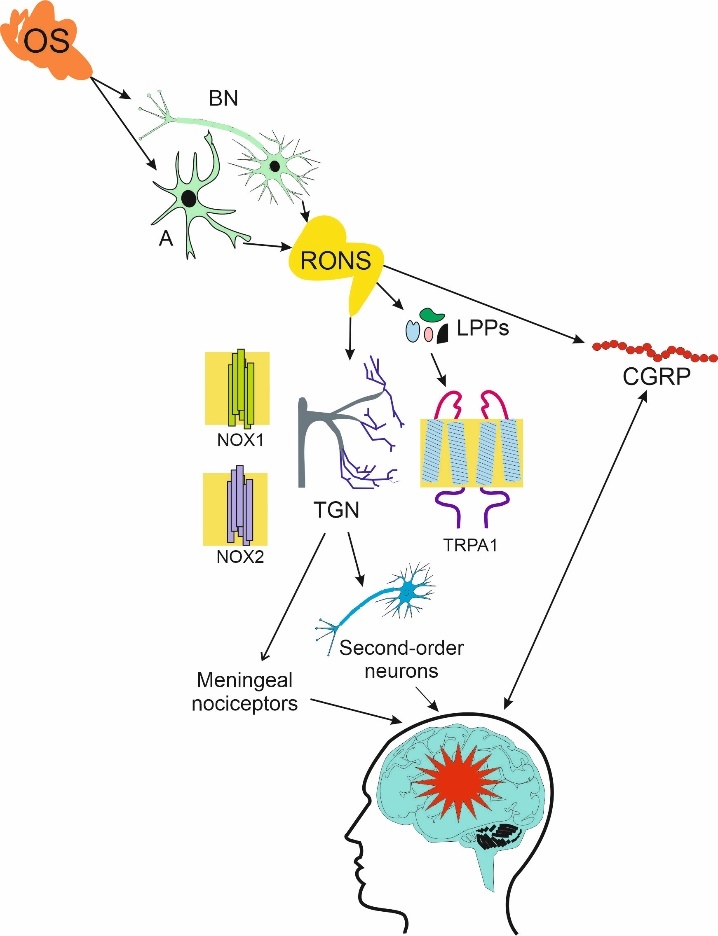
Oxidative stress (OS) in the brain affects brain neurons (BNs) and astrocytes (As). OS may release reactive oxygen and nitrogen species (RONS) that may interact with transient receptor potential cation channel subfamily A member 1 (TRPA1) and take an oxidative stress-related signaling function to activate reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1/2 (NOX1/2) in neurons of trigeminal nerve (TGN) and sensitize meningeal nociceptors and second-order TGN neurons, important in migraine, which is presented here as a red star. TRPA1 may induce the release of calcitonin gene related peptide (CGRP), strongly associated with migraine, from TGN neurons and dural tissue. Furthermore, RONS may also induce CGRP. Lipid peroxidation products (LPPs) are induced by RONS and may contribute to this cascade of events by TRPA1 stimulation.
MRI studies showed that migraine was associated with deficit in brain energy during attack and interictal phase (reviewed in [171]). It was a consequence of decreased energy production and/or increased energy need in the migraine brain and might enhance oxidative stress, which could lower the threshold for migraine triggers. Conversely, energy deficit in the brain lowers the threshold for migraine triggers to induce attack to counteract oxidative stress [95]. Cortical spreading depolarization was reported to reduce NADH content causing a decrease in mitochondrial NADPH and decreasing antioxidant defense [172]. This initial decrease is followed by RONS overproduction by the mitochondrial electron transport chain (ETC) resulting from local tissue hypoxia and NADH binding. As mentioned, hydrogen peroxide and other RONS induced the release of CGRP, a trigger of delayed sensitization of trigeminal neurons. Therefore, enhanced level of CGRP in migraine may result from defense mechanisms against oxidative stress associated with the brain energy deficit.
Oxidative stress occurring in may result in enhanced DNA damage in neurons, astrocytes, and glial cells, and impaired DNA repair in these cells may disturb brain homeostasis. An enhanced oxidative stress in nontarget tissues was associated with migraine [173]. That study reported enhanced levels of 7,8-dihydro-8-oxoguanine (8-oxoG), a major DNA-derived oxidative stress marker, in plasma of migraine patients. 8-oxoG has been positively correlated with both premature and normal aging in many studies (e.g., [174-176]).
A 1H-NMR study showed that the serum metabolic profile of migraine patients was different from controls [177]. The most pronounced differences in serum concentrations were observed in lipids, amino acids and products of glucose metabolism. These metabolites may be considered as predictive markers for migraine as the diagnosis of this disease is based on interview and physical examination.
Although the important role of CGRP in many aspects of migraine pathogenesis is well established, recent research suggests that CGRP may not be a kind of migraine trigger that could induce a complete migraine attack (reviewed in [158]). Some studies report antioxidant and anti-inflammatory actions of CGRP [178-180]. Therefore, CGRP release associated with migraine attack may support the role of migraine as an adaptive response to oxidative stress associated with brain energy deficit related to metabolic changes.
Some kinds of nutritional interventions may slow down metabolic aging through several mechanisms, including an improvement of mitochondrial functions and epigenetic regulation, which are important in migraine [181, 182]. Therefore, such intervention may be considered to prevent migraine and slow down metabolic aging, but nutrients that may be migraine triggers should be avoided, even when they show anti-aging properties [183]. Some nutrients that can be considered to delay metabolic aging in migraine are riboflavin, thiamine, magnesium ions, niacin; carnitine; coenzyme Q10, melatonin, lipoic acid, pyridoxine, folate and cobalamin (Fig. 4).
Figure 4.
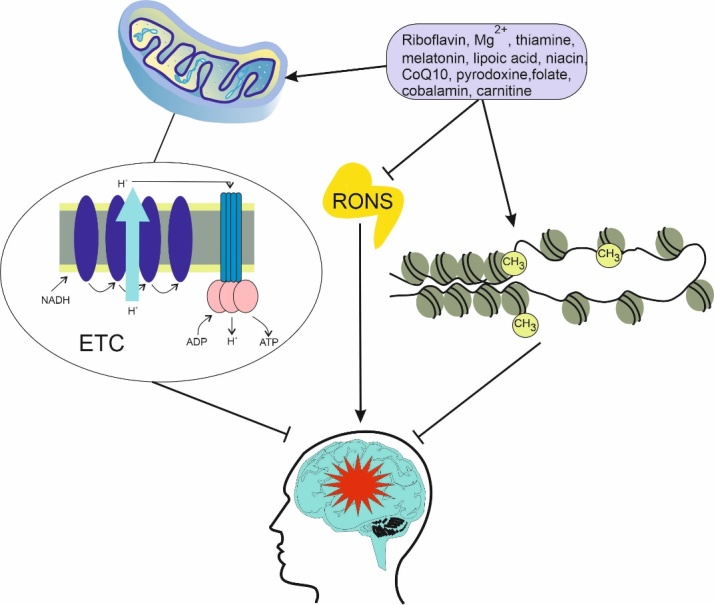
Metabolic aging can be slowed down by some nutritional interventions resulting in preventing or ameliorating migraine, symbolized here as a red star. These interventions may inhibit oxidative stress, represented by reactive oxygen and nitrogen species (RONS), improve mitochondrial metabolism and energy production through mitochondrial electron chain (ETC) and epigenetic changes in genes involved in energy metabolism, shown here as changes in the DNA methylation pattern and alteration in the chromatin structure. All these changes improve brain metabolism and compensate for brain energy deficit in migraine. Dietary interventions are represented by but not limited to some nutrients that were reported to display presented features. CoQ10 - coenzyme Q10, NADH - reduced nicotinamide adenine dinucleotide.
In the absence of glucose stores, astrocytes produce ketone bodies from fatty acids in the process of ketogenesis, which can be induced by the ketogenic diet, featured by a low carbohydrates content [184]. Therefore, this kind of diet, which would supplement energy deficit caused by lowered glucose metabolism, is considered as a dietary intervention in migraine [185]. However, the application of this kind of diet in clinical practice does not show long term beneficial effects in migraineurs and cannot be excluded that some positive results are only due to weight or/and fat mass loss [186].
Metabolic syndrome (MetS) is a set of metabolic risk factors including enlarged waist circumference, dyslipidemia, systemic hypertension, and hyperglycemia. Several works show that MetS coexists with migraine, but the pathophysiological mechanisms of such comorbidity are not known (reviewed in [187]). Some studies show an impairment of insulin sensitivity in migraine, even in young, non-obese, non-diabetic, normotensive patients, but again, no mechanistic explanation of this relationship has been proposed and verified. In summary, migraine is associated with metabolic changes, but at present it is difficult to select specific metabolic pathways and metabolites that would be causative in migraine pathogenesis. Many established and putative migraine risk factors can be related to cellular, or organ metabolism and metabolic aging may decide whether these factors may play a causative role in migraine. However, energy metabolism is strictly associated with brain homeostasis and its sensitivity to migraine triggers. Therefore, effects linked with metabolic aging may play a particularly important role in migraine pathogenesis. Moreover, it should be established whether metabolic aging is associated directly with migraine or through its secondary effects, such as obesity or metabolic syndrome.
10. Outstanding questions
At present, several problems wait for the explanation in the relationship between migraine and different aspects of aging and several outstanding questions can be asked, including the following:
What is the mechanism determining that some individuals are sensitive to migraine and the others are not?
Why do people in the most productive age have the highest prevalence of migraine?
Why the age characteristics of migraine in women is roughly the same as in men, despite the disease prevalence in the former is 2-3 times higher than in the latter?
Is stress associated with social aging correlated with migraine prevalence?
How does brain aging contribute to migraine susceptibility?
How are migraine triggers and genetic/epigenetic predisposition related to ging?
Does cognitive aging trigger migraine?
Does metabolic aging relate directly to migraine or rather via its secondary effects?
All these questions are addressed in the concluding section.
11. Conclusions, Hypotheses and Perspectives
Migraine is the second worldwide cause of disability, and first among young women, with the total number of affected individuals about 1 billion [8]. There are many migraine classifications presenting many migraine types, including menstrual, ocular/retinal, silent, vestibular, abdominal, hemiplegic migraines, migraine with brainstem aura and others [188]. Some of them are per definition associated with their primary cause, e.g., menstrual migraine, but others may be triggered by various factors. Therefore, it is tempting to ask whether specific aspects of aging are associated with specific types of migraine. There are not data on this subject, and it would be too speculative to hypothesize about such association. In particular, we are not able to determine or even speculate what migraine type, if any, is behind social aging. We have tried to provide arguments that social aging stress positively correlates with migraine prevalence and therefore may contribute to its pathogenesis. All other aspects of aging may differentially influence migraine occurrence and progression, but the intensity of stress related to those aspects does not strongly correlate with migraine prevalence, but rather with chronological aging.
Migraine as a disease has a few unusual features as compared with other human disorders. Despite its common occurrence and serious consequences for affected individuals and a significant burden for societies, mechanisms of its pathogenesis are poorly known. We do not know why some people develop migraine while others do not. This poor knowledge is underlined by several reasons, including, but not limited to, a restricted accessibility of human CNS, in particular the brain, to research and a limited usefulness of laboratory animals to model human migraine. However, despite this very limited knowledge on migraine pathogenesis, its relatively effective treatment, the anti-CGRP therapy, has been applied. The next extraordinal feature of migraine is its 2-3 times higher prevalence in women than in men, but this difference increases to 3-4 times in women after puberty. Chromosome X-linked events, hormonal homeostasis and mitochondrial transmission are considered to elucidate this difference, but no a satisfactory explanation has been provided so far [189]. The next unusual characteristic of migraine is the dependence of its prevalence on age with the peak around the range 20-40 years. How do these facts influence our conclusions and the outstanding questions?
Firstly, all effects related to aging may play a role in migraine pathogenesis only in migraine-susceptible individuals. In other words, migraine is not a classical age-related disease. And who is migraine-susceptible? Not a “migraine gene” has been identified so far and there is not a perspective to do so. Surely, the term “migraine gene” is equally non-sense as e.g., “breast cancer gene”. Although several migraine susceptibility genetic loci have been identified, the genetic picture of migraine is far from complete. Moreover, such pictures must be supplemented by the epigenetic profile. We assume that migraine susceptibility is determined by genetic and epigenetic factors as the main components. What else could be important? Traumas, shocks, complexes and other events affecting physical and mental health acquired during childhood and later life may contribute to this susceptibility. Then, at least three concepts can be regarded. One considers migraine as a disease with strictly pathological aspects, the other - as a defense mechanism against stress affecting the brain. Moreover, migraine may be associated with reproductive or survival advantages from the evolutionary point of view [190, 191]. In each concept, the brain is in its center, but it is not the only one element important in migraine pathogenesis [192]. This migraine susceptibility (migraine background) weakly depends on chronological aging, as genetic/epigenetic constitution is its dominant component. A migraine-susceptible individual is prone to various influences, including “classical” migraine triggers and those associated with different aspects of aging, as we presented above (Fig. 5).
Figure 5.
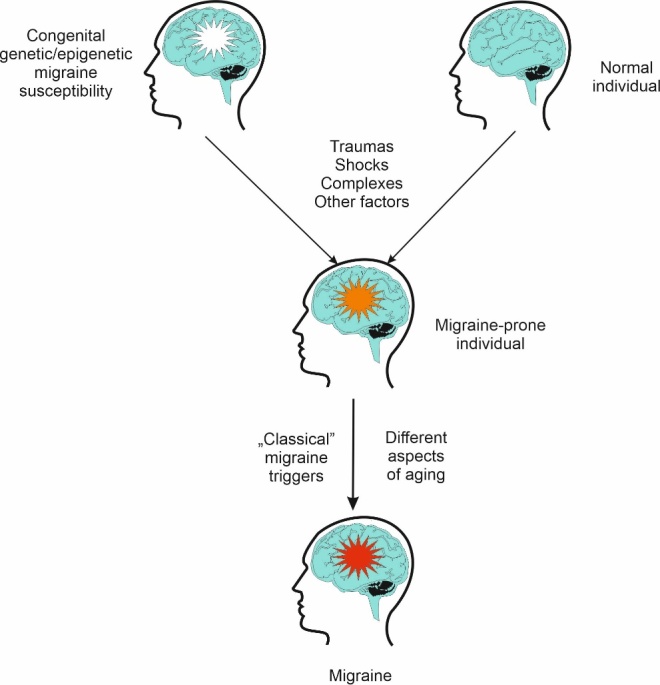
Different aspects of aging may play a key role in a selective migraine susceptibility. Migraine affects certain individuals, but for not fully known reasons, the others are not affected, although some of them are equally or even more exposed to “classical” migraine triggers (food component, odors, physiological complications etc.). We assume that migraines affect migraine-prone individuals. Such proneness can be congenital due to the changes in genetic and epigenetic constitution, symbolized here by a white star, or/and acquired due to traumatic events and other factors (orange star). Such migraine-prone person is susceptible for both “classical” migraine triggers and factors yielded by different aspects of aging presented in this work.
Which aspects of aging are the most important in migraine? If the migraine background is faintly dependent on age, these aspects should depend on chronological aging in a way similar to the relationship of migraine prevalence by age. We hypothesize that this is the stress associated with social aging, which may be mainly responsible for the induction and progression of migraine in migraine susceptible subjects. In many cases the most serious stress associated with everyday challenges occurs between 18 and 40 years as this time is linked to final and entrance exams, job hunting, professional challenges, starting a family, maternity/paternity and other stress-generating events. Before that time, a person is mainly dependent on his/her parents/guardians and after that stabilization time occurs in which many challenges no longer occur or are substantially weaker. The late period of life is very complex per se, and it is difficult to biologically link it to anything else than age-related impairment of life functions and diseases. There may be differences in facing these challenges by women and men, but it would be too speculative to relate them to migraine prevalence. Other aspects of aging may increase migraine susceptibility or directly induce migraine in migraine-susceptible individuals and metabolic aging may play an important role. This may follow from an important role of energy metabolism and significance of metabolism-related oxidative stress in brain homeostasis. Moreover, metabolic aging is strongly linked with other aspects of aging, including stem cell exhaustion, cellular senescence and epigenetic aging.
In this manuscript, we pointed out that migraine pathogenesis might contain two elements - inborn or acquired predisposition to migraine and aging related stress. It is tempting to consider the former in order to approach the answer to the question why some people develop migraine and others not, although they are exposed to the same migraine triggers. However, genetics of migraine, except rare familial hemiplegic migraine is poorly known and is supported by association rather than mechanistic studies [21, 193]. The epigenetics of migraine is even more poorly explored and still remains a “promising avenue” [125]. Therefore, there are not solid data to consider aging-related changes in the genetic and epigenetic profiles in migraine.
In a common understanding of the term age-dependence, “age” is considered as chronological age. Some mechanisms of migraine pathogenesis, underlined by physiological phenomena, are dependent on chronological age and may contribute to the age-dependence of migraine pathogenesis. Vasodilation and other vascular changes are progressively impaired with age and were considered to contribute to migraine pathogenesis, but nowadays they are treated as an epiphenomenon that is neither sufficient nor necessary to induce migraine [194-196]. As mentioned, migraine is associated with brain energy deficit, which can be translated into oxidative stress triggering a headache attack through oxidant-sensing nociceptive ion channels, including TRPA1, but aging is linked with a decrease in nicotinamide nucleotide transhydrogenase and therefore contribute to migraine occurrence [95, 164]. On the other hand, the brain may display a reduced sensitivity to oxidative stress when its basal levels rise with aging.
If we assume that the stress associated with social aging is the main age-related determinants of migraine occurrence and severity, the question is how remaining age-related factors influence migraine as their intensity increases with chronological age. This is an important problem in the elderly who accumulate consequences of such age-related factors, including migraine triggers to which the elderly is more susceptible than younger individuals. Cognitive aging, which may result in dementia and other mental disorders, is of a special significance not only in considering the mechanism behind migraine pathogenesis, but also in migraine diagnosis. Dementia may impede diagnosis of migraine as it is mainly based on self-reporting questionnaires. Other diseases typical for advanced age may be associated with non-migraine headaches and result in polypharmacy that may contribute to MOH, difficult to distinguish from migraine. Therefore, diagnostic criteria for migraine for the elderly may not be so adequate as for younger individuals and may require substantial modifications. Also, the prevalence of migraine in women after puberty is up to 4 times higher than in men, which provokes further questions about the age-dependence of migraine and diagnostic criteria in this disease.
In summary, many aspects of aging interwound in migraine pathogenesis, both molecular and cellular as well as social and cognitive. Migraine prevalence depends on aging in a way that fits the most social aging. However, effects attributed to that kind of aging cannot induce migraine in any person, but rather in individuals who inherited and/or acquired migraine susceptibility.
Acknowledgements
The authors thank Monika Kicinska for her help in figure preparation.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- [1].Burkhamer J, Kriebel D, Clapp R (2017). The increasing toll of adolescent cancer incidence in the US. PLoS One, 12:e0172986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Niccoli T, Partridge L (2012). Ageing as a Risk Factor for Disease. Current Biology, 22:R741-R752. [DOI] [PubMed] [Google Scholar]
- [3].Comfort A (1969). Test-battery to measure ageing-rate in man. Lancet, 2:1411-1414. [DOI] [PubMed] [Google Scholar]
- [4].Ahadi S, Zhou W, Schüssler-Fiorenza Rose SM, Sailani MR, Contrepois K, Avina M, et al. (2020). Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nature Medicine, 26:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, et al. (2021). Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front Neurol, 12:800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A (2022). Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol, 21:284-294. [DOI] [PubMed] [Google Scholar]
- [7].Stovner LJ, Hagen K, Linde M, Steiner TJ (2022). The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain, 23:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z (2020). Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. Journal of Headache and Pain, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moskowitz MA, Dodick DW, Scher AI, van den Maagdenberg AMJM (2022). Migraine research comes of age in the 21st century. The Lancet Neurology, 21:955-958. [DOI] [PubMed] [Google Scholar]
- [10].Schmauck-Medina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, et al. (2022). New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany NY), 14:6829-6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barzilai N, Cuervo AM, Austad S (2018). Aging as a Biological Target for Prevention and Therapy. JAMA, 320:1321-1322. [DOI] [PubMed] [Google Scholar]
- [12].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gems D, de Magalhães JP (2021). The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Res Rev, 70:101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crimmins EM (2020). Social hallmarks of aging: Suggestions for geroscience research. Ageing Research Reviews, 63:101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Committee on the Public Health Dimensions of Cognitive A, Board on Health Sciences P, Institute of M. 2015. The National Academies Collection: Reports funded by National Institutes of Health. In Cognitive Aging: Progress in Understanding and Opportunities for Action. Blazer D.G., Yaffe K., and Liverman C.T., editors. Washington (DC): National Academies Press (US) Copyright 2015 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- [16].Crouch J, Shvedova M, Thanapaul R, Botchkarev V, Roh D (2022). Epigenetic Regulation of Cellular Senescence. Cells, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li A, Koch Z, Ideker T (2022). Epigenetic aging: Biological age prediction and informing a mechanistic theory of aging. Journal of Internal Medicine, 292:733-744. [DOI] [PubMed] [Google Scholar]
- [18].Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, et al. (2022). Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduction and Targeted Therapy, 7:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, et al. (2022). Migraine. Nature Reviews Disease Primers, 8:2. [DOI] [PubMed] [Google Scholar]
- [20].Stewart WF, Ricci JA, Chee E, Morganstein D (2003). Lost Productive Work Time Costs from Health Conditions in the United States: Results from the American Productivity Audit. Journal of Occupational and Environmental Medicine, 45:1234-1246. [DOI] [PubMed] [Google Scholar]
- [21].Bron C, Sutherland HG, Griffiths LR (2021). Exploring the Hereditary Nature of Migraine. Neuropsychiatr Dis Treat, 17:1183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ishii R, Schwedt TJ, Dumkrieger G, Lalvani N, Craven A, Goadsby PJ, et al. (2021). Chronic versus episodic migraine: The 15-day threshold does not adequately reflect substantial differences in disability across the full spectrum of headache frequency. Headache: The Journal of Head and Face Pain, 61:992-1003. [DOI] [PubMed] [Google Scholar]
- [23].Torres-Ferrús M, Ursitti F, Alpuente A, Brunello F, Chiappino D, de Vries T, et al. (2020). From transformation to chronification of migraine: pathophysiological and clinical aspects. The Journal of Headache and Pain, 21:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ashina H, Christensen RH, Ashina M (2022). Provoked versus spontaneous migraine attacks: pathophysiological similarities and differences. J Headache Pain, 23:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kincses ZT, Veréb D, Faragó P, Tóth E, Kocsis K, Kincses B, et al. (2019). Are Migraine With and Without Aura Really Different Entities? Front Neurol, 10:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hansen JM, Charles A (2019). Differences in treatment response between migraine with aura and migraine without aura: lessons from clinical practice and RCTs. J Headache Pain, 20:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wijeratne T, Tang HM, Crewther D, Crewther S (2019). Prevalence of Migraine in the Elderly: A Narrated Review. Neuroepidemiology, 52:104-110. [DOI] [PubMed] [Google Scholar]
- [28].Hautakangas H, Winsvold BS, Ruotsalainen SE, Bjornsdottir G, Harder AVE, Kogelman LJA, et al. (2022). Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet, 54:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van den Maagdenberg A, Nyholt DR, Anttila V (2019). Novel hypotheses emerging from GWAS in migraine? J Headache Pain, 20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB (2014). Trigger factors and premonitory features of migraine attacks: summary of studies. Headache, 54:1670-1679. [DOI] [PubMed] [Google Scholar]
- [31].Peroutka SJ (2014). What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep, 18:454. [DOI] [PubMed] [Google Scholar]
- [32].Iyengar S, Johnson KW, Ossipov MH, Aurora SK (2019). CGRP and the Trigeminal System in Migraine. Headache, 59:659-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang L, Xu M, Bhuiyan SA, Li J, Zhao J, Cohrs RJ, et al. (2022). Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron, 110:1806-1821.e1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018). CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol, 14:338-350. [DOI] [PubMed] [Google Scholar]
- [35].(2019). Global, regional,national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 18:459-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kelman L (2006). Migraine changes with age: IMPACT on migraine classification. Headache, 46:1161-1171. [DOI] [PubMed] [Google Scholar]
- [37].Wöber-Bingöl C, Wöber C, Karwautz A, Auterith A, Serim M, Zebenholzer K, et al. (2004). Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia, 24:12-17. [DOI] [PubMed] [Google Scholar]
- [38].Fisher CM (1980). Late-life migraine accompaniments as a cause of unexplained transient ischemic attacks. Can J Neurol Sci, 7:9-17. [PubMed] [Google Scholar]
- [39].Ahn K, Lee S-J, Mook-Jung I (2022). White matter-associated microglia: New players in brain aging and neurodegenerative diseases. Ageing Research Reviews, 75:101574. [DOI] [PubMed] [Google Scholar]
- [40].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol, 15:565-581. [DOI] [PubMed] [Google Scholar]
- [41].Hedden T, Gabrieli JDE (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience, 5:87-96. [DOI] [PubMed] [Google Scholar]
- [42].Umarova RM (2017). Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: Implications for understanding neglect. Cortex, 97:327-338. [DOI] [PubMed] [Google Scholar]
- [43].Blinkouskaya Y, Caçoilo A, Gollamudi T, Jalalian S, Weickenmeier J (2021). Brain aging mechanisms with mechanical manifestations. Mechanisms of Ageing and Development, 200:111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA (2018). Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences of the United States of America, 115:E1896-E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, et al. (2021). White matter aging drives microglial diversity. Neuron, 109:1100-1117.e1110. [DOI] [PubMed] [Google Scholar]
- [47].Azam S, Haque ME, Balakrishnan R, Kim IS, Choi DK (2021). The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front Cell Dev Biol, 9:683459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kruit MC, van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD (2010). Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia, 30:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM, et al. (2012). Structural brain changes in migraine. Jama, 308:1889-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ashina S, Bentivegna E, Martelletti P, Eikermann-Haerter K (2021). Structural and Functional Brain Changes in Migraine. Pain Ther, 10:211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eikermann-Haerter K, Huang SY (2021). White Matter Lesions in Migraine. Am J Pathol, 191:1955-1962. [DOI] [PubMed] [Google Scholar]
- [52].Siracusa ER, Higham JP, Snyder-Mackler N, Brent LJN (2022). Social ageing: exploring the drivers of late-life changes in social behaviour in mammals. Biology Letters, 18:20210643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Buse DC, Armand CE, Charleston IV L, Reed ML, Fanning KM, Adams AM, et al. (2021). Barriers to care in episodic and chronic migraine: Results from the Chronic Migraine Epidemiology and Outcomes Study. Headache: The Journal of Head and Face Pain, 61:628-641. [DOI] [PubMed] [Google Scholar]
- [54].Stewart WF, Roy J, Lipton RB (2013). Migraine prevalence, socioeconomic status, and social causation. Neurology, 81:948-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liberatos P, Link BG, Kelsey JL (1988). The measurement of social class in epidemiology. Epidemiol Rev, 10:87-121. [DOI] [PubMed] [Google Scholar]
- [56].Bigal ME, Lipton RB, Winner P, Reed ML, Diamond S, Stewart WF (2007). Migraine in adolescents: association with socioeconomic status and family history. Neurology, 69:16-25. [DOI] [PubMed] [Google Scholar]
- [57].Barrett AE (2003). Socioeconomic status and age identity: the role of dimensions of health in the subjective construction of age. J Gerontol B Psychol Sci Soc Sci, 58:S101-109. [DOI] [PubMed] [Google Scholar]
- [58].Steptoe A, Zaninotto P (2020). Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proc Natl Acad Sci U S A, 117:14911-14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Le H, Tfelt-Hansen P, Skytthe A, Kyvik KO, Olesen J (2011). Association between migraine, lifestyle and socioeconomic factors: a population-based cross-sectional study. The Journal of Headache and Pain, 12:157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chu MK, Kim DW, Kim BK, Kim JM, Jang TW, Park JW, et al. (2013). Gender-specific influence of socioeconomic status on the prevalence of migraine and tension-type headache: the results from the Korean Headache Survey. J Headache Pain, 14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hammond NG, Stinchcombe A (2019). Health Behaviors and Social Determinants of Migraine in a Canadian Population-Based Sample of Adults Aged 45-85 Years: Findings From the CLSA. Headache: The Journal of Head and Face Pain, 59:1547-1564. [DOI] [PubMed] [Google Scholar]
- [62].Winter AC, Berger K, Buring JE, Kurth T (2012). Associations of socioeconomic status with migraine and non-migraine headache. Cephalalgia, 32:159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Belot RA, Bouteloup M, Bonnet M, Parmentier AL, Magnin E, Mauny F, et al. (2021). Evaluation of Attachment Style and Social Support in Patients With Severe Migraine. Applications in Doctor-Patient Relationships and Treatment Adherence. Front Neurol, 12:706639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wei D, Chang Y, Lu X, Fan X, Hu J, Manta O, et al. (2023). Association between Migraine and Workplace Social Support in the Social Context of China: Using a Validated Chinese Version of the DCSQ. Healthcare (Basel), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].González-Quintanilla V, Toriello-Suárez M, Gutiérrez-González S, Rojo-López A, González-Suárez A, Viadero-Cervera R, et al. (2015). Stress at work in migraine patients: differences in attack frequency. Neurologia, 30:83-89. [DOI] [PubMed] [Google Scholar]
- [66].Lin KC, Huang CC, Wu CC (2007). Association between stress at work and primary headache among nursing staff in Taiwan. Headache, 47:576-584. [DOI] [PubMed] [Google Scholar]
- [67].Magnavita N (2022). Headache in the Workplace: Analysis of Factors Influencing Headaches in Terms of Productivity and Health. Int J Environ Res Public Health, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Santos IS, Griep RH, Alves MG, Goulart AC, Lotufo PA, Barreto SM, et al. (2014). Job stress is associated with migraine in current workers: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Eur J Pain, 18:1290-1297. [DOI] [PubMed] [Google Scholar]
- [69].Vahtera J, Westerlund H, Hall M, Sjösten N, Kivimäki M, Sal OP, et al. (2009). Effect of retirement on sleep disturbances: the GAZEL prospective cohort study. Sleep, 32:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Westerlund H, Kivimäki M, Singh-Manoux A, Melchior M, Ferrie JE, Pentti J, et al. (2009). Self-rated health before and after retirement in France (GAZEL): a cohort study. Lancet, 374:1889-1896. [DOI] [PubMed] [Google Scholar]
- [71].Sjösten N, Nabi H, Westerlund H, Singh-Manoux A, Dartigues JF, Goldberg M, et al. (2011). Influence of retirement and work stress on headache prevalence: a longitudinal modelling study from the GAZEL Cohort Study. Cephalalgia, 31:696-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nicaise AM, Willis CM, Crocker SJ, Pluchino S (2020). Stem Cells of the Aging Brain. Front Aging Neurosci, 12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB (2009). Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology, 72:1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Magalhães JE, Sampaio Rocha-Filho PA (2018). Migraine and cerebrovascular diseases: Epidemiology, pathophysiological, and clinical considerations. Headache: The Journal of Head and Face Pain, 58:1277-1286. [DOI] [PubMed] [Google Scholar]
- [75].Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. (2003). Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med, 348:593-600. [DOI] [PubMed] [Google Scholar]
- [76].Liu J, Ding Y, Liu Z, Liang X (2020). Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Frontiers in Cell and Developmental Biology, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kaur P, Otgonbaatar A, Ramamoorthy A, Chua EHZ, Harmston N, Gruber J, et al. (2022). Combining stem cell rejuvenation and senescence targeting to synergistically extend lifespan. Aging (Albany NY), 14:8270-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kumari R, Jat P (2021). Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol, 9:645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jeyapalan JC, Sedivy JM (2008). Cellular senescence and organismal aging. Mech Ageing Dev, 129:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, et al. (2017). The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev, 31:172-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Carreno G, Guiho R, Martinez-Barbera JP (2021). Cell senescence in neuropathology: A focus on neurodegeneration and tumours. Neuropathol Appl Neurobiol, 47:359-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kursun O, Yemisci M, van den Maagdenberg A, Karatas H (2021). Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain, 22:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, et al. (2008). Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology, 70:1510-1517. [DOI] [PubMed] [Google Scholar]
- [84].Rodríguez-Osorio X, Sobrino T, Brea D, Martínez F, Castillo J, Leira R (2012). Endothelial progenitor cells: a new key for endothelial dysfunction in migraine. Neurology, 79:474-479. [DOI] [PubMed] [Google Scholar]
- [85].Bouland C, Philippart P, Dequanter D, Corrillon F, Loeb I, Bron D, et al. (2021). Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front Cell Dev Biol, 9:674084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. (2004). Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells, 22:377-384. [DOI] [PubMed] [Google Scholar]
- [87].Mauskop A, Rothaus KO (2017). Stem Cells in the Treatment of Refractory Chronic Migraines. Case Rep Neurol, 9:149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bright R, Bright M, Bright P, Hayne S, Thomas WD (2014). Migraine and tension-type headache treated with stromal vascular fraction: a case series. J Med Case Rep, 8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lv X, Chen Q, Zhang S, Gao F, Liu Q (2022). CGRP: A New Endogenous Cell Stemness Maintenance Molecule. Oxid Med Cell Longev, 2022:4107433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shi Z, Wang S, Deng J, Gong Z (2022). Neural Peptide α-CGRP Coregulated Angiogenesis and Osteogenesis via Promoting the Cross-Talk between Mesenchymal Stem Cells and Endothelial Cells. Biomed Res Int, 2022:1585840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhang Y, Yang J, Zhang P, Liu T, Xu J, Fan Z, et al. (2016). Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci Rep, 6:27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jia S, Zhang SJ, Wang XD, Yang ZH, Sun YN, Gupta A, et al. (2019). Calcitonin gene-related peptide enhances osteogenic differentiation and recruitment of bone marrow mesenchymal stem cells in rats. Exp Ther Med, 18:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Michot B, Casey SM, Gibbs JL (2021). Effects of CGRP-Primed Dental Pulp Stem Cells on Trigeminal Sensory Neurons. J Dent Res, 100:1273-1280. [DOI] [PubMed] [Google Scholar]
- [94].Xu G, Jiang D (2014). The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell Biochem Biophys, 69:369-378. [DOI] [PubMed] [Google Scholar]
- [95].Borkum JM (2021). Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem Res, 46:1913-1932. [DOI] [PubMed] [Google Scholar]
- [96].Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, et al. (2010). Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol, 6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Pilié PG, Tang C, Mills GB, Yap TA (2019). State-of-the-art strategies for targeting the DNA damage response in cancer. Nature Reviews Clinical Oncology, 16:81-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].da Silva PFL, Schumacher B (2019). DNA damage responses in ageing. Open Biol, 9:190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Fila M, Jablkowska A, Pawlowska E, Blasiak J (2022). DNA Damage and Repair in Migraine: Oxidative Stress and Beyond. Neuroscientist:10738584221090836. [DOI] [PubMed] [Google Scholar]
- [100].Horvath S (2013). DNA methylation age of human tissues and cell types. Genome Biol, 14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell, 49:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, et al. (2014). Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol, 15:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yang J, Yu L, Gaiteri C, Srivastava GP, Chibnik LB, Leurgans SE, et al. (2015). Association of DNA methylation in the brain with age in older persons is confounded by common neuropathologies. Int J Biochem Cell Biol, 67:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Winsvold BS, Palta P, Eising E, Page CM, van den Maagdenberg AM, Palotie A, et al. (2018). Epigenetic DNA methylation changes associated with headache chronification: A retrospective case-control study. Cephalalgia, 38:312-322. [DOI] [PubMed] [Google Scholar]
- [105].Pérez Pereda S, Toriello Suárez M, González Quintanilla V, Oterino A (2020). Methylation analysis of NPTX2 and SH2D5 genes in chronic migraine: A case-control study. Cephalalgia Reports, 3:2515816320923592. [Google Scholar]
- [106].Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017). Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev, 97:553-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gray EJ, Petsalaki E, James DA, Bagshaw RD, Stacey MM, Rocks O, et al. (2014). Src homology 2 domain containing protein 5 (SH2D5) binds the breakpoint cluster region protein, BCR, and regulates levels of Rac1-GTP. J Biol Chem, 289:35397-35408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang Z, Jin T, Le Q, Liu C, Wang X, Wang F, et al. (2020). Retrieval-Driven Hippocampal NPTX2 Plasticity Facilitates the Extinction of Cocaine-Associated Context Memory. Biol Psychiatry, 87:979-991. [DOI] [PubMed] [Google Scholar]
- [109].Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, et al. (2017). NPTX2 and cognitive dysfunction in Alzheimer's Disease. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Negro A, Martelletti P (2011). Chronic migraine plus medication overuse headache: two entities or not? J Headache Pain, 12:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Terlizzi R, Bacalini MG, Pirazzini C, Giannini G, Pierangeli G, Garagnani P, et al. (2018). Epigenetic DNA methylation changes in episodic and chronic migraine. Neurol Sci, 39:67-68. [DOI] [PubMed] [Google Scholar]
- [112].Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF (2008). Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci, 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sugiura L, Toyota T, Matsuba-Kurita H, Iwayama Y, Mazuka R, Yoshikawa T, et al. (2017). Age-Dependent Effects of Catechol-O-Methyltransferase (COMT) Gene Val158Met Polymorphism on Language Function in Developing Children. Cereb Cortex, 27:104-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Turan B, Sims T, Best SE, Carstensen LL (2016). Older age may offset genetic influence on affect: The COMT polymorphism and affective well-being across the life span. Psychol Aging, 31:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kwiatkowska KM, Bacalini MG, Sala C, Kaziyama H, de Andrade DC, Terlizzi R, et al. (2020). Analysis of Epigenetic Age Predictors in Pain-Related Conditions. Frontiers in Public Health, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gerring ZF, McRae AF, Montgomery GW, Nyholt DR (2018). Genome-wide DNA methylation profiling in whole blood reveals epigenetic signatures associated with migraine. BMC Genomics, 19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ayka A, Şehirli A (2020). The Role of the SLC Transporters Protein in the Neurodegenerative Disorders. Clin Psychopharmacol Neurosci, 18:174-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu J, Yang W, Li Y, Wei Z, Dan X (2020). ABCG2 rs2231142 variant in hyperuricemia is modified by SLC2A9 and SLC22A12 polymorphisms and cardiovascular risk factors in an elderly community-dwelling population. BMC Med Genet, 21:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kai M, Yamamoto E, Sato A, Yamano HO, Niinuma T, Kitajima H, et al. (2017). Epigenetic silencing of diacylglycerol kinase gamma in colorectal cancer. Mol Carcinog, 56:1743-1752. [DOI] [PubMed] [Google Scholar]
- [120].Stanton CM, Yates JR, den Hollander AI, Seddon JM, Swaroop A, Stambolian D, et al. (2011). Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci, 52:8828-8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xu J, Liu L, Ma R, Wang Y, Chen X, Liu H, et al. (2020). E2F1 Induces KIF26A Transcription and Promotes Cell Cycle Progression via CDK-RB-E2Fs Feedback Loop in Breast Cancer. Front Oncol, 10:530933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang ZY, Sun YY, Wang HC, Fu WN, Sun CF (2022). Overexpression of DOCK6 in oral squamous cell cancer promotes cellular migration and invasion and is associated with poor prognosis. Arch Oral Biol, 133:105297. [DOI] [PubMed] [Google Scholar]
- [123].Wan D, Hou L, Zhang X, Han X, Chen M, Tang W, et al. (2015). DNA methylation of RAMP1 gene in migraine: an exploratory analysis. J Headache Pain, 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Li Y-Y, O W-S, Tang F (2007). Effect of Aging on the Expression of Adrenomedullin and Its Receptor Component Proteins in the Male Reproductive System of the Rat. The Journals of Gerontology: Series A, 62:1346-1351. [DOI] [PubMed] [Google Scholar]
- [125].Fila M, Sobczuk A, Pawlowska E, Blasiak J (2022). Epigenetic Connection of the Calcitonin Gene-Related Peptide and Its Potential in Migraine. Int J Mol Sci, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rubino E, Boschi S, Giorgio E, Pozzi E, Marcinnò A, Gallo E, et al. (2022). Analysis of the DNA methylation pattern of the promoter region of calcitonin gene-related peptide 1 gene in patients with episodic migraine: An exploratory case-control study. Neurobiology of Pain, 11:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Xu M, Yan Y, Zhu M, Wang Z, Zhang X, Zhang D (2020). Effects of long non-coding RNA Gm14461 on pain transmission in trigeminal neuralgia. J Inflamm (Lond), 17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Park KY, Fletcher JR, Raddant AC, Russo AF (2011). Epigenetic regulation of the calcitonin gene-related peptide gene in trigeminal glia. Cephalalgia, 31:614-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Greco R, De Icco R, Demartini C, Zanaboni AM, Tumelero E, Sances G, et al. (2020). Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: towards the identification of a panel of peripheral biomarkers of migraine? The Journal of Headache and Pain, 21:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Deng T, Yang L, Zheng Z, Li Y, Ren W, Wu C, et al. (2017). Calcitonin gene-related peptide induces IL-6 expression in RAW264.7 macrophages mediated by mmu_circRNA_007893. Mol Med Rep, 16:9367-9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bertels Z, Singh H, Dripps I, Siegersma K, Tipton AF, Witkowski WD, et al. (2021). Neuronal complexity is attenuated in preclinical models of migraine and restored by HDAC6 inhibition. Elife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Guo X, Chen D, An S, Wang Z (2020). ChIP-seq Profiling Identifies Histone Deacetylase 2 Targeting Genes Involved in Immune and Inflammatory Regulation Induced by Calcitonin Gene-Related Peptide in Microglial Cells. J Immunol Res, 2020:4384696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, et al. (2014). TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell, 157:1023-1036. [DOI] [PubMed] [Google Scholar]
- [134].Wrigglesworth J, Ward P, Harding IH, Nilaweera D, Wu Z, Woods RL, et al. (2021). Factors associated with brain ageing - a systematic review. BMC Neurol, 21:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Morton RE, St John PD, Tyas SL (2019). Migraine and the risk of all-cause dementia, Alzheimer's disease, and vascular dementia: A prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry, 34:1667-1676. [DOI] [PubMed] [Google Scholar]
- [136].Qu H, Yang S, Yao Z, Sun X, Chen H (2022). Association of Headache Disorders and the Risk of Dementia: Meta-Analysis of Cohort Studies. Front Aging Neurosci, 14:804341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sharif S, Saleem A, Koumadoraki E, Jarvis S, Madouros N, Khan S (2021). Headache - A Window to Dementia: An Unexpected Twist. Cureus, 13:e13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gifford KA, Phillips JS, Samuels LR, Lane EM, Bell SP, Liu D, et al. (2015). Associations between Verbal Learning Slope and Neuroimaging Markers across the Cognitive Aging Spectrum. J Int Neuropsychol Soc, 21:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gil-Gouveia R, Martins IP (2018). Clinical description of attack-related cognitive symptoms in migraine: A systematic review. Cephalalgia, 38:1335-1350. [DOI] [PubMed] [Google Scholar]
- [140].Vuralli D, Ayata C, Bolay H (2018). Cognitive dysfunction and migraine. The Journal of Headache and Pain, 19:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wen K, Nguyen NT, Hofman A, Ikram MA, Franco OH (2016). Migraine is associated with better cognition in the middle-aged and elderly: the Rotterdam Study. Eur J Neurol, 23:1510-1516. [DOI] [PubMed] [Google Scholar]
- [142].Martins IP, Maruta C, Alves PN, Loureiro C, Morgado J, Tavares J, et al. (2020). Cognitive aging in migraine sufferers is associated with more subjective complaints but similar age-related decline: a 5-year longitudinal study. The Journal of Headache and Pain, 21:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rist PM, Dufouil C, Glymour MM, Tzourio C, Kurth T (2011). Migraine and cognitive decline in the population-based EVA study. Cephalalgia, 31:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Morton RE, St. John PD, Tyas SL (2019). Migraine and the risk of all-cause dementia, Alzheimer's disease, and vascular dementia: A prospective cohort study in community-dwelling older adults. International Journal of Geriatric Psychiatry, 34:1667-1676. [DOI] [PubMed] [Google Scholar]
- [145].Kostev K, Bohlken J, Jacob L (2019). Association Between Migraine Headaches and Dementia in More than 7,400 Patients Followed in General Practices in the United Kingdom. Journal of Alzheimer's Disease, 71:353-360. [DOI] [PubMed] [Google Scholar]
- [146].Islamoska S, Hansen ÅM, Wang H-X, Garde AH, Andersen PK, Garde E, et al. (2020). Mid- to late-life migraine diagnoses and risk of dementia: a national register-based follow-up study. The Journal of Headache and Pain, 21:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Huang L, juan Dong H, Wang X, Wang Y, Xiao Z (2017). Duration and frequency of migraines affect cognitive function: evidence from neuropsychological tests and event-related potentials. The Journal of Headache and Pain, 18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Costa-Silva MA, Prado ACA, de Souza LC, Gomez RS, Teixeira AL (2016). Cognitive functioning in adolescents with migraine. Dement Neuropsychol, 10:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Termine C, Bartoli B, Agosti MA, Cavanna AE, Balottin U (2018). Cognitive Impairment in Children and Adolescents With Migraine. Frontiers in Neurology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Echiverri K, Jicha GA, Smith JH (2018). Age-Related Changes in Headache Days across the Cognitive Spectrum. Pain Med, 19:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].van Beek JH, Kirkwood TB, Bassingthwaighte JB (2016). Understanding the physiology of the ageing individual: computational modelling of changes in metabolism and endurance. Interface Focus, 6:20150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Barzilai N, Huffman DM, Muzumdar RH, Bartke A (2012). The critical role of metabolic pathways in aging. Diabetes, 61:1315-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Fang Y, Wang X, Yang D, Lu Y, Wei G, Yu W, et al. (2021). Relieving Cellular Energy Stress in Aging, Neurodegenerative, and Metabolic Diseases, SIRT1 as a Therapeutic and Promising Node. Front Aging Neurosci, 13:738686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kokavec A (2016). Migraine: A disorder of metabolism? Medical Hypotheses, 97:117-130. [DOI] [PubMed] [Google Scholar]
- [155].Cuenoud B, Ipek Ö, Shevlyakova M, Beaumont M, Cunnane SC, Gruetter R, et al. (2020). Brain NAD Is Associated With ATP Energy Production and Membrane Phospholipid Turnover in Humans. Front Aging Neurosci, 12:609517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Younis S, Hougaard A, Vestergaard MB, Larsson HBW, Ashina M (2017). Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol, 30:246-262. [DOI] [PubMed] [Google Scholar]
- [157].Reyngoudt H, Achten E, Paemeleire K (2012). Magnetic resonance spectroscopy in migraine: What have we learned so far? Cephalalgia, 32:845-859. [DOI] [PubMed] [Google Scholar]
- [158].Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J (2019). The metabolic face of migraine — from pathophysiology to treatment. Nature Reviews Neurology, 15:627-643. [DOI] [PubMed] [Google Scholar]
- [159].Del Moro L, Rota E, Pirovano E, Rainero I (2022). Migraine, Brain Glucose Metabolism and the “Neuroenergetic” Hypothesis: A Scoping Review. The Journal of Pain, 23:1294-1317. [DOI] [PubMed] [Google Scholar]
- [160].Hölscher C (2019). Insulin Signaling Impairment in the Brain as a Risk Factor in Alzheimer's Disease. Front Aging Neurosci, 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cuenoud B, Ipek Ö, Shevlyakova M, Beaumont M, Cunnane SC, Gruetter R, et al. (2020). Brain NAD Is Associated With ATP Energy Production and Membrane Phospholipid Turnover in Humans. Frontiers in Aging Neuroscience, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ronchi JA, Francisco A, Passos LA, Figueira TR, Castilho RF (2016). The Contribution of Nicotinamide Nucleotide Transhydrogenase to Peroxide Detoxification Is Dependent on the Respiratory State and Counterbalanced by Other Sources of NADPH in Liver Mitochondria. J Biol Chem, 291:20173-20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kregel KC, Zhang HJ (2007). An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292:R18-R36. [DOI] [PubMed] [Google Scholar]
- [164].Borkum JM (2018). The Migraine Attack as a Homeostatic, Neuroprotective Response to Brain Oxidative Stress: Preliminary Evidence for a Theory. Headache, 58:118-135. [DOI] [PubMed] [Google Scholar]
- [165].Kozai D, Ogawa N, Mori Y (2014). Redox regulation of transient receptor potential channels. Antioxid Redox Signal, 21:971-986. [DOI] [PubMed] [Google Scholar]
- [166].Sies H (2017). Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol, 11:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, et al. (2018). TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain, 141:2312-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wang XL, Cui LW, Liu Z, Gao YM, Wang S, Li H, et al. (2019). Effects of TRPA1 activation and inhibition on TRPA1 and CGRP expression in dorsal root ganglion neurons. Neural Regen Res, 14:140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Jiang L, Ma D, Grubb BD, Wang M (2019). ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J Headache Pain, 20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, et al. (2015). Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal, 8:ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J (2019). The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol, 15:627-643. [DOI] [PubMed] [Google Scholar]
- [172].Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, et al. (2007). Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci, 10:754-762. [DOI] [PubMed] [Google Scholar]
- [173].Geyik S, Altunısık E, Neyal AM, Taysi S (2016). Oxidative stress and DNA damage in patients with migraine. J Headache Pain, 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gan W, Nie B, Shi F, Xu XM, Qian JC, Takagi Y, et al. (2012). Age-dependent increases in the oxidative damage of DNA, RNA, and their metabolites in normal and senescence-accelerated mice analyzed by LC-MS/MS: urinary 8-oxoguanosine as a novel biomarker of aging. Free Radic Biol Med, 52:1700-1707. [DOI] [PubMed] [Google Scholar]
- [175].Shi F, Nie B, Gan W, Zhou XY, Takagi Y, Hayakawa H, et al. (2012). Oxidative damage of DNA, RNA and their metabolites in leukocytes, plasma and urine of Macaca mulatta: 8-oxoguanosine in urine is a useful marker for aging. Free Radic Res, 46:1093-1098. [DOI] [PubMed] [Google Scholar]
- [176].Yu T, Slone J, Liu W, Barnes R, Opresko PL, Wark L, et al. (2022). Premature aging is associated with higher levels of 8-oxoguanine and increased DNA damage in the Polg mutator mouse. Aging Cell, 21:e13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Harder AVE, Vijfhuizen LS, Henneman P, Willems van Dijk K, van Duijn CM, Terwindt GM, et al. (2021). Metabolic profile changes in serum of migraine patients detected using 1H-NMR spectroscopy. The Journal of Headache and Pain, 22:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Bai YX, Fang F, Jiang JL, Xu F (2017). Extrinsic Calcitonin Gene-Related Peptide Inhibits Hyperoxia-Induced Alveolar Epithelial Type II Cells Apoptosis, Oxidative Stress, and Reactive Oxygen Species (ROS) Production by Enhancing Notch 1 and Homocysteine-Induced Endoplasmic Reticulum Protein (HERP) Expression. Med Sci Monit, 23:5774-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Dang HX, Li J, Liu C, Fu Y, Zhou F, Tang L, et al. (2017). CGRP attenuates hyperoxia-induced oxidative stress-related injury to alveolar epithelial type II cells via the activation of the Sonic hedgehog pathway. Int J Mol Med, 40:209-216. [DOI] [PubMed] [Google Scholar]
- [180].Holzmann B (2013). Antiinflammatory activities of CGRP modulating innate immune responses in health and disease. Curr Protein Pept Sci, 14:268-274. [DOI] [PubMed] [Google Scholar]
- [181].Fila M, Pawłowska E, Blasiak J (2019). Mitochondria in migraine pathophysiology - does epigenetics play a role? Arch Med Sci, 15:944-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kalache A, de Hoogh AI, Howlett SE, Kennedy B, Eggersdorfer M, Marsman DS, et al. (2019). Nutrition interventions for healthy ageing across the lifespan: a conference report. Eur J Nutr, 58:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Fila M, Chojnacki C, Chojnacki J, Blasiak J (2021). Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987). Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res, 18:551-561. [DOI] [PubMed] [Google Scholar]
- [185].Haslam RL, Bezzina A, Herbert J, Spratt N, Rollo ME, Collins CE (2021). Can Ketogenic Diet Therapy Improve Migraine Frequency, Severity and Duration? Healthcare (Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Valente M, Garbo R, Filippi F, Antonutti A, Ceccarini V, Tereshko Y, et al. (2022). Migraine Prevention through Ketogenic Diet: More than Body Mass Composition Changes. J Clin Med, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Rainero I (2015). Metabolism and headache. The Journal of Headache and Pain, 16:A33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].(2013). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia, 33:629-808. [DOI] [PubMed] [Google Scholar]
- [189].Al-Hassany L, Haas J, Piccininni M, Kurth T, Maassen Van Den Brink A, Rohmann JL (2020). Giving Researchers a Headache - Sex and Gender Differences in Migraine. Front Neurol, 11:549038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Loder E (2002). What is the evolutionary advantage of migraine? Cephalalgia, 22:624-632. [DOI] [PubMed] [Google Scholar]
- [191].Montagna P, Pierangeli G, Cortelli P (2010). The primary headaches as a reflection of genetic darwinian adaptive behavioral responses. Headache, 50:273-289. [DOI] [PubMed] [Google Scholar]
- [192].Karsan N, Goadsby PJ (2021). Migraine: beyond pain. Pract Neurol, 21:475-480. [DOI] [PubMed] [Google Scholar]
- [193].Cader MZ (2020). The genetics of migraine and the path to precision medicine. Prog Brain Res, 255:403-418. [DOI] [PubMed] [Google Scholar]
- [194].Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, et al. (2022). Migraine. Nat Rev Dis Primers, 8:2. [DOI] [PubMed] [Google Scholar]
- [195].Gerhard M, Roddy MA, Creager SJ, Creager MA (1996). Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension, 27:849-853. [DOI] [PubMed] [Google Scholar]
- [196].Mason BN, Russo AF (2018). Vascular Contributions to Migraine: Time to Revisit? Front Cell Neurosci, 12:233. [DOI] [PMC free article] [PubMed] [Google Scholar]



