Abstract
We investigated the transformation of six industrial azo and phthalocyanine dyes by ligninolytic peroxidases from Bjerkandera adusta and other white rot fungi. The dyes were not oxidized or were oxidized very little by Phanerochaete chrysosporium manganese peroxidase (MnP) or by a chemically generated Mn3+-lactate complex. Lignin peroxidase (LiP) from B. adusta also showed low activity with most of the dyes, but the specific activities increased 8- to 100-fold when veratryl alcohol was included in the reaction mixture, reaching levels of 3.9 to 9.6 U/mg. The B. adusta and Pleurotus eryngii MnP isoenzymes are unusual because of their ability to oxidize aromatic compounds like 2,6-dimethoxyphenol and veratryl alcohol in the absence of Mn2+. These MnP isoenzymes also decolorized the azo dyes and the phthalocyanine complexes in an Mn2+-independent manner. The reactions with the dyes were characterized by apparent Km values ranging from 4 to 16 μM and specific activities ranging from 3.2 to 10.9 U/mg. Dye oxidation by these peroxidases was not increased by adding veratryl alcohol as it was in LiP reactions. Moreover, the reaction was inhibited by the presence of Mn2+, which in the case of Reactive Black 5, an azo dye which is not oxidized by the Mn3+-lactate complex, was found to act as a noncompetitive inhibitor of dye oxidation by B. adusta MnP1.
A great variety of synthetic dyes are used for textile dyeing and other industrial applications. The structural diversity of dyes derives from the use of different chromophoric groups (e.g., azo, anthraquinone, triarylmethane, and phthalocyanine groups) and different application technologies (e.g., reactive, direct, disperse, and vat dyeing) (37). Azo dyes constitute the largest class of dyes used commercially. Phthalocyanines are used for the production of green and blue dyes. The total world colorant production is estimated to be on the order of 800,000 tons/year (37). Most of these compounds are highly resistant to microbial attack, and therefore they are hardly removed from effluents by conventional biological wastewater treatment, such as activated sludge treatment (20, 28). A number of laboratories are investigating the ability of the white rot fungus Phanerochaete chrysosporium to degrade xenobiotic compounds (reviewed in references 13 and 26). Decolorization of azo, anthraquinone, heterocyclic, triphenylmethane, and polymeric dyes (3, 19) and partial mineralization of azo dyes (25, 31) with this fungus have been reported.
White rot fungi produce various isoforms of extracellular oxidases and peroxidases, which are involved in the degradation of lignin in their natural lignocellulosic substrates (4, 10, 14). The first ligninolytic peroxidases were isolated from P. chrysosporium and called lignin peroxidase (LiP) (7, 33) and manganese peroxidase (MnP) (15). The catalytic cycles of LiP and MnP are similar to that of other peroxidases. Oxidation of the native enzyme by H2O2 yields an oxidized state (compound I), which is reduced in two steps to form a second oxidized state (compound II) and then the native enzyme. LiP catalyzes the oxidation of nonphenolic aromatic compounds like veratryl alcohol. MnP preferentially oxidizes Mn2+ to Mn3+, and the Mn3+ is responsible for the oxidation of many phenolic compounds (5). Although a variety of phenols are capable of directly reducing compound I of P. chrysosporium MnP, only Mn2+ efficiently reduces compound II to the native enzyme. Therefore, Mn2+ is necessary for completion of the catalytic cycle of P. chrysosporium MnP (35). The MnP isoenzymes from Pleurotus species and Bjerkandera adusta differ from the isoenzymes isolated from P. chrysosporium because they are able to oxidize 2,6-dimethoxyphenol (DMP) and veratryl alcohol in an Mn2+-independent reaction (1, 12, 16, 17, 27).
Several reports have shown that LiP or MnP from P. chrysosporium is directly involved in the degradation of various xenobiotic compounds and dyes. The decolorization of two azo dyes by LiP was strongly stimulated by the presence of veratryl alcohol in the reaction mixture (23). In the absence of veratryl alcohol compound II accumulated, indicating that these two azo dyes can only reduce compound I of LiP. In other studies preferential degradation of different sulfonated azo dyes either by MnP and Mn2+ or by LiP was demonstrated (22, 24). Furthermore, metabolic pathways for azo dye degradation by fungal peroxidases have been postulated (9, 32). Recently, we have demonstrated the excellent dye-degrading abilities of the white rot fungus B. adusta (11). The aim of the present study was to compare the ligninolytic peroxidases isolated from this fungus with similar enzymes from other fungal species with respect to their abilities to transform azo dyes and phthalocyanines of commercial interest.
MATERIALS AND METHODS
Chemicals.
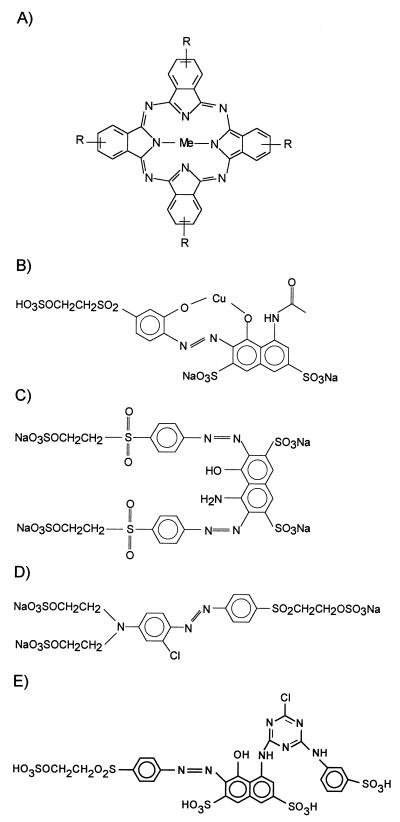
Reactive Blue 38 (RB38) (Fig. 1A), Reactive Violet 5 (RV5) (Fig. 1B), Reactive Black 5 (RB5) (Fig. 1C), Reactive Orange 96 (RO96) (Fig. 1D), and Reactive Red 198 (RR198) (Fig. 1E) were obtained from DyStar (Frankfurt, Germany). Reactive Blue 15 (RB15) (Fig. 1A), veratryl alcohol (3,4-dimethoxybenzyl alcohol), and DMP were purchased from Aldrich. The qualities of the dyes used were the same as the qualities of the dyes used in the textile industry.
FIG. 1.
Chemical structures of the phthalocyanine dye (A) and azo dyes (B through E) used. (A) RB38 (Me = Ni, R = −SO3H or −NH-D, D = phenylene unit with reactive group) and RB15 (Me = Cu, R = −SO3Na or −SO2-NH-C6H3SO3Na-NH-C3N3ClNH2). (B) RV5. (C) RB5 (D) RO96 (E) RR198.
Culture methods and enzyme preparation.
B. adusta B (= DSM 11310) was grown on glucose-ammonia medium as described previously (11). MnP and LiP were purified as described by Heinfling et al. (12). The culture supernatant was separated from the mycelium by filtration through a 1.2-μm-pore-size filter. Extracellular proteins were adsorbed to Q Sepharose (Pharmacia) at pH 6, eluted with 0.5 M NaCl, and dialyzed against 20 mM histidine (pH 6). The proteins were fractionated on a Mono-Q column (type HR5/5; Pharmacia) with a linear 0 to 300 mM NaCl gradient for 180 min (20 mM histidine buffer [pH 6]; flow rate, 1 ml/min), and LiP- and MnP-containing fractions were pooled separately. LiP isoenzymes were purified by Mono-Q chromatography at pH 4.6 in 20 mM histidine buffer (0 to 0.2 M NaCl gradient in 80 ml; flow rate, 1 ml/min). MnP isoenzymes were purified by Mono-Q chromatography in 10 mM tartrate at pH 4.6 (0 to 0.05 mM NaCl gradient in 28 ml; flow rate, 0.8 ml/min).
MnPL1 and MnPL2 of Pleurotus eryngii ATCC 90787 (= IJFM A169) grown on glucose-peptone medium were isolated by Q-cartridge chromatography (pH 4.5), Sephacryl S-200 chromatography, and Mono-Q chromatography (pH 5) as described by Martínez et al. (17). MnP1 of P. chrysosporium ATCC 24725 (= BKM-F-1767) was isolated from shaken cultures in N-limited B-III medium (containing 0.36 mM veratryl alcohol, 235 mM Mn2+, 0.5% Tween 80, and 20 mM sodium acetate; pH 4.5) (21) by consecutive Mono-Q chromatography in 10 mM tartrate (pH 5.7) and Superdex-75 chromatography in the same buffer supplemented with 150 mM NaCl. Isoenzyme identities were confirmed by N-terminal sequencing by using automated Edman degradation.
Enzyme and protein assays.
Manganese-independent peroxidase (MIP) activity was estimated by monitoring the oxidation of 1 mM DMP in 100 mM sodium tartrate (pH 3.25), using 0.1 mM H2O2. The reaction mixtures used to determine MnP activity contained 1 mM DMP, 0.1 mM H2O2, 1 mM MnSO4, and 100 mM sodium tartrate at pH 4.5. MnP activity was corrected for MIP activity by subtracting the activity obtained at pH 4.5 in the absence of MnSO4. Oxidation of DMP was measured by using an ɛ469 of 27,500 M−1 cm−1 for DMP as described by Martínez et al. (17). The reaction mixtures used to determine LiP activity contained 2 mM veratryl alcohol, 10 mM sodium tartrate (pH 3), and 0.1 mM H2O2. The formation of veratraldehyde was measured by using an ɛ310 of 9,300 M−1 cm−1. All enzyme reactions were started by adding enzyme, and the activities were calculated from the linear phases of the reactions. One unit of enzyme activity was defined as the amount of enzyme that transformed 1 μmol of substrate per min.
Protein concentrations were determined by using Bradford reagent (Bio-Rad) and bovine serum albumin as the standard.
Enzyme assays with dyes.
Unless stated otherwise, the reaction mixtures used to estimate dye-decolorizing activities contained dye, 0.1 mM H2O2, and 100 mM tartrate. Where indicated below, Mn2+ was added as MnSO4. For LiP reactions veratryl alcohol or tryptophan was used in the reaction mixtures at the concentrations given below. The reactions were monitored at the absorbance maximum of each dye. The molar extinction coefficients were estimated from a calibration curve by using the molecular weight of each specific dye and the dye content of each preparation and were as follows: ɛ558 = 30,000 M−1 cm−1 for RV5, ɛ598 = 50,000 M−1 cm−1 for RB5, ɛ460 = 25,000 M−1 cm−1 for RO96, ɛ520 = 35,000 M−1 cm−1 for RR198, ɛ675 = 70,000 M−1 cm−1 for RB15, and ɛ620 = 75,000 M−1 cm−1 for RB38. Experiments were carried out at dye concentrations at which the relationship between dye concentration and absorption was linear in the calibration curve.
Mn3+-lactate reactions.
A stock solution of Mn3+-acetate (5 mM) in 0.2 M lactate (pH 4.5) was prepared. Solutions were stored at 4°C and used within 1 h after preparation. Mn3+-lactate was added in aliquots over a period of 1 h to a reaction mixture containing dye (50 μM azo dye or 25 μM phthalocyanine dye) and 0.1 mM tartrate at pH 4.5. Dye decolorization was measured at the wavelength corresponding to the absorbance maximum of each dye.
RESULTS
Dye-decolorizing proteins secreted by B. adusta.
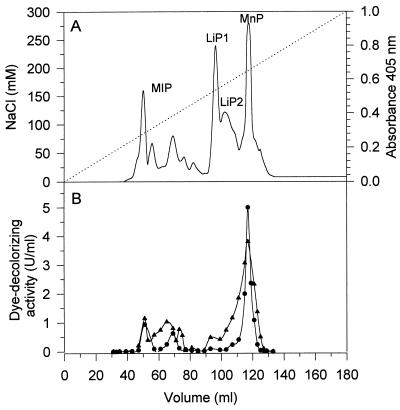
The mixture of B. adusta extracellular proteins obtained after an adsorption step (Q Sepharose, pH 6) was fractionated by anion-exchange chromatography on a Mono-Q column, and the fractions were tested for LiP activity, MnP activity, and MIP activity (Fig. 2A). Dye-decolorizing peroxidase activity was determined with RV5 and RB38 as substrates (Fig. 2B). Generally, the activity profiles for decolorization of the azo dye RV5 and the phthalocyanine dye RB38 were similar. The main peak of dye-decolorizing activity coincided with the main MnP peak around 118 ml. There was also dye-decolorizing activity between 50 and 75 ml, in fractions which did not contain LiP or MnP activity but contained MIP-like activity with DMP. The fractions corresponding to the major LiP peak exhibited little activity with the dyes in the absence of veratryl alcohol. The reaction rates with the dyes could not be increased by adding 1 mM Mn2+ to the reaction mixtures, indicating that the dyes are not degraded in an Mn2+-dependent reaction by MnP. This was tested for every individual fraction.
FIG. 2.
Activity profile of B. adusta extracellular proteins. Proteins were fractionated by anion-exchange chromatography on a Mono-Q HR5/5 column (20 mM histidine buffer, pH 6; flow rate, 1 ml/min). (A) Absorbance at 405 nm (——) and NaCl gradient (· · ·). (B) Profiles corresponding to RV5-decolorizing activity (•) and RB38-decolorizing activity (▴). The reaction mixtures contained 30 μM RV5 or 20 μM RB38, 0.1 mM H2O2, and 0.1 M tartrate at pH 3.5.
Mn3+-lactate reaction with commercial dyes.
The six commercial reactive dyes shown in Fig. 1 were tested for oxidation by Mn3+, which is the diffusible oxidizing agent produced by fungal MnP, in the form of the Mn3+-lactate complex. The azo dye RV5 was decolorized to some extent during incubation with Mn3+ (40% decolorization was observed after addition of 1 μmol of Mn3+ to 50 nmol of dye in a reaction volume of 1 ml). The three other azo dyes did not react with Mn3+-lactate. The nickel-phthalocyanine complexes RB38 and RB15 were decolorized to low extents (3 to 4% decolorization occurred after addition of 1 μmol of Mn3+ to 25 nmol of dye in a reaction volume of 1 ml).
Dye oxidation by MnP from B. adusta and P. eryngii.
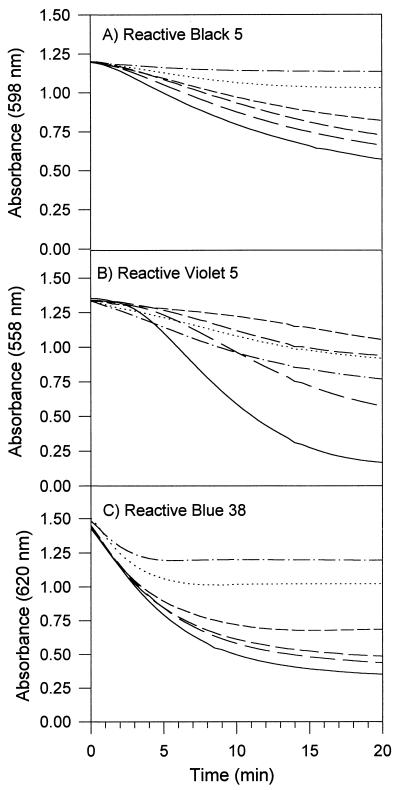
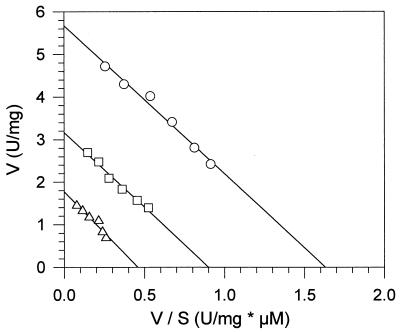
Purified MnP1 and MnP2 from B. adusta decolorized azo and phthalocyanine dyes in Mn2+-independent reactions. This activity was also exhibited by MnPL1 and MnPL2 from P. eryngii, which are similar to the B. adusta MnP isoenzymes with respect to Mn2+-independent oxidation of DMP and veratryl alcohol. The pH optima for oxidation of the different dyes by MnP1 were pH 3.5 (RV5 and RB5) and pH 3.75 (RB38). In contrast to the LiP reactions with these dyes, the MnP reactions were not stimulated by adding veratryl alcohol. Mn2+ in the reaction mixture inhibited the oxidation of the tested azo and phthalocyanine dyes by B. adusta MnP. Typical progress curves for the reactions of MnP1 with the dyes RB5, RV5, and RB38 in the presence of different concentrations of Mn2+ are shown in Fig. 3. In the case of RB5 (an azo dye which is not oxidized by Mn3+), addition of increasing amounts of Mn2+ had increasing inhibitory effects on the reaction rate (Fig. 3A). The influence of Mn2+ on the Km and Vmax values for the oxidation of RB5 by MnP1 was investigated by using several concentrations of Mn2+ and dye. Mn2+ affected the Vmax but did not affect the Km of the reaction, leading to parallel lines in the graph shown in Fig. 4. This result indicates that Mn2+ does not affect binding of RB5 to MnP but gives rise to a noncompetitive type of inhibition.
FIG. 3.
Time courses of RB5 (A), RV5 (B), and RB38 (C) decolorization by B. adusta MnP1 in the presence of the following concentrations of Mn2+: zero (——), 0.03 mM (——), 0.1 mM (–––), 0.3 mM (- - - -), 1 (· · ·), and 3 mM (–·–). The reaction mixtures contained dye, 0.1 mM H2O2, and 0.1 M tartrate at pH 3.5 (RV5 and RB5) or pH 3.75 (RB38).
FIG. 4.
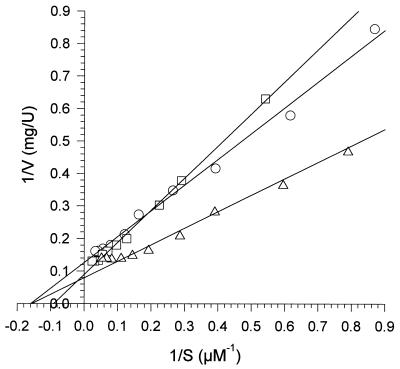
Mn2+ is a noncompetitive inhibitor for the oxidation of RB5 catalyzed by B. adusta MnP1. The reaction rate with RB5 (V) was plotted against the reaction rate with RB5 divided by the RB5 concentration (S). The Mn2+ concentrations used were zero (○), 0.3 mM (□), and 1 mM (▵). The reaction mixtures contained RB5, 0.1 mM H2O2, and 0.1 M tartrate at pH 4.
The case of RV5 is more complicated (Fig. 3B). Whereas in the absence of Mn2+ decolorization started after a lag phase, addition of 3 mM Mn2+ resulted in acceleration of the initial reaction rate. However, the maximum reaction rate achieved was lower (leading to 42% decolorization after 20 min with 3 mM Mn2+) than the maximum reaction rate observed in the absence of Mn2+ (87% decolorization). Moreover, addition of a lower amount of Mn2+ (0.3 mM) resulted in a very slow reaction rate (21% decolorization). These results suggest that RV5 can be oxidized either directly by MnP1 (in experiments without Mn2+) or via Mn3+ produced by oxidation of Mn2+ (in experiments with high levels of Mn2+). The low reaction rate in the presence of low amounts of Mn2+ may be explained by Mn2+ inhibition of dye oxidation by the first mechanism together with low efficiency of the second mechanism because of mediator concentrations that are too low. Finally, addition of increasing amounts of Mn2+ to the RB38-decolorizing reaction resulted in an earlier termination of the reaction (Fig. 3C).
Kinetic constants of MnP isoenzymes with dye substrates.
To calculate the kinetic constants for the different dyes, the reciprocals of reaction rates with B. adusta MnP1 were plotted against the reciprocals of dye concentrations (Lineweaver-Burk plots are shown in Fig. 5). With RV5 the reaction rates during the linear phase (after the initial lag) were used to do this and to calculate Km values. The Km values obtained for the reactions of B. adusta and P. eryngii MnP isoenzymes with RB5, RV5, and RB38 are shown in Table 1. All of the Km values were in the range from 4 to 16 μM, and there were only minor differences among the three enzymes.
FIG. 5.
Lineweaver-Burk plots for B. adusta MnP1 oxidation of RB5 (○), RV5 (□), and RB38 (▵). The reciprocals of reaction rates (V) were plotted against the reciprocals of dye concentrations (S). The reaction mixtures contained dye, 0.1 mM H2O2, and 0.1 M tartrate at pH 3.5 (RV5 and RB5) or pH 3.75 (RB38).
TABLE 1.
Apparent Km values for oxidation of the azo dyes RV5 and RB5 and the phthalocyanine complex RB38 by MnP isoenzymes from B. adusta and P. eryngiia
| Isoenzyme |
Km (μM)
|
||
|---|---|---|---|
| RV5 | RB5 | RB38 | |
| B. adusta MnP1 | 11 | 6 | 6 |
| P. eryngii MnPL1 | 16 | 4 | 8 |
| P. eryngii MnPL2 | 12 | 4 | 7 |
The reaction mixtures contained dye, 0.1 mM H2O2, and 0.1 M tartrate at pH 3.5 (RV5 and RB5) or pH 3.75 (RB38).
Dye oxidation by LiP from B. adusta.
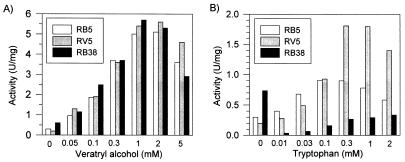
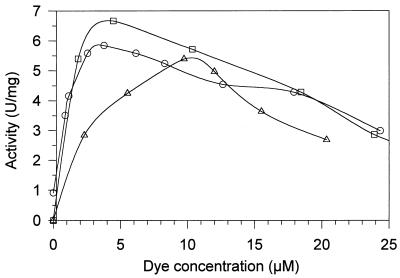
RV5, RB5, and RB38 were decolorized efficiently by LiP1 and LiP2 from B. adusta in the presence of veratryl alcohol. The pH optima for the reactions of LiP2 with the dyes in the presence of veratryl alcohol were pH 3.25 (RV5), pH 3.5 (RB5), and pH 3.75 (RB38). Saturation of the reaction mixture with veratryl alcohol was achieved at concentrations of approximately 1 mM (Fig. 6). Tryptophan also enhanced the LiP reactions with RV5 and RB5, but veratryl alcohol more efficiently promoted dye oxidation by LiP. As shown in Fig. 7, oxidation of dyes by B. adusta LiP isoenzymes in the presence of 2 mM veratryl alcohol was inhibited by concentrations of RV5 and RB5 of more than 5 μM and by concentrations of RB38 of more than 10 μM.
FIG. 6.
Influence of veratryl alcohol (A) and tryptophan concentration (B) on oxidation of three dyes by B. adusta LiP2. The reaction mixtures contained 10 μM dye, 0.1 mM H2O2, 0.1 M tartrate at pH 3.5 (RV5 and RB5) or pH 3.75 (RB38), and veratryl alcohol or tryptophan at different concentrations.
FIG. 7.
Influence of substrate concentration on oxidation of RB5 (○), RV5 (□), and RB38 (▵) by B. adusta LiP2. The reaction mixtures contained dye, 0.1 mM H2O2, 2 mM veratryl alcohol, and 0.1 M tartrate at pH 3.5 (RV5 and RB5) or pH 3.75 (RB38).
Comparison of dye-decolorizing activities of different ligninolytic peroxidases.
The specific activities of LiP from B. adusta and MnP from B. adusta, P. eryngii, and P. chrysosporium with the different dyes are compared in Table 2. P. eryngii and B. adusta MnP isoenzymes oxidized the dyes with specific activities of 3.2 to 10.9 U/mg. Only minor differences were detected between the MnP isoenzymes from these two fungi, but some dye-specific differences were observed (including lower biodegradability of RR198). LiP decolorized the dyes in the presence of veratryl alcohol with similar specific activities (3.9 to 9.6 U/mg), but in this case RR198 was the best substrate. In the absence of veratryl alcohol the LiP specific activities with dyes were between 1 and 12% the specific activities observed in the presence of veratryl alcohol. Finally, it is interesting that P. chrysosporium MnP oxidized RV5, RB38, and RB15 with initial specific activities that were 30- to 90-fold lower than the specific activities observed with MnP from B. adusta and P. eryngii, but with the phthalocyanine dyes the reaction rates of P. chrysosporium MnP were reduced by 50% after 60 s. The specific activity of P. chrysosporium MnP with RV5 can be increased by a factor of 5 by adding Mn2+ to the reaction mixture, but it still is much lower than the specific activities observed with B. adusta and P. eryngii MnP. RB5, RO96, and RR198 were not oxidized by P. chrysosporium MnP in the presence or in the absence of Mn2+. Moreover, we found that the reaction rates with MnP and Mn2+ were not increased by increasing the pH of the reaction mixture to 4.5 (a pH value that increases Mn2+ oxidation by MnP).
TABLE 2.
Specific activities of B. adusta, P. eryngii, and P. chrysosporium peroxidases with selected azo and phthalocyanine dyesa
| Prepn | Sp act (U/mg) with:
|
|||||
|---|---|---|---|---|---|---|
| Azo dyes
|
Phthalocyanine dyes
|
|||||
| RV5 | RB5 | RO96 | RR198 | RB38 | RB15 | |
| B. adusta MnP1 | 6.6 | 6.1 | 5.8 | 3.2 | 7.2 | 9.7 |
| P. eryngii MnPL1 | 8.2 | 5.4 | 6.8 | 3.7 | 9.3 | 10.8 |
| P. eryngii MnPL2 | 8.1 | 5.4 | 8.1 | 3.2 | 10.0 | 10.9 |
| P. chrysosporium MnP1 | 0.09 | Nb | N | N | 0.22 | 0.12 |
| P. chrysosporium MnP1 + 0.3 mM MnSO4 | 0.46 | N | N | N | N | N |
| B. adusta LiP2 | 0.2 | 0.3 | 0.13 | 0.09 | 0.67 | 1.1 |
| B. adusta LiP2 + 2 mM veratryl alcohol | 5.6 | 5.1 | 3.9 | 9.6 | 5.3 | 9.5 |
The reaction mixtures contained 0.1 mM H2O2 and 0.1 M tartrate at pH 3.5 (azo dyes) or pH 3.75 (phthalocyanines). For the MnP reactions dye concentrations of 30 μM (azo dyes) and 20 μM (phthalocyanines) were used. In the case of LiP reactions a lower dye concentration (10 μM) was used to avoid inhibition by high substrate concentrations.
N, negligible (<10−3 U/mg).
DISCUSSION
In this work dye oxidation by peroxidases from B. adusta, a white rot fungus which recently was found to be an excellent degrader of synthetic dyes (11), was investigated and compared with transformation of dyes by MnP isoenzymes from P. eryngii and P. chrysosporium. Using a crude extract from P. chrysosporium cultures, Paszczynski et al. described dye degradation under MnP- or LiP-specific reaction conditions (24). These authors found that some differently substituted sulfonated azo dyes were better oxidized in the presence of Mn2+ at pH 5 (MnP conditions), whereas others were better oxidized in the absence of Mn2+ at pH 3 (LiP conditions). On the other hand, Ollikka et al. (19) reported that LiP isoenzymes from P. chrysosporium have different specificities for dyes belonging to different structural classes. An isoenzyme with a pI of 3.85 (which could correspond to isoenzyme H6) had greater veratryl alcohol-independent activity with the dyes methylene blue, methyl orange, and toluidine blue than the other LiP isoenzymes tested (19).
The reactivities of P. chrysosporium MnP (with or without Mn2+) or Mn3+-lactate with the dyes used in the present work were low. These results, together with those reported by Young and Yu (36), suggest that many commercial reactive dyes cannot be efficiently decolorized by P. chrysosporium MnP and Mn2+.
The MnP from B. adusta oxidizes dyes in an Mn2+-independent manner, and, judging from activity estimates obtained with anion-exchange chromatography fractions, this is the most important dye-decolorizing extracellular activity produced by the fungus. B. adusta MnP also exhibits Mn2+-independent activity with other aromatic substrates, like DMP and veratryl alcohol (12), as first described for MnP isoenzymes from Pleurotus species (1, 16, 17, 27). Mn2+-independent oxidation of DMP or veratryl alcohol has not been described for the well-known MnP from P. chrysosporium, but some Mn2+-independent activity against pinacyanol has been reported (5, 6). Moreover, paper pulp bleaching by Bjerkandera sp. has been described, suggesting that there is an Mn-independent activity (18). In the present study, dye decolorization in the absence of Mn2+ was also obtained with P. eryngii MnP isoenzymes but not with P. chrysosporium MnP1, suggesting that the Pleurotus and B. adusta enzymes may form a subclass of MnP isoenzymes that oxidize both Mn2+ and a variety of aromatic compounds. The fact that Mn2+ acted as a noncompetitive inhibitor for oxidation of the azo dye RB5 by B. adusta MnP suggests that the peroxidases mentioned above may have at least two substrate binding sites.
Dye oxidation by B. adusta and P. eryngii MnP is characterized by low Km values (in the micromolar range) and specific activities between 3.2 and 10.9 U/mg for the dyes investigated in this study. The Km values reported here for the dyes are low compared with those reported for peroxidase reactions with different aromatic substrates. For example, a Km of 200 μM was obtained for P. eryngii MnP1 with DMP in the absence of Mn2+; the Km for the same enzyme with veratryl alcohol was 3.5 mM (17), the Km values for P. chrysosporium LiP isoenzymes with veratryl alcohol were 83 to 200 μM (8), and the Km for horseradish peroxidase with ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] was 270 μM (30). A dye-decolorizing peroxidase recently found in Pleurotus ostreatus (29) also has high affinity for some dyes (e.g., the Km values for crystal violet and Remazol brilliant blue R are 6 and 11 μM, respectively), but it does not oxidize veratryl alcohol and no information about eventual oxidation of Mn2+ is available. The Mn-independent oxidation of o-dianisidine and p-anisidine by MnP isoenzymes from Poria subvermispora (synonym, Ceriporiopsis subvermispora) was characterized by high Km values (0.5 to 1 and 4.5 to 42 mM, respectively) (34).
As described for oxidation of different synthetic dyes by LiP from P. chrysosporium, the B. adusta LiP2 efficiently decolorized azo and phthalocyanine dyes only when veratryl alcohol was present in the reaction mixture (specific activities, 3.9 to 9.6 U/mg). Stabilization of LiP by tryptophan, as suggested by Collins et al. (2), was less efficient than veratryl alcohol stabilization when B. adusta LiP and the dyes used in this work were examined. Moreover, this peroxidase was inhibited by relatively low substrate concentrations of the azo dyes RV5 or RB5, as also shown for P. chrysosporium LiP and RB5 (36). In contrast, B. adusta MnP was less sensitive to high dye concentrations than LiP.
The enlarged substrate spectrum of the MnP produced by B. adusta and Pleurotus species may open new possibilities for biotechnological applications of ligninolytic peroxidases. The new MnP isoenzymes directly catalyzed decolorization of the reactive dyes studied in this work more efficiently than the MnP-Mn2+ or LiP-veratryl alcohol enzyme mediator system, and the use of a mediator is not necessary. On the other hand, any MnP can catalyze various reactions of biotechnological interest via diffusible Mn3+ chelates, which can penetrate substrates that are not accessible to enzymes due to steric hindrance. With the MnP described here, the two reaction strategies (i.e., direct oxidation and Mn3+-mediated oxidation) can be applied alternatively or sequentially with the same enzyme preparation.
ACKNOWLEDGMENTS
We thank Carolyn Palma (University of Santiago de Compostela, Santiago de Compostela, Spain) for the sample of MnP1 from P. chrysosporium and Susanne Pollter and Robert Opitz (Technical University of Berlin) for skillful technical assistance.
This work was funded by Deutsche Forschungsgemeinschaft grant Sfb 193. A.H. is grateful to the Gesellschaft von Freunden der Technischen Universität Berlin e.V. for partially financing her stay at the Centro de Investigaciones Biológicas in Madrid. The work at the Centro de Investigaciones Biológicas was supported in part by the EU project AIR2-CT93-1219 and by the Spanish Biotechnology Programme.
REFERENCES
- 1.Camarero S, Böckle B, Martínez M J, Martínez A T. Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol. 1996;62:1070–1072. doi: 10.1128/aem.62.3.1070-1072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins P J, Field J A, Teunissen P, Dobson A D W. Stabilization of lignin peroxidases in white rot fungi by tryptophan. Appl Environ Microbiol. 1997;63:2543–2548. doi: 10.1128/aem.63.7.2543-2548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cripps C, Bumpus J A, Aust S D. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990;56:1114–1118. doi: 10.1128/aem.56.4.1114-1118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson K-E L, Blanchette R A, Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 5.Glenn J K, Akileswaran L, Gold M H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986;251:688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 6.Glenn J K, Gold M H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin degrading basidiomycete Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;242:329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- 7.Glenn J K, Morgan M A, Mayfield M B, Kuwahara M, Gold M H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white-rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983;114:1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- 8.Glumoff T, Harvey P, Molinari S, Goble M, Frank G, Palmer J M, Smit J D G, Leisola M S A. Lignin peroxidase from Phanerochaete chrysosporium. Molecular kinetics and characterization of isoenzymes. Eur J Biochem. 1990;187:515–520. doi: 10.1111/j.1432-1033.1990.tb15333.x. [DOI] [PubMed] [Google Scholar]
- 9.Goszczynski S, Paszczynski A, Pasti-Grigsby M B, Crawford R L, Crawford D L. New pathway for degradation of sulfonated azo dyes by microbial peroxidases of Phanerochaete chrysosporium and Streptomyces chromofuscus. J Bacteriol. 1994;176:1339–1347. doi: 10.1128/jb.176.5.1339-1347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattaka A. Lignin-modifying enzymes from selected white-rot fungi—production and role in lignin degradation. FEMS Microbiol Rev. 1994;13:125–135. [Google Scholar]
- 11.Heinfling A, Bergbauer M, Szewzyk U. Biodegradation of azo and phthalocyanine dyes by Trametes versicolor and Bjerkandera adusta. Appl Microbiol Biotechnol. 1997;48:261–266. [Google Scholar]
- 12.Heinfling, A., M. J. Martínez, A. T. Martínez, M. Bergbauer, and U. Szewzyk. Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol. Lett., in press. [DOI] [PubMed]
- 13.Higson F K. Degradation of xenobiotics by white-rot fungi. Rev Environ Contam Toxicol. 1991;122:111–151. doi: 10.1007/978-1-4612-3198-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 15.Kuwahara M, Glenn K J, Morgan M A, Gold M H. Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 1984;169:247–250. [Google Scholar]
- 16.Martínez M J, Böckle B, Camarero S, Ruiz-Dueñas F J, Guillén F A, Martínez A T. MnP isoenzymes produced by two Pleurotus species in liquid culture and during wheat-straw solid-state fermentation. Am Chem Soc Symp Ser. 1996;655:183–196. [Google Scholar]
- 17.Martínez M J, Ruiz-Dueñas F J, Guillén F A, Martínez A T. Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem. 1996;237:424–432. doi: 10.1111/j.1432-1033.1996.0424k.x. [DOI] [PubMed] [Google Scholar]
- 18.Moreira M T, Feijoo G, Sierra-Alvarez R, Lema J, Field J A. Manganese is not required for biobleaching of oxygen-delignified kraft pulp by the white rot fungus Bjerkandera sp. strain BOS55. Appl Environ Microbiol. 1997;63:1749–1755. doi: 10.1128/aem.63.5.1749-1755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ollikka P, Alhonmäki K, Leppänen V-M, Glumoff T, Raijola T, Suominen L. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:4010–4016. doi: 10.1128/aem.59.12.4010-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagga U, Brown D. The degradation of dyestuffs. II. Behavior of dyestuffs in aerobic biodegradation tests. Chemosphere. 1986;15:479–491. [Google Scholar]
- 21.Palma, C., A. T. Martínez, J. Lema, and M. J. Martínez. A comparison of manganese peroxidase isoenzymes from Bjerkandera sp. and Phanerochaete chrysosporium. Submitted for publication.
- 22.Pasti-Grigsby M B, Paszczynski A, Goszczynski S, Crawford D L, Crawford R L. Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3605–3613. doi: 10.1128/aem.58.11.3605-3613.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paszczynski A, Crawford R L. Degradation of azo compounds by ligninase from P. chrysosporium. Biochem Biophys Res Commun. 1991;178:1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- 24.Paszczynski A, Pasti M B, Goszczynski S, Crawford D L, Crawford R L. New approach to improve degradation of recalcitrant azo dyes by Streptomyces spp. and Phanerochaete chrysosporium. Enzyme Microb Technol. 1991;13:378–384. [Google Scholar]
- 25.Paszczynski A, Pasti-Grigsby M B, Goszczynski S, Crawford R L, Crawford D L. Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol. 1992;58:3598–3604. doi: 10.1128/aem.58.11.3598-3604.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy C A. The potential for white-rot fungi in the treatment of pollutants. Curr Biol. 1995;6:320–328. [Google Scholar]
- 27.Sarkar S, Martínez A T, Martínez M J. Biochemical and molecular characterization of manganese peroxidase from Pleurotus ostreatus. Biochim Biophys Acta. 1997;1339:23–30. doi: 10.1016/s0167-4838(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 28.Shaul G M, Holdsworth T J, Dempsey C R, Dostal K A. Fate of water soluble azo dyes in the activated sludge process. Chemosphere. 1991;22:107–119. [Google Scholar]
- 29.Shin K-S, Oh I-K, Kim C J. Production and purification of Remazol brilliant blue R decolorizing peroxidase from the culture filtrate of Pleurotus ostreatus. Appl Environ Microbiol. 1997;63:1744–1748. doi: 10.1128/aem.63.5.1744-1748.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A T, Sanders S A, Thorneley R N F, Burke J F, Bray R R C. Characterisation of a haem active-site mutant of horseradish peroxidase, Phe41→Val, with altered reactivity towards hydrogen peroxide and reducing substrates. Eur J Biochem. 1992;207:507–519. doi: 10.1111/j.1432-1033.1992.tb17077.x. [DOI] [PubMed] [Google Scholar]
- 31.Spadaro J T, Gold M H, Renganathan V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:2397–2401. doi: 10.1128/aem.58.8.2397-2401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spadaro J T, Renganathan V. Peroxidase-catalyzed oxidation of azo dyes: mechanism of Disperse Yellow 3 degradation. Arch Biochem Biophys. 1994;312:301–307. doi: 10.1006/abbi.1994.1313. [DOI] [PubMed] [Google Scholar]
- 33.Tien M, Kirk T K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- 34.Urzúa U, Larrondo L F, Lobos S, Larraín J, Vicuña R. Oxidation reactions catalyzed by manganese peroxidase isoenzymes from Ceriporiopsis subvermispora. FEBS Lett. 1995;371:132–136. doi: 10.1016/0014-5793(95)00874-9. [DOI] [PubMed] [Google Scholar]
- 35.Wariishi H, Akileswaran L, Gold M H. Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochemistry. 1988;27:5365–5370. doi: 10.1021/bi00414a061. [DOI] [PubMed] [Google Scholar]
- 36.Young L, Yu J. Ligninase catalyzed decolorization of synthetic dyes. Water Res. 1997;31:1187–1193. [Google Scholar]
- 37.Zollinger H. Color chemistry—syntheses, properties and applications of organic dyes and pigments. New York, N.Y: VCH Publications; 1991. [Google Scholar]