Abstract
Almost all stroke survivors suffer physical disabilities and neuropsychiatric disturbances, which can be briefly divided into post-stroke neurological diseases and post-stroke psychiatric disorders. The former type mainly includes post-stroke pain, post-stroke epilepsy, and post-stroke dementia while the latter one includes post-stroke depression, post-stroke anxiety, post-stroke apathy and post-stroke fatigue. Multiple risk factors are related to these post-stroke neuropsychiatric complications, such as age, gender, lifestyle, stroke type, medication, lesion location, and comorbidities. Recent studies have revealed several critical mechanisms underlying these complications, namely inflammatory response, dysregulation of the hypothalamic pituitary adrenal axis, cholinergic dysfunction, reduced level of 5-hydroxytryptamine, glutamate-mediated excitotoxicity and mitochondrial dysfunction. Moreover, clinical efforts have successfully given birth to many practical pharmaceutic strategies, such as anti-inflammatory medications, acetylcholinesterase inhibitors, and selective serotonin reuptake inhibitors, as well as diverse rehabilitative modalities to help patients physically and mentally. However, the efficacy of these interventions is still under debate. Further investigations into these post-stroke neuropsychiatric complications, from both basic and clinical perspectives, are urgent for the development of effective treatment strategies.
Keywords: Complications, neurological diseases, pathogenesis, post-stroke, psychiatric disorders
1. Introduction
Stroke is one of the leading causes of death and disability worldwide, and has become a major economic burden owing to the costs of long-term rehabilitative care [1, 2]. With the rapid growth of the elderly population, the incidence of stroke is increasing at an alarming trend, increasing age remains the most threatening risk factor for patients suffering from stroke in both men and women [1]. Although improved drug treatment and advances in healthcare have reduced stroke mortality, poor outcomes are common in long-term survivors, accompanied by secondary complications, including cognitive impairment and dementia, pain, anxiety, depression, fatigue, and epilepsy [3, 4]. These complications substantially increase the risk of subsequent recurrence and later mortality. Here, we sought to review the available evidence to elucidate these different types of complications, and refine the definition, risk factors, prevalence, potential mechanisms, and therapeutic interventions, despite their overlap and interrelationship with each other. The mechanisms underlying post-stroke neuropsychiatric complications (PSNCs) are complex, involving multiple neurobiological cytokines and neurotransmitters systems [5]. Evidence has supported that inflammatory response, dysregulation of the hypothalamic pituitary adrenal (HPA) axis, cholinergic dysfunction, reduced level of 5-hydroxytryptamine (5-HT, also called serotonin), glutamate-mediated excitotoxicity, and mitochondrial dysfunction are involved in PSNCs, of which inflammation, dysfunction of the HPA axis and mitochondrial dysfunction affect almost all of the types of complications [6, 7]. While glutaminergic, cholinergic, and serotoninergic dysfunction have been suggested as key determinants of PSNCs, it must be noted that PSNCs, similar to other psychiatric disorders, are the consequence of abnormal brain functional network involving diverse neurotransmitter systems. Interestingly, as the most well-established neurotransmitter involved in mood disorders, dopamine has been found to play a role in the comorbidity of cardiovascular diseases with mood disorders, and thus the dopaminergic contribution to PSNCs is definitely worth further clarification [5, 7]. The prevention and treatment of post-stroke secondary symptoms remain challenging, partially attributable to multiple pre-stroke risk factors, diverse types, and characteristics of stroke [8, 9]. The discussion of these possible secondary complications provides physicians, nurses, and allied health professionals with comprehensive awareness of acute and long-term PSNCs, which enables them to provide stroke survivors with appropriate preventative care and intervention during acute care and rehabilitation [4].
2. Methods
In this narrative review, we searched stroke-related studies in the English language only, with no restrictions on research objective or publication type. The search terms in the “Therapy” section are restricted to human studies. Databases including PubMed, Medline, and Web of Science were searched for all relevant documents, mainly in the previous two decades, with the exception of several older representative studies. The search terms were as follows: ("stroke" OR "ischemia stroke" OR "hemorrhagic stroke") AND ("cognitive impairment" OR "cognition" OR "dementia" OR "memory impairment"). Additionally, we searched for publications on stroke therapy using the following search terms: ("stroke" OR "ischemia stroke" OR "hemorrhagic stroke") AND ("cognitive impairment" OR "cognition" OR "dementia" OR "memory impairment ") AND ("therapy" OR "intervention" OR "treatment"). The search terms for other PSNCs are similar to those listed above. The search terms used for the other PSNCs were as follows: "pain", or "epilepsy", "seizure", "epileptogenesis", "epilepsia", or "depression", "depressive", "depressed", or "anxiety", or "apathy", or "fatigue". Moreover, we reviewed the reference lists in retrieved publications.
3. Classification and clinical features of post-stroke neuropsychiatric complications
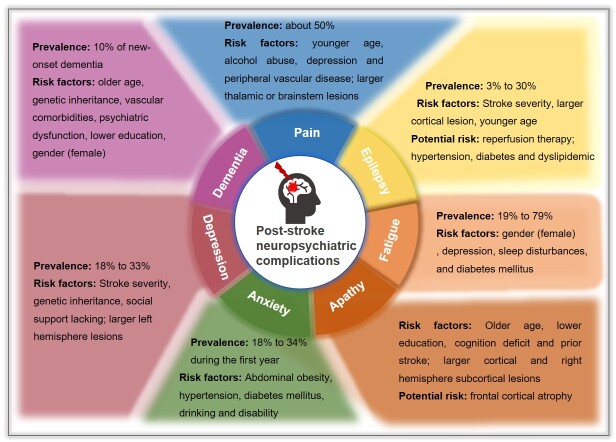
We generally categorize PSNCs into neurological diseases, including pain, epilepsy, dementia, and psychiatric disorders, including depression, anxiety, apathy and fatigue. We will elaborate on the prevalence, occurrence characteristics, risk factors, vulnerability, and disease progression of different PCNCs. The risk factors of these different complications are summarized in Figure 1.
Figure 1.
The prevalence, risk factors and potential risk of different post-stroke neuropsychiatric complications.
3.1. Neurological diseases
3.1.1. Post-stroke cognitive impairment and dementia
Previous reports confirmed that long-term functional deficits occur in most stroke survivors, causing chronic disability. Post-stroke cognitive impairment and dementia (PSCID) are fairly common in stroke survivors. Generally, dementia is defined as moderate-to-severe cognitive impairment that occurs within 3 months after incident stroke. Cognitive impairment often occurs as both a predictor and consequence of stroke incidence [10]. According to extensive data, over 50% of stroke survivors develop varying degrees of cognitive impairment, and approximately 25% develop dementia [10, 11]. To be more precise, reliable data have refered that the incidence of developing new-onset dementia after the initial stroke is 10%, and more than one-third of patients develop dementia after recurrent stroke [12]. Otherwise, a study aimed at US adults showed that women were more likely to develop post-stroke dementia than men, especially during the early period after stroke onset [13]. Moreover, it was suggested that the impact of acute stroke on cognition showed no racial differences in older individuals [14].
Risk factors for PSCID are multifactorial, including increasing age, genetic inheritance, undereducated status, socioeconomic disparity, vascular comorbidities, and psychiatric dysfunction. It has been well documented that older age is the leading risk factor for PSCID, which is consistent with the high morbidity of stroke-related dementia in elderly [15]. Most notably, elderly individuals who have any comorbidity with vascular risk factors, such as diabetes and atrial fibrillation, are at higher risk of PSCID [16]. Interestingly, hypertension, high cholesterol, smoking, and alcohol use, which strongly contribute to stroke, have no direct effect on increasing the risk of PSCID, yet the intervention focused on these factors still preserves cognition decline through preventing recurrent stroke [16, 17]. Additionally, studies have found that longer educational history gives rise to less PSCID, which brings about a favorable long-term survival [12, 13].
Currently, it is widely accepted that PSCID is associated with psychiatric disorders. Available data have suggested that experiencing delirium in the acute stage of stroke predicted more cognitive impairment, and PSCID often co-exists with mood disorders and apathy[18]. Also, it is well-known that dementia is closely associated with memory decrements, research has confirmed that stroke onset triggers memory impairment, and those who suffered stroke experienced faster memory decline in the years prior to stroke compared with stroke-free populations [19]. Although PSCID occurs frequently, it is often overlooked when it coincides with other worrisome symptoms. Thus, it is critically important to specifically assess for PSCID during follow-up care, which may reduce the risk of permanent institutionalization and prolong survival [11].
3.1.2. Post-stroke pain
Pain almost runs through all stages of stroke. Indeed, post-stroke pain (PSP) affects up to 50% of stroke survivors [20]. Most clinical management after stroke focuses on reducing the risk of recurrence and recovering motor and neurologic functions, as well as treating aphasia, dementia or neglect syndrome, causing a direct consequence of underdiagnosis and undertreatment of PSP [21]. The prevalence and annual incidence of PSP is difficult to define accurately given the different types, intensities, and post-stroke phases and follow-up periods. Some research shows that almost half of patients suffer various degrees and types of pain in different period, and the highest incidence of PSP occurs in the subacute phase after stroke [20-22]. Common PSP subtypes include central post-stroke pain (CPSP), regional pain syndromes, joint pain, spasticity-related pain, headache, and musculoskeletal pain, such as the most common hemiplegic shoulder pain, all of which negatively affect daily functional abilities of stroke survivors [23].
Among the various types of PSP, CPSP is the most common and unbearable pain. CPSP is a dynamic condition that occurs as constant or intermittent neuropathic pain symptoms accompanied by sensory hyposensitivity, such as temperature dysesthesia [24]. Since pain caused by ischemic injury to the central nervous system (CNS) is difficult or vague to characterize or classify, patients may describe painful sensations that are indefinitely positioned or that vary over time. As a consequence of ischemia stroke, headache emergence can also be considered as a precursor of stroke, and several studies have demonstrated that headache occurs mainly in the acute stage after stroke, and that a primary headache disorder doubles the risk of ischemic stroke [25]. While hemiplegic shoulder pain is a typically delayed complication that usually occurs 2-3 months after stroke, it is often negligible in the acute phase and then markedly amplified in the subacute phase [26].
Older age and female gender are well-recognized demographic characteristics related to any PSP syndrome [20, 25]. However, it is interesting to note that younger patients are at higher risk for CPSP [27]. Pre-stroke features, such as alcohol abuse, statin use, depression and peripheral vascular disease, are identified as independent risk factors of aggravating PSP [28]. Clinically, PSP is always accompanied by symptoms of increased muscle tone, sensory deficits, and upper limb motor paralysis. Additionally, stroke site and mechanism, including thalamic and brainstem localization, also play an essential role in the initiation and development of PSP [23]. Accordingly, follow-up records have shown that pain can lead to a series of comorbidities, such as depression[29], fatigue [30], sleep disturbances [31], and in severe cases, even suicidal tendencies. With the aim to ameliorate long-term outcomes and quality of life of stroke survivors, clinicians’ active acquirement and patients’ spontaneous complaints of pain sensation will contribute to earlier identification and intervention of PSP.
3.1.3. Post-stroke epilepsy
Stroke is the leading cause of epilepsy in older adults, accounting for nearly half of all epilepsy cases, and post-stroke epilepsy (PSE) is closely related to increased morbidity and mortality [32]. Over the last decades, progress in post-stroke healthcare and management has allowed more patients to survive, correspondingly, though, the occurrence of PSE is also observed raised. The International League Against Epilepsy (ILAE) defines early or acute symptomatic seizures (ASSs) as those that occur within 7 days after stroke, whereas remote symptomatic seizures are those that occur after 7 days [33]. Generally, only late post-stroke seizures are considered as PSE because ASSs have a low relapse rate, whereas late-onset seizures have a high relapse rate of approximately 70%, which is essential basis for the diagnosis of PSE [34]. In addition, patients with ASSs occurring between 4-7 days after stroke-onset were at higher risk of developing PSE [32].
The cumulative incidence rate of PSE ranges from 3% to 30%, and no significant difference in incidence rate has been observed between men and women [34-36]. Patients who suffer a hemorrhagic stroke are more likely to develop epilepsy than patients who have an ischemic stroke, occurring in approximately 6% and 10%, respectively [35, 37]. Currently, generally accepted risk factors of PSE include stroke severity, degree of neurological damage, large lesion volumes (especially in cortex), younger age at stroke onset, and hemorrhage [38]. It is noteworthy that considerable proportion of younger, even neonate, suffer ischemic stroke, who has a greater propensity for epileptogenesis and long-term unfavorable functional outcomes [39]. Finally, two studies have reported that the ALDH2 rs671and T allele of the D40-1C/T polymorphism are associated with PSE susceptibility [40, 41].
The association between reperfusion therapy and risk of post-stroke epileptic seizures presents conflict. An earlier study suggested that seizures might be a marker of successful reperfusion [42]. A meta-analysis concluded that there is insufficient evidence to prove that reperfusion therapy, either using intravenous thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) or mechanical thrombectomy (MT), directly increased risk of seizures [35], although it may augment the risk of seizures by causing hemorrhagic transformation (HT) [43]. Otherwise, some studies held that patients undergoing reperfusion therapy with intravenous rt-PA or intra-arterial therapies were susceptible to developing PSE, with a phenomenon of lowering seizure threshold, and early data from several animal studies have also supported this finding [44, 45]. Of note, the aforementioned clinical studies achieved consensus that combined therapy of rt-PA and MT had no additive negative effect on the occurrence of seizures. The contribution of reperfusion therapy to acquired PSE awaits further investigation. Moreover, whether stroke-related diseases, such as hypertension, diabetes, and dyslipidemia, are responsible for PSE has not been definitively concluded. Several studies have reported that depression and dementia are involved in PSE, and that patients with depression or impaired cognition who encountered a stroke had an increased likelihood of experiencing PSE seizures [34, 45].
3.2. Psychiatric disorders
Considering the often-devastating impact of stroke on physical function, clinicians can overlook the impact of stroke on the mental health of patients, thus normalizing psychiatric disorders, and causing delayed (or even non-existent) diagnosis and treatment.
3.2.1. Post-stroke depression
Depression is the most frequent post-stroke psychiatric disorder, with an estimated prevalence range from 18% to 33% [46, 47]. Patients who suffer post-stroke depression (PSD) are more vulnerable to cognitive deficits, suicidal tendencies and long-term disability, ultimately resulting in poor quality of life and higher mortality. Recent reports have shown that the incidence rate of PSD is higher within the first year after stroke onset, which is reported by approximately 33% and then decreases to about 25% after 1 year [46, 47]. An accumulating body of evidence focused on the analyzing risk factors of PSD has emerged to help clinicians identify and prevent PSD symptoms. Several lines of research have indicated that females are more susceptible to depression after stroke, yet are less likely to receive treatment than males [46, 48]. However, some studies considered no connection between gender and PSD, thus, whether incidence of PSD is differentiated by gender remains controversial [49]. Stroke severity including various lesion type, volume, location and laterality, is judged to be the most robust facilitator for PSD[50]. It is generally recognized that patients with large infarct volumes, left hemisphere lesions, and infarct in the anterior/frontal areas and basal ganglia have a higher likelihood of developing PSD [46]. In addition, no difference in the incidence and mechanism of PSD has been found between hemorrhagic stroke and ischemic stroke. Furthermore, both individual and familial history of psychiatric diseases, and lack of social support during stroke may aggravate the possibility of PSD [51].
3.2.2. Post-stroke apathy
Patients with depression who suffer a stroke frequently suffer from apathy, a common neuropsychiatric symptom that occurs in about one-third of stroke patients. Apathy refers to the simultaneous decrease of goal-oriented activities in the cognitive, behavioral, emotional, or social fields of a patient's life due to diminished motivation [52]. Post-stroke apathy (PSAp) is often misdiagnosed as depression partially attributed to overlapping symptoms with depression, and both manifesting as an inactive mood. Although sharing some symptomatic characteristics, PSAp and depression differ in prevalence, pathogenesis, comorbidities, and outcomes [53]. Emotional distress is the key point to distinguish between apathy and depression: patients with PSD usually accompanied by pessimism and hopeless emotion, which rarely occur in patients with PSAp, who, conversely, often present with indifference and passive engagement with daily behaviors. Furthermore, neuroimaging findings have indicated that PSAp lesions are mainly distributed in the right hemispheric subcortical region, whereas PSD is linked to left anterior lesions [53]. More detailed, greater basal ganglia engage in initiation apathy, prefrontal cortex engages in executive apathy, and orbitofrontal cortex engages in emotional apathy [54]. Crucially, substantial reductions in white matter integrity were observed in patients with apathy [55]. It is of utmost importance for clinicians and caregivers to distinguish PSAp from depression to ensure patients receive appropriate treatment and effective subsequent management.
Intriguingly, except for well-established risk factors such as older age, lower education, and cognition deficit, PSAp was found to be independent of stroke severity or prior stroke [56]. Nevertheless, it has been found that frontal cortical atrophy is a radiological predictor of PSAp[57]. On the other hand, recent studies presented idea that apathy, instead of depression, might be a prodromal symptom of dementia in stroke, which provides some guidance for recognizing patients at-risk of mild cognitive impairment [52]. Considerable evidence has demonstrated that PSAp is associated with other adverse consequences of stroke, including functional impairment, delirium, and fatigue, and particularly with difficulty in concentrating and reduced information processing speed [58].
3.2.3. Post-stroke anxiety
Post-stroke anxiety (PSAn), a consequence of poor motor functioning and substantial decline in quality of life, is a common mental disorder that compromises patient rehabilitation, and, in turn, results in a lasting and steady deterioration of life quality. Previous accumulative studies have reported that PSAn occurs in 18% to 34% of survivors during the first year after stroke, and the rates did not lower meaningfully up to 5 years after stroke [59-62]. Nevertheless, as a serious psychological and physiological problem, PSAn is relatively neglected, presently, compared to other post-stroke psychological disorders. PSAn typically manifests as excessive concern on personal prognosis including recurrence of stroke, returning to work, falling, and ability to maintain independence, which bare considered normal responses to stroke, and are considerable challenges for the accurate diagnosis of PSAn [59]. An analytical study reported that patients who had a hemorrhagic stroke were at higher risk of developing PSAn and that hemorrhagic stroke was the main predictor of anxiety in the first year after stroke[61], yet no connection was found between lesion location or lesion side and PSAn [59]. Accordingly, the authors invoked that clinicians pay close attention to anxiety symptoms in patients with hemorrhagic stroke. Similar to PSD, reports in terms of associations between gender and PSAn are inconsistent. Some studies have deemed that female stroke survivors are more likely to develop PSAn, possibly due to female patients being more vulnerable to social pressure and other psychological factors, yet other researchers who disagreed considered no statistical link between gender and PSAn, or to the convert, men are more likely to experience anxiety after a stroke than women [59, 60, 62].
PSAn usually coexists with other threatening events such as cognitive impairment, depression, and insomnia, forming a chronic interaction in stroke patients. PSAn can exacerbate depression and cognitive impairment, and degrade patient prognosis, which possibly attributes to reduced social engagement and increased functional dependence in daily activities [63]. Most studies involving the relationship between age and PSAn conclude that age is not a risk factor of PSAn [59]. A preliminary study of obesity and PSAn proposed that abdominal obesity was independently related to PSAn but not to PSD, which was supported by the study of DeJesus et al. [64]. In addition, an analysis using machine learning models found that diabetes mellitus, hypertension, alcohol use, and disability increase the risk of PSAn, and that higher serum high-density lipoprotein cholesterol (HDL-C) level can decrease the risk of PSAn [59]. It has also been reported that vitamin D deficiency and serum levels of oxidative markers such as MDA, SOD, and CAT at admission for stroke can be predictors of PSAn at 1 month after the event. Cumulatively, these correlations could provide a guide in identifying and diagnosing PSAn in clinical practice.
3.2.4. Post-stroke fatigue
Fatigue is one of the most common and persistent consequences after stroke. Given substantial heterogeneity exists among various studies, the prevalence of post-stroke fatigue (PSF) has been roughly estimated at 19% to 79% [65-67]. Until recently, PSF was still a neglected issue since it is difficult to quantify and measure. Also, it is often characterized by early exhaustion, subjective lack of energy, and chronically existence that causes burnout towards exercise, emotion and cognition, and usually cannot be ameliorated by rest [67]. It also negatively affects functional outcomes in stroke survivors, leading to worse quality of life, less social participation, lower likelihood to return to work and higher mortality [68]. In line with this, PSF not only affects psychological disorders, such as depression and insomnia, but also causes sarcopenia [69]. PSF is affected by multiple risk factors, including age, gender, depression, lesion location, sleep disturbances, and diabetes mellitus [70]. On the flip side, chronic PSF appears to largely promote multiple comorbidities, such as depression, and it can be a crucial feature of depression. Depression and fatigue can be mutual predictors, the relationship between PSD and PSF does not necessarily mean that one factor will induce the other, nor does it rule out the possibility that a third factor leads to both [71]. Previous analyses reported that depression and anxiety can accelerate fatigue within half a year after stroke, and sometimes they were considered as consequences of fatigue rather than causative factors [63, 68]. Age is the most widely studied factor, however, conclusions regarding these findings are still controversial. Relatively more evidence supported that patients with older age are more likely to suffer PSF [72], whereas other studies shown that younger stroke survivors had a higher incidence of PSF [73, 74]. Moreover, a recent study conducted in Asian and European populations showed that female increased susceptibility to PSF, which is in parallel with the data that female predicts long-term PSF [65, 70]. In addition, there is no definite evidence indicates that cardiovascular disease is responsible for PSF [65, 70]. Thus far, data regarding whether lesion location is associated with PSF are inconclusive, although basal ganglia, brainstem, or thalamus lesions were more observed in stroke survivors with PSF [67, 71, 75]. Also, a higher proportion of patients with recurrent stroke had PSF compared to patients after a first stroke [76]. Considering the serious burden of PSF and its negative impact on patient survival, a better understanding of its etiology and pathogenesis is meaningful to develop new prevention strategies and treatments.
4. Mechanisms underlying post-stroke complications
Interestingly, although the symptoms of PSNCs vary, the studies to date seem to support some general mechanisms underlying these complications, including inflammatory response, dysregulation of the HPA axis, cholinergic dysfunction, reduced level of 5-hydroxytryptamine, glutamate-mediated excitotoxicity, and mitochondrial dysfunction (Fig. 2).
Figure 2.
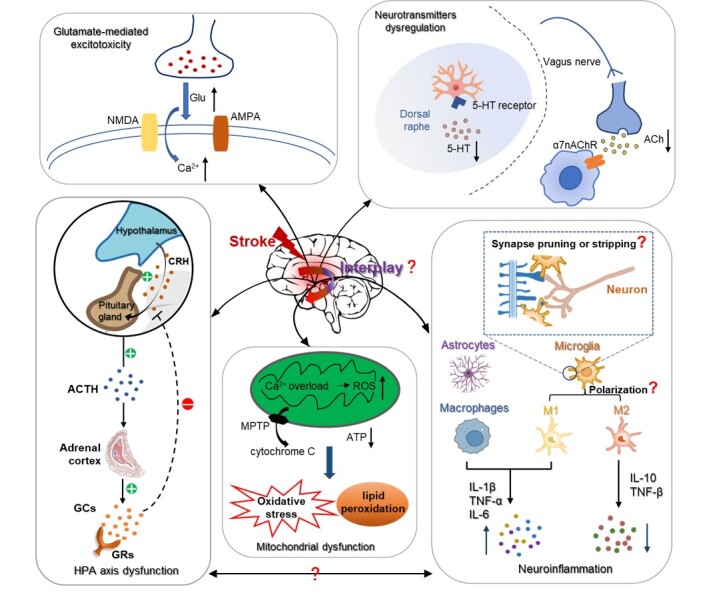
Schematic overview of mechanisms underlying post-stroke neuropsychiatric complications. In the acute phase, stroke as a stressor initiates the activation of the hypothalamic-pituitary-adrenal (HPA) axis and simultaneously stimulates the secretion of pro-inflammatory cytokines from immune cells, e.g., TNF-α, IL-1 and IL-6. The burst of systematic inflammation after stroke attenuates the inhibitory feedback of glucocorticoids (GCs) on hypothalamus and thus prolongs HPA axis activation. Moreover, stroke cascades affect diverse neurotransmitter pathways resulting in the reduced level of 5-HT and cholinergic dysfunction. Stroke also leads to the impaired release and reuptake of glutamate, as well as the overload of intracellular Ca2+ that promotes the rapid rise of glutamate levels in the cerebrospinal fluid. The activation of NMDA and AMPA GluR further induces glutamate toxicity. Excess ROS and Ca2+ accumulation induce mitochondrial dysfunction that leads to oxidative stress and lipid peroxidation. The MPTP open, followed by cytochrome C releasing from mitochondria into the cytoplasm. These dysregulations contribute to PSNCs, e.g., cognitive impairment, pain, epilepsy, depression, apathy and anxiety. However, the key brain areas responsible for serotoninergic and cholinergic dysfunction are still murky. Moreover, although microglia-mediate neuroinflammation has long been believed to play an essential role in stroke, the changes of microglia (like polarization, synapse pruning and synapse stripping) have not been dynamically observed in vivo in the context of PSNCs. Furthermore, the interplay between these pathological events awaits further study.
4.1. Neuroinflammation
Several meta-analyses or longitudinal analyses have described findings of pro-inflammatory markers in patients with PSCID, PSD or PSP, and conversely, the increments of inflammatory markers can be predictors of these complications [77-79]. Additionally, both human and animal studies indicate that oxidative stress induced by inflammatory response is involved in neuronal death and seizures [80]. Patients with depression present higher blood concentrations of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6, and other proteins such as C-reactive protein during the acute phase. In turn, a stroke-induced immune reaction in the brain can also influence the progression of PSD [81]. Long-term neuroinflammation can strongly induce gray-matter and white-matter damage, resulting in long-term sensorimotor and cognitive impairment [82]. Pain can be driven by neuroinflammation via central sensitization in periphery and the CNS [79]. These results establish that inflammation and immune responses, as pivotal biological events, are highly relevant to stroke rehabilitation and neuropsychiatric complications [83].
The inflammatory processes can be further divided into peripheral and central inflammation. Microglia and astrocytes, as the main immunocompetent cells, engage in neuroinflammatory processes [84]. Abnormal neuroplasticity can be observed in several PSNCs, including depression and anxiety, pain, and cognitive impairment [16, 78, 85]. Microglia regulate neuroplasticity in various ways. After ischemic brain injury, microglia can be activated within minutes, and they can be recruited to the lesion sites and rapidly respond to injury signals by releasing pro-inflammatory cytokines [86]. Released cytokines, such as IL-1β and TNF-α, may account for hyperexcitability in neural networks underlying PSE [87]. Moreover, microglial activation has also been shown to suppress hippocampal neurogenesis, which aggravates cognitive dysfunction after stroke [88]. Furthermore, microglia may modulate synaptic plasticity directly by pruning or stripping synapses, both of which are worthy of further investigation in the context of PSNCs [89]. Nevertheless, there are contradictory findings supporting the beneficial effects of microglia in PSNCs. Activated microglia can attenuate neuronal apoptosis and enhance neurogenesis through engulfing cellular debris, and by secreting IL-10 and TGF-β cytokines [6]. More detailed, microglia can differentiate into two phenotypes—the detrimental pro-inflammatory M1 type and the protective and anti-inflammatory M2 type—which represents the dual role of microglia (Figure 2). The M1 type microglia can promote the release of a range of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and nitric oxide (NO) as well as proteolytic enzymes, such as matrix metalloproteinase-9 (MMP-9) and MMP-2, which eventually aggravate neuronal injury and inhibit neurogenesis in the hippocampus [90]. The M2 type microglia can express protective cytokines such as CD206, TGF-β, and IL-10 as well as scavenge receptors, which is instrumental in inhibiting inflammation and promoting tissue repair. Within 7 days after stroke, microglia mainly polarize into the M1 proinflammatory phenotype, whereas the M2 anti-inflammatory phenotype subsequently predominates during the following 2 weeks [90, 91]. The polarization of microglia may explain the contradictory findings above; however, direct evidence is lacking.
In addition to microglia, astrocytes are also activated and proliferated following stroke, with the main characteristic of hypertrophy and ion channel remodeling, which may lead to secondary neurological disease such as epilepsy [80]. In the early phase of stroke, astrocytes can produce excess inflammatory cytokines, such as IL-1β or release glutamate and MMP-2 that causes degradation of matrix protein and subsequent blood-brain barrier (BBB) disruption, which eventually damages neuronal viability and causes encephaledema [92]. Furthermore, astrogliosis is also involved in the formation of glial scar that compromises neuronal regeneration, which is closely associated with neuroinflammation [90]. During stroke rehabilitation, astrocytes can also exert protective effects by uptaking glutamate and releasing neurotrophins to promote angiogenesis and neuronal plasticity [92, 93].
Accumulating evidence has demonstrated that CNS communicates with periphery through various mechanisms. Stress in stroke stimulates peripheral immune cells to secrete pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, which can cross the BBB and further activate microglia and astrocytes in the CNS to secrete more pro-inflammatory cytokines. Ultimately, this causes widespread neuroinflammation and produces neurodegenerative disorders, such as dementia and epilepsy [84, 94]. Macrophages, as the main cells of the peripheral immune system, have been proved to participate in the prognosis of stroke via secreting pro-inflammatory cytokines, such as TNF-α and IL-6, and anti-inflammatory cytokines, such as IL-10 [83]. Similar to central inflammation, peripheral inflammation also has a biphasic effect, and the specific role of peripheral inflammation in determining outcomes after stroke requires further study.
4.2. Dysregulation of the HPA axis
The HPA axis is an important parcel of the neuroendocrine system and is involved in controlling stress responses and regulating many physiological activities, such as metabolic, cardiovascular, immune, and behavioral processes [85]. The connection between increased HPA axis activity and prognosis after ischemic stroke has long been confirmed. Olsson et al. reported that HPA axis hyperactivation has several negative effects on organism function activity and may be a predictor of a poorer functional prognosis after acute ischemic stroke [95]. Also, HPA axis hyperactivation may be critical in shaping pathophysiological reactions to ischemia. It has been well established that a series of psychiatric disorders, including panic, depression, phobias and anxiety, are affected by hyperactivation of the HPA axis [96]. Moreover, the HPA axis passively affects the structure and function of the temporal lobe of the brain, which is widely involved in epilepsy [97]. Stroke-induced seizures damage temporal lobe structures, which can further dis-restrain the HPA axis, trigger a vicious circle of neuronal injury, and predispose to subsequent seizures and psychiatric disorder comorbidities [97, 98].
In the acute phase, stroke as a stressor initiates a hyperactive sequence of the HPA axis: hypophysiotropic neurons secrete more corticotropin-releasing hormone (CRH), the pituitary releases more adrenocorticotropic hormone (ACTH), and then ACTH induces the adrenal cortex to synthesize and secrete more glucocorticoids (GCs), including cortisol (Fig. 2) [98]. Both clinical data and results from a study in an ischemia model show that GCs participate in stroke induced by brain dysfunction, indicating that GCs could potentially aggravate brain injury via activation of GC receptors and increase the vulnerability of neurons [96]. Many studies have shown that activation of these hormones after ischemic stroke could directly lead to neurohormonal dysfunction and neuronal and cell damage, which culminate in poor long-term prognosis [99]. Furthermore, abnormal responses with GCs over-release are regulated by low-affinity glucocorticoid receptors (GRs), which cause reduced inhibitory feedback (Fig. 2). Subsequently, GCs over-release brings about saturation of high-affinity mineralocorticoid receptors that are mainly distributed in the septa and hippocampus, and are responsible for the fundamental secretion of GCs, thereby augmenting neurohormonal dysfunction [100].
In addition to the positive and negative feedback mechanisms, modulation of the HPA axis can affect prognosis after stroke through GC-independent pathways, such as the neurotransmitter pathway. It was reported that, in acute ischemic stroke patients, elevated cortisol in plasma was negatively correlated with brain-derived neurotrophic factor (BDNF) levels, cognition, neurological status, functional responses, and emotional conditions, prompting a link between the decrease of clinical, behavioral, and blood biochemical parameters as well as stress-induced cortisol increase [101, 102]. Additionally, concentration of GCs resulted by HPA axis dysfunction impairs hippocampal neurogenesis, and reduced levels of BDNF in the hippocampus have also been observed [101]. Many studies have reported that patients with depression had lower BDNF levels in blood and hippocampus [102]. Stroke cascades involve hyper-glutamatergic transmission associated with excessive glutamate release, and the excitotoxicity caused by glutamate is reported to be of great importance in several neurological and psychiatric disorders [96]. Some researchers have proposed that glutamate might act on several brain regions, including hypothalamus, hippocampus and temporal cortex, and that glutamate may directly activate the HPA axis, which sequentially affect the secretion of hormones, such as CRH, ACTH, and GCs [103]. Abundant GRs in the hippocampus are essential for the physiological control of executive functions and behavioral responses to stressogenic factors. These effects accelerate post-stroke hippocampal degeneration and culminate in cognitive impairment. Moreover, it is well documented that the functioning of the HPA system is also related to neuroinflammation, and that progressive neuroinflammation caused by HPA axis dysfunction stimulates the expression of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which could further activate the HPA axis and cause increased levels of corticosterone [103]. In brief, inflammation, neurotransmitters and the HPA axis influence each other after stroke, and elicit a wide array of complications.
4.3. Cholinergic dysfunction
It is widely known that the central cholinergic system plays a pivotal role in learning and memory functions, but it is also involved in various pathological processes, such as epilepsy, depression, and anxiety [53, 104]. The broad effects of the cholinergic system may be due to its potent regulatory role in inflammatory immune responses [105]. There is substantial evidence that central cholinergic dysfunction is involved in vascular dementia pathogenesis [8]. Previous studies investigating the relationship between stroke and cholinergic activity have demonstrated a cholinergic deficit in stroke, indicated by a reduction of cholinergic markers. It has further been demonstrated that, the activity of acetylcholine esterase (AChE) dimers decreases with age, which provides additional evidence that aging patients are more likely to suffer post-stroke dementia [106]. At the onset of stroke, ischemia rapidly triggers a cascade of reactions, including the inflammatory response, oxidative stress, and apoptosis, which can lead to central cholinergic dysfunction in selectively vulnerable regions such as hippocampus and cerebral cortex, thereby exacerbating neuronal injury and cognitive decline [107]. Experimental evidence suggests that cerebral ischemia/reperfusion injury (CI/RI) or hypoperfusion also causes many sequelae that include loss of hippocampal neurons and behavioral deficits [8, 107]. Acetylcholine (ACh) is an important brain-body signaling route, the damaged tissue can secrete inflammatory cytokines to activate afferent signals through ascending vagus nerve fibers. In addition, there is a “cholinergic anti-inflammatory pathway” in which ACh interacts with α7 subunit-containing nicotinic receptors (α7nAChR) in immunocytes and promptly suppresses the release/synthesis of TNF-α and other inflammatory mediators, then preventing tissue damage [108]. Also, it was reported that vagus nerve stimulation has a neuroprotective effect in suppressing inflammation and apoptosis via activation of cholinergic pathways in acute CI/RI [109].
Reduced ACh levels and AChE activity have been observed in the brains of patients with cognitive impairment. Correspondingly, the use of cholinomimetic agents, such as cholinesterase inhibitors and ACh receptor agonist, has been an effective strategy for PSCID, and these drugs are also assumed to be a therapeutic strategy in the treatment of acute cerebral damage [108]. However, cholinomimetic agents have not consistently shown efficacy in cognitive impairment, and it is possible that they are only beneficial for individuals with an established cholinergic deficit [110]. Additionally, the clinical use of AChE inhibitors may reduce the risk of ischemic stroke and death in patients with dementia [111]. In summary, activating the cholinergic anti-inflammatory pathway may be a potential strategy for post-stroke therapy. However, detailed characterization of the molecular mechanism of ACh in stroke is lacking and requires further investigation.
4.4. Lower levels of 5-hydroxytryptamine
Presently, antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) are the main therapeutic prescribed for post-stroke depression and apathy, as well as for pain and cognitive impairment, which underscores the importance of 5-HT in the pathogenesis of PSNCs [112, 113]. Serotoninergic neurons reside mainly in the dorsal raphe nucleus and can project to the entire brain and neuraxis. Previous studies have shown substantial upregulation of serotonergic receptors after the onset of stroke [9]. The injured projection could cause diminished levels of 5-HT in the left frontal cortex, resulting in depressed mood and cognitive impairment [46]. Likewise, neuroinflammation is involved in 5-HT metabolism. BDNF interacts with 5-HT to regulate neuronal plasticity, and exogenous BDNF increases the level of 5-HT[9]. Furthermore, there is evidence that 5-HT can mitigate glutamate efflux induced by ischemic human cerebrocortical slices [46]. Also, the HPA axis and 5-HT influence each other: HPA axis dysfunction followed by stroke affects the release of 5-HT, and decreased 5-HT further activates the HPA axis, which is additionally evidenced by higher cortisol levels in patients with depression [114].
4.5. Glutamate-mediated excitotoxicity
Glutamate is the major excitatory neurotransmitter in the CNS. Numerous studies have shown that excitotoxicity caused by pathological excessive release of glutamate is involved in stroke [115]. Following on, dysfunction of the glutamate transmission system is closely associated with the pathogenesis of neurodegenerative and neuro-psychiatric diseases after stroke, including depression, epilepsy, and cognitive impairment [116]. After the onset of stroke, brain tissue becomes acutely ischemic/hypoxic, resulting in ion transporter dysfunction and ion homeostasis disruption, which, in turn, lead to the impaired release and reuptake of glutamate, and overload of intracellular Ca2+ that further promotes the rapid rise of glutamate levels in the cerebrospinal fluid (CSF). These cascades ultimately lead to neuronal death [117, 118]. Moreover, the excessive release of glutamate could cause synaptic excitotoxicity by aggravating oxidative stress and inflammation, and inflammatory mediators might regulate extracellular glutamate levels by reducing the glutamate scavenging ability of glial cells, including microglia and astrocytes [7, 119].
Extensive activation of N-methyl-D-aspartate (NMDA), a glutamate-gated ion channel, is one of the major causes of glutamate toxicity. NMDA activation induces glutamate toxicity by suppressing the synthesis and release of neurotrophic factors, such as BDNF and other synaptic proteins [119]. It was shown that another α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor (GluR) increases the release of inflammatory cytokines [120], which, conversely, increases synaptic glutamate and potentially contributes to excitotoxicity, followed by a succession of events, including synaptic plasticity and memory impairment [121]. Simply put, inappropriate activation of GluRs eventually destroys the delicate balance of neuroprotective versus neurotoxic effects of glutamate in brain, substantially increasing the likelihood of mental disorders, such as depression. In support of this idea, many studies have reported that higher levels of glutamate and its metabolite were measured in the blood and CSF of patients with PSD, particularly in the frontal cortex [122, 123]. Also, the alteration of glutamate levels in the epileptic brain is well established. A microdialysis study by Çavuş et al. showed that, compared to the non-epileptogenic cortex, glutamate levels were elevated in epileptogenic, non-localized and lesional cortical sites [124]. With the increasingly clear role of glutamate in post-stroke damage, glutamate excitotoxicity might be a potential therapeutic target for these neuropsychiatric complications.
4.6. Mitochondrial dysfunction
The importance of mitochondrial dysfunction in the development of stroke-related brain damage is widely recognized [125]. Stroke induces depolarization of mitochondrial membrane potential (MMP), which initiates a succession of disastrous events, including aberrant reactive oxygen species (ROS) generation, reduced mitochondrial adenosine triphosphate (ATP) production, and Ca2+ accumulation, which exacerbates the dysregulation of mitochondrial biogenesis [126]. Deficiency of energy supply and metabolism is the most immediate cause of mitochondrial dysfunction in cerebral ischemia [127]. Furthermore, mitochondrial dysfunction-induced excess ROS leads to oxidative stress and lipid peroxidation, which activates microglia and, subsequently leads to neuroinflammation [128, 129]. Moreover, Ca2+ overload and the irreversible collapse of MMP potentially activate the mitochondrial permeability transition pore (MPTP) opening, followed by cytochrome C releasing from mitochondria into the cytoplasm, which, in turn, initiates the apoptotic cascade and results neuronal death [130]. Thus, these cascades induced by mitochondrial dysfunction affect complications of stroke involving dementia, epilepsy, and psychiatric disorder. The crucial role of mitochondrial dysfunction in post-stroke sequelae is further supported by the finding of impaired energy metabolism in patients with depression [130], as well as by evidence that enhanced cerebral ATP availability may have antidepressant effects [131]. Previous data also showed that ameliorating oxidative stress and mitochondrial dysfunction can promote the recovery of learning and memory function [132, 133], and transplantation of exogenous mitochondria exert the function of rescue neurons caused by ischemia damage [134].
5. Therapeutic intervention
5.1. Pharmacological treatment
Currently, multiple rehabilitative modalities are used to intervene in post-stroke complications, including pharmacological agents (Western and traditional Chinese medicine [TCM]), and non-pharmacological treatments such as acupuncture, TMS, and psychological counseling. Here, we discuss the mechanisms and effects of these therapies and explore more efficient or combined therapeutic strategies. The general characteristics of various treatments for post-stroke complications are shown in Table 1.
Table 1.
The general characteristics of various treatments for post-stroke neuropsychiatric complications
| Types of PSNCs | Study ID | Inclusion criteria | Treatment | Dose range | Type or mechanism of drug | Outcomes or conclusion | Adverse effects |
|---|---|---|---|---|---|---|---|
| Cognitive impairment and Dementia | Narasimhalu et al [165] | Ischemic stroke patients aged 55-85 years, with CI no dementia | Rivastigmine | 1.5-4.5 mg twice a day. | Cholinesterase inhibitors | Well tolerated, potentially improve executive functioning. | Nausea, sleeping difficulties, headache, gastrointestinal upse, chest pain, giddiness, etc |
| Dichgans et al [166] | Men and women aged 25-70 years, with CADASIL, meet the CI criteria: (1) a description of cognitive problems given by others. (2)a MMSE score of 10-27, or a TMT B time score 1.5 SDs below the mean | Donepezil | 5 mg daily for the first 6 weeks and 10 mg daily thereafter | Cholinesterase inhibitor | Had no effect on the primary endpoint of the V-ADAS-cog score, had some improvements on executive function. | Gastrointestinal disorders, nausea, diarrhoea and vomiting, muscle cramps, dizziness, insomnia | |
| Kos et al [58] | Stroke in the right hemisphere with cognitive impairment | Donepezil | 5 mg/day | Increased recruitment of the parieto-frontal networks | Significant improvements in the Mini-Mental Status Examination | / | |
| Jorge et al [113] | Patients aged 50-90 years, within 3 months following stroke | Escitalopram | 10 mg/day for patients <65 years and 5 mg/day for patients ≥65 years | Selective serotonin reuptake inhibitor | Significant improvement of RBANS score | Dry mouth, constipation, indigestion, anorexia, etc | |
| Black et al [167] | Patients mean aged 73.9 years; with probable or possible VaD, according to criteria of NINDS and AIREN | Donepezil | 5-10 mg/day | Cholinesterase inhibitor | Significant improvement in cognition, global function and activities of daily living | Nausea, diarrhea, cramps and leg cramps, anorexia, vomiting, headache, abnormal dreams, hypertension, etc | |
| Erkinjuntti et al[168] | Patients met the clinical criteria of probable vascular dementia | Galantamine | 24 mg/day | Inhibits acetylcholinesterase and modulates nicotinic receptors | Great efficacy on ADAS-cog and CIBIC-plus, activities of daily living and behavioural symptoms | Nausea, vomiting | |
| Whyte et al [169] | Stroke within the last 30 days, and cognitive impairment | (1)Galantamine; (2)donepezil |
(1)8-24 mg/day; (2)5-10 mg/day |
Acetylcholinesterase inhibitors | Donepezil had better functional recovery than galantamine. | Gastrointestinal side effects | |
| Pain | Vick et al [170] | A 68 years old female with central pain syndrome following an ICH |
Ketamine | 50 mg/night to 50 mg three times/day | NMDA antagonist | Beneficial in decreasing allodynia and hyperalgesia, improving functional capabilities | No side effect |
| Lampl et al [171] | Patients aged 36 to 68 years, with central post-stroke pain | Amitriptyline | 10-75 mg in extended-release form within 3 weeks then kept for 365 days | Elevate levels of serotonin and noradrenaline, NMDA receptor blockers | Reduces but does not completely prevent CPSP | No new side effect | |
| Kruszewski et al [172] | Patients aged over 18 years, with central neuropathic pain associated with spinal cord injury | Pregabalin | 150-600 mg/day | Reduced calcium influx into hyperexcited neurons | Efficacious in relieving central neuropathic pain, improving sleep, anxiety, and overall status | Somnolence and dizziness | |
| Vestergaard et al [173] | Patients range 37 to 77 years, with CPSP |
Lamotrigine | 25-200 mg/day | Anti-glutamatergic and block sodium channel | A well tolerated and moderately effective treatment for CPSP | Mild rashes | |
| Pellicane et al [174] | Patients with CPSP | Methylprednisolone | 4-12 mg/day | Not mentioned | Nearly equivalent effects versus other drugs (pregabalin, amitriptyline, lamotrigine) | / | |
| Hesami et al [175] | Patients with stroke in the thalamic area and diagnosed with CPSP | Gabapentin | 300 mg twice daily for one month | Probably elevates GABA levels in the brain | Safe, efficacious, well tolerated and lack of interaction with other drugs | No adverse effects | |
| Shimodozono et al [112] | Patients with hemiplegia and intractable CPSP | Fluvoxamine | 25-125 mg/day | Selective serotonin reuptake inhibitor | Useful for the control of CPSP in the early stage | Headache, itching of skin, appetite loss | |
| Jungehulsing et al [176] | Patients, age > 18 years, CPSP persisted > 3 months, and a pain score ⩾4 on the 11-point Likert scale | Levetiracetam | 1000 mg BID to 3000 mg/day | / | Not effective in treatment for CPSP | Tiredness, pain increase, dizziness, pruritus and headache | |
| Epilepsy | Gilad et al [138] | Patients with a first single epileptic seizure after an ischemic stroke | (1)Lamotrigine (LTG) (2)Carbamazepine (CBZ) |
(1)25-100 mg every week (2)100-300 mg/day |
(1)Inhibition of the voltage-dependent sodium channel and the release of glutamate. (2)/ | LTG is a relatively better-tolerated drug than CBZ, and can be acceptable as initial treatment in post-stroke seizures | (1)Somnolence and dizziness (2)Somnolence, dizziness, nausea and vomiting, skin eruption, confusion and overdose symptoms |
| Belcastro et al [177] | Patients, aged 71.9±7.3 years, with late-onset post-stroke seizures | Levetiracetam (LEV) | 1000-2000 mg/day | / | A safe and effective candidate as a first-choice drug against post-stroke seizures. | Drowsiness, aggressive behaviour | |
| Kutlu et al [178] | Patients aged 60 or older with at least two late-onset post-stroke seizures. | Levetiracetam | 1000-3000 mg/day | / | An efficacious and well tolerated therapy in elderly patients with late-onset post-stroke seizures | Somnolence, headache, dizziness | |
| Hsieh et al [153] | Human; hospitalized for the first-ever stroke | Statins include atorvastatin, rosuvastatin, fluvastatin, simvastatinand lovastatin | Not mentioned in detail | Possibly decrease isoprenoids of the β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase pathway | A modest drug with non-significant effect in preventing post-stroke epilepsy, decreasing post-stroke mortality only in men. | / | |
| Matsubara et al [140] | patients with a first-ever ischemic stroke and no history of epilepsy before stroke | Statins included pitavastatin, atorvastatin, simvastatin, rosuvastatin and pravastatin. | Not mentioned in detail | Probably inhibit HMG-CoA reductase and its neuroprotective properties | Reduction the risk of post-stroke early onset seizures, preventing the progression of initial post-stroke seizure-induced neurodegeneration into chronic epilepsy. |
/ | |
| Gilad et al [179] | Patients with spontaneous non-traumatic and non-aneurysmatic ICH | Valproic acid | 800 mg/day | / | Improve neurological outcome and reduce early seizures, but did not prevent the occurrence of seizures post ICH | Mild liver dysfunction | |
| Alvarez-Sabín et al | Patients aged 18 years or older, with a first post-stroke late epileptic [180] seizures | Gabapentin | 900-1800 mg/day | / | Useful and safe for late post-stroke epileptic seizures | Drowsiness and dizziness, headache, fatigue, nausea or vomiting, dyspepsia, weight increase and peripheral edema | |
| Depression | Choi-kwon et al [152] | Patients with post-stroke depression, emotional incontinence, or anger proneness | Fluoxetine | 20 mg/day | Selective serotonin reuptake inhibitor | Efficacious in the treatment of PSEI and PSAP; effect on PSD is not solidly confirmed. | Nausea, headache, insomnia, sexual dysfunction, etc |
| Kim et al [181] | Older than 20 years, with an acute stroke within the past 21 days, had a modified Rankin Scale score of two or greater | Escitalopram | 10 mg/day for 3 months | Selective serotonin reuptake inhibitor | Did not significantly reduce moderate or severe depressive symptoms in patients with acute stroke. | Constipation, muscle pain, insomnia, diarrhoea | |
| Ribeiro et al [182] | An 82-year-old man with no psychiatric history, severely depressed after stroke | (1)Sertraline (2)quetiapine |
(1) 100 mg/day (2) 50 mg/day | Acting on frontal-subcortical circuits and related regions. | Full remission of depressed symptoms | Not mentioned | |
| Ding et al [151] | Aged 18-75 years; not taken antidepressants in the last 2weeks; meet the diagnostic criteria of stroke and depression, and HAMD>18 points; | (1)Danzhi Xiaoyao Powder (2)escitalopram oxalate | (1)3 bag/day (2) 10 mg/day |
Probably inhibit hyperactivity of HPA and regulate neurotransmitters in hippocampus | / | Without increasing adverse effects | |
| Palomäki et al [183] | Ischemic stroke occurred not more than 30 days earlier, under 71years old | Mianserin | 10-60 mg/day | Tetracyclic antidepressant Drug | Unable to prevent PSD | Diarrhea and nausea | |
| Rasmussen et al [184] | Diagnosed with stroke without current depression, significant aphasia or dementia, history of schizophrenia, psychosis, or severe drug abuse and antidepressants treatment | Sertraline | 50-100 mg/day | Selective serotonin reuptake inhibitor | Have significantly superior prophylactic efficacy | Tiredness, weight gain, dry mouth, cheek smarting, and rash | |
| Robinson et al [185] | Nondepressed patients within 3 months following acute stroke | Escitalopram | 10 mg/day < age 65, 5 mg/day > age 65 | / | Significantly lower incidence of depression | Gastrointestinal adverse effects such as dry mouth, constipation, indigestion, etc; Sexual adverse effects such as decreased libido; Cardiovascular adverse effects such as tachycardia; Other adverse effects such as dizziness, fatigue, etc | |
| Apathy | Tay et al [186] | Patients ⩾18 years between 2 and 15 days after stroke onset, with persisting neurological deficit | Fluoxetine | 20 mg/day | Selective serotonin reuptake inhibitor | Ineffective in preventing post-stroke apathy | / |
| Kohno et al [187] | An 80-year-old right-handed man with severe apathy after cerebral infarction in the prefrontal cortex | Ropinirole | 0.75 mg/day | Selective agonist of dopamine D2/3 receptors | Remarkably improved verbal output and spontaneity in daily life | / | |
| Aragona et al [188] | A 62-year-old man affected by hemorrhagic stroke in the left thalamus, presented with marked apathy | Bupropion | 150 mg/day | Improve the mesocortical pathway | Have an overall reduction of apathy | / | |
| Auret et al [189] | A 44-year-old patient with severe and disabling apathy nearly 2 years after a right hemisphere hemorrhagic stroke | Zolpidem | A single-dose of 10 mg/day on days 2, 4 and 5 over a 9-day period | / | Dramaticly improved apathy | No adverse effects | |
| Robinson et al [190] | Diagnosed with apathy | Nefiracetam | 600 or 900 mg/day | / | 900 mg nefiracetam had a significantly greater change in Apathy Scale scores | Gastrointestinal, nervous system side effects, etc | |
| Mikami et al [191] | Patients 50-90 years old within 3 months of an index stroke who did not meet DSM-IV diagnostic criteria for major or minor depression and did not have a serious comorbid physical illness |
Escitalopram | 10 mg patients ≤ 65 years; 5 mg patients ≥ 65 years | / | Effective in preventing new onset of apathy following stroke | Constipation, indigestion, anorexia, anorgasmia, ejaculation disorders, chest pain and tachycardia | |
| Watanabe et al [192] | A 38-year-old right-handed man with prominent apathy after subcortical infarcts | Methylphenidate | 5mg bid | / | Selective improvement of frontal system function | / | |
| Starkstein et al [193] | Age 40 to 90 years, apathy onset 8 weeks after stroke | Nefiracetam | 900 mg bid | / | Did not prove to be efficacious in post-stroke apathy | Fatigue, muscle or joint pain, etc. It did not produce more adverse events than placebo | |
| Anxiety | Rao et al [194] | Diagnosed as post-stroke anxiety | Sertraline | 25-200 mg/day | Selective serotonin reuptake inhibitor | Well tolerant, had improvement on the Clinical Anxiety Scale | No adverse events |
| Mikami et al [191] | Aged 50 to 90 years old, an index stroke within 3 months and did not meet DSM-IV diagnostic criteria for depressive disorder | Escitalopram | 10 mg/day ≤ age 65, 5 mg/day ≥ age 65 | / | Effective in preventing new onset of post-stroke generalized anxiety disorder | No significant differences among different group | |
| Fatigue | Choi-Kwon et al [152] | Patients with PSF | Fluoxetine | 20 mg/day for 3 months | Selective serotonin reuptake inhibitor | Not effective in improving PSF | Not mentioned |
Abbreviations: CI, cognitive impairment; CADASIL, subcortical infarcts and leucoencephalopathy; MMSE, mini-mental state examination; TMT, trail making test; V-ADAS-cog, vascular AD assessment scale cognitive subscale; RBANS, Assessment of Neuropsychological Status; VaD, vascular dementia; NINDS, National In-stitute of Neurological Disorders and Stroke; AIREN, the Association International pour la Recherche et l’Enseignement en Neurosciences; ICH, intracerebral hemorrhage; NMDA, N-methyl D-aspartate; GABA, gamma-Aminobutyric acid; PSEI, post-stroke emotional incontinence; PSEI, post-stroke anger proneness; HAMD; Hamilton Depression Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorder
5.1.1. PSCID treatment
Thus far, there are no specific FDA approved pharmacological treatments for PSCID [16]. Several agents have been proved to be effective for vascular dementia, such as cholinesterase inhibitors (galantamine, donepezil, or rivastigmine), memantine and gingko biloba[135]. Clinical studies presented contradictory results on the effects of cholinesterase inhibitors. Stroke is a cerebrovascular accident, and PSCID is often the consequence of small cerebrovascular diseases. The treatment of PSCID should not be confined to anti-dementia medication, it may also benefit from therapy of anti-cerebrovascular disease [136].
5.1.2. PSE treatment
Current stroke guidelines do not recommend the administration of anti-epileptic drugs (ADs) for prophylactic treatment of PSE, owing to common adverse effects and drug-drug interactions, and evidence regarding use of ADs in PSE is scarce [137, 138]. A paucity of basic research and clinical trials investigating post-stroke has resulted in the situation that no valid medication is approved for the treatment of PSE. Further complicating the field, there is a lack of animal models that faithfully recapitulate post-stroke spontaneous epilepsy to enable robust preclinical studies. It is not surprising, however, that there is a divergence between evidence-based recommendations and clinical practice. Many clinicians prescribe ADs that are well-tolerated and lack significant drug interactions to prevent recurrent seizures when patients suffer an acute symptomatic seizure after stroke. Currently, clinical management of PSE is guided mainly by patient age, co-morbidities, and co-medications[139]. Over the last decades, several studies have shown that statins could decrease the incidence of epilepsy after stroke, although the results were inconsistent [137, 140].
5.1.3. CPSP treatment
A growing number of studies and trials have demonstrated the efficacy of pharmacological management in CPSP, including intravenous lidocaine and opioids as well as tricyclic antidepressants (TCAs), such as gabapentin, amitriptyline, and lamotrigine, which are recommended as first-line drugs [141]. In recent years, studies have found that aliphatic acid products and peptides may have potential in the treatment of CPSP by modulating the function of hippocampus [142, 143]. To date, the pharmacologic interventions available for CPSP are inadequate due to both drug resistance and side effects, particularly in elderly patients [143]. Furthermore, patients with CPSP often have co-morbidities, thus fully considering the necessity of prompt pain relief, side effects, and potential beneficial effects, as well as individualized medication selection is crucial to ensure appropriate management. Certainly, future drug development programs for CPSP should consider the high incidence of co-morbidities in this patient population.
5.1.4. PSD treatment
Pharmacological intervention is the main therapeutic approach to PSD, particularly in the sub-acute phase after stroke, and effective strategies are defined. Antidepressants used to treat PSD mainly include TCAs, SSRIs, dual serotonin and noradrenergic reuptake inhibitors, and these are the basis of current PSD intervention. Compelling clinical and preclinical evidence suggests that various phytochemical compounds extracted from natural products and compound prescriptions of TMC have strong antidepressant activities and few side effects, and these may represent promising new interventions for PSD [144-146]. Unlike clinically available agents, the mechanism of drugs being evaluated in basic research of PSD mainly focused on the regulation of the HPA axis and inflammation [147]. In addition, prophylactic use of antidepressants may be a feasible management strategy in stroke patients.
5.1.5. PSAp treatment
Available pharmacological treatments for PSAp are currently limited. Additionally, little basic research is being conducted on PSAp, and most published clinical studies are limited to anecdotal case reports and mainly focus on dopaminergic agents, cholinesterase inhibitors, and stimulants. Owing to the shared symptomatology with depression, many antidepressants, such as SSRIs, are prescribed for the treatment of apathy [148]. However, evidence showing that antidepressants have effects on patients without additional depressive symptoms is limited. More animal studies and controlled clinical trials are urgently needed to investigate the efficacy of current therapeutic options and to test other agents in PSAp.
5.1.6. PSAn treatment
Similar to the other post-stroke psychiatric disorders after stroke, no specific guidelines have been developed for the treatment of anxiety in stroke patients, and few clinical trials and no animal studies have specifically targeted PSAn symptoms. SSRIs, TCAs, benzodiazepines, and Z-drugs, such as zopiclone, zolpidem, and zaleplon, can, however, be used to treat general anxiety [149]. In addition, several studies have suggested that SSRIs might be effective for several anxiety disorders after stroke, and preliminarily evidence suggests that herbal medicine (HM) or HM combined with conventional pharmacotherapy may be a safer and more effective strategy for the treatment of PSAn when it is accompanied by other symptoms [150, 151].
5.1.7. PSF treatment
To date, there are no effective interventions for the treatment or prevention of PSF. Most current clinical practice guidelines rely on expert experience and consensus, which is partly attributed to the lack of understanding of its underlying mechanisms. An important consideration that has not been settled in the field is whether PSF actually requires medical therapy. Currently, pharmacological treatments for fatigue have been frequently investigated in patients with multiple sclerosis but rarely in patients with stroke. A considerable proportion of PSF management has focused on environmental suggestions and physical interventions. Even if depression and fatigue are generally dissociated, a few studies have reported relieving PSF with antidepressants, but these have achieved few definite effects [152]. Additional studies are required to clarify the causative factors of PSF and to explore effective treatments for PSF. Another noteworthy point is the application of statins: due to neuroprotective effects, statins are commonly prescribed to stroke patients, yet emerging studies have shown that statins result in a higher frequency of PSF, and part of the blame lied with their neuromuscular side effects [66]. It is necessary to raise awareness of this symptom among professionals and patients, and the use of statins should be vigilant when the evaluation of Fatigue Severity Scale (FSS) ≥4. Moreover, a retrospective cohort study has shown that statins only significantly reduce the long-term mortality of male patients after stroke [153]. Given the pro-PSF effect of statins and the truth that females predict long-term PSF more frequently, gender should be considered when prescribing statins.
5.2. Non-pharmacological treatment
5.2.1. Acupuncture treatment
Acupuncture and moxibustion are prevalent in medicine, particularly in rehabilitation medicine, and show good efficacy and few adverse effects. In the last decade, there has been a marked increase in studies of acupuncture for intervention in PSNCs, most of which present positive outcomes. An overview of systematic reviews regarding acupuncture in post-stroke cognitive impairment and depression management concluded that acupuncture is safe and effective in improving cognitive function and depressive disorder without obvious serious side events for post-stroke patients [154]. Consistently, a systematic review and meta-analysis reported that acupuncture has a greater effect on PSD and a better safety profile than antidepressants [155]. Also, acupuncture can be an adjunctive treatment for post-stroke epilepsy, and patients who received acupuncture treatment experienced a decreased incidence of and a reduced risk of epilepsy [156]. Moreover, it has been shown that acupuncture is an effective treatment for post-stroke shoulder pain and central pain [157], and animal researches provide further support that electroacupuncture can effectively relieve CPSP by inhibiting autophagy in the hippocampus [93]. Nevertheless, the mechanism by which acupuncture affects the recurrence and rehabilitation of ischemic stroke remains poorly understood, and additional well-designed studies are required to elucidate its efficacy and mechanism.
5.2.2. Other non-pharmacological treatment of PSNCs
Therapy for post-stroke complications is an unmet medical need [158]. Emerging technologies such as, transcranial magnetic stimulation (TMS), psychotherapy, behavioral therapy, educational programs and music or art therapy, have shown good efficacy in the treatment of post-stroke sequelae. Stroke patients often experience a strong psychological deterioration as a result of the sudden onset and potential loss of physical functioning, and psychosocial interventions are thought to be necessary for stroke survivors for whom monotherapy is ineffective, most notably those with psychiatric disorders. A randomized controlled trial revealed that a brief psychosocial-behavioral intervention plus pharmaco-therapy achieved better depression reduction and remission than antidepressant treatment alone in the short term and at a 2-years follow-up [159]. Strengthening the psychological expertise of healthcare professionals working on stroke units, and combining psychological counseling with nursing care, can effectively promote the rehabilitation of stroke patients. TMS has been shown to be beneficial for patients who are pharmaco-resistant. One randomized trial involving 13 patients with chronic stroke showed that high-frequency repetitive TMS over the dorsal anterior cingulate cortex to the medial prefrontal cortex ameliorated apathy compared to sham stimulation [160]. A meta-analysis by Shao et al. [161] showed that TMS could be an effective treatment for PSD and was more effective in Asians. In addition, using TMS to increase cortical excitability in patients with high levels of PSF resulted in improved fatigue symptoms that lasted for a week later. In recent years, music interventions have been used in post-stroke rehabilitation to contribute to the recovery of brain functions involved in movement, cognition, mood, and sensory perceptions. Several studies have indicated that music interventions have a positive impact on gait, extremity motor function, communication outcomes, and general well-being after stroke [162, 163]. To confirm these promising results, more high-quality studies including a larger number of patients are needed before specific recommendations can be made for clinical practice.
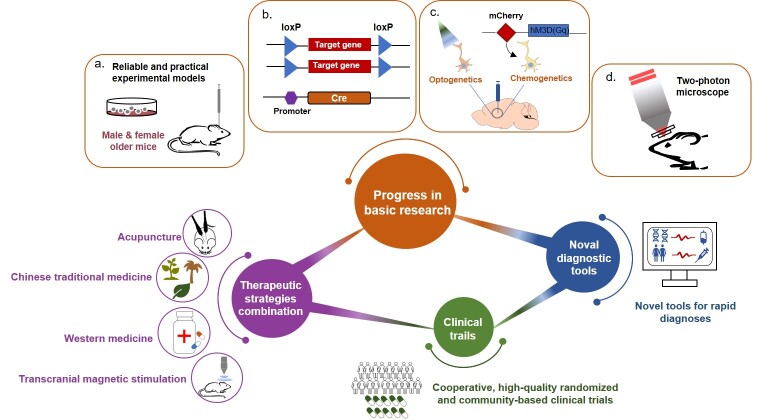
6. Conclusion and perspective
Although substantial progress has been made in the past two decades, the risk factors and the interplay of the underlying mechanisms mentioned above are insufficiently understood. More importantly, therapeutic interventions that are both effective and safe are lacking. Furthermore, the side effects of unsuitable treatments, probably resulting from the untimely diagnosis and complex clinical symptoms, should not be ignored. Taken all, further investigations into PSNCs, from both basic and clinical perspective, are urgent for the development of novel strategies for PSNCs (Fig. 3).
-
(1)Progress in basic research:
-
a.Establish reliable and practical experimental models for PSNCs. So far, the basic research of mechanisms underlying PSNCs jog on slowly largely due to lack of repeatable animal models of PSNCs. Notably, existing laboratory models often use healthy young male animals, but stroke patients often have concomitant hypertension, diabetes, atherosclerosis, and other conditions. Many comorbidities must be taken into account when investigating novel animal models.
-
b.Employ advanced pharmacological technologies. Currently, it is generally accepted that inflammation is involved in stroke-associated complications. However, how inflammation or inflammatory cytokines/ chemokines affect post-stroke phenotypes specifically remains unclear. Intriguingly, recent studies found that microglial synapse engulfment can be specifically promoted by IL-33 derived from astrocytes [164]. Therefore, it may be worth trying to dissect the cell-type-specific functions in the context of PSNCs by employing Cre-Loxp technology. In addition, many brain regions can provide cholinergic or serotoninergic effects to the cortex and hippocampus. However, it is still unclear which brain areas are responsible for cholinergic and serotoninergic dysfunction after stroke. The combination of optogenetics or chemogenetics with PSNCs animal models may be necessary to clarify this issue. Furthermore, in vivo two-photon imaging is an optional way to observe dynamic changes of immune cells after stroke; Detailed records of their movements (e.g., synaptic pruning or stripping by microglia) and morphology (e.g., microglial polarization) will help address the contradictory role of inflammation.
-
a.
-
(2)
Develop novel tools for rapid diagnoses. Stroke survivors often also have aphasia and limb incoordination, plus a strong social-psychological gap, which can make it difficult, or can make them reluctant, to articulate their needs and express their feelings. This can explain why some post-stroke symptoms are easily ignored by caregivers. Current instruments can already accurately locate the stroke lesions and provide high-definition images of the injured brain. These images can be re-used to establish the association of lesion patterns and post-stroke symptoms by a series of computational analyses, which will provide a valuable tool for predicting PSNCs.
-
(2)
Conduct cooperative, high-quality randomized and community-based clinical trials. As mentioned above, most existing studies related to PSNCs have unignored limitations, including biased sampling, relatively small sample sizes and short follow-up periods, thus producing relatively unconvincing evidence. Hence, high-quality randomized clinical trials and community-based studies are urgently needed to fill existing current knowledge gaps.
-
(2)
Combine multiple therapeutic strategies. Many approaches in addition to pharmaceutical interventions are also shown impressive protective effects against PSNCs. Therefore, the integrative applications of medicines and these treatment strategies, such as acupuncture and moxibustion, TMS, and environmental enrichment, are promising for the effective rehabilitation of stroke. However, standard clinical guidelines should also be proposed in time to avoid overtreatment.
Figure 3.
The perspective of future research in post-stroke neuropsychiatric complications. (1) Progress in basic research: a. Establish reliable and practical experimental models for PSNCs and b. Employ advanced pharmacological technologies. (2) Develop novel tools for rapid diagnoses. (3) Conduct cooperative, high-quality randomized and community-based clinical trials. (4) Combine multiple therapeutic strategies.
Acknowlegements
This project was supported by grants from the National Natural Science Foundation of China (U21A20418, 82273903, 82003727), Natural Science Foundation of Zhejiang Province (LQ21H310002) and the Research Project of Zhejiang Chinese Medical University (2021JKJNTZ011B).
Funding Statement
This project was supported by grants from the National Natural Science Foundation of China (U21A20418, 82273903, 82003727), Natural Science Foundation of Zhejiang Province (LQ21H310002) and the Research Project of Zhejiang Chinese Medical University (2021JKJNTZ011B).
Footnotes
Author Contributions
Conceptualization: J.Z. and Y.Z.; Writing: J.Z., Y.F. and Y.Z.; Supervision: Z.C. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- [1].Hankey GJ (2014). Secondary stroke prevention. Lancet Neurol, 13:178-194. [DOI] [PubMed] [Google Scholar]
- [2].Zaidi SK, Hoda MN, Tabrez S, Khan MI (2022). Pharmacological Inhibition of Class III Alcohol Dehydrogenase 5: Turning Remote Ischemic Conditioning Effective in a Diabetic Stroke Model. Antioxidants(Basel), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferro JM, Caeiro L, Figueira ML (2016). Neuropsychiatric sequelae of stroke. Nat Rev Neurol, 12:269-280. [DOI] [PubMed] [Google Scholar]
- [4].Esenwa C, Gutierrez J (2015). Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag, 11:437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fornaro M, Solmi M, Veronese N, De Berardis D, Buonaguro EF, Tomasetti C, et al. (2017). The burden of mood-disorder/cerebrovascular disease comorbidity: essential neurobiology, psychopharmacology, and physical activity interventions. Int Rev Psychiatry, 29:425-435. [DOI] [PubMed] [Google Scholar]
- [6].Chamorro Á, Dirnagl U, Urra X, Planas AM (2016). Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol, 15:869-881. [DOI] [PubMed] [Google Scholar]
- [7].Tomasetti C, Iasevoli F, Buonaguro EF, De Berardis D, Fornaro M, Fiengo AL, et al. (2017). Treating the Synapse in Major Psychiatric Disorders: The Role of Postsynaptic Density Network in Dopamine-Glutamate Interplay and Psychopharmacologic Drugs Molecular Actions. Int J Mol Sci, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].O'Sullivan MJ, Oestreich LKL, Wright P, Clarkson AN (2022). Cholinergic and hippocampal systems facilitate cross-domain cognitive recovery after stroke. Brain, 145:1698-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gu S, He Z, Xu Q, Dong J, Xiao T, Liang F, et al. (2022). The Relationship Between 5-Hydroxytryptamine and Its Metabolite Changes With Post-stroke Depression. Front Psychiatry, 13:871754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gottesman RF, Hillis AE (2010). Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol, 9:895-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sibolt G, Curtze S, Jokinen H, Pohjasvaara T, Kaste M, Karhunen PJ, et al. (2021). Post-stroke dementia and permanent institutionalization. J Neurol Sci, 421:117307. [DOI] [PubMed] [Google Scholar]
- [12].Pendlebury ST, Rothwell PM (2009). Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol, 8:1006-1018. [DOI] [PubMed] [Google Scholar]
- [13].Levine DA, Gross AL, Briceño EM, Tilton N, Giordani BJ, Sussman JB, et al. (2021). Sex Differences in Cognitive Decline Among US Adults. JAMA Netw Open, 4:e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levine DA, Kabeto M, Langa KM, Lisabeth LD, Rogers MA, Galecki AT (2015). Does Stroke Contribute to Racial Differences in Cognitive Decline? Stroke, 46:1897-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, et al. (2011). Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain, 134:3716-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Droś J, Klimkowicz-Mrowiec A (2021). Current view on post-stroke dementia. Psychogeriatrics, 21:407-417. [DOI] [PubMed] [Google Scholar]
- [17].Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, et al. (2017). Post-stroke dementia - a comprehensive review. BMC Med, 15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Droś J, Kowalska K, Pasińska P, Szyper-Maciejowska A, Gorzkowska A, Klimkowicz-Mrowiec A (2020). Delirium Post-Stroke-Influence on Post-Stroke Dementia (Research Study-Part of the PROPOLIS Study). J Clin Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Q, Capistrant BD, Ehntholt A, Glymour MM (2012). Long-term rate of change in memory functioning before and after stroke onset. Stroke, 43:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harrison RA, Field TS (2015). Post stroke pain: identification, assessment, and therapy. Cerebrovasc Dis, 39:190-201. [DOI] [PubMed] [Google Scholar]
- [21].Choi HR, Aktas A, Bottros MM (2021). Pharmacotherapy to Manage Central Post-Stroke Pain. CNS Drugs, 35:151-160. [DOI] [PubMed] [Google Scholar]
- [22].Atalan P, Bērziņa G, Sunnerhagen KS (2021). Influence of mobility restrictions on post-stroke pain. Brain Behav, 11:e02092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Delpont B, Blanc C, Osseby GV, Hervieu-Bègue M, Giroud M, Béjot Y (2018). Pain after stroke: A review. Rev Neurol (Paris), 174:671-674. [DOI] [PubMed] [Google Scholar]
- [24].Klit H, Finnerup NB, Andersen G, Jensen TS (2011). Central poststroke pain: a population-based study. Pain, 152:818-824. [DOI] [PubMed] [Google Scholar]
- [25].Paolucci S, Iosa M, Toni D, Barbanti P, Bovi P, Cavallini A, et al. (2016). Prevalence and Time Course of Post-Stroke Pain: A Multicenter Prospective Hospital-Based Study. Pain Med, 17:924-930. [DOI] [PubMed] [Google Scholar]
- [26].Huang YC, Chang KH, Liou TH, Cheng CW, Lin LF, Huang SW (2017). Effects of Kinesio taping for stroke patients with hemiplegic shoulder pain: A double-blind, randomized, placebo-controlled study. J Rehabil Med, 49:208-215. [DOI] [PubMed] [Google Scholar]
- [27].Pollak L, Shlomo N, Korn Lubetzki I (2017). Headache in stroke according to National Acute Stroke Israeli Survey. Acta Neurol Scand, 135:469-475. [DOI] [PubMed] [Google Scholar]
- [28].Plecash AR, Chebini A, Ip A, Lai JJ, Mattar AA, Randhawa J, et al. (2019). Updates in the Treatment of Post-Stroke Pain. Curr Neurol Neurosci Rep, 19:86. [DOI] [PubMed] [Google Scholar]
- [29].Bair MJ, Robinson RL, Katon W, Kroenke K (2003). Depression and pain comorbidity: a literature review. Arch Intern Med, 163:2433-2445. [DOI] [PubMed] [Google Scholar]
- [30].Naess H, Lunde L, Brogger J, Waje-Andreassen U (2012). Fatigue among stroke patients on long-term follow-up. The Bergen Stroke Study. J Neurol Sci, 312:138-141. [DOI] [PubMed] [Google Scholar]
- [31].Ma G, Sun L, Qie Z, He J, Cui F (2021). Factors Associated with Benzodiazepines Prolonged-Term Use in Post-Stroke Subjective Sleep Disturbance: A Single-Centre Retrospective Study from China. Drug Des Devel Ther, 15:2469-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lin R, Yu Y, Wang Y, Foster E, Kwan P, Lin M, et al. (2021). Risk of Post-stroke Epilepsy Following Stroke-Associated Acute Symptomatic Seizures. Front Aging Neurosci, 13:707732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. (2014). ILAE official report: a practical clinical definition of epilepsy. Epilepsia, 55:475-482. [DOI] [PubMed] [Google Scholar]
- [34].Pitkänen A, Roivainen R, Lukasiuk K (2016). Development of epilepsy after ischaemic stroke. Lancet Neurol, 15:185-197. [DOI] [PubMed] [Google Scholar]
- [35].Lekoubou A, Fox J, Ssentongo P (2020). Incidence and Association of Reperfusion Therapies With Poststroke Seizures: A Systematic Review and Meta-Analysis. Stroke, 51:2715-2723. [DOI] [PubMed] [Google Scholar]
- [36].Zhang C, Wang X, Wang Y, Zhang JG, Hu W, Ge M, et al. (2014). Risk factors for post-stroke seizures: a systematic review and meta-analysis. Epilepsy Res, 108:1806-1816. [DOI] [PubMed] [Google Scholar]
- [37].Zou S, Wu X, Zhu B, Yu J, Yang B, Shi J (2015). The pooled incidence of post-stroke seizure in 102-008 patients. Top Stroke Rehabil, 22:460-467. [DOI] [PubMed] [Google Scholar]
- [38].Brondani R, de Almeida AG, Cherubini PA, Secchi TL, de Oliveira MA, Martins SCO, et al. (2019). Risk Factors for Epilepsy After Thrombolysis for Ischemic Stroke: A Cohort Study. Front Neurol, 10:1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Billinghurst LL, Beslow LA, Abend NS, Uohara M, Jastrzab L, Licht DJ, et al. (2017). Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology, 88:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang B, Chen M, Yang H, Wu T, Song C, Guo R (2014). Evidence for involvement of the CD40/CD40L system in post-stroke epilepsy. Neurosci Lett, 567:6-10. [DOI] [PubMed] [Google Scholar]
- [41].Yang H, Song Z, Yang GP, Zhang BK, Chen M, Wu T, et al. (2014). The ALDH2 rs671 polymorphism affects post-stroke epilepsy susceptibility and plasma 4-HNE levels. PLoS One, 9:e109634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodan LH, Aviv RI, Sahlas DJ, Murray BJ, Gladstone JP, Gladstone DJ (2006). Seizures during stroke thrombolysis heralding dramatic neurologic recovery. Neurology, 67:2048-2049. [DOI] [PubMed] [Google Scholar]
- [43].Thevathasan A, Naylor J, Churilov L, Mitchell PJ, Dowling RJ, Yan B, et al. (2018). Association between hemorrhagic transformation after endovascular therapy and poststroke seizures. Epilepsia, 59:403-409. [DOI] [PubMed] [Google Scholar]
- [44].Brigo F, Schneider M, Wagenpfeil G, Ragoschke-Schumm A, Fousse M, Holzhoffer C, et al. (2020). Intravenous thrombolysis with tPA and cortical involvement increase the risk of early poststroke seizures: Results of a case-control study. Epilepsy Behav, 104:106312. [DOI] [PubMed] [Google Scholar]
- [45].Tsirka SE, Gualandris A, Amaral DG, Strickland S (1995). Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature, 377:340-344. [DOI] [PubMed] [Google Scholar]
- [46].Medeiros GC, Roy D, Kontos N, Beach SR (2020). Post-stroke depression: A 2020 updated review. Gen Hosp Psychiatry, 66:70-80. [DOI] [PubMed] [Google Scholar]
- [47].Guo J, Wang J, Sun W, Liu X (2022). The advances of post-stroke depression: 2021 update. J Neurol, 269:1236-1249. [DOI] [PubMed] [Google Scholar]
- [48].Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CD (2011). Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke, 42:1907-1911. [DOI] [PubMed] [Google Scholar]
- [49].De Ryck A, Brouns R, Geurden M, Elseviers M, De Deyn PP, Engelborghs S (2014). Risk factors for poststroke depression: identification of inconsistencies based on a systematic review. J Geriatr Psychiatry Neurol, 27:147-158. [DOI] [PubMed] [Google Scholar]
- [50].Zhang E, Liao P (2020). Brain-derived neurotrophic factor and post-stroke depression. J Neurosci Res, 98:537-548. [DOI] [PubMed] [Google Scholar]
- [51].Shi Y, Yang D, Zeng Y, Wu W (2017). Risk Factors for Post-stroke Depression: A Meta-analysis. Front Aging Neurosci, 9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tay J, Morris RG, Tuladhar AM, Husain M, de Leeuw FE, Markus HS (2020). Apathy, but not depression, predicts all-cause dementia in cerebral small vessel disease. J Neurol Neurosurg Psychiatry, 91:953-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Douven E, Staals J, Freeze WM, Schievink SH, Hellebrekers DM, Wolz R, et al. (2020). Imaging markers associated with the development of post-stroke depression and apathy: Results of the Cognition and Affect after Stroke - a Prospective Evaluation of Risks study. Eur Stroke J, 5:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Horne KS, Gibson EC, Byrne J, Bender JR, Robinson GA (2022). Post-stroke apathy: A case series investigation of neuropsychological and lesion characteristics. Neuropsychologia, 171:108244. [DOI] [PubMed] [Google Scholar]
- [55].Saleh Y, Le Heron C, Petitet P, Veldsman M, Drew D, Plant O, et al. (2021). Apathy in small vessel cerebrovascular disease is associated with deficits in effort-based decision making. Brain, 144:1247-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tay J, Lisiecka-Ford DM, Hollocks MJ, Tuladhar AM, Barrick TR, Forster A, et al. (2020). Network neuroscience of apathy in cerebrovascular disease. Prog Neurobiol, 188:101785. [DOI] [PubMed] [Google Scholar]
- [57].Mihalov J, Mikula P, Budiš J, Valkovič P (2016). Frontal Cortical Atrophy as a Predictor of Poststroke Apathy. J Geriatr Psychiatry Neurol, 29:171-176. [DOI] [PubMed] [Google Scholar]
- [58].Kos C, van Tol MJ, Marsman JB, Knegtering H, Aleman A (2016). Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci Biobehav Rev, 69:381-401. [DOI] [PubMed] [Google Scholar]
- [59].Wang J, Zhao D, Lin M, Huang X, Shang X (2021). Post-stroke Anxiety Analysis via Machine Learning Methods. Front Aging Neurosci, 13:657937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chun HY, Whiteley WN, Dennis MS, Mead GE, Carson AJ (2018). Anxiety After Stroke: The Importance of Subtyping. Stroke, 49:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ojagbemi A, Akinyemi J, Owolabi M, Akinyemi R, Arulogun O, Gebregziabher M, et al. (2020). Predictors and prognoses of new onset post-stroke anxiety at one year in black Africans. J Stroke Cerebrovasc Dis, 29:105082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yao M, Li H, Luo Y, Li L, Yu J (2021). High Prevalence of Post-stroke Anxiety in Elderly Patients Following COVID-19 Outbreak. Front Psychiatry, 12:699869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Raju RS, Sarma PS, Pandian JD (2010). Psychosocial problems, quality of life, and functional independence among Indian stroke survivors. Stroke, 41:2932-2937. [DOI] [PubMed] [Google Scholar]
- [64].DeJesus RS, Breitkopf CR, Ebbert JO, Rutten LJ, Jacobson RM, Jacobson DJ, et al. (2016). Associations Between Anxiety Disorder Diagnoses and Body Mass Index Differ by Age, Sex and Race: A Population Based Study. Clin Pract Epidemiol Ment Health, 12:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang S, Cheng S, Zhang Z, Wang C, Wang A, Zhu W (2021). Related risk factors associated with post-stroke fatigue: a systematic review and meta-analysis. Neurol Sci, 42:1463-1471. [DOI] [PubMed] [Google Scholar]
- [66].Vitturi BK, Mitre LP, Kim AIH, Gagliardi RJ (2021). Prevalence and Predictors of Fatigue and Neuropsychiatric Symptoms in Patients with Minor Ischemic Stroke. J Stroke Cerebrovasc Dis, 30:105964. [DOI] [PubMed] [Google Scholar]
- [67].Acciarresi M, Bogousslavsky J, Paciaroni M (2014). Post-stroke fatigue: epidemiology, clinical characteristics and treatment. Eur Neurol, 72:255-261. [DOI] [PubMed] [Google Scholar]
- [68].Gurková E, Štureková L, Mandysová P, Šaňák D (2023). Factors affecting the quality of life after ischemic stroke in young adults: a scoping review. Health Qual Life Outcomes, 21:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Su Y, Asamoto M, Yuki M, Saito M, Hasebe N, Hirayama K, et al. (2021). Predictors and short-term outcomes of post-stroke fatigue in initial phase of transition from hospital to home: A prospective observational study. J Adv Nurs, 77:1825-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pedersen A, Almkvist E, Holmegaard L, Lagging C, Redfors P, Blomstrand C, et al. (2022). Fatigue 7-years post-stroke: Predictors and correlated features. Acta Neurol Scand, 146:295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wei C, Zhang F, Chen L, Ma X, Zhang N, Hao J (2016). Factors associated with post-stroke depression and fatigue: lesion location and coping styles. J Neurol, 263:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [72].Barbour VL, Mead GE (2012). Fatigue after Stroke: The Patient's Perspective. Stroke Res Treat, 2012:863031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Parks NE, Eskes GA, Gubitz GJ, Reidy Y, Christian C, Phillips SJ (2012). Fatigue impact scale demonstrates greater fatigue in younger stroke survivors. Can J Neurol Sci, 39:619-625. [DOI] [PubMed] [Google Scholar]
- [74].Snaphaan L, van der Werf S, de Leeuw FE (2011). Time course and risk factors of post-stroke fatigue: a prospective cohort study. Eur J Neurol, 18:611-617. [DOI] [PubMed] [Google Scholar]
- [75].Tang WK, Chen YK, Mok V, Chu WC, Ungvari GS, Ahuja AT, et al. (2010). Acute basal ganglia infarcts in poststroke fatigue: an MRI study. J Neurol, 257:178-182. [DOI] [PubMed] [Google Scholar]
- [76].Glader EL, Stegmayr B, Asplund K (2002). Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke, 33:1327-1333. [DOI] [PubMed] [Google Scholar]
- [77].Kim KY, Shin KY, Chang KA (2022). Potential Biomarkers for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Int J Mol Sci, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014). Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry, 71:1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gold MS, Gebhart GF (2010). Nociceptor sensitization in pain pathogenesis. Nat Med, 16:1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pitkänen A, Lukasiuk K (2011). Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol, 10:173-186. [DOI] [PubMed] [Google Scholar]
- [81].Levada OA, Troyan AS (2018). Poststroke Depression Biomarkers: A Narrative Review. Front Neurol, 9:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Liu W, Wang X, Yang S, Huang J, Xue X, Zheng Y, et al. (2016). Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sci, 151:313-322. [DOI] [PubMed] [Google Scholar]
- [83].Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019). Global brain inflammation in stroke. Lancet Neurol, 18:1058-1066. [DOI] [PubMed] [Google Scholar]
- [84].Wardlaw JM, Smith C, Dichgans M (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol, 18:684-696. [DOI] [PubMed] [Google Scholar]
- [85].Juruena MF, Eror F, Cleare AJ, Young AH (2020). The Role of Early Life Stress in HPA Axis and Anxiety. Adv Exp Med Biol, 1191:141-153. [DOI] [PubMed] [Google Scholar]
- [86].Wang R, Pu H, Ye Q, Jiang M, Chen J, Zhao J, et al. (2020). Transforming Growth Factor Beta-Activated Kinase 1-Dependent Microglial and Macrophage Responses Aggravate Long-Term Outcomes After Ischemic Stroke. Stroke, 51:975-985. [DOI] [PubMed] [Google Scholar]
- [87].Iori V, Frigerio F, Vezzani A (2016). Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol, 26:118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F (2017). Regulation of microglial activation in stroke. Acta Pharmacol Sin, 38:445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wan Y, Feng B, You Y, Yu J, Xu C, Dai H, et al. (2020). Microglial Displacement of GABAergic Synapses Is a Protective Event during Complex Febrile Seizures. Cell Rep, 33:108346. [DOI] [PubMed] [Google Scholar]
- [90].Lima RR, Santana LN, Fernandes RM, Nascimento EM, Oliveira AC, Fernandes LM, et al. (2016). Neurodegeneration and Glial Response after Acute Striatal Stroke: Histological Basis for Neuroprotective Studies. Oxid Med Cell Longev, 2016:3173564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M (2016). MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials, 91:151-165. [DOI] [PubMed] [Google Scholar]
- [92].Zhao Y, Rempe DA (2010). Targeting astrocytes for stroke therapy. Neurotherapeutics, 7:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zheng L, Li XY, Huang FZ, Zhang XT, Tang HB, Li YS, et al. (2020). Effect of electroacupuncture on relieving central post-stroke pain by inhibiting autophagy in the hippocampus. Brain Res, 1733:146680. [DOI] [PubMed] [Google Scholar]
- [94].Kalaria RN, Akinyemi R, Ihara M (2016). Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta, 1862:915-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Olsson T (1990). Urinary free cortisol excretion shortly after ischaemic stroke. J Intern Med, 228:177-181. [DOI] [PubMed] [Google Scholar]
- [96].Gulyaeva NV, Onufriev MV, Moiseeva YV (2021). Ischemic Stroke, Glucocorticoids, and Remote Hippocampal Damage: A Translational Outlook and Implications for Modeling. Front Neurosci, 15:781964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Basu T, Maguire J, Salpekar JA (2021). Hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Neurosci Lett, 746:135618. [DOI] [PubMed] [Google Scholar]
- [98].Maguire J, Salpekar JA (2013). Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav, 26:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Radak D, Resanovic I, Isenovic ER (2014). Changes in hypothalamus-pituitary-adrenal axis following transient ischemic attack. Angiology, 65:723-732. [DOI] [PubMed] [Google Scholar]
- [100].Zhou L, Wang T, Yu Y, Li M, Sun X, Song W, et al. (2022). The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed Pharmacother, 151:113146. [DOI] [PubMed] [Google Scholar]
- [101].Casas S, Perez AF, Mattiazzi M, Lopez J, Folgueira A, Gargiulo-Monachelli GM, et al. (2017). Potential Biomarkers with Plasma Cortisol, Brain-derived Neurotrophic Factor and Nitrites in Patients with Acute Ischemic Stroke. Curr Neurovasc Res, 14:338-346. [DOI] [PubMed] [Google Scholar]
- [102].Pang C, Cao L, Wu F, Wang L, Wang G, Yu Y, et al. (2015). The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology, 97:447-456. [DOI] [PubMed] [Google Scholar]
- [103].Kim S, Park ES, Chen PR, Kim E (2022). Dysregulated Hypothalamic-Pituitary-Adrenal Axis Is Associated With Increased Inflammation and Worse Outcomes After Ischemic Stroke in Diabetic Mice. Front Immunol, 13:864858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS, et al. (2012). Roles of p75(NTR), long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biol Psychiatry, 71:75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Conner JM, Chiba AA, Tuszynski MH (2005). The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron, 46:173-179. [DOI] [PubMed] [Google Scholar]
- [106].Saldanha C (2017). Human Erythrocyte Acetylcholinesterase in Health and Disease. Molecules, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ray RS, Rai S, Katyal A (2014). Cholinergic receptor blockade by scopolamine and mecamylamine exacerbates global cerebral ischemia induced memory dysfunction in C57BL/6J mice. Nitric Oxide, 43:62-73. [DOI] [PubMed] [Google Scholar]
- [108].Winek K, Soreq H, Meisel A (2021). Regulators of cholinergic signaling in disorders of the central nervous system. J Neurochem, 158:1425-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C (2014). Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One, 9:e102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Barrett KM, Brott TG, Brown RD, Jr., Carter RE, Geske JR, Graff-Radford NR, et al. (2011). Enhancing recovery after acute ischemic stroke with donepezil as an adjuvant therapy to standard medical care: results of a phase IIA clinical trial. J Stroke Cerebrovasc Dis, 20:177-182. [DOI] [PubMed] [Google Scholar]
- [111].Tan ECK, Johnell K, Garcia-Ptacek S, Haaksma ML, Fastbom J, Bell JS, et al. (2018). Acetylcholinesterase inhibitors and risk of stroke and death in people with dementia. Alzheimers Dement, 14:944-951. [DOI] [PubMed] [Google Scholar]
- [112].Shimodozono M, Kawahira K, Kamishita T, Ogata A, Tohgo S, Tanaka N (2002). Reduction of central poststroke pain with the selective serotonin reuptake inhibitor fluvoxamine. Int J Neurosci, 112:1173-1181. [DOI] [PubMed] [Google Scholar]
- [113].Jorge RE, Acion L, Moser D, Adams HP, Jr., Robinson RG (2010). Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry, 67:187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Khodanovich M, Kisel A, Kudabaeva M, Chernysheva G, Smolyakova V, Krutenkova E, et al. (2018). Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats. Int J Mol Sci, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shen Z, Xiang M, Chen C, Ding F, Wang Y, Shang C, et al. (2022). Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed Pharmacother, 151:113125. [DOI] [PubMed] [Google Scholar]
- [116].Liu Y, Wang S, Kan J, Zhang J, Zhou L, Huang Y, et al. (2020). Chinese Herbal Medicine Interventions in Neurological Disorder Therapeutics by Regulating Glutamate Signaling. Curr Neuropharmacol, 18:260-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Suzuki H, Kawakita F, Asada R, Nakano F, Nishikawa H, Fujimoto M (2022). Old but Still Hot Target, Glutamate-Mediated Neurotoxicity in Stroke. Transl Stroke Res, 13:216-217. [DOI] [PubMed] [Google Scholar]
- [118].Gruenbaum BF, Kutz R, Zlotnik A, Boyko M (2020). Blood glutamate scavenging as a novel glutamate-based therapeutic approach for post-stroke depression. Ther Adv Psychopharmacol, 10:2045125320903951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Haroon E, Miller AH, Sanacora G (2017). Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology, 42:193-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cheng SY, Zhao YD, Li J, Chen XY, Wang RD, Zeng JW (2014). Plasma levels of glutamate during stroke is associated with development of post-stroke depression. Psychoneuroendocrinology, 47:126-135. [DOI] [PubMed] [Google Scholar]
- [121].Wang CJ, Wu Y, Zhang Q, Yu KW, Wang YY (2019). An enriched environment promotes synaptic plasticity and cognitive recovery after permanent middle cerebral artery occlusion in mice. Neural Regen Res, 14:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Madeira C, Vargas-Lopes C, Brandão CO, Reis T, Laks J, Panizzutti R, et al. (2018). Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients With Probable Alzheimer's Disease and Depression. Front Psychiatry, 9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sanacora G, Treccani G, Popoli M (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology, 62:63-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Çavuş I, Romanyshyn JC, Kennard JT, Farooque P, Williamson A, Eid T, et al. (2016). Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the yale epilepsy surgery program. Ann Neurol, 80:35-45. [DOI] [PubMed] [Google Scholar]
- [125].Ng YS, Lax NZ, Blain AP, Erskine D, Baker MR, Polvikoski T, et al. (2022). Forecasting stroke-like episodes and outcomes in mitochondrial disease. Brain, 145:542-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Prentice H, Modi JP, Wu JY (2015). Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid Med Cell Longev, 2015:964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Shukla V, Fuchs P, Liu A, Cohan CH, Dong C, Wright CB, et al. (2019). Recurrent Hypoglycemia Exacerbates Cerebral Ischemic Damage in Diabetic Rats via Enhanced Post-Ischemic Mitochondrial Dysfunction. Transl Stroke Res, 10:78-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Petrovic S, Arsic A, Ristic-Medic D, Cvetkovic Z, Vucic V (2020). Lipid Peroxidation and Antioxidant Supplementation in Neurodegenerative Diseases: A Review of Human Studies. Antioxidants(Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Scaini G, Mason BL, Diaz AP, Jha MK, Soares JC, Trivedi MH, et al. (2022). Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol Psychiatry, 27:1095-1102. [DOI] [PubMed] [Google Scholar]
- [130].Bansal Y, Kuhad A (2016). Mitochondrial Dysfunction in Depression. Curr Neuropharmacol, 14:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, Frederick Bde B, et al. (2001). Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry, 158:2048-2055. [DOI] [PubMed] [Google Scholar]
- [132].Andrabi SS, Parvez S, Tabassum H (2020). Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma, 257:335-343. [DOI] [PubMed] [Google Scholar]
- [133].Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018). Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis, 9:924-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heiβ-Lückemann L, et al. (2021). Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics, 11:3109-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kavirajan H, Schneider LS (2007). Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol, 6:782-792. [DOI] [PubMed] [Google Scholar]
- [136].Sun JH, Tan L, Yu JT (2014). Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med, 2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. (2018). 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 49:e46-e110. [DOI] [PubMed] [Google Scholar]
- [138].Gilad R, Sadeh M, Rapoport A, Dabby R, Boaz M, Lampl Y (2007). Monotherapy of lamotrigine versus carbamazepine in patients with poststroke seizure. Clin Neuropharmacol, 30:189-195. [DOI] [PubMed] [Google Scholar]
- [139].Holtkamp M, Beghi E, Benninger F, Kälviäinen R, Rocamora R, Christensen H (2017). European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur Stroke J, 2:103-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Matsubara S, Tanaka T, Tomari S, Fukuma K, Ishiyama H, Abe S, et al. (2020). Statin treatment can reduce incidence of early seizure in acute ischemic stroke: A propensity score analysis. Sci Rep, 10:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Attal N, Gaudé V, Brasseur L, Dupuy M, Guirimand F, Parker F, et al. (2000). Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study. Neurology, 54:564-574. [DOI] [PubMed] [Google Scholar]
- [142].Chen X, Li Z, Zhang B, Liu T, Yao W, Wan L, et al. (2022). Antinociception role of 14,15-epoxyeicosatrienoic acid in a central post-stroke pain model in rats mediated by anti-inflammation and anti-apoptosis effect. Neurochem Int, 154:105291. [DOI] [PubMed] [Google Scholar]
- [143].Sasaki K, Halder SK, Matsunaga H, Ueda H (2020). Beneficial actions of prothymosin alpha-mimetic hexapeptide on central post-stroke pain, reduced social activity, learning-deficit and depression following cerebral ischemia in mice. Peptides, 126:170265. [DOI] [PubMed] [Google Scholar]
- [144].Fang C, Zhang Z, Xu H, Liu Y, Wang X, Yuan L, et al. (2022). Natural Products for the Treatment of Post-stroke Depression. Front Pharmacol, 13:918531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Du Y, Ruan J, Zhang L, Fu F (2020). Jieyu Anshen Granule, a Chinese Herbal Formulation, Exerts Effects on Poststroke Depression in Rats. Evid Based Complement Alternat Med, 2020:7469068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhao A, Ma B, Xu L, Yao M, Zhang Y, Xue B, et al. (2021). Jiedu Tongluo Granules Ameliorates Post-stroke Depression Rat Model via Regulating NMDAR/BDNF Signaling Pathway. Front Pharmacol, 12:662003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Baune BT (2017). Are Non-steroidal Anti-Inflammatory Drugs Clinically Suitable for the Treatment of Symptoms in Depression-Associated Inflammation? Curr Top Behav Neurosci, 31:303-319. [DOI] [PubMed] [Google Scholar]
- [148].Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S (2009). A longitudinal view of apathy and its impact after stroke. Stroke, 40:3299-3307. [DOI] [PubMed] [Google Scholar]
- [149].Chun HY, Newman R, Whiteley WN, Dennis M, Mead GE, Carson AJ (2018). A systematic review of anxiety interventions in stroke and acquired brain injury: Efficacy and trial design. J Psychosom Res, 104:65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Shyu BC, He AB, Yu YH, Huang ACW (2021). Tricyclic antidepressants and selective serotonin reuptake inhibitors but not anticonvulsants ameliorate pain, anxiety, and depression symptoms in an animal model of central post-stroke pain. Mol Pain, 17:17448069211063351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ding C, Xu M, Gao L, Wang X, Xu W, Guo M, et al. (2021). Clinical efficacy of Danzhi Xiaoyao Powder in the treatment of post-stroke depression: A protocol for randomized, double-blind clinical study. Medicine (Baltimore), 100:e27318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Choi-Kwon S, Han SW, Kwon SU, Kang DW, Choi JM, Kim JS (2006). Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double-blind, placebo-controlled study. Stroke, 37:156-161. [DOI] [PubMed] [Google Scholar]
- [153].Hsieh PF, Tung H, Lin CH (2020). Statin effects on post-stroke epilepsy and mortality - Taiwan population-based study. Neurol Res, 42:422-429. [DOI] [PubMed] [Google Scholar]
- [154].Hung CY, Wu XY, Chung VC, Tang EC, Wu JC, Lau AY (2019). Overview of systematic reviews with meta-analyses on acupuncture in post-stroke cognitive impairment and depression management. Integr Med Res, 8:145-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Liu R, Zhang K, Tong QY, Cui GW, Ma W, Shen WD (2021). Acupuncture for post-stroke depression: a systematic review and meta-analysis. BMC Complement Med Ther, 21:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhang Y, Zhao M, Zhang B, Zhang K, Zhou Z (2021). Acupuncture as an Adjunctive Treatment for Post-stroke Epilepsy: Protocol for a Randomized Controlled Trial. Front Neurol, 12:711390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lee JA, Park SW, Hwang PW, Lim SM, Kook S, Choi KI, et al. (2012). Acupuncture for shoulder pain after stroke: a systematic review. J Altern Complement Med, 18:818-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Liao J, Lu X, Shao X, Zhu L, Fan X (2021). Uncovering an Organ's Molecular Architecture at Single-Cell Resolution by Spatially Resolved Transcriptomics. Trends Biotechnol, 39:43-58. [DOI] [PubMed] [Google Scholar]
- [159].Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, et al. (2009). Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke, 40:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sasaki N, Hara T, Yamada N, Niimi M, Kakuda W, Abo M (2017). The Efficacy of High-Frequency Repetitive Transcranial Magnetic Stimulation for Improving Apathy in Chronic Stroke Patients. Eur Neurol, 78:28-32. [DOI] [PubMed] [Google Scholar]
- [161].Shao D, Zhao ZN, Zhang YQ, Zhou XY, Zhao LB, Dong M, et al. (2021). Efficacy of repetitive transcranial magnetic stimulation for post-stroke depression: a systematic review and meta-analysis of randomized clinical trials. Braz J Med Biol Res, 54:e10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Raglio A, Zaliani A, Baiardi P, Bossi D, Sguazzin C, Capodaglio E, et al. (2017). Active music therapy approach for stroke patients in the post-acute rehabilitation. Neurol Sci, 38:893-897. [DOI] [PubMed] [Google Scholar]
- [163].Jones C (2020). The Use of Therapeutic Music Training to Remediate Cognitive Impairment Following an Acquired Brain Injury: The Theoretical Basis and a Case Study. Healthcare(Basel), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, et al. (2018). Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science, 359:1269-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Narasimhalu K, Effendy S, Sim CH, Lee JM, Chen I, Hia SB, et al. (2010). A randomized controlled trial of rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurol Scand, 121:217-224. [DOI] [PubMed] [Google Scholar]
- [166].Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. (2008). Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol, 7:310-318. [DOI] [PubMed] [Google Scholar]
- [167].Black S, Román GC, Geldmacher DS, Salloway S, Hecker J, Burns A, et al. (2003). Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke, 34:2323-2330. [DOI] [PubMed] [Google Scholar]
- [168].Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV (2002). Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet, 359:1283-1290. [DOI] [PubMed] [Google Scholar]
- [169].Whyte EM, Lenze EJ, Butters M, Skidmore E, Koenig K, Dew MA, et al. (2008). An open-label pilot study of acetylcholinesterase inhibitors to promote functional recovery in elderly cognitively impaired stroke patients. Cerebrovasc Dis, 26:317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Vick PG, Lamer TJ (2001). Treatment of central post-stroke pain with oral ketamine. Pain, 92:311-313. [DOI] [PubMed] [Google Scholar]
- [171].Lampl C, Yazdi K, Röper C (2002). Amitriptyline in the prophylaxis of central poststroke pain. Preliminary results of 39 patients in a placebo-controlled, long-term study. Stroke, 33:3030-3032. [DOI] [PubMed] [Google Scholar]
- [172].Kruszewski SP, Shane JA (2007). Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology, 68:2158-2159; author reply 2159-2160. [DOI] [PubMed] [Google Scholar]
- [173].Vestergaard K, Andersen G, Gottrup H, Kristensen BT, Jensen TS (2001). Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology, 56:184-190. [DOI] [PubMed] [Google Scholar]
- [174].Pellicane AJ, Millis SR (2013). Efficacy of methylprednisolone versus other pharmacologic interventions for the treatment of central post-stroke pain: a retrospective analysis. J Pain Res, 6:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hesami O, Gharagozli K, Beladimoghadam N, Assarzadegan F, Mansouri B, Sistanizad M (2015). The Efficacy of Gabapentin in Patients with Central Post-stroke Pain. Iran J Pharm Res, 14:95-101. [PMC free article] [PubMed] [Google Scholar]
- [176].Jungehulsing GJ, Israel H, Safar N, Taskin B, Nolte CH, Brunecker P, et al. (2013). Levetiracetam in patients with central neuropathic post-stroke pain--a randomized, double-blind, placebo-controlled trial. Eur J Neurol, 20:331-337. [DOI] [PubMed] [Google Scholar]
- [177].Belcastro V, Costa C, Galletti F, Autuori A, Pierguidi L, Pisani F, et al. (2008). Levetiracetam in newly diagnosed late-onset post-stroke seizures: a prospective observational study. Epilepsy Res, 82:223-226. [DOI] [PubMed] [Google Scholar]
- [178].Kutlu G, Gomceli YB, Unal Y, Inan LE (2008). Levetiracetam monotherapy for late poststroke seizures in the elderly. Epilepsy Behav, 13:542-544. [DOI] [PubMed] [Google Scholar]
- [179].Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y (2011). Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res, 95:227-231. [DOI] [PubMed] [Google Scholar]
- [180].Alvarez-Sabín J, Montaner J, Padró L, Molina CA, Rovira R, Codina A, et al. (2002). Gabapentin in late-onset poststroke seizures. Neurology, 59:1991-1993. [DOI] [PubMed] [Google Scholar]
- [181].Kim JS, Lee EJ, Chang DI, Park JH, Ahn SH, Cha JK, et al. (2017). Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Psychiatry, 4:33-41. [DOI] [PubMed] [Google Scholar]
- [182].Ribeiro NF, Madruga L (2021). A sudden and severe depressive episode after a left cingulate gyrus stroke: a case report of post-stroke depression and review of literature. J Neural Transm (Vienna), 128:711-716. [DOI] [PubMed] [Google Scholar]
- [183].Palomäki H, Kaste M, Berg A, Lönnqvist R, Lönnqvist J, Lehtihalmes M, et al. (1999). Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mianserin with 6 month follow up after therapy. J Neurol Neurosurg Psychiatry, 66:490-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rasmussen A, Lunde M, Poulsen DL, Sørensen K, Qvitzau S, Bech P (2003). A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics, 44:216-221. [DOI] [PubMed] [Google Scholar]
- [185].Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, et al. (2008). Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. Jama, 299:2391-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Tay J, Mårtensson B, Markus HS, Lundström E (2022). Does fluoxetine reduce apathetic and depressive symptoms after stroke? An analysis of the Efficacy oF Fluoxetine-a randomized Controlled Trial in Stroke trial data set. Int J Stroke:17474930221124760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kohno N, Abe S, Toyoda G, Oguro H, Bokura H, Yamaguchi S (2010). Successful treatment of post-stroke apathy by the dopamine receptor agonist ropinirole. J Clin Neurosci, 17:804-806. [DOI] [PubMed] [Google Scholar]
- [188].Aragona B, De Luca R, Piccolo A, Le Cause M, Destro M, Casella C, et al. (2018). Is bupropion useful in the treatment of post-stroke thalamic apathy? A case report and considerations. Funct Neurol, 33:213-216. [PubMed] [Google Scholar]
- [189].Autret K, Arnould A, Mathieu S, Azouvi P (2013). Transient improvement of poststroke apathy with zolpidem: a single-case, placebo-controlled double-blind study. BMJ Case Rep, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Robinson RG, Jorge RE, Clarence-Smith K, Starkstein S (2009). Double-blind treatment of apathy in patients with poststroke depression using nefiracetam. J Neuropsychiatry Clin Neurosci, 21:144-151. [DOI] [PubMed] [Google Scholar]
- [191].Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, et al. (2013). Prevention of poststroke apathy using escitalopram or problem-solving therapy. Am J Geriatr Psychiatry, 21:855-862. [DOI] [PubMed] [Google Scholar]
- [192].Watanabe MD, Martin EM, DeLeon OA, Gaviria M, Pavel DG, Trepashko DW (1995). Successful methylphenidate treatment of apathy after subcortical infarcts. J Neuropsychiatry Clin Neurosci, 7:502-504. [DOI] [PubMed] [Google Scholar]
- [193].Starkstein SE, Brockman S, Hatch KK, Bruce DG, Almeida OP, Davis WA, et al. (2016). A Randomized, Placebo-Controlled, Double-Blind Efficacy Study of Nefiracetam to Treat Poststroke Apathy. J Stroke Cerebrovasc Dis, 25:1119-1127. [DOI] [PubMed] [Google Scholar]
- [194].Rao V, Bergey A, Rosenberg P (2012). Sertraline for treatment of post-stroke anxiety. J Neuropsychiatry Clin Neurosci, 24:E22. [DOI] [PubMed] [Google Scholar]