To the editor,
Aging is related to cancer, and an increasing incidence of malignant tumors among the older population is observed [1]. Urinary tumors, such as prostate cancer, bladder cancer (BC), and renal cancer, frequently occur in the aging population [2-10]. Urothelial carcinoma (UC) ranks as the fourth most common tumor, with an estimated 160,000 new diagnoses and 31,000 deaths each year [11]. Due to the rarity of upper tract UC (5%-10%), high-level evidence is often scarce to provide strong recommendations, and in many cases, guidelines are extrapolated from existing evidence on bladder UC (90%-95%) [12, 13]. Muscle-invasive BC (MIBC) accounts for about 25% of newly diagnosed BC patients [14]. Non-MIBC (NMIBC) patients usually undergo transurethral resection followed by intravesical chemotherapy or Bacillus Calmette-Guérin (BCG) therapy, while MIBC cases are usually treated with radical cystectomy (RC) plus pelvic node dissection [6, 14-16]. However, MIBC is prone to relapse after RC, and only about 5% of metastatic cancer patients survive for at least 5 years post-diagnosis [17]. Thus, metastasis is one of the most important causes of death for such patients.
The standard of care for the treatment of metastatic UC for more than 20 years has been cisplatin-based combinations [18]. However, a number of sizable studies that looked at the effectiveness of immunotherapy employing immune checkpoint inhibitors (ICIs) have cast doubt on this time-honored strategy. Moreover, immunotherapy has shown significant progress in other solid tumors, such as lung cancer [19]. We aimed to summarize the many novel agents and trials for UC presented at the 2023 ASCO-GU Cancers Symposium.
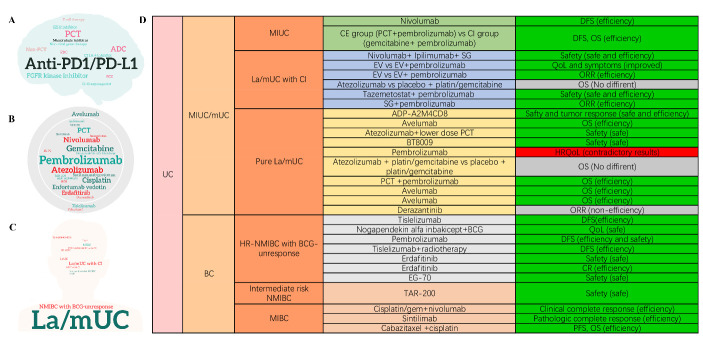
In terms of presented novel agents and trials for UC in this congress, PD-1/PD-L1-based ICIs (Pem-brolizumab, Atezolizumab, Nivolumab, and Avelumab), platinum-based chemotherapy (PCT), antibody-drug conjugates (ADCs), (Enfortumab vedotin (EV) and Sacituzumab govitecan (SG)), and fibroblast growth factor receptor kinase inhibitors (Erdafitinib and Derazantinib) were most presented (Fig. 1A-B). UC patients mainly focused on locally advanced or metastatic UC (la/mUC), NMIBC, and MIBC (Fig. 1C). We summarized these trials in Supplementary Table 1 and described several interesting trials here.
Figure 1.
Novel agents and trials for UC. (A) word cloud showing medication clusters; (B) word cloud showing specific treatments; (C) word cloud showing patient types; (D) summary of novel agents and trials for UC in this meeting. UC: urothelial carcinoma; MIBC: muscle-invasive bladder cancer; BCG: Bacillus Calmette Guérin; EV: enfortumab vedotin; la/mUC: locally advanced or metastatic UC; SG: Sacituzumab govitecan PCT: platinum-based chemotherapy; ORR: objective response rate; MIUC: muscle-invasive urothelial carcinoma; PFS: progression-free survival; OS: overall survival; HR: high-risk. QoL: quality of life; HRQoL: health-related QoL.
EV-103 Cohort K (NCT03288545) evaluated first-line EV and Pembrolizumab or EV alone in patients with la/mUC who were cisplatin-ineligible. EV and Pembrolizumab led to EV dose reduction and showed a clinically meaningful objective response rate (ORR: 64.5%; 95% CI, 52.7-75.1) with a manageable safety profile compared to EV alone (45.2%; 95% CI: 33.5-57.3). Moreover, EV and Pembrolizumab contributed to clinically meaningful reductions in quality of life (QoL). Based on these results, three Phase 3 trials (NCT04223856, NCT04700124, NCT03924895) are being conducted for EV and Pembrolizumab in the first-line la/mUC and MIBC patients.
IMVIGOR 130 phase III trial (NCT02807636) reported final overall survival (OS) data, showing that improved OS with Atezolizumab plus platinum/ gemcitabine vs placebo plus platinum/gemcitabine did not reach statistical significance in patients with la-/mUC. Two large Cohort trials (Javelin Bladder100 (NCT02603432) and NCT04822350) supported the usage of Avelumab as maintenance therapy in la/mUC patients without progression after PCT due to benefits of OS. In this meeting, CheckMate 274 trial (NCT02632409) reported extended follow-up data, indicating that Nivolumab continued to show disease-free survival (DFS), non-urothelial tract recurrence-free survival, and distant metastasis-free survival benefits versus placebo.
Cohort B of KEYNOTE-057 (NCT02625961) reported that Pembrolizumab monotherapy showed notable antitumor activity in patients with BCG-unresponsive non-CIS papillary high-risk (HR) NMIBC after ~45 months of follow-up (12-month DFS rate: 43.5 (34.9-51.9); 12-month OS rate: 96.2 (91.1-98.4)). FIDES-02 study (NCT04045613) explored the activity of Derazantinib in patients with mUC and FGFR1-3 genetic aberrations. Confirmed ORR was 8.2% (95% CI 2.2, 19.6) and the disease control rate was 28.6% (95% CI: 16.6, 43.3). Other emerging agents showed various promising results and deserve expectation in the future. The drugs and trials were presented in Figure 1D.
For trials in progress, researchers still mainly concentrate on patients with mUC, HR BCG-unresponsive NMIBC or MIBC. What's different from the published trials is that researchers are commencing on investigating the effect of ADCs with or without immunotherapy on such patients, suggesting that ADCs are receiving more attention. The ongoing trials are summarized in Table 1.
Table 1.
Trials in progress for UC.
| Author | Country | Clinical trials | Patients | Therapeutic regimen | Patient feature |
|---|---|---|---|---|---|
| Po-Jung Su et al. | Asia-Pacific (APAC) region | NA | 286 | Avelumab first-line (1L) maintenance after 1L platinum-based chemotherapy | Unresectable locally advanced or metastatic (stage IV) measurable UC |
| Massimo Lazzeri et al. | Italy | (EudraCT 2021-003751-42_studio ICH-013 (MMC)) | 160 | Neoadjuvant MMC vs standard of care (RCT) | Primary treatment-naive NMIBC |
| Christopher Gaffney et al. | USA | (NCT04179162) | 25 | Gemcitabine and BCG | BCG-relapsed/BCG-exposed NMIBC |
| Sarmad Sadeghi et al. | USA | (NCT04579224) | 465 | Randomized 3 arm study comparing eribulin vs. gemcitabine plus eribulin vs. SOC (docetaxel, paclitaxel, or gemcitabine) | Metastatic UC refractory to or ineligible for PD/PDL1 antibody (Ab) |
| Ashish M. Kamat et al. | International | (NCT05014139) | NA | Intravesical enfortumab vedotin | High-risk BCG-unresponsive NMIBC |
| Neal D. Shore et al. | International | (NCT02625961) | 60 | Pembrolizumab and vibostolimab vs pembrolizumab and favezelimab (RCT) | High-risk BCG-unresponsive NMIBC |
| Guru P. Sonpavde et al. | USA | (NCT05574504) | 36 | Lurbinectedin plus avelumab as maintenance therapy | Metastatic UC with stable or responding disease following platinum-based chemotherapy |
| Himanshu Nagar et al. | USA | (NCT04936230) | 144 | Atezolizumab versus atezolizumab and radiation therapy (RCT) |
Platinum-ineligible/refractory metastatic UC |
| Andrea Necchi et al. | International | (NCT03924895) | 857 | Neoadjuvant pembrolizumab plus RC+PLND plus adjuvant pembrolizumab vs RC+PLND vs Neoadjuvant pembrolizumab+enfortumab vedotin plus RC+PLND plus adjuvant pembrolizumab+enfortumab vedotin (RCT) | Cisplatin ineligible or decline cisplatin-based treatment with treatment-naive MIBC |
| Evan Y. Yu et al. | International | (NCT04579380) | 30 | Tucatinib and trastuzumab | HER2+ locally advanced or metastatic disease, with progression during or after, or intolerance of, the most recent line of systemic therapy. |
| Christopher J et al. | International | (NCT04700124) | 784 | Neoadjuvant enfortumab vedotin + pembrolizumabo plus RC+PLND plus adjuvant enfortumab vedotin+ pembrolizumab vs neoadjuvant chemotherapy [gemcitabine + cisplatin] plus RC+PLND) (RCT) | Cisplatin-eligible patients with MIBC |
| Jean H. Hoffman-Censits et al. | (NCT05312671) | 17-63 | Neoadjuvant atezolizumab+etoposide+investigator choice cisplatin or carboplatin chemotherapy+ cystectomy+ adjuvant atezolizumab | Localized small cell neuroendocrine bladder cancer | |
| Thomas Powles et al. | International | (NCT03547973) | 158 | Sacituzumab govitecan plus zimberelimab vs avelumab vs zimberelimab (RCT) | Cisplatin-eligible patients with unresectable or metastatic UC |
| Ignacio Duran et al. | International | (NCT03547973) | 226 | Sacituzumab govitecan (SG), SG plus zimberelimab (ZIM), SG plus ZIM plus domvanalimab, or carboplatin + gemcitabine (RCT) |
Cisplatin ineligible patients with treatment-naive metastatic UC |
| Benjamin Garmezy et al. | USA | (NCT05242822) | 120 | KIN-3248, a next-generation, irreversible pan-FGFR inhibitor |
Advanced UC harboring FGFR2 and/or FGFR3 gene alterations. |
| Thomas Powles et al. | International | (NCT04879329) | NA | Disitamab vedotin with or without pembrolizumab | HER2-expressing UC |
| Andrea Necchi et al. | International | NA | 30 | Neoadjuvant nivolumab + visugromab vs nivolumab + Placebo | MIBC |
| Seth P. Lerner et al. | USA | (NCT05483868) | 23 | Intramural injection - belzupacap sarotalocan vs intratumoral injection - belzupacap sarotalocan+Laser vs post-injection cystectomy vs post-Injection TURBT | NMIBC |
| Gopa Iyer et al. | USA | (NCT05103358) | NA | Albumin-bound (nab)-sirolimus, a novel mTOR inhibitor (mTORi) | Patients with alterations in TSC1 (Arm A) and TSC2 (Arm B). |
IL: first line; RCT: randomized controlled trials; NMIBC: non-muscle-invasive bladder cancer; MIBC: muscle-invasive bladder cancer; TURBT: transurethral resection of bladder tumor; UC: urothelial carcinoma; RC: radical cystectomy; PLND: pelvic lymph node dissection; MMC: mitomycin C. BCG: Bacillus Calmette Guérin.
Median OS for advanced UC patients undergoing PCT followed by immunotherapy is still smaller than 1 year and there is an urgent need for alternative therapies[20]. With the advent of next-generation sequencing, The Cancer Genome Atlas project reported comprehensive molecular alterations of high-grade MIUC and found that numerous genomic aberrations, such as FGFR3 (15%), HER2 (8%), and EGFR (6%)[20-22]. The presence of multiple oncogenic alterations in UC, together with the low efficacy of first-line immunotherapy in this scenario, suggests that oncogenic alterations may have potential as a predictive biomarker for therapeutic decision-making. In this case, ADCs may offer new avenues for biomarker-driven treatment in advanced UC, especially for patients with oncogenic alterations [22]. In addition, rapid advancements have taken place in gene therapy technology and gene therapy that targets genes related to aging represents an exciting research direction with tremendous potential[23]. Nanomedicines have been widely studied in cancer therapy in recent years [24].
Based on the current evidence and the advancements in this meeting, we proposed the following management strategy of UC. PCT is the first-line standard therapy for all patients who are candidates for either cisplatin or carboplatin. Patients positive for PD-L1 and ineligible for cisplatin may receive immunotherapy (Atezolizumab or Pembrolizumab) or EV and Pembrolizumab, among which Erdafitinib could be considered in la/mUC patients with FGFR alterations. If the disease does not progress after PCT, maintenance immunotherapy (Avelumab) is suggested. Immuno-therapy (Pembrolizumab) is the recommended second-line therapy for patients who do not have maintenance therapy. Later-line treatments like Derazantinib, EZH2 inhibitor (Tazemetostat), T-cell therapy (ADP-A2M 4CD8) and non-viral gene therapy (EG-70) could be tried in specific cases with the patient’s consent. Pembrolizumab monotherapy might be recommended in patients with HR NMIBC unresponsive to BCG who declined or were ineligible to undergo radical resection. In future trials, oncogenic alterations can be considered in trial designs and PCT-based target therapy or ADCs with immunotherapy should be conducted in more mUC patients or MIBC candidates for RC.
Supplementary Materials
The Supplementary data can be found online at: www.aginganddisease.org/EN/10.14336/AD.2023.0502.
Acknowledgement
This program was supported by the National Key Research and Development Program of China (2021YFC2009303), Project of Health Commission of Sichuan Province (21PJ041) and the Key Research and Development Support Plan of Chengdu Science and Technology Bureau (2022-YF05-01568-SN). The funders had no role in the study design, data collection or analysis, preparation of the manuscript, or the decision to publish.
Funding Statement
This program was supported by the National Key Research and Development Program of China (2021YFC2009303), Project of Health Commission of Sichuan Province (21PJ041) and the Key Research and Development Support Plan of Chengdu Science and Technology Bureau (2022-YF05-01568-SN). The funders had no role in the study design, data collection or analysis, preparation of the manuscript, or the decision to publish.
References
- [1].Shen W, He J, Hou T, Si J, Chen S (2022). Common Pathogenetic Mechanisms Underlying Aging and Tumor and Means of Interventions. Aging Dis, 13:1063-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feng D, Bai Y, Liu S, Yang Y, Han P, Wei W (2021). Risk of renal cancer in patients with inflammatory bowel disease: A pooled analysis of population-based studies. Urol Oncol, 39:93-9. [DOI] [PubMed] [Google Scholar]
- [3].Feng D, Li D, Shi X, et al. (2022). A gene prognostic index from cellular senescence predicting metastasis and radioresistance for prostate cancer. J Transl Med, 20:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feng D, Shi X, You J, Wei Q, et al. (2022). A cellular senescence-related gene prognostic index for biochemical recurrence and drug resistance in patients with prostate cancer. Am J Cancer Res, 12:3811-28. [PMC free article] [PubMed] [Google Scholar]
- [5].Feng D, Shi X, Zhang F, Xiong Q, Wei Q, Yang L (2022). Energy Metabolism-Related Gene Prognostic Index Predicts Biochemical Recurrence for Patients With Prostate Cancer Undergoing Radical Prostatectomy. Front Immunol, 13:839362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feng D, Tang Y, Yang Y, Han P, Wei W (2020). Intracorporeal versus extracorporeal urinary diversion after robotic-assisted radical cystectomy: evidence from a systematic review and pooled analysis of observational studies. Minerva Urol Nefrol, 72:519-30. [DOI] [PubMed] [Google Scholar]
- [7].Feng D, Zhu W, Shi X, et al. (2023). Immune-related gene index predicts metastasis for prostate cancer patients undergoing radical radiotherapy. Exp Hematol Oncol, 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feng D, Zhu W, Shi X, et al. (2022). Spindle and kinetochore-associated complex subunit 3 could serve as a prognostic biomarker for prostate cancer. Exp Hematol Oncol, 11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feng DC, Zhu WZ, Shi X, Xiong Q, You J, Wei Q, et al. (2022). Identification of senescence-related molecular subtypes and key genes for prostate cancer. Asian J Androl, 25:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weitao Zheng DF, Xingyu Xiong, et al. (2023). The Role of cGAS-STING in Age-Related Diseases from Mechanisms to Therapies. Aging Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siegel RL, Miller KD, Fuchs HE, Jemal A (2022). Cancer statistics, 2022. CA Cancer J Clin, 72:7-33. [DOI] [PubMed] [Google Scholar]
- [12].Freifeld Y, Krabbe LM, Clinton TN, Woldu SL, Margulis V (2018). Therapeutic strategies for upper tract urothelial carcinoma. Expert Rev Anticancer Ther, 18(8):765-74. [DOI] [PubMed] [Google Scholar]
- [13].Roupret M, Babjuk M, Burger M, et al. (2021). European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol, 79:62-79. [DOI] [PubMed] [Google Scholar]
- [14].Liu S, Chen X, Lin T (2022). Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J Adv Res, 39:187-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feng D, Li A, Hu X, Lin T, Tang Y, Han P (2020). Comparative effectiveness of open, laparoscopic and robot-assisted radical cystectomy for bladder cancer: a systematic review and network meta-analysis. Minerva Urol Nefrol, 72:251-64. [DOI] [PubMed] [Google Scholar]
- [16].Shih KW, Chen WC, Chang CH, Tai TE, Wu JC, Huang AC, et al. (2021). Non-Muscular Invasive Bladder Cancer: Re-envisioning Therapeutic Journey from Traditional to Regenerative Interventions. Aging Dis, 12(3):868-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feng D, Bai Y, Yang Y, Han P, Wei W (2020). Clinicopathological characteristics and treatment outcomes of 162 Chinese patients with metastatic bladder cancer: results from a tertiary teaching hospital. Transl Cancer Res, 9:4870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cathomas R, Lorch A, Bruins HM, Comperat EM, et al. (2022). The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur Urol, 81:95-103. [DOI] [PubMed] [Google Scholar]
- [19].Xing S, Hu K, Wang Y (2022). Tumor Immune Microenvironment and Immunotherapy in Non-Small Cell Lung Cancer: Update and New Challenges. Aging Dis, 13:1615-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katims AB, Reisz PA, Nogueira L, et al. (2022). Targeted Therapies in Advanced and Metastatic Urothelial Carcinoma. Cancers, 14:5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ikeda S, Hansel DE, Kurzrock R. Beyond conventional chemotherapy: Emerging molecular targeted and immunotherapy strategies in urothelial carcinoma. Cancer Treat Rev. 2015. Sep; 41(8):699-706. [DOI] [PubMed] [Google Scholar]
- [22].Kartolo A, Robinson A, Vera Badillo FE (2023). Can Oncogenic Driver Alterations be Responsible for the Lack of Immunotherapy Efficacy in First-line Advanced Urothelial Carcinoma? Eur Urol, 83(1):1-2. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Li T, Zhu J (2023). Gene Therapy Strategies Targeting Aging-Related Diseases. Aging Dis, 14:398-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zheng YH CT, Han X, Cao P, Zhan QC (2022). Structurally diverse polydopamine-based nanomedicines for cancer therapy. Acta Materia Medica, 4:427-44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.