Abstract
This article is part of the Theo Murphy meeting issue ‘Strongyloides: omics to worm-free populations’.
Keywords: Strongyloides, soil-transmitted helminth, Neglected Tropical Disease, nematode, parasite
The nematode genus Strongyloides consists of more than 50 different species that are all small intestinal parasites of vertebrates other than fish [1] and are of variable veterinary and medical importance [2–5]. The threat for human health caused by the species Strongyloides stercoralis is increasingly appreciated [3–5] after it had been grossly neglected for a long time. Human strongyloidiasis is included in the WHO list of the Neglected Tropical Diseases (NTD) [6,7] and the estimated number of people infected with S. stercoralis has recently been raised to about 600 million people [3]. The prevalence of S. stercoralis was, and probably still is, underestimated since specific diagnostic methodology is required and all such methodology has issues with sensitivity and/or specificity [8–10]. Another reason that infections are frequently missed is that, although S. stercoralis infections can be fatal, most infections show only mild or no clinical symptoms and if there are symptoms, they are rather unspecific [4]. Strongyloides stercoralis has a cosmopolitan distribution but is strongly enriched in tropical and subtropical socioeconomically disadvantaged regions [3,11]. The recommended treatment for S. stercoralis is ivermectin, which is highly effective but unfortunately not available in all countries. Mass drug administration (MDA) is under evaluation by the World Health Organization (WHO) to control strongyloidiasis in endemic areas [4,12].
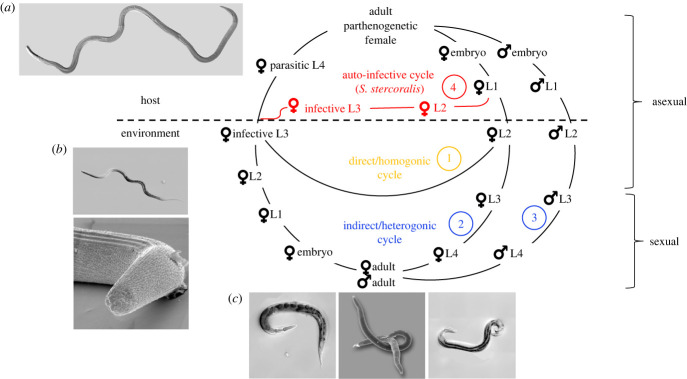
Strongyloides spp. is also an emerging model system for translational, basic biological and evolutionary research [13–17]. Both the medical threat that it poses and its attractiveness for basic research are connected to its rather complicated life cycle. The life cycle of Strongyloides spp. has been reviewed repeatedly (e.g. [16,18,19]) and is summarized here (figure 1). AlI infective third-stage larvae (iL3) are females and they enter a new host by skin penetration. After migrating through the blood and the lungs or nose (dependent on the species), the larvae are swallowed and eventually reach the small intestine of the host where they complete their development to parthenogenetically reproducing parasitic adults. Whether alternative migration paths through the host's body are also possible is a matter of debate (see article by Al-Jawabreh and colleagues [] in this special issue). Dependent on the species, the progeny of the parasitic females have three or four developmental options. 1) They may become female, leave the host as embryonated eggs or first-stage larvae (dependent on the species), develop in the environment into iL3 and search for a new host (called direct or homogonic development), closing an asexual reproductive cycle. 2) They may become female and leave the host as embryonated eggs or first-stage larvae but develop into free-living, non-infective third-stage larvae and subsequently into adult females (indirect or heterogonic development). 3) They may become male and leave the host as embryonated eggs or first-stage larvae and develop into free-living adult males (indirect or heterogonic development). The free-living adults mate and reproduce in the environment and all their progeny are females and develop to iL3s, completing a sexual reproductive cycle (as the only exception, S. planiceps has been described to be capable of undergoing up to nine consecutive free-living generations [21]). 4) They may become female, and develop into autoinfective third-stage larvae (aiL3) within the host and re-infect the same host individual (autoinfective cycle, asexual). While all species of Strongyloides (but not necessarily all isolates of these species [22–24]) may undergo homogonic or heterogonic development, the autoinfective cycle (option 4) appears to be specific for S. stercoralis and maybe a few other less well-investigated species [18]. The species-specific existence of this autoinfective cycle is the reason why strongyloidiasis is a serious threat to human health [3–5] but only of moderate veterinary concern, except for animals—such as dogs and monkeys—that can also carry S. stercoralis [2]. The autoinfective cycle allows the parasite to persist in an individual host for much longer than an individual worm can live outside a host (chronic strongyloidiasis). Usually, healthy individuals tolerate chronic infections well and control them at very low worm burdens [4]. Because such people have only mild or no symptoms and the worm burdens are so low, much of the routine parasitological diagnostic methodology is not suitable to detect S. stercoralis and chronic strongyloidiasis goes frequently unnoticed [9]. However, if a chronically infected patient becomes immunodeficient due to disease or immunosuppressive treatment (i.e. steroids, cancer chemotherapy or organ transplantation), the control of the autoinfective cycle may fail, leading to hyperinfection syndrome and disseminated strongyloidiasis, which are usually lethal if not treated in time due to late recognition and/or uncertainty about the best treatment strategy [4].
Figure 1.
Life cycle of Strongyloides stercoralis. For explanations see text. The circled numbers refer to the developmental option numbers in the text. This life cycle also applies, with small modifications, to other species of Strongyloides. i.e. the autoinfective cycle appears specific for S. stercoralis; in some species the young larvae hatch while still in the host, while in other species embryonated eggs are passed; in S. planiceps multiple consecutive free-living generations are possible (for more information and references see text). The images show Strongyloides papillosus (a) adult parasitic female (top left): in this differential interference contrast (DIC) image, the worm is about 5 mm long (the size of adult females varies between species of Strongyloides; in S. stercoralis they are about 2.5 mm [1]); (b) infective L3 (bottom left): DIC image (upper panel) and scanning electron microscopic (SEM) image (lower panel); the worm is about 0.6 mm long; (c) free-living adults (bottom), DIC image of a female (left panel), DIC image of a male (right panel), SEM image of a mating couple (middle panel). The free-living adults are about 1 mm long.
For the basic biologist, Strongyloides spp. is an attractive system because of the availability of a free-living sexual generation of adults that provides, for a parasite, a quite unique opportunity for experimental manipulation, combined with a short generation time of a few days to a few weeks, dependent on the species [14,15]. A further advantage of Strongyloides spp. is the small size of the genome for members of this genus [25], Kounosu et al. this issue [26]). Several species of Strongyloides can be maintained in the laboratory relatively easily, either in their natural hosts (S. ratti and S. venezuelensis in rats [14]) or in permissive laboratory hosts (S. papillosus in rabbits [27] and S. stercoralis in dogs or gerbils [17,28]). While S. ratti and S. venezuelensis in particular provide attractive animal models to study Strongyloides biology in their natural host [14], the absence of the auto-infective cycle limits the study of pathogenicity in these species such that studies on the human pathogen itself are indispensable.
We had long felt that there are insufficient interactions between more applied, health-care oriented Strongyloides researchers and basic biologists working with this group of parasites and that both sides could profit from the expertise of the other. We had entertained the idea for a joint meeting for quite some time. Finally, the Royal Society enabled us to organize a Theo Murphy meeting entitled ‘Strongyloides: omics to worm-free populations’. On 28th and 29th November 2022 about 50 people interested in Strongyloides spp., including clinicians, diagnosticians, epidemiologists, geneticists, molecular biologists, bioinformaticians and immunologists, met in in Frome, England to discuss the biology and the control of Strongyloides spp., with a strong emphasis on S. stercoralis.
Philosophical Transactions B offered to publish a special issue related to the Theo Murphy Meeting and invited us, the scientific organizers of this conference, to guest edit it. Twelve papers were accepted for publication in this special issue. They are briefly mentioned below.
Overall, we have to admit that, although Strongyloides spp. has been studied for more than 160 years, there are still substantial gaps in our understanding of these parasites, some of which are almost embarrassing because they concern very basic aspects of Strongyloides biology and pathogenicity. For the first article in this special issue, to which all meeting participants were invited to contribute, Mark Viney compiled a list of open questions in Strongyloides biology, immunology, pathogenesis, diagnostics and control (Al-Jawabreh et al. [20]).
Dora Buonfrate, Antonio Montresor, Zeno Bisoffi, Francesca Tamarozzi and Donal Bisanzio estimate the global number of adults who should be included in MDA for strongyloidiasis, which could be used by endemic countries to calculate sources and funds needed to implement control programmes (Buonfrate et al. [29]).
Pockets of poverty can lead to disproportionately high prevalence of strongyloidiasis even in populations living in one of the world's wealthiest countries, Australia. Kirstin Ross describes vividly the issues leading to high strongyloidiasis rates in First Nation communities, and advocates for action to fight this situation (Ross [30]).
Benjamin Collyer and Roy Anderson present a stochastic individual-based model that is aimed at evaluating the impact of MDA for strongyloidiasis, although some knowledge gaps (e.g. dynamics of post-treatment re-infection) still limit its application (Collyer & Anderson [31]).
It had already been noticed in very early reports about the human-infective S. stercoralis that dogs carry Strongyloides spp. that are similar to the human ones. This followed a decade-long discussion over whether the Strongyloides spp. in dogs is the same or just very similar to the one in humans—and with this, if dogs are a reservoir for zoonotic strongyloidiasis. In their article, Richard Bradbury and Adrian Streit discuss this issue, which is still not resolved (Bradbury & Streit [32]).
Eva Nosková, Kelly Sambucci, Klara Petrzelkova, Barbora Cervena, David Modry and Barbora Pafco discuss Strongyloides infections in humans and non-human primates, and highlight gaps in the currently available data and the importance of this information for understanding zoonosis transmission and pathogenicity (Pafko et al. [33]).
A crucial step in the life cycle of Strongyloides is finding and percutaneously entering a host individual. Courtney McClure, Ruhi Patel and Elissa Hallem review the current knowledge of skin-penetration behaviour and the underlaying mechanisms for Strongyloides and for hookworms, which are phylogenetically rather distant nematode parasites with similar infection biology (McClure et al. [34]).
In their article, Minka Breloer and Lara Linnemann review what is known about the immune response that S. ratti and S. stercoralis elicit in their natural hosts and in mice that are permissive laboratory hosts, and they provide the unique tools of mouse genetics and immunology to the study of Strongyloides infection biology. The authors also discuss the strategies that the parasite employs to cope with the host's defence mechanism (Breloer & Linnemann [35]).
In the next contribution, Reem Al-Jawabreh, Dominika Lastik, Darrin McKenzie, Kieran Reynolds, Mona Suleiman, Angela Mousley, Louise Atkinson and Vicky Hunt discuss the state of -omics data and resources for Strongyloides spp. and compare them to the model nematode Caenorhabditis elegans (Al-Jawabreh et al. [36]).
Asuka Kounosu, Simo Sun, Yasunobu Maeda, Mehmet Dayi, Akemi Yoshida, Haruhiko Maruyama, Vicky Hunt, Asako Sugimoto and Taisei Kikuchi report chromosomally complete or near complete genome assemblies of two species of Strongyloides with different numbers of chromosomes (S. ratti and S. venezuelensis) and Rhabditophanes diuinus the phylogenetically closest non-parasitic relative of Strongyloides spp. currently known. They investigate the syntenic relationships and discuss the genome evolution in these species (Kounosu et al. [26]).
Natalia Tiberti, Marcello Manfredi, Chiara Piubelli and Dora Buonfrate discuss Strongloides proteomics data and present results for the first study on serum proteomics from patients suffering from strongyloidiasis (Tiberti et al. [37]).
Astra Bryant, Damia Akimori, Jonathan Stoltzfus and Elissa Hallem highlight gene annotation errors in the Strongyloides genomes and present a workflow for improving gene annotations and correcting errors (Bryant et al. [38]).
Acknowlegements
We thank the Royal Society and its staff for supporting and administratively organizing the Theo Murphy Meeting ion 'Strongyloides: -omics to worm free populations'. In particular we thank Annabel Sturgess and Sarah-Kate Lewis for their help before and during the meeting. We thank the editors of Philosophical Transactions B, in particular Helen Eaton for the opportunity to compile this special edition.
Contributor Information
Dora Buonfrate, Email: dora.buonfrate@sacrocuore.it.
Vicky L. Hunt, Email: bs1vlh@bath.ac.uk.
Peter Odermatt, Email: peter.odermatt@swisstph.ch.
Adrian Streit, Email: adrian.streit@tuebingen.mpg.de.
Data accessibility
This article has no additional data.
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
D.B.: conceptualization, writing—review and editing; V.L.H.: conceptualization, writing—review and editing; P.O.: conceptualization, writing—review and editing; A.S.: conceptualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
This theme issue was put together by the Guest Editor team under supervision from the journal's Editorial staff, following the Royal Society's ethical codes and best-practice guidelines. The Guest Editor team invited contributions and handled the review process. Individual Guest Editors were not involved in assessing papers where they had a personal, professional or financial conflict of interest with the authors or the research described. Independent reviewers assessed all papers. Invitation to contribute did not guarantee inclusion.
Funding
For D.B., IRCCS Sacro Cuore Don Calabria hospital received funding from the Italian Ministry of Health - 'Ricerca corrente'; V.L.H. was funded by a Wellcome Trust/Royal Society Sir Henry Dale Fellowship (grant no. 211227/Z/18/Z); work in A.S.'s laboratory is funded by the Max Planck Society.
References
- 1.Speare R. 1989. Identification of species of Strongyloides. In Strongyloidiasis: a major roundworm infection of man (ed. Grove DI), pp. 11-83. London, UK: Taylor & Francis. [Google Scholar]
- 2.Thamsborg SM, Ketzis J, Horii Y, Matthews JB. 2017. Strongyloides spp. infections of veterinary importance. Parasitology 144, 274-284. ( 10.1017/S0031182016001116) [DOI] [PubMed] [Google Scholar]
- 3.Buonfrate D, et al. 2020. The global prevalence of Strongyloides stercoralis infection. Pathogens 9, 468. ( 10.3390/pathogens9060468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nutman TB. 2017. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144, 263-273. ( 10.1017/S0031182016000834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross KE, et al. 2017. The National Strongyloides Working Group in Australia 10 workshops on: commendations and recommendations. Aust. N Z J. Public Health 41, 221-223. ( 10.1111/1753-6405.12611) [DOI] [PubMed] [Google Scholar]
- 6.Bisoffi Z, et al. 2013. Strongyloides stercoralis: a plea for action. PLoS Negl. Trop. Dis. 7, e2214. ( 10.1371/journal.pntd.0002214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen A, et al. 2009. Strongyloidiasis – the most neglected of the neglected tropical diseases? Trans. R Soc. Trop. Med. Hyg. 103, 967-972. ( 10.1016/j.trstmh.2009.02.013) [DOI] [PubMed] [Google Scholar]
- 8.Buonfrate D, Tamarozzi F, Paradies P, Watts MR, Bradbury RS, Bisoffi Z. 2022. The diagnosis of human and companion animal Strongyloides stercoralis infection: challenges and solutions. A scoping review. Adv. Parasitol. 118, 1-84. ( 10.1016/bs.apar.2022.07.001) [DOI] [PubMed] [Google Scholar]
- 9.Page W, Speare R. 2016. Chronic strongyloidiasis - Don't look and you won't find. Aust. Fam. Physician 45, 40-44. [PubMed] [Google Scholar]
- 10.Watts MR, Robertson G, Bradbury RS. 2016. The laboratory diagnosis of Strongyloides stercoralis. Microbiol. Australia 37, 4-9. ( 10.1071/MA16003) [DOI] [Google Scholar]
- 11.Beknazarova M, Whiley H, Ross K. 2016. Strongyloidiasis: A Disease of Socioeconomic Disadvantage. Int. J. Environ. Res. Public Health 13, 517. ( 10.3390/ijerph13050517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonfrate D, Rodari P, Barda B, Page W, Einsiedel L, Watts MR. 2022. Current pharmacotherapeutic strategies for Strongyloidiasis and the complications in its treatment. Expert Opin. Pharmacother. 23, 1617-1628. ( 10.1080/14656566.2022.2114829) [DOI] [PubMed] [Google Scholar]
- 13.Streit A. 2014. How to become a parasite without sex chromosomes: a hypothesis for the evolution of Strongyloides spp. and related nematodes. Parasitology 141, 1244-1254. ( 10.1017/S003118201400064X) [DOI] [PubMed] [Google Scholar]
- 14.Viney M, Kikuchi T. 2017. Strongyloides ratti and S. venezuelensis - rodent models of Strongyloides infection. Parasitology 144, 285-294. ( 10.1017/S0031182016000020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viney ME. 1999. Exploiting the life cycle of Strongyloides ratti. Parasitol. Today 15, 231-235. ( 10.1016/S0169-4758(99)01452-0) [DOI] [PubMed] [Google Scholar]
- 16.Viney ME, Lok JB.2015. The biology of Strongyloides spp. (16 July, 2015). In Wormbook (ed. The C. elegans Research Community). ( 10.1895/wormbook.1.141.2) See http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lok JB. 2007. Strongyloides stercoralis: a model for translational research on parasitic nematode biology (February 17, 2007). In WormBook (ed. The C. elegans Research Community). ( 10.1895/wormbook.1.134.1) See http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schad GA. 1989. Morphology and life history of Strongyloides stercoralis. In Strongyloidiasis: A major roundworm infection of man (ed. Grove DI), pp. 85-104. London, UK: Taylor & Francis. [Google Scholar]
- 19.Streit A. 2017. Genetics: modes of reproduction and genetic analysis. Parasitology 144, 316-326. ( 10.1017/S0031182016000342) [DOI] [PubMed] [Google Scholar]
- 20.Al-Jawabreh R, et al. 2023. Strongyloides questions—a research agenda for the future. Phil. Trans. R. Soc. B 379, 20230004. ( 10.1098/rstb.2023.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada M, Matsuda S, Nakazawa M, Arizono N. 1991. Species-specific differences in heterogonic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J. Parasitol. 77, 592-594. ( 10.2307/3283165) [DOI] [PubMed] [Google Scholar]
- 22.Viney ME, Matthews BE, Walliker D. 1992. On the biological and biochemical nature of cloned populations of Strongyloides ratti. J. Helminthol. 66, 45-52. ( 10.1017/S0022149X00012554) [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, et al. 2019. Characterization of a non-sexual population of Strongyloides stercoralis with hybrid 18S rDNA haplotypes in Guangxi, Southern China. PLoS Negl. Trop. Dis. 13, e0007396. ( 10.1371/journal.pntd.0007396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole R, Holroyd N, Tracey A, Berriman M, Viney M. 2023. The parasitic nematode Strongyloides ratti exists predominantly as populations of long-lived asexual lineages. Nat. Commun. 14, 6427. ( 10.1038/s41467-023-42250-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt VL, et al. 2016. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat. Genet. 48, 299-307. ( 10.1038/ng.3495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kounosu A, Sun S, Maeda Y, Dayi M, Yoshida A, Maruyama H, Hunt V, Sugimoto A, Kikuchi T. 2023. Syntenic relationship of chromosomes in Strongyloides species and Rhabditophanes diutinus based on the chromosome-level genome assemblies. Phil. Trans. R. Soc. B 379, 20220446. ( 10.1098/rstb.2022.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberhardt AG, Mayer WE, Streit A. 2007. The free-living generation of the nematode Strongyloides papillosus undergoes sexual reproduction. Int. J. Parasitol. 37, 989-1000. ( 10.1016/j.ijpara.2007.01.010) [DOI] [PubMed] [Google Scholar]
- 28.Nolan TJ, Megyeri Z, Bhopale VM, Schad GA. 1993. Strongyloides stercoralis: the first rodent model for uncomplicated and hyperinfective strongyloidiasis, the Mongolian gerbil (Meriones unguiculatus). J. Infect. Dis. 168, 1479-1484. ( 10.1093/infdis/168.6.1479) [DOI] [PubMed] [Google Scholar]
- 29.Buonfrate D, Montresor A, Bisoffi Z, Tamarozzi F, Bisanzio D. 2023. Progress towards the implementation of control programmes for strongyloidiasis in endemic areas: estimation of number of adults in need of ivermectin for strongyloidiasis. Phil. Trans. R. Soc. B 379, 20220433. ( 10.1098/rstb.2022.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross K. 2023. Locally acquired strongyloidiasis in remote Australia: why are there still cases? Phil. Trans. R. Soc. B 379, 20220435. ( 10.1098/rstb.2022.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collyer BS, Anderson R. 2023. The transmission dynamics of Strongyloides stercoralis and the impact of mass drug administration. Phil. Trans. R. Soc. B 379, 20220442. ( 10.1098/rstb.2022.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradbury RS, Streit A. 2023. Is strongyloidiasis a zoonosis from dogs? Phil. Trans. R. Soc. B 379, 20220445. ( 10.1098/rstb.2022.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosková E, Sambucci KM, Petrželková KJ, Červená B, Modrý D, Pafčo B. 2023. Strongyloides in non-human primates significance for public health control. Phil. Trans. R. Soc. B 379, 20230006. ( 10.1098/rstb.2023.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClure CR, Patel R, Hallem EA. 2023. Invade or die: behaviours and biochemical mechanisms that drive skin penetration in Strongyloides and other skin-penetrating nematodes. Phil. Trans. R. Soc. B 379, 20220434. ( 10.1098/rstb.2022.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breloer M, Linnemann L. 2023. Strongyloides ratti infection in mice: immune response and immune modulation. Phil. Trans. R. Soc. B 379, 20220440. ( 10.1098/rstb.2022.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Jawabreh R, Lastik D, McKenzie D, Reynolds K, Suleiman M, Mousley A, Atkinson L, Hunt V. 2023. Advancing Strongyloides omics data: bridging the gap with Caenorhabditis elegans. Phil. Trans. R. Soc. B 379, 20220437. ( 10.1098/rstb.2022.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiberti N, Manfredi M, Piubelli C, Buonfrate D. 2023. Progresses and challenges in Strongyloides spp. proteomics. Phil. Trans. R. Soc. B 379, 20220447. ( 10.1098/rstb.2022.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryant AS, Akimori D, Stoltzfus JDC, Hallem EA. 2023. A standard workflow for community-driven manual curation of Strongyloides genome annotations. Phil. Trans. R. Soc. B 379, 20220443. ( 10.1098/rstb.2022.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.