Abstract
Interleukin-(IL)-11 is a cytokine involved in hematopoiesis, cancer metastasis, and inflammation. IL-11 belongs to the IL-6 cytokine family, binding to the complex of receptors glycoprotein gp130 and the ligand-specific-receptor subunits (IL-11Rα or their soluble counterpart sIL-11R). IL-11/IL-11R signaling enhances osteoblast differentiation and bone formation and mitigates osteoclast-induced bone resorption and cancer bone metastasis. Recent studies have shown that systemic and osteoblast/osteocyte-specific IL-11 deficiency leads to reduced bone mass and formation, but also adiposity, glucose intolerance, and insulin resistance. In humans, mutations of IL-11 and the receptor IL-11RA genes are associated with height reduction, osteoarthritis, and craniosynostosis. In this review, we describe the emerging role of IL-11/IL-11R signaling in bone metabolism by targeting osteoblasts, osteoclasts, osteocytes, and bone mineralization. Furthermore, IL-11 promotes osteogenesis and suppresses adipogenesis, thereby influencing the fate of osteoblast/adipocyte differentiation derived from pluripotent mesenchymal stem cells. We have newly identified IL-11 as a bone-derived cytokine that regulates bone metabolism and the link between bone and other organs. Thus, IL-11 is vital in bone homeostasis and could be considered a potential therapeutic strategy.
Keywords: Interleukin (IL)-11, bone formation, osteoblast differentiation, osteoclast, bone-derived hormones, adipogenesis
Introduction
Interleukin-11 (IL-11) is a member of the cytokine interleukin IL-6 family. It binds to the receptor complex of a ligand-specific receptor subunit (IL-11Rα or its soluble counterpart sIL-11R) and the transmembrane glycoprotein β-subunit gp130. It shares gp130 with the other cytokines from the IL-6 family (IL-6, leukemia inhibitor factor (LIF), oncostatin M (OSM), and ciliary neurotrophic factor (CNTF)) [1].
IL-11 is involved in hematopoiesis. In synergy with other cytokines, it promotes bone marrow hematopoiesis, megakaryocyte maturation, and platelet formation [2]. It is currently used as a platelet-stimulating factor in clinical practice. Recombinant IL-11 (Oprelvekin) has been approved by the US Food and Drug Administration for the treatment of thrombocytopenia in humans [3]. IL-11 has a potential therapeutic effect on inflammatory bowel disease [4] and rheumatoid arthritis based on its anti-inflammatory effects as revealed by small-scale clinical and animal studies [5, 6]. Recently, the role of IL-11 in various physiological and pathological processes has been investigated.
In this review, we aimed at updating and summarizing the role of IL-11 in bone metabolism and homeostasis, including bone development, formation and resorption, and cancer bone metastasis. We identified IL-11 as a novel bone-derived cytokine that affects bone remodeling and the link between bone and other organs. It enhances osteoblastogenesis and suppresses adipogenesis. Thus, this insight provides a novel perspective on the therapeutic potentials of IL-11.
Structure of IL-11/IL-11R
IL-11 is secreted by several mesenchymal-origin cells, including osteoblasts, osteoclasts, chondrocytes, fibroblasts, leukocytes, epithelial cells, keratinocytes, and synoviocytes [7]. The protein is encoded by the IL-11 gene, which contains five coding exons and four introns and is located on chromosome 19q13. The promoter region of IL-11 contains binding sites for several transcriptional factors, including activator protein-1 (AP-1), Runt-related transcription factor 2 (Runx2), and Smad [8]. The transcription and expression of IL-11 are mainly regulated by extracellular signal-regulated kinases (ERKs) and p38 mitogen-activated protein kinase (MAPK) signaling pathways via the AP-1 family of transcriptional factors [9, 10].
IL-11 binds to IL-11Rα and sIL-11R, thereby influencing the physiological and pathological processes. The formation of IL-11/sIL-11R complexes two molecules of gp130 dimerization, leading to the activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) and MAPK signaling cascades. This procress is characterized as “trans-signaling” [11, 12]. ADAM10 is a metalloprotease that releases the IL-11R ectodomain from the cells. The serine proteases neutrophil elastase (NE) and autoantigen proteinase 3 (PR3) also cleave IL-11R, in combination with IL-11, and induce trans-signaling downstream [13]. In addition, IL-11 trans-signaling is actived via Rhomboid-Like 2 (RHBDL2)-derived sIL-11R [11]. Soluble forms of both IL-6 receptor (IL-6R) and IL-11Rα have been identified. Signaling can be initiated through membrane-bound IL-6R (classic signaling) and soluble forms of IL-6R (trans-signaling) [14, 15]. Transmembrane gp130 is involved in some cytokine-mediated cellular responses and acts as a signal-transducing receptor subunit. IL-11 “trans-presentation” signaling has been identified to occur through IL-11R binding to gp130. IL-11 binds to cleaved sIL-11R and induces cellular proliferation via gp130 in a STAT3-dependent manner. IL-11: sIL-11R takes the overlapping binding site of the common signal transduction gp130 to activate STAT3 and STAT1 via the JAK/STAT pathway [16,17]. Studies in vitro demonstrate that IL-11 promotes gastric tumorigenesis and affects fertility through classic signaling pathways [18, 19]. Therefore, the role of IL-11 trans signal transduction in vivo requires further investigation.
Downstream signaling of IL-11/IL-11R
IL-11 regulates cellular function mainly through three downstream signaling pathways: the JAK/STAT3, Ras/Raf/MAPK and phosphatidyl-inositol 3 kinase (PI3K/Akt) pathways [20-22].
JAK/STAT signaling is the most well-known downstream pathway of IL-11. IL-11 signal transduction that involves gp130 mediated by the activation of STAT3 and a relatively low level of STAT1 [1, 23]. After IL-11 binds to its receptors, JAKs form a complex with the adapter protein. STATs then become tyrosine-phosphorylated, leading to their dimerization and dissociation from the receptor complex. Activated JAK kinase may lead to tyrosine phosphorylation and stimulation of the STAT family transcriptional factors [22]. Tyrosine phosphorylation of STAT3 and STAT1 are induced by IL-11, though the latter occurs at higher concentrations in a dose-dependent manner [24]. In multiple sclerosis, the activation of STAT3 by IL-11 influences oligodendrocytes, whereas the activation of STAT1 predominates in DCs, leading to apoptosis and damping of the inflammatory response. STAT is phosphorylated by JAK on specific tyrosine residues, and STAT homo- and heterodimers dock via the SH2 domain [25]. Phosphorylated STATs dimerize, translocate into the nucleus, and trigger the transcriptional modulation of target genes [26].
IL-11 and its receptor IL-11R are expressed in fibroblasts. They drive fibrogenic protein synthesis via non-canonical ERK-dependent autocrine signaling [27, 28]. Administering a high concentration of rhIL-11 promotes the formation of the active GTP-bound form Ras and coupled with the growth factor receptor binding protein 2 (Grb2)/son of sevenless complex, thereby initiating the Ras signaling pathway in adipocytes [29]. This pathway promotes the activation of Raf kinases, which, in turn, phosphorylate MEK kinases resulting in MAPK activation and subsequent regulation of intracellular targets [30]. Thus, IL-11 is transduced in part through the Ras/Raf/MAPK signaling pathway [30, 31].
IL-11 also activates the PI3K/AKT pathway independent of the tyrosine phosphorylation of gp130. This mechanism is shared by IL-11 and IL-6. PI3K inhibitor and siRNA-STAT3 mitigate the expression of matrix metalloproteinase (MMP)-13 induced by rhIL-11, revealing the involvement of PI3K/AKT and JAK/STAT3 signal transduction pathways [32]. IL-11 increases cell proliferation via MEK- and PI3K-dependent signaling pathways, but attenuates cell apoptosis only through the PI3K-dependent signaling pathway [33]. The expression of p-Akt and the apoptosis-related proteins Bcl-2 (anti-apoptotic protein), Bcl-xl (anti-apoptotic protein), and Bax (pro-apoptotic protein) are downregulated in IL-11 knockdown cells, revealing that IL-11 activates the PI3K/Akt signaling pathway in the treatment of radiotherapy-resistant cervical cancer [32, 34]. IL-11 upregulates MMP-13 expression by activating PI3K, Akt, and AP-1 signaling pathways that subsequently enhances MMP-13-induced tumor metastasis [33]. Thus, IL-11 is involved in tumorigenesis via the PI3K/Akt pathway (Fig. 1). Furthermore, the PI3K/Akt pathway is activated by IL-11, particularly in cancer metastasis and tumorigenesis.
Figure 1.
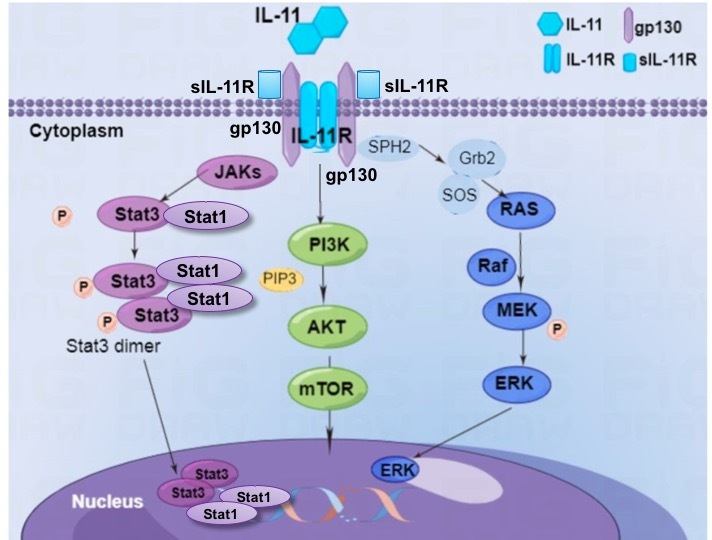
The signaling pathway downstream of IL-11/IL-11R. In classic signaling, IL-11 binds to the unique receptor IL-11Rα and signal-transducing receptor gp130. The IL-11/IL-11R complex recruits a homodimer of the signal-transducing receptor gp130, resulting in activation of downstream signaling. In trans-signaling, the gp130 dimer is induced by IL-11 bound to the soluble IL-11R (sIL-11R). After IL-11 binds to receptors, JAKs forms a complex with the adapter protein. The STATs then become tyrosine phosphorylated and translocates into the nucleus and trigger the transcriptional modulation of target genes. IL-11 drives non-canonical ERK-dependent autocrine signaling for fibrogenic protein synthesis, then promotes the formation of the active Grb2/SOS complex, then phosphorylate MEK kinases, and results in MAPK activation. IL-11 can also activate the PI3K/AKT pathway, independent of the tyrosine-phosphorylation of gp130, in tumor metastasis. The downstream signaling cascades are activated, including JAK/STAT1/STAT3, PI3K/Akt, and Ras/Raf/MAPK signaling in physiological and pathological condition, to promote target gene transcription.
The emerging role of IL-11 in bone homeostasis
IL-11 plays a vital role in bone development and remodeling [35]. Bone remodeling is essential for homeostasis and renewal and occurs in cortical and trabecular bones. It consists of two phases: bone formation and bone resorption. Osteoclasts lead to resorption of damaged bone, and osteoblasts induce bone formation. forming the multicellular unit termed as “bone remodeling unit”. The initial remodeling signal recruits osteoclast precursors to the remodeling site. In the following reversal phase, osteoclasts disappear, and osteoblasts replace osteoclasts in the formation phase. During the remodeling cycle, the resting bone surface is maintained until the next wave of remodeling. This complex interaction between osteoblast-associated bone formation and osteoclast-induced bone resorption is important for maintaining bone remodeling and homeostasis. The balance between bone formation and resorption is crucial for sustaining bone mass and homeostasis [36]. Bone remodeling is regulated by local and systemic factors, including estrogen, calcitonin, parathyroid hormone and 1,25(OH)2-vitamin D3, those hormones involving in bone resorption and formation [37]. In a recent study, IL-11 regulates all types of cells involved in bone homeostasis, including osteoblast, osteoclast, and osteocyte.
IL-11 in osteoblast, osteocyte, bone formation and bone mineralization
IL-11/IL-11R is involved in osteoblast-mediated osteogenesis, thereby influencing bone and skeletal structure and shape. IL-11R knockout (IL-11R-/-) mice exhibited a decline in osteoblasts count and bone formation, but showed an increase in trabecular bone volume [38]. In contrast, ex vivo differentiation of osteoblast precursors derived from the bone marrow of IL-11R-/- mice revealed similar osteoblast maturation or osteoblast-mediated mineralization. This indicates the involvement of other cells within the bone microenvironment [38]. Similar to the other subunits of the IL-11 receptor, gp130 knockout (Gp130-/-) mice also showed reduced osteoblast count and bone formation. However, they exhibited decreased trabecular bone volume [39]. Gp130-/- mice died perinatally and exhibited delayed bone development and short skeleton in the embryo [39]. Interestingly, a conditional knockout of gp130 in osteoblasts and osteocytes also resulted in decreased bone formation and reduced trabecular bone mass, despite a normal number of osteoblasts [40]. This reveals that bone formation and remodeling require IL-11 signaling through the Gp130/IL-11Rα receptor complex.
Recently, Dong et al. demonstrated that systemic IL-11 knockout (IL-11-/-) result in reduced vertebral and femoral bone mineral density (BMD), bone formation, osteoblast count, and expression of osteoblastogenic genes. However, the osteoclast count, serum bone resorption markers, and expression of osteoclastogenic genes were comparable to those in WT mice. Thus, the reduced bone mass in IL-11-/- mice results from decreased bone formation, but not bone resorption. In addition, IL-11-/- mice showed suppressed bone formation in response to mechanical loading due to the enhanced expression of Wnt inhibitors and suppression of Wnt signaling. Furthermore, general adiposity and bone marrow adipose tissue were increased in IL-11-/- mice [41]. Osteoblast/osteocyte-specific IL-11 deletion in osteocalcin-Cre; IL-11fl/fl mice resulted in reduced serum IL-11 levels, blunted bone formation under mechanical loading, and increased systemic adiposity, similar to systemic IL-11-/- mice. Moreover, systemic and osteoblast/osteocyte-specific IL-11 deficiencies led to the reduced bone mass and bone formation in response to mechanical loading, as well as increased adiposity, glucose intolerance, and insulin resistance [41]. Therefore, bone-derived IL-11 acts as an osteokine, participating in systemic regulation of bone metabolism and other organs. On contrast, IL-11 overexpressed-transgenic (Tg) mice exhibited enhanced bone formation, increased cortical thickness and bone strength, indicating that IL-11 is involved in endochondral bone formation [21, 42]. IL-11 Tg mice showed greater bone mass preservation and resistance to age-related bone loss [42] (Table 1). IL-11-/- mice showed reduced bone mass, osteoblast count, and bone formation, similar to the phenotypes of gp130-/-mice, but contrary to the phenotypes of IL-11 Tg mice. However, IL-11R-/- mice showed increased bone mass. These differences as well as the mechanisms underlying them are unclear. This could be because IL-11R null compensates the other subunit gp130 function, or it alters its competition for the shared subunit. However, further investigation is required to ascertain this hypothesis.
Table 1.
The phenotypes of mutant IL-11 ligand and receptors in animal models and human diseases.
| Bone mass | Bone formation | Bone resorption | Adiposity | Human diseases | ||
|---|---|---|---|---|---|---|
| Ligand IL-11 | ||||||
| IL-11 deletion | IL-11-/- global [41] | Bone mass↓ | Bone formation↓ Osteoblast cell number↓ Osteoblast differentiation ↓ |
Bone resorption→ Osteoclast Number→ |
Systemic AT↑ Bone marrow adiposity↑ Impaired glucose tolerance and insulin resistance |
Height↓ [96-98] |
| Osteoblast specific IL-11-/- (Ocn-Cre) [41] | Bone mass↓ | Bone formation↓ Osteoblastogenesis↓ |
Bone resorption→ Osteoclast Number→ |
Systemic AT↑ Impaired glucose tolerance and insulin resistance |
-- | |
| Adipocyte specific IL-11-/- (Adipo-Cre) [41] | Trabecular and cortical BMD→ | Bone formation→ | Bone resorption→ | Adipose tissue→ Normal glucose metabolism |
-- | |
| IL-11 overexpression | IL-11 Transgenic [42] | Bone mass↑ against aging, cortical thickness↑ | Bone formation↑ Osteoblast number↑ Osteoblastogenesis↑ and bone mineralization↑ ex vivo |
Bone resorption→ Osteoclast Number→ |
Adipogenesis↓ from BMSC ex vivo | -- |
| Receptor | ||||||
| IL-11Receptor | IL-11R null [7,35,38] | Trabecular bone mass↑ Bone length↓ |
Bone formation↓ Osteoblast number↓ Osteoblast differentiation→ in vitro |
Bone resorption↓ osteoclastogenesis↓ osteoclast number↓ maturation↓ |
Bone marrow adiposity↓ |
Craniosynostosis, dental abnormalities, and digit malformations [100-102] |
| Gp130 | Gp130 null, gp130-STAT3 deletion [39] | Trabecular bone mass↓ | Bone formation↓ Osteoblast number↓ |
Large osteoclast | -- | |
| osteoblast/osteocyte specific -/- (Osx-Cre, Dmp1-Cre) [40] | Trabecular bone mass↓ | Bone formation↓ Ob number→ |
Bone resorption→ Osteoclast Number→ |
-- | ||
| Osteoclast specific -/- (Ctsk-Cre) [69] | Trabecular bone mass↓ Cortical growth↓ |
Bone formation↓ | Bone resorption→ Osteoclast number→ |
-- |
Previous studies have also demonstrated that IL-11 is an important regulator of mechanical stress-induced osteoblast differentiation through canonical Wnt/β-catenin signaling [43]. Wnt/β-catenin signaling promotes osteoblast differentiation and proliferation, maintains bone marrow stromal cell (BMSC) self-renewal, and mediating the crosstalk between chondrocytes and osteoblasts in bone growth plates [44, 45]. Wnt inhibitors, including sclerostin (SOST) and Dickkopf (DKK) 1/2, bind to the Wnt protein or prevent the interaction between Wnt protein and its co-receptors, thereby preventing β-catenin translocation and inhibiting osteogenic differentiation and bone formation [46]. IL-11 promotes bone formation in response to mechanical stress in part. The expression of IL-11 in bone tissues is upregulated by mechanical loading [35]. Mechanical stress rapidly enhances FosB transcription into deltaFosB, then forms a heterodimer with JunD and binds to the IL-11 gene promoter, enhancing IL-11 gene transcription and expression [47, 48]. Mechanical stress also activates the Smad-1 pathway through protein kinase C (PKC), and crosstalk between Smad1 and deltaFosB/JunD pathways synergistically stimulate IL-11 gene transcription [48]. IL-11 suppresses the expression of the mechanical-sensitive gene SOST (encoding sclerostin), activates the Wnt signaling pathway, and enhances mechanical stress induced bone formation. The response of bone formation to mechanical loading is significantly reduced in global and osteoblast/osteocyte-specific IL-11-/- mice [41]. The expression of the Wnt inhibitor sclerostin was increased in IL-11-/- mice. Sclerostin expression was upregulated by mechanical unloading in WT mice. However, sclerostin levels remained high at baseline and under unloading and reloading conditions in IL-11-/- mice. Bone formation is blunted by sustained high expression of Wnt inhibitors in IL-11-/- mice under mechanical loading (32). This implies that IL-11 is a mechano-sensitive cytokine, regulating bone formation in response to mechanical loading (Fig. 2).
Figure 2.
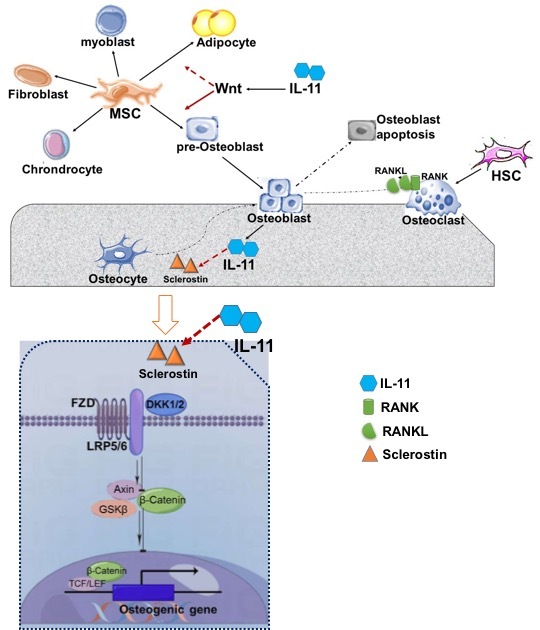
The effect of IL-11 on shifting cell fates from mesenchymal stem cells. Mesenchymal stem cells can be differentiated into osteoblast, adipocyte, fibroblast, chondrocyte, myoblast and so on. IL-11 promotes osteoblast differentiation and osteogenesis, while suppresses adipocyte differentiation and adipogenesis via activation of Wnt/β-catenin signaling pathway. IL-11 suppresses the Wnt inhibitors sclerostin and Dkks. IL-11 regulates the osteoblast/adipocyte differentiational cell-fate from mesenchymal stromal cells.
PTH is another stimulator of osteoblast differentiation and bone formation. PTH upregulates the expression of gp130 cytokines in osteoblasts, including IL-6, IL-11, OSMR and CRLF1 [49]. It activates the downstream signaling cascade to upregulate IL-11 expression in a time- and dose-dependent manner by stimulating osteoblast differentiation and bone anabolism in part. The effect of PTH on IL-11 transcription is mediated by FosB/deltaFosB expression and Smad1 phosphorylation in response to PKC activation [50]. In addition, IL-11 influences endochondral bone formation during bone fracture healing. Together with bone morphogenetic protein-2, IL-11 induced osteoblast differentiation in a rabbit model of bone healing [51]. Glucocorticoid (GC) therapy is an established cause to osteoporosis [52]. It is worthwhile to note that IL-11 also suppresses osteoblast differentiation and apoptosis induced by GCs. GC downregulates IL-11 mRNA expression in osteoblasts in vitro, while IL-11 attenuates the inhibitory effect of GC on osteoblast differentiation [52]. However, the effect of IL-11 on ameliorating bone loss in an animal model of glucocorticoid-induced bone loss requires further investigation. Those results indicate that IL-11/IL-11R signaling is essential for osteoblast differentiation and osteogenesis.
Osteocytes have a lifespan of decades in the bone matrix, and they represent over 90% of the bone cells [53]. Their main role is to maintain bone strength. They also interact signalings with cells on the bone surface [54] and modify the local environment [55, 56]. In addition, osteocytes are sensors in response to mechanical loading, and regulate bone formation and bone mass. IL-11 expression was upregulated by mechanical stress in vitro. IL-11 suppressed the expression of sclerostin, an osteoblast differentiation inhibitor produced by osteocytes [43]. Thus, IL-11 promotes osteogenesis.
In osteogenesis process, mature osteoblasts produce and deposit an organic bone matrix called osteoid. The bone matrix mainly contains collagen type I. IL-11 is involved in the regulation of bone mineralization. In the human osteoblast cell line hFOB/ER, the regulation of IL-11 expression was more pronounced at the late mineralization stage of differentiation than at the earlier stages [57]. Ex vivo study using bone marrow stromal cells obtained from IL-11 Tg mice showed an enhanced expression of osteoblastic markers and mineralization compared to those of wild-type littermates [42]. Moreover, Monnouchi et al. demonstrated that rhIL-11 significantly increased the proportion of mineralized nodules as revealed by positive Alizarin Red staining. This implies that IL-11 promotes bone mineralization. IL-11 stimulates osteoblast differentiation through the JAK/STAT signaling pathway, leading to increased alkaline phosphatase activity and bone mineralization [58].
IL-11 in osteoclast and bone resorption
Osteoclasts originate from hematopoietic progenitor cells and fuse into multinuclear cells during maturation. They migrate to the bone surface and undergo active bone resorption. They attach to the bone surface and form a sealing zone that regulates resorption through fission and fusion [59, 60]. Bone resorption occurs when acids and proteolytic enzymes are released to remove minerals, thereby degrading the bone matrix. The NF-kappa B (RANK)/RANK ligand signaling pathway mediates osteoclast formation and maturation. The pre-mature osteoblasts produce RANKL, which combines with RANK expressed in osteoclasts and stimulates osteoclast differentiation [61, 62].
IL-11 is secreted by osteoclasts. In an in vitro study, stimulating primary cultured osteoblasts with exogenous IL-11 resulted in the upregulation of RANKL expression, indicating that IL-11 is involved in osteoclastogenesis [63]. IL-11 stimulates osteoclast formation by upregulating RANKL generation in osteoblast lineage [64]. However, a recent study demonstrated that IL-11 stimulates osteoclastogenesis via a RANKL-independent mechanism [65]. IL-11 activates osteoclastogenesis in breast cancer bone metastasis-associated osteolysis through the JAK1/STAT3/c-Myc pathway [66]. IL-11 also prolongs the survival of osteoclast progenitors [67] and inhibits osteoclast maturation [38].
Although previous reports have demonstrated that IL-11 enhances osteoclastogenesis, systemic overexpression of IL-11 in Tg mice resulted in increased bone mass, specifically due to enhanced bone formation in vivo [42]. The IL-11R and gp130 receptors are highly expressed in mature osteoclasts; thus, mature osteoclasts respond to IL-11 [40, 68]. IL-11R-deficient mice exhibit reduced osteoclast counts, leading to decreased bone resorption [38]. Furthermore, osteoclastogenesis derived from the bone marrow of IL-11R-deficient mice shows a weaker response to exogenous RANKL stimulation than WT mice, indicating that osteoclastogenesis derived from hematopoietic lineage retards the deficiency of IL-11 signaling [38]. However, IL-11 deficient mice exhibit a slightly reduced tendency of osteoclast cell number, RANKL and CathepsinK mRNA expression in bone, but show no significant difference from the WT littermates [41]. In the IL-11 overexpressed transgenic mice, bone mass unexpectedly increased without changes in osteoclastic bone resorption [42]. Interestingly, osteoclast-specific deletion of gp130 in Ctsk-gp130fl/fl mice showed no differences in osteoclast number or bone resorption activity, but showed a reduction in trabecular bone and cortical growth [69]. The mechanism of gp130-mediated signaling in osteoclasts may be associated with the coupling regulation of bone modeling linking osteoblast-induced bone formation [69]. It suggests that gp130 plays a physiological role in the bone resorptive process not only through osteoclast-induced bone resorption, but also by promoting the release of coupling factors such as IL-6. IL-11 affects osteoclast formation both directly, and indirectly depending on the osteoblastic cells, just as the same family member of cytokine IL-6 acts on osteoclastogenesis directly and indirectly [42, 66, 70]. The effect of the IL-11/IL-11R axis and the hemicomplex subunit of receptor gp130 on osteoclast-associated bone resorption requires further investigation.
IL-11 in chondrocyte
IL-11 and IL-11R are also expressed in chondrocytes, growth plates, and cartilage [71]. IL-11 stimulates cartilage damage by inducing aggrecanase activity. It causes cartilage damage encountered in pathogenesis of rheumatoid and osteoarthritis [72]. IL-11R null mice exhibit shorter length of bones, indicating that IL-11R signaling stimulates longitudinal growth in the growth plate [38]. In an animal model of rheumatoid arthritis, transduced fibroblasts were injected into the knee joints of the mice. Those animals treated with IL-11-transfected cells show reduced cartilage damage. In addition, transduction of IL-11 inhibited apoptosis in chondrocytes [73].
IL-11 in cancer bone metastasis
IL-11 participates in the osteolytic cycle and interacts between cancer and bone cells. IL-11 is considered as osteolytic factor expressed in human breast cancer cells. Breast cancer cells with high IL-11 expression also show higher occurrences of bone metastasis [7, 66, 74]. Osteolytic bone injury process contains bone marrow homing and exosmosis, pericellular proteolysis and invasion, angiogenesis, osteoclast production, growth factor regulation, and extracellular matrix changes [75]. In a mice model of cancer-induced bone metastasis, overexpression of IL-11 in breast cancer cell lines increased tumor burden and osteolytic lesions [75]. Osteoclasts are direct mediators of bone resorption in osteolytic bone metastasis. IL-11 is a potent inducer of osteoclast formation. Notably, breast cancer cell lines target osteoblasts to stimulate IL-11 production from osteoblast, further increasing the concentration of IL-11 in the bone microenvironment [67]. It also stimulates the development and survival of osteoclast progenitor cells [67]. IL-11 may not be involved in homing of the disseminated cancer cells to bone [76]. In the bone-specific metastatic breast cancer cell line MDA-MB-231, ectopic IL-11 expression interacts with overexpression of the chemokine receptor CXC motif chemokine receptor type 4 (CXCR4) to drive osteolytic metastasis [75]. The combined overexpression of IL-11 and osteopontin (OPN, a secretory protein that stimulates the adhesion of osteoclasts to the bone matrix), significantly increases the incidence of bone metastasis [75].
Cyclooxygenase-2 (COX-2)-mediated production of IL-11 in poorly metastatic (MCF-7) and highly metastatic (MDA-MB231) breast cancer cell lines are necessary for osteolytic bone metastases to occur from breast cancer [76]. The transforming growth factor-β (TGF-β) signaling pathway also stimulates IL-11 production in combination with the COX-2 pathway [75]. TGF-β1 induces the secretion of IL-11 by activating p38-MAPK, which then enhances the transcription factor AP-1 and its binding to the promoter of IL-11 [74, 77]. Overexpression of IL-11 induces osteolytic and angiogenic factors, and further promotes the upregulation of TGF-β. TGF-β rapidly induces the binding of Smad2/3 and Smad4 to the relevant regions of IL-11 and connective tissue growth factor via the canonical TGF-β /Smad pathway in metastatic cells and then participates in the bone metastasis cycle [75]. Bone matrix-induced TGF-β enhances the expression of IL-11 and other osteoclast differentiation factors in breast cancer cells, thereby further increasing the rate of bone loss [20].
Patients with breast cancer-related bone metastasis exhibit increased serum levels and mRNA expression of IL-11, suggesting that IL-11 is involved in bone metastasis via STAT3 phosphorylation [74]. IL-11 activates STAT3 induced c-Myc expression and further increases the expression of c-Fos and the nuclear factor of activated T cells 1 (NFATc1), the major regulator of osteoclastogenesis [66]. Coupled with the RANKL/RANK/OPG system, IL-11 promotes osteoclastogenesis indirectly via the stimulation of osteoblast-derived RANKL [67]. Thus, breast cancer cells produce IL-11, which in turn stimulates RANKL production in the bone microenvironment. IL-11 is essential for promoting osteolysis in breast cancer bone metastasis via RANKL-independent osteoclastogenesis by activating the JAK1/STAT3 signaling pathway [66]. Moreover, a monoclonal antibody against IL-11 blocked the osteoclastogenic effect. RANKL-dependent and IL-11 mediated osteoclastogenesis require STAT3 induced c-Myc expression. Thus, IL-11 is a possible target for the therapeutic strategy of bone metastasis.
IL-11 in adipocyte and bone marrow
Osteoblasts originate from the pluripotent stem lineage, which also has the potential for differentiation into chondrocytes, adipocytes, and fibroblasts [78]. The Wnt signaling pathway promotes osteoblastogenesis and suppresses adipogenesis, thereby shifting the fate of MSC. Recent studies have provided insight into the role of cytokine IL-11 in adipogenesis.
IL-11 was identified as an adipogenesis inhibitor factor early since 1990s [79-82]. In in vitro study, it significantly inhibited lipoprotein lipase activity and adipogenesis in 3T3-L1 cells. This suppression is controlled by tyrosine phosphorylation during the initiation of IL-11R-mediated transmembrane signaling [81]. Yang et al. demonstrated that IL-11 induces adipose-derived stem cell proliferation, migration, and anti-apoptotic effects via STAT3 signaling pathway [83]. However, research in this field has been dumped for decades. In a recent study, Dong et al. demonstrated that IL-11 suppressed adipocyte differentiation and adipogenesis via the Wnt signaling pathway. In the IL-11 systemic deletion (IL-11-/-) mice model, the systemic adiposity significantly increased (with increased adipocyte size and cell number), but with reduced bone mass and formation. In ex vivo study, bone marrow stromal cell derived from IL-11-/- mice showed greater potential for adipocyte differentiation but not for osteoblast differentiation and osteogenesis. This phenomenon was reversed by the administration of exogenous IL-11. The expression of Wnt pathway inhibitors, SOST and Dkk1/2, was upregulated in IL-11-/- mice, resulting in the suppression of Wnt signaling in adipose and bone tissues of IL-11 deletion mice. Those findings indicate that IL-11 was involved in shifting the fate of osteoblast/adipocyte differentiation cells from MSC via Wnt signaling pathway [41]. In addition, IL-11-/- mice exhibited glucose intolerance, insulin resistance, fatty liver, and inflammatory infiltration in adipose tissue. Therefore, IL-11 influences systemic metabolism and is involved in the crosstalk between bone and other organs (Fig. 3).
Figure 3.
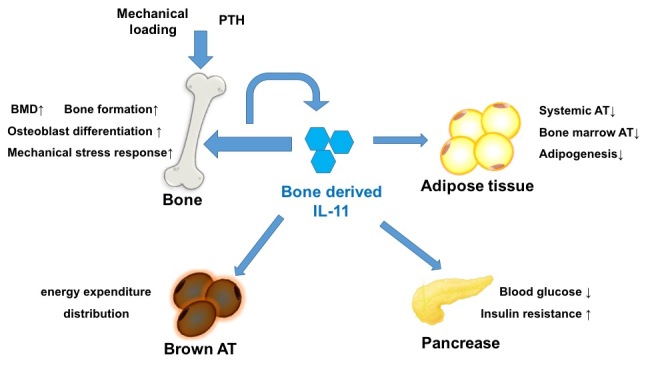
Bone-derived IL-11 acts as an osteokine. The osteokine IL-11 acts as bone-derived hormones, promotes bone formation and osteoblast differentiation, and increases bone mineral density (BMD) and bone enhancement response to mechanical stress. On other hand, IL-11 suppresses adipogenesis and decreases systemic and bone marrow adipose tissue (BMAT). IL-11 also improves insulin resistance and glucose homeostasis and regulates energy expenditure. The bone-derived cytokine IL-11 acts as an osteokine, linking the crosstalk among bones and other organs.
IL-11, the cytokine acts as an osteokine
The bone is considered an endocrine organ that participates in homeostasis. Bone-derived hormones, also referred as “osteokines,” link bone to adipose tissue, kidney, muscle, central sympathetic nerous system, immune system, pancreas, glucose metabolism and insulin synthesis, calcium-phosphonate homeostasis, energy metabolism, and reproduction [84].
For instance, osteocalcin is secreted by osteoblasts, and it regulates bone mineralization. Osteocalcin is an osteoblast-derived endocrine hormone involved in the regulation of multiple target organs such as pancreas, liver, muscle, testes, and nervous system [85]. Osteocalcin stimulates β-cell proliferation and insulin synthesis and increases insulin sensitivity in the liver and adipose tissues. Furthermore, osteocalcin promotes male fertility by increasing testosterone synthesis [86]. Osteocyte-derived FGF23 interacts with Klotho to influence urinary phosphate excretion, PTH, and 1,25(OH)2D3 synthesis. Thus, bone-derived FGF23 is involved in the progression of chronic kidney disease and vascular calcification [87]. Sclerostin, encoded by SOST, is an osteocyte-secreted protein that suppresses the canonical Wnt/β-catenin signaling pathway. It binds to the LRP5/6 receptor and inhibits bone morphogenetic protein (BMP)-2-mediated osteoblast activity [88], and bone formation response to mechanical loading [89]. Animal studies have shown that inhibiting sclerostin (using a monoclonal antibody (Scl-Ab)) increases bone formation, mineral density, and strength [88]. The monoclonal antibody of sclerostin, “Romosozumab,” is widely used for the treatment of osteoporosis in postmenopasul women [90]. Furthermore, sclerostin promotes adipogenesis [91]. In a cross-sectional study, serum sclerostin levels in patients with type 2 diabetes mellitus (T2DM) were significantly higher than those without T2DM [92]. The axis of RANK/RANKL/OPG influences bone remodeling and homeostasis [93]. RANKL forms a homotrimer and interacts with its receptor, RANK, on osteoclasts to regulate bone resorption. The RANKL/RANK/OPG axis influences bone/skeletal homeostasis, glucose homeostasis and insulin resistance. Skeletal muscles also express RANK, RANKL, and osteoprotegerin (OPG). RANKL overexpression leads to muscle atrophy. Moreover, the RANKL/OPG ratio induces cardiac hypertrophy, heart failure, and vascular calcification [94].
In a recent study, Dong et al. demonstrated that systemic IL-11 deletion mice exhibited reduced bone mass and bone formation, resulting from the suppression of the Wnt signaling pathway (41). Systemic IL-11 deletion also results in increased visceral, subcutaneous, and bone marrow adiposity. Interestingly, the conditional knockout of IL-11 in bone also results in reduced bone mass with decreased osteoblastogenesis and bone formation, similar to conventional IL-11-/- mice. However, adipocyte-specific IL-11 deletion mice showed no difference in cortical or cancellous BMD compared to control mice. Increased adiposity, impaired glucose metabolism, and insulin sensitivity were observed in osteoblast/osteocyte-specific IL-11 deletion only [41]. The increased adipose tissue mass and decreased bone mass caused by the systemic deletion of IL-11 was due to a reduced osteoblast/osteocyte-derived IL-11. Those results suggest that IL-11, the cytokine derived from bone, participates as an “osteokine” in regulating bone, adipose tissue, and glucose metabolism (Fig. 3).
Osteokines are derived from bone, and they regulate bone metabolism. Furthermore, they act as hormones that link bones to other organs. IL-11 is emerging as a novel target in the treatment of bone diseases such as osteoporosis and is also involved in weight loss and glucose intolerance.
IL-11 in bone-related human diseases
IL-11 is involved in bone-related human diseases. Tachmazidou et al. explored novel candidate genes and therapeutic targets for osteoarthritis through a genome-wide analysis of the UK BioBank database [95]. Genome-wide association analysis was performed to analyze the genetic enrichment of single-gene forms of bone diseases, as well as pathways underlying collagen formation and extracellular matrix organization. The results showed that IL-11 is associated with an increased risk of osteoarthritis and disease progression, and its expression is upregulated in osteoarthritic tissue [95]. In humans, single nucleotide polymorphisms of IL-11 (rs4252548) and IL-11R (rs11575580) lead to decreased adult height [96-98], demonstrating the role of the IL-11/IL-11R axis in skeletal development. Genetic variants in IL-11 with mutation p.R112H are associated with osteoarthritis and a reduction in adult height [99].
Mutations of IL-11RA result in hereditary disorders associated with craniosynostosis, dental abnormalities, and digital malformations [100-102]. Craniosynostosis is characterized by premature synostosis of skull bone plates, with limited space available for brain tissue growth, resulting in facial and skull malformations as well as intellectual disability [103]. Generally, it occurs because of missense mutations in the extracellular domains of IL-11Rα [104-106], and these mutations are located in regions distant from the putative cytokine or receptor binding sites. Mutations in IL-11R impair the processing and surface expression of its receptor. Genetic analysis reveals that patients with craniosynthesis carrying homozygous high-frequency pathogenic mutations of c.662C>G (p.Pro221Arg), c.734C>G (p.Ser245Cys), c.886C>T (p.Arg296Trp) in IL11RA on chromosome 9p13.3 [107]. Most variants are located in the IL11Rα extracellular domains and cluster in the second Fibronectin III domain, adjacent to the C-terminal transmembrane domain. Molecular dynamics of the structure indicate that mutations in IL-11R destabilize the receptor and disturb the cytokine-binding region [11]. The loss-of-function of Arg296Trp mutation impairs the activation of downstream STAT3-mediated intracellular signal transduction by IL11Rα [102, 104]. Interestingly, a patient carrying a homozygous mutation in IL6ST (encoding gp130, p.N404Y) exhibited craniosynostosis [103]. However, no related gp130 mutant phenotypes have been identified in animal models.
In an animal study, IL-11R-/- mice showed reduced long-bone length, leading to reduced body length [108]. Interestingly, 40-50% of IL-11RA-/- mice exhibited craniosynostosis-like phenotypes and snout deformities, whereas no snout deformities were observed in IL-11-/-mice [100, 104]. In IL-11KO mice, no craniosynostosis were observed, and the BMD of the calvaria did not differ between the IL-11KO and WT mice [41].
Conclusion
In this review, we discuss the mechanisms of IL-11 in bone metabolism and homeostasis using current knowledge from human diseases. We highlight that IL-11 acts as a bone-derived hormone, or termed as “osteokine”, and is involved in the systemic regulation linking the crosstalk between bone and adiposity. We also put insight that IL-11 promotes osteogenesis, inhibits adipogenesis, and shifts the cell fate from stromal cells. Thus, the therapeutic potential of IL-11 in osteoporosis and metabolic syndrome needs further verification.
Acknowledgements
This work is supported by National Natural Science Foundation of China (Grant No. 81600691), China Postdoctoral Science Foundation funded project (Grant No.2018M640615). Dr. Bingzi Dong thanks Prof. Toshio Matsumoto (Fujii Memorial Institute of Medical Science, Tokushima University, Japan) for long-term guidance and helpful advice. We thank Prof. Toshio Matsumoto for the perspective regarding the emerging role of IL-11 in bone metabolism.
Funding Statement
This work is supported by National Natural Science Foundation of China (Grant No. 81600691), China Postdoctoral Science Foundation funded project (Grant No.2018M640615). Dr. Bingzi Dong thanks Prof. Toshio Matsumoto (Fujii Memorial Institute of Medical Science, Tokushima University, Japan) for long-term guidance and helpful advice. We thank Prof. Toshio Matsumoto for the perspective regarding the emerging role of IL-11 in bone metabolism.
References
- [1].Metcalfe RD, Putoczki TL, Griffin MDW (2020). Structural Understanding of Interleukin 6 Family Cytokine Signaling and Targeted Therapies: Focus on Interleukin 11. Front Immunol, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nguyen PM, Abdirahman SM, Putoczki TL (2019). Emerging roles for Interleukin-11 in disease. Growth Factors, 37:1-11. [DOI] [PubMed] [Google Scholar]
- [3].Kaye JA (1998). FDA licensure of NEUMEGA to prevent severe chemotherapy-induced thrombocytopenia. Stem Cells, 16 Suppl 2:207-223. [DOI] [PubMed] [Google Scholar]
- [4].Lim WW, Ng B, Widjaja A, Xie C, Su LP, Ko N, et al. (2020). Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice. Plos One, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang X, Zhu G, Ren Q, Wu J, Gu B, Su D, et al. (2022). Increased interleukin-11 associated with disease activity and development of interstitial lung disease in patients with rheumatoid arthritis. Clin Exp Rheumatol, 40:135-141. [DOI] [PubMed] [Google Scholar]
- [6].Elshabrawy H, Volin M, Essani A, Chen ZL, McInnes I, Van Raemdonck K, et al. (2018). IL-11 facilitates a novel connection between RA joint fibroblasts and endothelial cells. Angiogenesis, 21:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maroni P, Bendinelli P, Ferraretto A, Lombardi G (2021). Interleukin 11 (IL-11): Role(s) in Breast Cancer Bone Metastases. Biomedicines, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang XH, Wu H, Dobson JR, Browne G, Hong D, Akech J, et al. (2015). Expression of the IL-11 Gene in Metastatic Cells Is Supported by Runx2-Smad and Runx2-cJun Complexes Induced by TGF1. J Cell Biochem, 116:2098-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tohjima E, Inoue D, Yamamoto N, Kido S, Ito Y, Kato S, et al. (2003). Decreased AP-1 activity and interleukin-11 expression by bone marrow stromal cells may be associated with impaired bone formation in aged mice. J Bone Miner Res, 18:1461-1470. [DOI] [PubMed] [Google Scholar]
- [10].Bamba S, Andoh A, Yasui H, Makino J, Kim S, Fujiyama Y (2003). Regulation of IL-11 expression in intestinal myofibroblasts: role of c-Jun AP-1- and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol, 285:G529-G538. [DOI] [PubMed] [Google Scholar]
- [11].Koch L, Kespohl B, Agthe M, Schumertl T, Dusterhoft S, Lemberg MK, et al. (2021). Interleukin-11 (IL-11) receptor cleavage by the rhomboid protease RHBDL2 induces IL-11 trans-signaling. FASEB J, 35. [DOI] [PubMed] [Google Scholar]
- [12].Monhasery N, Moll J, Cuman C, Franke M, Lamertz L, Nitz R, et al. (2016). Transcytosis of IL-11 and Apical Redirection of gp130 Is Mediated by IL-11 alpha Receptor. Cell Rep, 16:1067-1081. [DOI] [PubMed] [Google Scholar]
- [13].Lokau J, Nitz R, Agthe M, Monhasery N, Aparicio-Siegmund S, Schumacher N, et al. (2016). Proteolytic Cleavage Governs Interleukin-11 Trans-signaling. Cell Rep, 14:1761-1773. [DOI] [PubMed] [Google Scholar]
- [14].Lokau J, Flynn CM, Garbers C (2017). Cleavage of the Interleukin-11 receptor induces processing of its C-terminal fragments by the gamma-secretase and the proteasome. Biochem Biophys Res Commun, 491:296-302. [DOI] [PubMed] [Google Scholar]
- [15].Lamertz L RF, Polz R, et al. (2018). Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci Signal. 2018; 11(550):eaar7388. [DOI] [PubMed] [Google Scholar]
- [16].Dams-Kozlowska H, Gryska K, Kwiatkowska-Borowczyk E, Izycki D, Rose-John S, Mackiewicz A (2012). A designer hyper interleukin 11 (H11) is a biologically active cytokine. Bmc Biotechnology, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dahmen H, Horsten U, Kuster A, Jacques Y, Minvielle S, Kerr IM, et al. (1998). Activation of the signal transducer gp130 by interleukin-11 and interleukin-6 is mediated by similar molecular interactions. Biochem J, 331:695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Balic JJ, Garbers C, Rose-John S, Liang Y, Jenkins BJ (2017). Interleukin-11-driven gastric tumourigenesis is independent of trans-signalling. Cytokine, 92:118-123. [DOI] [PubMed] [Google Scholar]
- [19].Agthe M, Garbers Y, Putoczki T, Garbers C (2017). Interleukin-11 classic but not trans-signaling is essential for fertility in mice. Placenta, 57:13-16. [DOI] [PubMed] [Google Scholar]
- [20].Putoczki TL, Ernst M (2015). IL-11 signaling as a therapeutic target for cancer. Immunotherapy, 7:441-453. [DOI] [PubMed] [Google Scholar]
- [21].Lokau J, Nitz R, Agthe M, Monhasery N, Aparicio-Siegmund S, Schumacher N, et al. (2016). Proteolytic Cleavage Governs Interleukin-11 Trans-signaling. Cell Rep, 14:1761-1773. [DOI] [PubMed] [Google Scholar]
- [22].Fiebelkow J, Guendel A, Guendel B, Mehwald N, Jetka T, Komorowski M, et al. (2021). The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling. Cell Commun Signal, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, et al. (2008). STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest, 118:1727-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mahboubi K, Biedermann BC, Carroll JM, Pober JS (2000). IL-11 activates human endothelial cells to resist immune-mediated injury. J Immunol, 164:3837-3846. [DOI] [PubMed] [Google Scholar]
- [25].Zhang J, Zhang Y, Dutta DJ, Argaw AT, Bonnamain V, Seto J, et al. (2011). Proapoptotic and antiapoptotic actions of Stat1 versus Stat3 underlie neuroprotective and immunoregulatory functions of IL-11. J Immunol, 187:1129-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buzzelli JN, O'Connor L, Scurr M, Chin SCN, Catubig A, Ng GZ, et al. (2019). Overexpression of IL-11 promotes premalignant gastric epithelial hyperplasia in isolation from germline gp130-JAK-STAT driver mutations. Am J Physiol Gastrointest Liver Physiol, 316:G251-G262. [DOI] [PubMed] [Google Scholar]
- [27].Widjaja AA, Viswanathan S, Jinrui D, Singh BK, Tan J, Ting JGW, et al. (2021). Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front Mol Biosci, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, et al. (2017). IL-11 is a crucial determinant of cardiovascular fibrosis. Nature, 552:110-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yin T, Yang YC (1994). Mitogen-activated protein kinases and ribosomal S6 protein kinases are involved in signaling pathways shared by interleukin-11, interleukin-6, leukemia inhibitory factor, and oncostatin M in mouse 3T3-L1 cells. J Biol Chem, 269:3731-3738. [PubMed] [Google Scholar]
- [30].Vogiatzi A, Mavrothalassitis G (2019). Craniofacial, orofacial and dental disorders: the role of the RAS/ERK pathway. Expert Rev Mol Med, 21. [DOI] [PubMed] [Google Scholar]
- [31].Wang XY, Fuhrer DK, Marshall MS, Yang YC (1995). Interleukin-11 induces complex formation of Grb2, Fyn, and JAK2 in 3T3L1 cells. J Biol Chem, 270:27999-28002. [DOI] [PubMed] [Google Scholar]
- [32].Yang GL, Ma F, Zhong MX, Fang L, Peng Y, Xin XM, et al. (2014). Interleukin-11 induces the expression of matrix metalloproteinase 13 in gastric cancer SCH cells partly via the PI3K-AKT and JAK-STAT3 pathways. Mol Med Rep, 9:1371-1375. [DOI] [PubMed] [Google Scholar]
- [33].Sun RG, Chen CL, Deng XZ, Wang FQ, Song SM, Cai Q, et al. (2021). IL-11 mediates the Radioresistance of Cervical Cancer Cells via the PI3K/Akt Signaling Pathway. J Cancer, 12:4638-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu CY, Liu JF, Tsai HC, Tzeng HE, Hsieh TH, Wang M, et al. (2022). Interleukin-11/gp130 upregulates MMP-13 expression and cell migration in OSCC by activating PI3K/Akt and AP-1 signaling. J Cell Physiol, 237:4551-4562. [DOI] [PubMed] [Google Scholar]
- [35].Kespohl B, Schumertl T, Bertrand J, Lokau J, Garbers C (2021). The cytokine interleukin-11 crucially links bone formation, remodeling and resorption. Cytokine Growth Factor Rev, 60:18-27. [DOI] [PubMed] [Google Scholar]
- [36].Siddiqui JA, Partridge NC (2016). Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology (Bethesda), 31:233-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang L, You X, Zhang L, Zhang C, Zou W (2022). Mechanical regulation of bone remodeling. Bone Res, 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sims NA, Jenkins BJ, Nakamura A, Quinn JMW, Li RL, Gillespie MT, et al. (2005). Interleukin-11 receptor signaling is required for normal bone remodeling. J Bone Miner Res, 20:1093-1102. [DOI] [PubMed] [Google Scholar]
- [39].Shin HI, Divieti P, Sims NA, Kobayashi T, Miao DS, Karaplis AC, et al. (2004). gp130-mediated signaling is necessary for normal osteoblastic function in vivo and in vitro. Endocrinology, 145:1376-1385. [DOI] [PubMed] [Google Scholar]
- [40].Johnson RW, Brennan HJ, Vrahnas C, Poulton IJ, McGregor NE, Standal T, et al. (2014). UThe Primary Function of gp130 Signaling in Osteoblasts Is To Maintain Bone Formation and Strength, Rather Than Promote Osteoclast Formation. J Bone Miner Res, 29:1492-1505. [DOI] [PubMed] [Google Scholar]
- [41].Dong BZ, Hiasa M, Higa Y, Ohnishi Y, Endo I, Kondo T, et al. (2022). Osteoblast/osteocyte-derived interleukin-11 regulates osteogenesis and systemic adipogenesis. Nat Commun, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Takeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, et al. (2002). Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem, 277:49011-49018. [DOI] [PubMed] [Google Scholar]
- [43].Dzialo E, Czepiel M, Tkacz K, Siedlar M, Kania G, Blyszczuk P (2021). WNT/beta-Catenin Signaling Promotes TGF-beta-Mediated Activation of Human Cardiac Fibroblasts by Enhancing IL-11 Production. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wan Y, Lu C, Cao JJ, Zhou RJ, Yao YY, Yu J, et al. (2013). Osteoblastic Wnts differentially regulate bone remodeling and the maintenance of bone marrow mesenchymal stem cells. Bone, 55:258-267. [DOI] [PubMed] [Google Scholar]
- [45].Lu C, Wan Y, Cao JJ, Zhu XM, Yu J, Zhou RJ, et al. (2013). Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone, 53:566-574. [DOI] [PubMed] [Google Scholar]
- [46].Marini F, Giusti F, Palmini G, Brandi ML (2022). Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos Int, 34:213-238. [DOI] [PubMed] [Google Scholar]
- [47].Kido S, Kuriwaka-Kido R, Imamura T, Ito Y, Inoue D, Matsumoto T (2009). Mechanical stress induces Interleukin-11 expression to stimulate osteoblast differentiation. Bone, 45:1125-1132. [DOI] [PubMed] [Google Scholar]
- [48].Kido S, Kuriwaka-Kido R, Umino-Miyatani Y, Endo I, Inoue D, Taniguchi H, et al. (2010). Mechanical Stress Activates Smad Pathway through PKC delta to Enhance Interleukin-11 Gene Transcription in Osteoblasts. Plos One, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Walker EC, Poulton IJ, McGregor NE, Ho PW, Allan EH, Quach JM, et al. (2012). Sustained RANKL response to parathyroid hormone in oncostatin M receptor-deficient osteoblasts converts anabolic treatment to a catabolic effect in vivo. J Bone Miner Res, 27:902-912. [DOI] [PubMed] [Google Scholar]
- [50].Kuriwaka-Kido R, Kido S, Miyatani Y, Ito Y, Kondo T, Omatsu T, et al. (2013). Parathyroid Hormone (1-34) Counteracts the Suppression of Interleukin-11 Expression by Glucocorticoid in Murine Osteoblasts: A Possible Mechanism for Stimulating Osteoblast Differentiation Against Glucocorticoid Excess. Endocrinology, 154:1156-1167. [DOI] [PubMed] [Google Scholar]
- [51].Suga K, Saitoh M, Kokubo S, Fukushima S, Kaku S, Yasuda S, et al. (2003). Interleukin-11 acts synergistically with bone morphogenetic protein-2 to accelerate bone formation in a rat ectopic model. J Interferon Cytokine Res, 23:203-207. [DOI] [PubMed] [Google Scholar]
- [52].Rauch A, Baschant U, Rauner M, Amling M, Hofbauer L, De Bosscher K, et al. (2011). Glucocorticoids Suppress Bone Formation by Attenuating Osteoblast Differentiation Via the Monomeric Glucocorticoid Receptor. Osteoporos Int, 22:170-171. [DOI] [PubMed] [Google Scholar]
- [53].Buenzli PR, Sims NA (2015). Quantifying the osteocyte network in the human skeleton. Bone, 75:144-150. [DOI] [PubMed] [Google Scholar]
- [54].Johanna I, David JJD, Amy JN, Berno D, Corinna W (2020). Importance of osteocyte-mediated regulation of bone remodelling in inflammatory bone disease. Swiss Med Wkly, 150. [DOI] [PubMed] [Google Scholar]
- [55].Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF (2018). Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact, 18:292-303. [PMC free article] [PubMed] [Google Scholar]
- [56].Blank M, Sims NA (2019). Cellular Processes by Which Osteoblasts and Osteocytes Control Bone Mineral Deposition and Maturation Revealed by Stage-Specific EphrinB2 Knockdown. Curr Osteoporos Rep, 17:270-280. [DOI] [PubMed] [Google Scholar]
- [57].Waters KM, Rickard DJ, Riggs BL, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, et al. (2001). Estrogen regulation of human osteoblast function is determined by the stage of differentiation and the estrogen receptor isoform. J Cell Biochem, 83:448-462. [DOI] [PubMed] [Google Scholar]
- [58].Monnouchi S, Maeda H, Yuda A, Hamano S, Wada N, Tomokiyo A, et al. (2015). Mechanical induction of interleukin-11 regulates osteoblastic/cementoblastic differentiation of human periodontal ligament stem/progenitor cells. J Periodontal Res, 50:231-239. [DOI] [PubMed] [Google Scholar]
- [59].Martin TJ, Sims NA (2015). RANKL/OPG; Critical role in bone physiology. Rev Endocr Metab Disord, 16:131-139. [DOI] [PubMed] [Google Scholar]
- [60].McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, et al. (2021). Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell, 184:1330-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sims NA, Martin TJ (2020). Osteoclasts Provide Coupling Signals to Osteoblast Lineage Cells Through Multiple Mechanisms. Annu Rev Physiol, Vol 82, 82:507-529. [DOI] [PubMed] [Google Scholar]
- [62].Thomas GP, Baker SUK, Eisman JA, Gardiner EM (2001). Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol, 170:451-460. [DOI] [PubMed] [Google Scholar]
- [63].Horwood NJ, Elliott J, Martin TJ, Gillespie MT (1998). Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology, 139:4743-4746. [DOI] [PubMed] [Google Scholar]
- [64].Hill PA, Tumber A, Papaioannou S, Meikle MC (1998). The cellular actions of interleukin-11 on bone resorption in vitro. Endocrinology, 139:1564-1572. [DOI] [PubMed] [Google Scholar]
- [65].Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA (2003). Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone, 32:1-7. [DOI] [PubMed] [Google Scholar]
- [66].Liang MM, Ma QY, Ding N, Luo F, Bai Y, Kang F, et al. (2019). IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mccoy EM, Hong HX, Pruitt HC, Feng X (2013). IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. Bmc Cancer, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Standal T, Johnson RW, McGregor NE, Poulton IJ, Ho PWM, Martin TJ, et al. (2014). gp130 in late osteoblasts and osteocytes is required for PTH-induced osteoblast differentiation. J Endocrinol, 223:181-190. [DOI] [PubMed] [Google Scholar]
- [69].Johnson RW, McGregor NE, Brennan HJ, Crimeen-Irwin B, Poulton IJ, Martin TJ, et al. (2015). Glycoprotein130 (Gp130)/interleukin-6 (IL-6) signalling in osteoclasts promotes bone formation in periosteal and trabecular bone. Bone, 81:343-351. [DOI] [PubMed] [Google Scholar]
- [70].Kaushansky K, Broudy VC, Lin N, Jorgensen MJ, Mccarty J, Fox N, et al. (1995). Thrombopoietin, the Mpl Ligand, Is Essential for Full Megakaryocyte Development. Proc Natl Acad Sci U S A, 92:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Maier R, Ganu V, Lotz M (1993). Interleukin-11, an Inducible Cytokine in Human Articular Chondrocytes and Synoviocytes, Stimulates the Production of the Tissue Inhibitor of Metalloproteinases. J Biol Chem, 268:21527-21532. [PubMed] [Google Scholar]
- [72].Liu X, Croker BA, Campbell IK, Gauci SJ, Alexander WS, Tonkin BA, et al. (2014). Key Role of Suppressor of Cytokine Signaling 3 in Regulating gp130 Cytokine-Induced Signaling and Limiting Chondrocyte Responses During Murine Inflammatory Arthritis. Arthritis Rheumatol, 66:2391-2402. [DOI] [PubMed] [Google Scholar]
- [73].Sack U, Sehm B, Kahlenberg F, Murr A, Lehmann J, Tannapfel A, et al. (2005). Investigation of arthritic joint destruction by a novel fibroblast-based model. Ann N Y Acad Sci, 1051:291-298. [DOI] [PubMed] [Google Scholar]
- [74].Ren L, Wang X, Dong ZL, Liu J, Zhang SW (2013). Bone metastasis from breast cancer involves elevated IL-11 expression and the gp130/STAT3 pathway. Med Oncol, 30. [DOI] [PubMed] [Google Scholar]
- [75].Kang YB, Siegel PM, Shu WP, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 3:537-549. [DOI] [PubMed] [Google Scholar]
- [76].Singh B, Berry JA, Shoher A, Lucci A (2006). COX-2 induces IL-11 production in human breast cancer cells.J Surg Res, 131:267-275. [DOI] [PubMed] [Google Scholar]
- [77].Gupta J, Robbins J, Jilling T, Seth P (2011). TGF beta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther, 11:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen X, Wang ZQ, Duan N, Zhu GY, Schwarz EM, Xie C (2018). Osteoblast-osteoclast interactions. Connect Tissue Res, 59:99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ohsumi J, Miyadai K, Kawashima I, Ishikawa-Ohsumi H, Sakakibara S, Mita-Honjo K, et al. (1991). Adipogenesis inhibitory factor. A novel inhibitory regulator of adipose conversion in bone marrow. FEBS Lett, 288:13-16. [DOI] [PubMed] [Google Scholar]
- [80].Kawashima I, Ohsumi J, Mita-Honjo K, Shimoda-Takano K, Ishikawa H, Sakakibara S, et al. (1991). Molecular cloning of cDNA encoding adipogenesis inhibitory factor and identity with interleukin-11. FEBS Lett, 283:199-202. [DOI] [PubMed] [Google Scholar]
- [81].Yin T, Miyazawa K, Yang YC (1992). Characterization of interleukin-11 receptor and protein tyrosine phosphorylation induced by interleukin-11 in mouse 3T3-L1 cells. J Biol Chem, 267:8347-8351. [PubMed] [Google Scholar]
- [82].Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, et al. (1998). Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res, 13:1370-1377. [DOI] [PubMed] [Google Scholar]
- [83].Yang WL, Zhang SN, Ou TT, Jiang H, Jia DI, Qi ZY, et al. (2020). Interleukin-11 regulates the fate of adipose-derived mesenchymal stem cells via STAT3 signalling pathways. Cell Prolif, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Li Y, Gu Z, Wang J, Wang Y, Chen X, Dong B (2022). The Emerging Role of Bone-Derived Hormones in Diabetes Mellitus and Diabetic Kidney Disease. Front Endocrinol (Lausanne), 13:938830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mizokami A, Kawakubo-Yasukochi T, Hirata M (2017). Osteocalcin and its endocrine functions. Biochem Pharmacol, 132:1-8. [DOI] [PubMed] [Google Scholar]
- [86].Wang JS, Mazur CM, Wein MN (2021). Sclerostin and Osteocalcin: Candidate Bone-Produced Hormones. Front Endocrinol (Lausanne), 12:584147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martin A, David V, Quarles LD (2012). Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev, 92:131-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ke HZ, Richards WG, Li XD, Ominsky MS (2012). Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr Rev, 33:747-783. [DOI] [PubMed] [Google Scholar]
- [89].Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, et al. (2014). Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res, 29:2456-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shakeri A, Adanty C (2020). Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. J Popul Ther Clin Pharmacol, 27:e25-e31. [DOI] [PubMed] [Google Scholar]
- [91].Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, et al. (2017). Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res, 32:373-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Napoli N, Strollo R, Defeudis G, Leto G, Moretti C, Zampetti S, et al. (2018). Serum Sclerostin and Bone Turnover in Latent Autoimmune Diabetes in Adults. J Clin Endocrinol Metab, 103:1921-1928. [DOI] [PubMed] [Google Scholar]
- [93].Yasuda H (2021). Discovery of the RANKL/RANK/OPG system (vol 39, pg 1, 2021). J Bone Miner Metab, 39:12-12.33439336 [Google Scholar]
- [94].Marcadet L, Bouredji Z, Argaw A, Frenette J (2022). The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease.Front Cell Dev Biol, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. (2019). Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet, 51:230-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, et al. (2017). Rare and low-frequency coding variants alter human adult height. Nature, 542:186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. (2014). Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet, 46:1173-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lanktree MB, Guo Y, Murtaza M, Glessner JT, Bailey SD, Onland-Moret NC, et al. (2011). Meta-analysis of Dense Genecentric Association Studies Reveals Common and Uncommon Variants Associated with Height. Am J Hum Genet, 88:6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lokau J, Gottert S, Arnold P, Dusterhoft S, Massa Lopez D, Grotzinger J, et al. (2018). The SNP rs4252548 (R112H) which is associated with reduced human height compromises the stability of IL-11. Biochim Biophys Acta Mol Cell Res, 1865:496-506. [DOI] [PubMed] [Google Scholar]
- [100].Ng B, Widjaja AA, Viswanathan S, Dong JR, Chothani SP, Lim S, et al. (2021). Similarities and differences between IL11 and IL11RA1 knockout mice for lung fibro-inflammation, fertility and craniosynostosis. Sci Rep, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, et al. (2011). Inactivation of IL11 Signaling Causes Craniosynostosis, Delayed Tooth Eruption, and Supernumerary Teeth. Am J Hum Genet, 89:67-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Keupp K, Li Y, Vargel I, Hoischen A, Richardson R, Neveling K, et al. (2013). Mutations in the interleukin receptor IL11RA cause autosomal recessive Crouzon-like craniosynostosis. Mol Genet Genomic Med, 1:223-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Schwerd T, Twigg SRF, Aschenbrenner D, Manrique S, Miller KA, Taylor IB, et al. (2017). A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med, 214:2547-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Agthe M, Brugge J, Garbers Y, Wandel M, Kespohl B, Arnold P, et al. (2018). Mutations in Craniosynostosis Patients Cause Defective Interleukin-11 Receptor Maturation and Drive Craniosynostosis-like Disease in Mice. Cell Rep, 25:10-+. [DOI] [PubMed] [Google Scholar]
- [105].Feng W, Liu HR, Luo TT, Liu D, Du J, Sun J, et al. (2022). Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-kappa B, ERK and JNK signaling pathways (vol 27, 41411, 2022). Sci Rep, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Miller KA, Twigg SRF, McGowan SJ, Phipps JM, Fenwick AL, Johnson D, et al. (2017). Diagnostic value of exome and whole genome sequencing in craniosynostosis. J Med Genet, 54:260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wilkie AO (2000). Epidemiology and genetics of craniosynostosis. Am J Med Genet, 90:82-84. [DOI] [PubMed] [Google Scholar]
- [108].Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T, et al. (1996). The role of gp130-mediated signals in osteoclast development: Regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med, 183:2581-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]


