Abstract
The Strongyloides genus of parasitic nematodes have a fascinating life cycle and biology, but are also important pathogens of people and a World Health Organization-defined neglected tropical disease. Here, a community of Strongyloides researchers have posed thirteen major questions about Strongyloides biology and infection that sets a Strongyloides research agenda for the future.
This article is part of the Theo Murphy meeting issue ‘Strongyloides: omics to worm-free populations’.
Keywords: Strongyloides, questions, research
1. Introduction
Strongyloides is a genus of parasitic nematode that infects a wide variety of terrestrial vertebrates, including humans. Strongyloides is one of the soil-transmitted helminthiases, and so a WHO-defined Neglected Tropical Disease, and it is estimated that some 100–600 million people are infected with Strongyloides worldwide [1,2]. Strongyloides infection is also of some clinical veterinary relevance [3]. A recent ‘Strongyloides: omics to worm-free populations' meeting brought together a diverse, international group of people interested in Strongyloides. Directly after the meeting this community posed questions that they had about Strongyloides, in part inspired by similar question-setting by other research communities [4]. This resulted in 93 questions (see electronic supplementary material, table S1), of which 90 could be grouped into 13 main questions, divided into two main themes, Basic Biology and Immunology (8 main questions), and Human Infection and Disease (5 main questions). Many of these questions relate to aspects of the Strongyloides life cycle, shown in figure 1. In what follows, for each main question the current state of knowledge is used to give a context to the question and then state outstanding questions, so articulating a Strongyloides research agenda for the future. The questions considered here are clearly not an exhaustive list, and others will have additional, different questions.
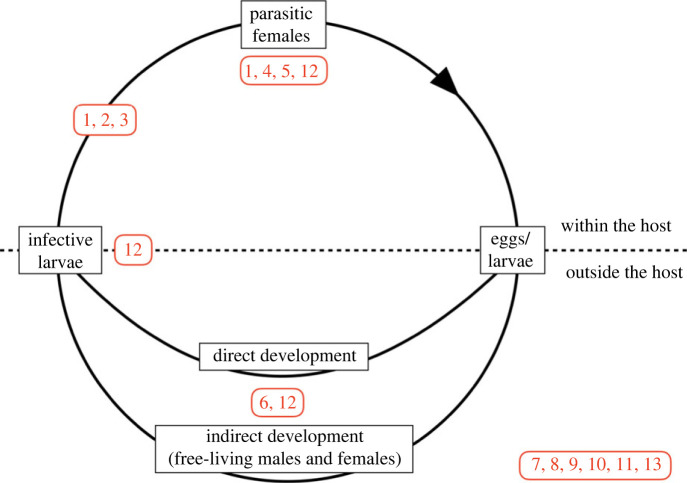
Figure 1.
The life cycle of Strongyloides, with parasitic female worms inside hosts that produce eggs that pass out of the host, where larvae either develop (i) directly to infective larvae that infect a host and migrate to the host gut, or (ii) indirectly into free-living adult males and females, whose progeny develop into infective larvae, which then infect a host. Strongyloides stercoralis, the parasite of people, also undergoes internal autoinfection. Questions 1, 2, 3, 4, 5, 6 and 12 (shown in red) are about specific aspects of the Strongyloides life cycle and are shown at the point in the life cycle where they pertain; Questions 7, 8, 9, 10, 11 and 13 do not directly apply to the life cycle, and are shown separately.
2. Theme 1: basic biology and immunology
Question 1. What is the biology of host infection and within-host physiological and behavioural adaptation? (8 questions, electronic supplementary material, table S1)
The enduring fascination of parasites is that they live inside other animals, an environment that to us might seem hugely inhospitable. But, of course, they have evolved to live in these environments and have a suite of adaptations enabling them to do so. For Strongyloides, as for many other parasitic nematodes, there are a raft of questions about how this adaptation is actually achieved. Strongyloides infective third-stage larvae are developmentally arrested and non-feeding, and live in the soil. When these penetrate a host, host-derived signals allow them to detect that they are inside a host, which then initiates physiological and gene expression changes enabling larvae to exit developmental arrest and resume reproductive growth as the parasitism programme of development is initiated. There is a long history of studying what signals change the behaviour of parasitic nematode larvae, and these can be expanded to more fully understand the signals that Strongyloides uses to detect the within-host environment, beyond those already known [5–7]. An interesting question here is to what extent host-derived signals contribute to Strongyloides' host species-specificity (and see Question 7, below). Studying the signals that Strongyloides uses within hosts is more challenging because this would likely need to be addressed using ex vivo experiments. For the same reason, studying the within-host behavioural biology is also challenging, though the development of remote imaging of worms in vivo (possibly including transgenic worms that report signal reception) can develop this area. Strongyloides ratti parasitic females migrate through host intestinal tissue, laying eggs as they go, and disperse themselves along the gut. These phenomena and the underling behavioural biology are completely unexplored.
It is likely that the larval head sensory neurons are an important part of the sensory process of host infection, within-host migration, and other within-host biology [7,8]. The structure and function of nematode head neurons have been studied extensively in the free-living nematode Caenorhabditis elegans, and the structure of Strongyloides stercoralis head neurons have been determined, showing some anatomical similarity to those of C. elegans [5,9,10]. Moreover, functional studies have revealed that Strongyloides' head sensory neurons confer responses to the same sensory modalities as C. elegans [5,8,11–14], and at least some of the signal transduction pathways that mediate sensory responses are also conserved between Strongyloides and C. elegans. However, how transduction of these signals leads to changes in gene expression that result in within-host adapted biology remains unknown.
Outstanding questions include: what host signals do Strongyloides use to detect their within-host environment; how does the detection of these signals then lead to the initiation and then exposition of its parasitism programme; and what is the behavioural biology of Strongyloides within the host?
Question 2. What is Strongyloides’ within-host migration route? (3 questions, electronic supplementary material, table S1)
Strongyloides, in common with many parasitic nematodes, undergoes a within-host migration from the site of infection to the final within-host site. A phylogenetically controlled analysis of parasitic nematodes shows that such within-host migration allows developing worms to grow more (compared with those that do not migrate), with this greater adult size resulting in greater fecundity, and so likely greater comparative fitness [15]. For Strongyloides, the canonical route of migration has been thought to be from skin penetration, via the blood, lungs, trachea, to the intestinal tract, where parasitic adults establish [16]. But, working out the route of migration is less than straightforward. A substantial proportion of infective stages that infect a host never make it to the final within-host site. If larvae are found in various sites in a host shortly post-infection, are these larvae that are lost and will never get to the gut, or are they larvae en route to the gut? The logic of being able to confirm which larvae are en route to the gut was laid out by Tindal & Wilson, and for S. ratti in rats they and others convincingly showed that the naso-frontal region was a key part of the migration route of worms en route to the gut [17–19]. Investigating routes of within-host migration can be addressed by direct parasitological methods in some host species, though obviously not in human hosts. Knowing how, when, and where Strongyloides migrates in people is relevant not only to better understand pathology but also to diagnose infection.
Outstanding questions include: is there diversity in migration route (or routes) for different Strongyloides species or for the same species when in different hosts?
Question 3. What is the diversity of routes of infection? (6 questions, electronic supplementary material, table S1)
Successfully infecting a host is central to parasites’ evolutionary fitness, and for Strongyloides this occurs by infective larvae penetrating host skin, which has been studied in the laboratory. However, rather little of this is known in natural conditions, in no small part because of the substantial difficulty of finding Strongyloides infective larvae in nature or studying their infection of hosts. Understanding how Strongyloides infective larvae invade hosts under natural conditions is critical for an understanding of the basic biology of host infection and the epidemiological parameters that affect infection in host populations. Other routes of infection need to be considered, and there is some evidence of transmammary transmission in humans [20] and other animals, though inconsistently (e.g. [21–23]).
Outstanding questions include: what is the natural biology of Strongyloides infection and are there means of host infection other than skin penetration by infective larvae?
Question 4. What is the role of certain pathways and of parasite-derived products? (8 questions, electronic supplementary material, table S1)
Parasitic nematodes inside hosts release molecules and other products that can affect the host to the parasite's benefit. For example, helminth immunomodulation of hosts is now known to be due to host excreted/secreted products (ES) [24,25]. Other parasitic nematodes have been shown to release extracellular vesicles (EVs) that in vitro can affect host cells [26], and that contain proteins and non-coding RNAs that can directly influence host gene expression [27]. Strongyloides also produces ES [28–30] and EVs (V. Hunt 2023, personal communication), but understanding the nature of the contents of the EVs is an area of active work, as too is elucidating the function of the contents, as well as of the wider ES. The ES of Strongyloides parasitic females seem prodigious (shown empirically both by in vitro studies and by inference given the size of the gene families whose products are likely secreted inside hosts [29]), and one can envisage that these are involved in Strongyloides' residence in, and feeding on, hosts. Strongyloides parasitic females lie within, and migrate through, host tissue, and parasite-derived products likely play a role in regulating these processes. Genome sequencing projects have also identified various molecular pathways and other molecules in Strongyloides—such as the endocannabinoid pathway [31]—that may play a role in Strongyloides within-host biology.
Apart from understanding the normal role of parasite-derived products in hosts, the very fact that these factors are in the host could be exploited for diagnosis of infection or parasite control. Such approaches have not yet been developed for Strongyloides, and there remains the challenge of genus- or species-specific diagnoses (see Question 10).
Outstanding questions include: what molecules do Strongyloides release into their hosts, and what effects do these molecules have on the hosts and so on Strongyloides itself, and can these molecules be used to diagnose infection; what are the roles of a range of Strongyloides genetic pathways in its parasitic lifestyle?
Question 5. What is the immunological relationship of Strongyloides and its host? (3 questions, electronic supplementary material, table S1)
Many parasitic nematodes immunomodulate their hosts for their own benefit [24,25] and the mechanisms of how they do this remain under intense study. Several lines of evidence suggest that Strongyloides follows in this pattern; for example, S. ratti infection in mice expands T lymphocytes with regulatory function and induces the expression of regulatory receptors on effector T cells [32–35]. Importantly, abrogation of parasite immunomodulation through immunization-induced antibody blockade could be a viable vaccination strategy [36]. Most work on Strongyloides immunobiology in this area has been done in laboratory models, particularly with S. ratti and Strongyloides venezuelensis, but there is a comparative dearth of studies in people and livestock [37]. However, studies in humans show that there is protective immunity to S. stercoralis and that these immune responses include many general features of anti-helminth immune responses seen with other helminth infections [37,38].
Outstanding questions include: does Strongyloides immunomodulate its hosts (and if so how), and what is the functional effect of host anti-Strongyloides immune responses in natural human and animal infections; what is the extent of molecular communication from parasite to host, and host to parasite?
Question 6. What is the Strongyloides life cycle, particularly the free-living generation, and does it vary among species? (15 questions, electronic supplementary material, table S1)
Strongyloides has a complex and fascinating life cycle, and one that sets it apart from most other parasitic nematodes. Specifically, in Strongyloides external to the host there are direct (homogonic) and indirect (heterogonic) routes of development that ultimately result in infective larvae. Direct development is larval only, whereas indirect development involves free-living adult nematodes (figure 1). Details of this free-living development have only been studied in detail in a few laboratory-maintained species [39], though free-living adult stages have been described for a wider range of species. So, at best, our understanding of this phase of the Strongyloides life cycle is taxonomically restricted and thus the potential diversity of this life cycle in different species is unknown. Strongyloides’ nearest relative, Parastrongyloides, has multiple free-living adult generations [40], suggesting that the ability to have one or more free-living generations may vary among Strongyloides species, as has been shown at least once [41].
Strongyloides' direct development appears to be similar to the development of many other parasitic nematodes, but indirect development is quite distinct, which raises the question of why Strongyloides has been selected for this apparently rare life cycle. It is notable that among parasitic nematodes of vertebrates virtually all reproduce sexually, which contrasts with the wide range of sexual, hermaphroditic, and parthenogenetic reproduction among nematodes more widely. One possibility is that the parthenogenetic reproduction of Strongyloides parasitic females, and so the absence of sexual reproduction, has been compensated for by sexual reproduction in its indirect route of development.
Strongyloides larvae have a developmental choice between direct and indirect development, and some of the cues that affect this, such as environmental temperature, are known but how these developmental choices are molecularly specified remains to be discovered. Here, the fate of the offspring of parasitic and free-living females clearly differs. In the well-studied S. ratti system the progeny of free-living females always develop into infective larvae, whereas the progeny of parasitic females can be mixed, developing into infective larvae directly and into free-living adults. The control of these different fates is fascinating, and Strongyloides may be a very good system in which to investigate control of development fate in nematodes. The lifespan of the two adult female stages also differs, with the parasitic female living for a maximum of about a year, some eighty times the maximum 5 day lifespan of the free-living female [42]. The mechanistic basis of this difference is not understood [43], though the evolutionary theory of ageing would suggest that the parasitic phase of the life cycle is one with the lowest effective extrinsic mortality rate, either directly or because of the facultative nature of the free-living adult stage [42]. Analogies have been made between the direct and indirect development of Strongyloides and the dauer versus non-dauer choice of free-living nematodes (including C. elegans) [44], which have been explored for a range of parasitic nematodes including Strongyloides, though probably with little ultimate benefit [45,46]. However, recently it has been shown that Δ7-dafachronic acid specifies the development of S. stercoralis infective larvae, a mechanism that is directly analogous with C. elegans dauer larva development [47]. But there are also other levels of control that are not understood, for example how the sex of Strongyloides infective larvae is always female.
Strongyloides stercoralis undergoes autoinfection, where apparently precocious development occurs inside the host so that infective stages internally infect the host. How this is controlled and whether or not it is unique to S. stercoralis remain to be studied.
Outstanding questions include: what diversity (if any) is there in the free-living life cycle of different Strongyloides species, or genotypes within species; what are the cues that initiate direct or indirect development and how are these developmental pathways controlled, including the control of sex determination; what are the selection pressures acting on the free-living developmental route, and can they help explain autoinfection of S. stercoralis?
Question 7. What is a Strongyloides species and what are species' host ranges? (14 questions, electronic supplementary material, table S1)
There are currently more than 50 species of Strongyloides described, all based on morphological characters and/or the host from which they were derived. Strongyloides taxonomy is challenging: there are limited morphological characters, the adult parasitic female stages are not always available, and a number of putatively useful morphological characters can be altered by fixation and downstream processing [48]. More recently there have been molecular analyses of Strongyloides, commonly by sequence analysis of single loci, but with some whole genome sequencing too [49]. While molecular analyses may be a step forward in understanding Strongyloides populations and species, there is a substantial challenge in linking sequence data to Strongyloides species names. Often there is no explicit taxonomic basis for the species names that are attached to sequence data in databases, so these names should be treated with caution. Several Strongyloides species have been erected largely (or solely) on the basis of the host species in which the parasite was found. Almost analogously, recent molecular approaches often use host species to provide a Strongyloides species name to the parasite.
While the aim of taxonomy is to identify and distinguish species that are biologically meaningful, the very concept of a Strongyloides species may be unclear. Specifically, a species can be considered a group of inter-breeding individuals (though there are other species concepts), but in Strongyloides ‘interbreeding’ relies on the facultative free-living adult generation. Cleary these do exist, but outside of laboratory-maintained Strongyloides lines how often (if at all) they occur is unknown. In the absence of free-living sexual stages, Strongyloides will consist of lineages of parthenogenically reproducing genotypes, and in this scenario what is a Strongyloides species is a moot point.
Putting this together, for Strongyloides the whole species concept may be in doubt, but also there is no good taxonomic assignment of most species, and molecular identification and naming of species is even less clear.
Beyond the whole question of defining a Strongyloides species, the host range of Strongyloides species or genotypes is little known. Several Strongyloides species have been erected largely (or solely) on the basis of the host species in which the parasite was found. Almost analogously, recent molecular approaches often use host species to provide a Strongyloides species name to the parasite (e.g. [50,51], but see [52]). For Strongyloides, the common, implicit, assumption is that there is a one-to-one relationship between host species and Strongyloides species. This assumption is challenged when the same parasites are found in multiple host species—for example S. stercoralis in people, carnivores and great apes; Strongyloides papillosus in sheep and rabbits—and it is notable that these putative exceptions are in well-studied taxa such that one might wonder if they are rather the rule than the exception.
Studying the host range of parasites can be done by cross-infection studies, that is taking a parasite from host species X and attempting to infect host species Y. While possible for some hosts, it is clearly not possible or desirable for all hosts, and most certainly not for humans. Experimental models—S. papillosus, S. ratti, S. stercoralis, S. venezuelensis—have had their host species range examined experimentally in this way. This is not the place to review this literature, but the general pattern is that (i) some Strongyloides species can infect more than one host species, though with varying success, and (ii) severe immune suppression or modification is needed to break down host species barriers [53], suggesting that Strongyloides' host range is determined by a wider set of host and parasite physiological characters.
An alternative approach to understanding host range is population genetics; this is, identifying genetically defined Strongyloides populations and then asking how those align to host species. The complication here is that, given the potential asexual nature of the Strongyloides life cycle, the population genetic structure of populations may deviate from those expected under patterns of random sexual mating. Beyond the basic biological interest of understanding species’ niche breadth and parasite host range, the host range of Strongyloides species is of epidemiological importance for species infecting humans and livestock. Specifically, to control infection in a focal host species, one needs to know the source of infection, and whether that is just the focal host species or other host species.
To make progress our community needs to agree how to refer to Strongyloides genotypes, with a system that encompasses both molecular and non-molecular approaches. Given that it is now possible to whole genome sequence Strongyloides straight from the wild, there are great prospects for widely studying its population genomics, to bring an unrivalled understanding of the genetic diversity in Strongyloides and its association with hosts.
Outstanding questions include: can we apply the biological species concept to Strongyloides (which might only sexually reproduce occasionally, if at all) or should other species concepts be considered; how should we define and use species names (or, even, should we use species names) with Strongyloides; how are genetically related Strongyloides genotypes distributed among host populations and host species, and from this what are the sources of infection of humans and of livestock?
Question 8. What laboratory methods do we need to improve, to better study Strongyloides? (3 questions, electronic supplementary material, table S1)
This article presents outstanding questions in Strongyloides biology, and the development of new methods will help make progress with answering these questions. Understanding the within-host biology (Questions 1 and 2) will benefit from remote sensing or other tracking methods. Ultimately, understanding the role of molecular pathways and parasite-secreted molecules, and the control of Strongyloides' free-living generation (Questions 4 and 6) will require using reverse genetic approaches. Strongyloides is one of the few parasitic nematode species where there are established CRISPR-Cas9 and RNAi methods [54–56], though they remain technically challenging, especially for propagation through hosts. Study of S. stercoralis in humans will always be limited, though dogs and gerbils are available as experimental models (though see Question 7), and so the use of in vitro approaches for work with S. stercoralis could also be beneficial. Applying the latest ‘omics technologies and long read sequencing to Strongyloides will allow full completion and annotation of Strongyloides genome assemblies, which will then underpin discovering the genomic and molecular basis of Strongyloides infection phenotypes, and facilitate reverse genetic approaches.
Outstanding questions include: can there be further improvement in using CRISPR-Cas9 and other reverse genetic methods with Strongyloides; is it possible to maintain the whole Strongyloides life cycle in vitro; how can the Parastrongyloides system be used to improve the study of Strongyloides?
3. Theme 2: human infection and disease
Question 9. What are the different types of Strongyloides infections of people and animals? (8 questions, electronic supplementary material, table S1)
While human Strongyloides infection is common, with estimates of 100–600 million people being infected worldwide [1,2,57,58], we know rather little of the nature of most of these infections, with studies instead focusing on people with complex and highly pathogenic infections that receive medical attention. Most people infected with Strongyloides likely have very low-intensity infections, which can be hard to diagnose (see Question 10). Further, disseminated infection (or hyperinfection) is also likely rare, or if it is not rare, then it is commonly undiagnosed. The duration of human infection is not well known, though it is thought to be chronic, but whether this is due to the lifespan of the parasitic females or due to autoinfection is unclear. Immunosuppression, particularly because of steroid treatment, can induce disseminated infection, as (less commonly) can severe malnutrition, alcoholism, immunodeficiency, and human T-lymphotropic virus type 1 infection [38,59], but it is unknown if there are other causes of dissemination, and so difficult to establish the multiple risk factors for disseminated infections (see Question 6). Most human infection is with S. stercoralis, but in New Guinea there is also infection with Strongyloides fuelleborni kellyi [60] and in central Africa and Asia there are reports of zoonotic S. fuelleborni infection (e.g. [50,61]) (see Question 7).
Outstanding questions include: what is the range of types and duration of human Strongyloides infection in endemic populations; what are the causes and risk factors for disseminated infection, and for which species does this occur?
Question 10. How can one best diagnose Strongyloides infection? (5 questions, electronic supplementary material, table S1)
Compared with other soil-transmitted helminths (STHs; Ascaris, hookworm, Trichuris), Strongyloides is hard to diagnose, which has probably led to an underappreciation of its importance in endemic human populations. Faecal diagnosis using Baermann funnels or culture is the most sensitive, non-molecular diagnostic method, but is hard to do at large scale in epidemiological surveys. Overall, there is no agreed ‘gold standard’ diagnostic test available The development of improved molecular methods to diagnose and quantify Strongyloides and other STHs would be a very welcome development. If such methods were available then this could also be applied to understanding the distribution of Strongyloides infective stages in the wider environment, and so understanding the spatial and temporal infection risk (Question 3). Immunological diagnosis of infection is also possible, though with such methods there can be cross-reactions to other nematode infections; it can be difficult to clearly separate current and historical infection in some regions; antibodies may be maintained for up to 18 months after successful treatment; and cases of serologically negative individuals passing microscopically detectable Strongyloides larvae do occur [62].
Outstanding questions include: can a rapid, accurate, and easy-to-use diagnostic test be developed to diagnose infection at the population level and in clinical settings?
Question 11. What is the best treatment for Strongyloides infection? (6 questions, electronic supplementary material, table S1)
There are a range of anthelmintic drugs available that are used to treat STHs, including Strongyloides. The current US Centers for Disease Control and Prevention recommendation for treating Strongyloides infection is ivermectin, with albendazole as an alternative [63]. Commonly when people are infected with Strongyloides they will also be infected with other STHs, and in these settings a broad-spectrum anthelmintic is desirable; the same applies in veterinary settings. Human Strongyloides infections can be disseminated, and here therapy apart from anthelmintic treatment is required, but what is ideal and optimum is not well established [38], and given the relative rarity of disseminated infections it is difficult to see how controlled trials of different therapies can be established. As with all anti-parasitic drug treatment, drug resistance will evolve, with the only question being when and where. Within Strongyloides populations there is the possibility of natural variation in anthelmintic susceptibility, but drugs will also act as a selection pressure, changing populations' anthelmintic sensitivity and genetic diversity. Linking-back to the earlier consideration of Strongyloides species and populations (see Question 7), the population genetic structure of Strongyloides populations and the extent to which they interbreed will also affect the spread of resistance to anthelmintic drugs.
Outstanding questions include: what is the best treatment for uncomplicated and for disseminated Strongyloides infections; how does genetic variation in Strongyloides populations contribute to the evolution of drug resistance and its spread?
Question 12. What are the life cycle and infection parameters that we need to know to better understand Strongyloides transmission and epidemiology? (6 questions, electronic supplementary material, table S1)
Epidemiological science can model and predict patterns of infection in populations, and how those infections will respond to control measures that perturb the system. The underlying theory is now well established, though critical to applying this to make real-world predictions is having accurate parameters of aspects of infection. The challenges of estimating these parameters have been met for many STHs, but remain poorly studied for Strongyloides. Much of what needs to be known concerns within-host processes (see Question 1, 3 and 6), which are challenging, though possible, to study in laboratory systems, but much harder in human infections.
Outstanding questions include: what is the effective fecundity of the parasitic generation (both daily rate and lifetime), and what is the effective fecundity of the free-living generation, and free-living adults’ contribution to that; what are the source, rate and magnitude of reinfection post-treatment?
Question 13. How can we promote the importance and interest of Strongyloides within the context of it being one of the soil-transmitted helminthiases (a WHO Neglected Tropical Disease) and the One Health agenda? (5 questions, electronic supplementary material, table S1)
Strongyloides as an STH is one of the WHO-defined Neglected Tropical Diseases, and arguably it is the most neglected of the STHs. This may be because it is rarer than other STHs and/or it is harder to diagnose, and so under-diagnosed (see Question 10). The source of human (and livestock) infection is intimately tied in with understanding what a Strongyloides species is and what host ranges Strongyloides species have (see Question 7), which is important to resolve to fully understand the source of human infection, and so how to control it.
The biological interest in Strongyloides and its life cycle has provoked two-thirds of the questions collected here, but answering these can be directly applied to understanding infection and disease in people and in livestock.
The outstanding challenge to all of us is to address and answer the questions we have posed ourselves, and to be advocates for the interest, importance and reward of studying the biology of Strongyloides.
Data accessibility
The data are provided in electronic supplementary material [64].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
R.A.-J.: writing—review and editing; R.A.: writing—review and editing; L.E.A.: writing—review and editing; J.B.-S.: writing—review and editing; R.S.B.: writing—review and editing; M.B.: writing—review and editing; A.S.B.: writing—review and editing; D.B.: writing—review and editing; L.C.C.: writing—review and editing; B.C.: writing—review and editing; M.D.: writing—review and editing; W.G.: writing—review and editing; E.H.: writing—review and editing; S.M.H.: writing—review and editing; V.H.: writing—review and editing; V.K.: writing—review and editing; T.K.: writing—review and editing; A.K.: writing—review and editing; D.L.: writing—review and editing; L.L.: writing—review and editing; Y.L.: writing—review and editing; H.J.M.: writing—review and editing; P.M.: writing—review and editing; A.M.: writing—review and editing; B.M.: writing—review and editing; W.D.N.: writing—review and editing; E.N.: writing—review and editing; E.P.: writing—review and editing; K.Re.: writing—review and editing; K.Ro.: writing—review and editing; A.S.: writing—review and editing; M.S.: writing—review and editing; N.T.: writing—review and editing; M.V.: conceptualization, project administration, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Bethany J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521-1532. ( 10.1016/S0140-6736(06)68653-4) [DOI] [PubMed] [Google Scholar]
- 2.Buonfrate D, et al. 2020. The global prevalence of Strongyloides stercoralis infection. Pathogens 9, 468. ( 10.3390/pathogens9060468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thamsborg SM, Ketzis J, Horii Y, Matthews JB. 2017. Strongyloides spp. infections of veterinary importance. Parasitology 144, 274-284. ( 10.1017/S0031182016001116) [DOI] [PubMed] [Google Scholar]
- 4.Morgan ER, et al. 2019. 100 Questions in livestock helminthology research. Trends Parasitol. 35, 52-71. ( 10.1016/j.pt.2018.10.006) [DOI] [PubMed] [Google Scholar]
- 5.Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. 2007. Strongyloides stercoralis: amphidial neuron pair ASJ triggers significant resumption of development by lnfective larvae under host-mimicking in vitro conditions. Exp. Parasitol. 115, 92-97. ( 10.1016/j.exppara.2006.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoltzfus JD, Bart SM, Lok JB. 2014. cGMP and NHR signaling co-regulate expression of insulin-like peptides and developmental activation of infective larvae in Strongyloides stercoralis. PLoS Pathog. 10, e1004235. ( 10.1371/journal.ppat.1004235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gang SS, Castelletto ML, Yang E, Ruiz F, Brown TM, Bryant AS, Grant WN, Hallem EA. 2020. Chemosensory mechanisms of host seeking and infectivity in skin-penetrating nematodes. Proc. Natl Acad. Sci. USA 117, 17 913-17 923. ( 10.1073/pnas.1909710117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant AS, Ruiz F, Lee J, Hallem EA. 2022. The neural basis of heat seeking in a human-infective parasitic worm. Curr. Biol. 32, 2206-2221. ( 10.1016/j.cub.2022.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashton FT, Schad GA. 1996. Amphids in Strongyloides stercoralis and other parasitic nematodes. Parasitol. Today 12, 187-194. ( 10.1016/0169-4758(96)10012-0) [DOI] [PubMed] [Google Scholar]
- 10.Bryant AS, Hallem EA. 2018. Temperature-dependent behaviors of parasitic helminths. Neurosci. Lett. 687, 290-303. ( 10.1016/j.neulet.2018.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. 1998. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J. Parasitol. 84, 691-695. ( 10.2307/3284571) [DOI] [PubMed] [Google Scholar]
- 12.Lopez PM, Boston R, Ashton FT, Schad GA. 2000. The neurons of class ALD mediate thermotaxis in the parasitic nematode Strongyloides stercoralis . Int. J. Parasitol. 30, 1115-1121. ( 10.1016/S0020-7519(00)00087-4) [DOI] [PubMed] [Google Scholar]
- 13.Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. 2004. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet. Parasitol. 120, 189-198. ( 10.1016/j.vetpar.2004.01.005) [DOI] [PubMed] [Google Scholar]
- 14.Nolan TJ, Brenes M, Ashton FT, Zhu X, Forbes WM, Boston R, Schad GA. 2004. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology 129, 753-759. ( 10.1017/S0031182004006092) [DOI] [PubMed] [Google Scholar]
- 15.Read AF, Skorping A. 1995. The evolution of tissue migration by parasitic nematode larvae. Parasitology 111, 359-371. ( 10.1017/S0031182000081919) [DOI] [PubMed] [Google Scholar]
- 16.Schad GA, Aikens LM, Smith G. 1989. Strongyloides stercoralis: is there a canonical migratory route through the host? J. Parasitol. 75, 740-749. ( 10.2307/3283059) [DOI] [PubMed] [Google Scholar]
- 17.Tada I, Mimori T, Nakai M. 1979. Migration route of Strongyloides ratti in albino rats. Jpn. J. Parasitol. 28, 219-227. [Google Scholar]
- 18.Tindall N, Wilson P. 1988. Criteria for a proof of migration routes of immature parasites inside hosts exemplified by studies of Strongyloides ratti in the rat. Parasitology 96, 551-563. ( 10.1017/S0031182000080185) [DOI] [PubMed] [Google Scholar]
- 19.Tindall N, Wilson P. 1990. A basis to extend the proof of migration routes of immature parasites inside hosts: estimated time of arrival of Nippostrongylus brasiliensis and Strongyloides ratti in the gut of the rat. Parasitology 100, 275-280. ( 10.1017/S0031182000061278) [DOI] [PubMed] [Google Scholar]
- 20.Brown RC, Girardeau HF. 1977. Transmammary passage of Strongyloides sp. larvae in the human host. Am. J. Trop. Med. Hyg. 26, 215-219. ( 10.4269/ajtmh.1977.26.215) [DOI] [PubMed] [Google Scholar]
- 21.Wilson P, Cameron M, Scott D. 1978. Patterns of milk transmission of Strongyloides ratti. Parasitology 77, 87-96. ( 10.1017/S0031182000048745) [DOI] [PubMed] [Google Scholar]
- 22.Mansfield LS, Schad GA. 1995. Lack of transmammary transmission of Strongyloides stercoralis from a previously hyperinfected bitch to her pups. J. Helminthol. Soc. Wash. 62, 80-83. [Google Scholar]
- 23.Shoop WL, Michael BF, Eary CH, Haines HW. 2002. Transmammary transmission of Strongyloides stercoralis in dogs. J. Parasitol. 88, 536-539. ( 10.1645/0022-3395(2002)088[0536:TTOSSI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 24.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. 2004. Helminth parasites – masters of regulation. Immunol. Rev. 201, 89-116. ( 10.1111/j.0105-2896.2004.00191.x) [DOI] [PubMed] [Google Scholar]
- 25.Maizels RM, Smits HH, Mcsorley HJ. 2018. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity 49, 801-818. ( 10.1016/j.immuni.2018.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck AH, et al. 2014. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 5, 5488. ( 10.1038/ncomms6488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Pan W. 2022. Host–parasite interactions mediated by cross-species microRNAs. Trends Parasitol. 38, 478-488. ( 10.1016/j.pt.2022.02.011) [DOI] [PubMed] [Google Scholar]
- 28.Soblik H, Younis AE, Mitreva M, Renard BY, Kirchner M, Geisinger F, Steen H, Brattig NW. 2011. Life cycle stage-resolved proteomic analysis of the excretome/secretome from Strongyloides ratt—identification of stage-specific proteases. Mol. Cell. Proteom. 10, M111.010157. ( 10.1074/mcp.M111.010157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt VL, et al. 2016. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat. Genet. 48, 299-307. ( 10.1038/ng.3495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda Y, Palomares-Rius JE, Hino A, Afrin T, Mondal SI, Nakatake A, Maruyama H, Kikuchi T. 2019. Secretome analysis of Strongyloides venezuelensis parasitic stages reveals that soluble and insoluble proteins are involved in its parasitism. Parasites Vectors 12, 21. ( 10.1186/s13071-018-3266-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooks BA, Mckenzie D, Cadd LC, Mccoy CJ, Mcveigh P, Marks NJ, Maule AG, Mousley A, Atkinson LE. 2022. Pan-phylum in silico analyses of nematode endocannabinoid signalling systems highlight novel opportunities for parasite drug target discovery. Front. Endocrinol. 13, 892758. ( 10.3389/fendo.2022.892758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankenhaus B, Klemm U, Eschbach M-L, Sparwasser T, Huehn J, Kühl AA, Loddenkemper C, Jacobs T, Breloer M. 2011. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J. Immunol. 186, 4295-4305. ( 10.4049/jimmunol.1001920) [DOI] [PubMed] [Google Scholar]
- 33.Blankenhaus B, et al. 2014. Foxp3+ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathog. 10, e1003913. ( 10.1371/journal.ppat.1003913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breloer M, Hartmann W, Blankenhaus B, Eschbach ML, Pfeffer K, Jacobs T. 2015. Cutting edge: the BTLA–HVEM regulatory pathway interferes with protective immunity to intestinal helminth infection. J. Immunol. 194, 1413-1416. ( 10.4049/jimmunol.1402510) [DOI] [PubMed] [Google Scholar]
- 35.Hartmann W, Blankenhaus B, Brunn ML, Meiners J, Breloer M. 2021. Elucidating different pattern of immunoregulation in BALB/c and C57BL/6 mice and their F1 progeny. Scient. Rep. 11, 1536. ( 10.1038/s41598-020-79477-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nisbet AJ, et al. 2013. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine 31, 4017-4023. ( 10.1016/j.vaccine.2013.05.026) [DOI] [PubMed] [Google Scholar]
- 37.Breloer M, Linnemann L. 2023. Strongyloides ratti infection in mice: immune response and immune modulation. Phil. Trans. R. Soc. B 378, 20220442. ( 10.1098/rstb.2022.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nutman TB. 2017. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144, 263-273. ( 10.1017/S0031182016000834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viney ME, Lok JB. 2007. The biology of Strongyloides spp. Wormbook, pp. 1–17. ( 10.1895/wormbook.1.141.1) See http://www.wormbook.org. [DOI]
- 40.Grant WN, Stasiuk S, Newton-Howes J, Ralston M, Bisset SA, Heath DD, Shoemaker CB. 2006. Parastrongyloides trichosuri, a nematode parasite of mammals that is uniquely suited to genetic analysis. Int. J. Parasitol. 36, 453-466. ( 10.1016/j.ijpara.2005.11.009) [DOI] [PubMed] [Google Scholar]
- 41.Yamada M, Matsuda S, Nakazawa M, Arizono N. 1991. Species-specific differences in heterogonic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J. Parasitol. 77, 592-594. ( 10.2307/3283165) [DOI] [PubMed] [Google Scholar]
- 42.Gardner MP, Gems D, Viney ME. 2006. Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution. Aging Cell 5, 315-323. ( 10.1111/j.1474-9726.2006.00226.x) [DOI] [PubMed] [Google Scholar]
- 43.Thompson FJ, Barker GL, Nolan T, Gems D, Viney ME. 2009. Transcript profiles of long- and short-lived adults implicate protein synthesis in evolved differences in ageing in the nematode Strongyloides ratti. Mech. Ageing Dev. 130, 167-172. ( 10.1016/j.mad.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 44.Hotez P, Hawdon J, Schad GA. 1993. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans daf-c paradigm. Parasitol. Today 9, 23-26. ( 10.1016/0169-4758(93)90159-D) [DOI] [PubMed] [Google Scholar]
- 45.Crook M. 2014. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 44, 1-8. ( 10.1016/j.ijpara.2013.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viney M, Morris R. 2022. Approaches to studying the developmental switch of Strongyloides – moving beyond the dauer hypothesis. Mol. Biochem. Parasitol. 249, 111477. ( 10.1016/j.molbiopara.2022.111477) [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, et al. 2021. Characterization of the endogenous DAF-12 ligand and its use as an anthelmintic agent in Strongyloides stercoralis. eLife 10, e73535. ( 10.7554/eLife.73535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speare R. 1989. Identification of species of Strongyloides. In Strongyloidiasis: a major infection of man (ed. Grove DI), pp. 11-83. London, UK: Taylor & Francis. [Google Scholar]
- 49.Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, Odermatt P, Lok JB, Streit A. 2017. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 11, e0005752. ( 10.1371/journal.pntd.0005752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasegawa H, et al. 2010. Molecular identification of the causative agent of human strongyloidiasis acquired in Tanzania: dispersal and diversity of Strongyloides spp. and their hosts. Parasitol. Int. 59, 407-413. ( 10.1016/j.parint.2010.05.007) [DOI] [PubMed] [Google Scholar]
- 51.Ko PP, et al. 2023. Population genetics study of Strongyloides fuelleborni and phylogenetic considerations on primate-infecting species of Strongyloides based on their mitochondrial genome sequences. Parasitol. Int. 92, 102663. ( 10.1016/j.parint.2022.102663) [DOI] [PubMed] [Google Scholar]
- 52.Barratt JLN, Sapp SGH. 2020. Machine learning-based analyses support the existence of species complexes for Strongyloides fuelleborni and Strongyloides stercoralis. Parasitology 147, 1184-1195. ( 10.1017/S0031182020000979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patton JB, et al. 2018. Methylprednisolone acetate induces, and Δ7-dafachronic acid suppresses, Strongyloides stercoralis hyperinfection in NSG mice. Proc. Natl Acad. Sci. USA 115, 204-209. ( 10.1073/pnas.1712235114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gang SS, Castelletto ML, Bryant AS, Yang E, Mancuso N, Lopez JB, Pellegrini M, Hallem EA. 2017. Targeted mutagenesis in a human-parasitic nematode. PLoS Pathog. 13, e1006675. ( 10.1371/journal.ppat.1006675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lok JB, Shao H, Massey HC, Li X. 2017. Transgenesis in Strongyloides and related parasitic nematodes: historical perspectives, current functional genomic applications and progress towards gene disruption and editing. Parasitology 144, 327-342. ( 10.1017/S0031182016000391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dulovic A, Streit A. 2019. RNAi-mediated knockdown of daf-12 in the model parasitic nematode Strongyloides ratti. PLoS Pathog. 15, e1007705. ( 10.1371/journal.ppat.1007705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 3, e412. ( 10.1371/journal.pntd.0000412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleitas PE, Travacio M, Martí-Soler H, Socías ME, Lopez WR, Krolewiecki AJ. 2020. The Strongyloides stercoralis-hookworms association as a path to the estimation of the global burden of strongyloidiasis: a systematic review. PLoS Negl. Trop. Dis. 14, e0008184. ( 10.1371/journal.pntd.0008184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z. 2013. Severe strongyloidiasis: a systematic review of case reports. BMC Infect. Dis. 13, 78. ( 10.1186/1471-2334-13-78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashford RW, Barnish G, Viney ME. 1992. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol. Today 8, 314-318. ( 10.1016/0169-4758(92)90106-C) [DOI] [PubMed] [Google Scholar]
- 61.Hira PR, Patel BG. 1977. Strongyloides fülleborni infections in man in Zambia. Am. J. Trop. Med. Hyg. 26, 640-643. ( 10.4269/ajtmh.1977.26.640) [DOI] [PubMed] [Google Scholar]
- 62.Buonfrate D, Tamarozzi F, Paradies P, Watts MR, Bradbury RS, Bisoffi Z. 2022. The diagnosis of human and companion animal Strongyloides stercoralis infection: challenges and solutions. A scoping review. Adv. Parasitol. 118, 1-84. ( 10.1016/bs.apar.2022.07.001) [DOI] [PubMed] [Google Scholar]
- 63.CDC 2023. Resources for health professionals. Parasites - Strongyloides. See https://www.cdc.gov/parasites/strongyloides/health_professionals/index.html.
- 64.Al-Jawabreh R, et al. 2023. Strongyloides questions–a research agenda for the future. Figshare. ( 10.6084/m9.figshare.c.6908983) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Al-Jawabreh R, et al. 2023. Strongyloides questions–a research agenda for the future. Figshare. ( 10.6084/m9.figshare.c.6908983) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [64].