Abstract
Background
CAB+RPV LA is the first complete long-acting regimen for virologically suppressed people with HIV (PWH) and demonstrated non-inferiority to standard of care antiretroviral regimens in the Phase 3/3b trials FLAIR, ATLAS, ATLAS-2M, and SOLAR. Implementation of a provider administered regimen poses new delivery challenges and real-world evidence is essential to understand utilization and clinical outcomes. The BEYOND study describes the demographics and month 6 (M6) clinical outcomes of patients initiating CAB+RPV LA in the US.
Methods
BEYOND is a 2-year observational real-world study of utilization, outcomes, and experience of people with HIV (PWH) initiating CAB+RPV LA (monthly or every 2 months) across 30 US sites. Healthcare providers (HCPs) completed an electronic case report form (eCRF) at baseline and M6 to capture demographics, medical and treatment history, and clinical outcomes.
Results
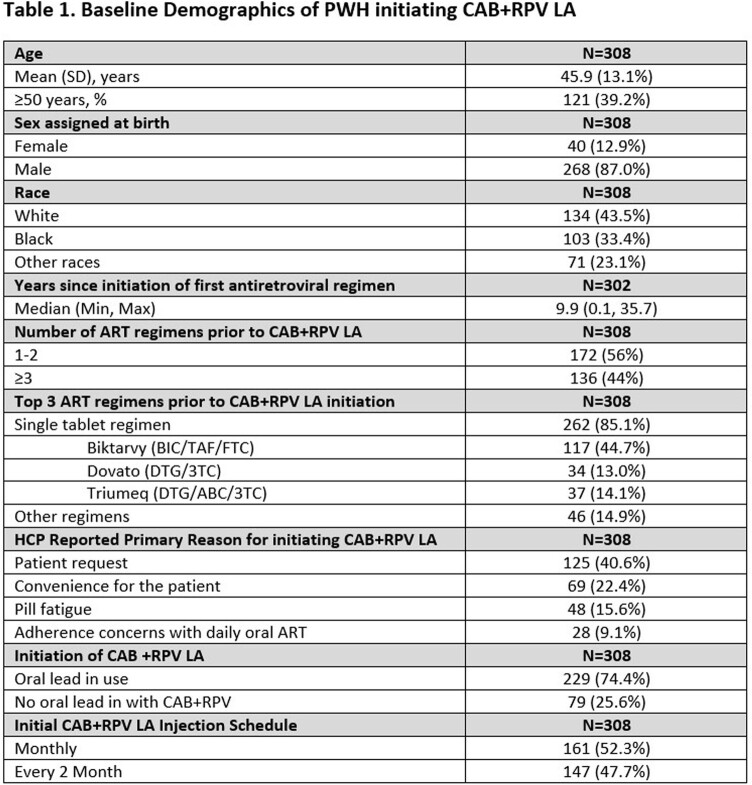
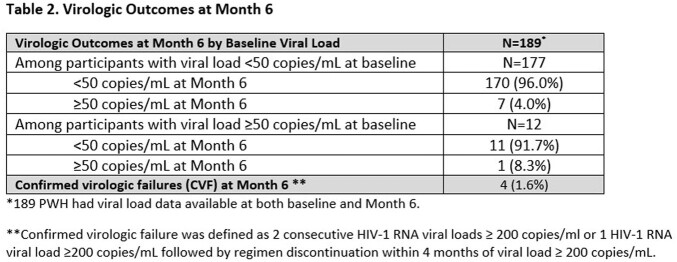
A total of 308 PWH (Table 1) were enrolled between Sep 2021- Jul 2022 and initiated on CAB+RPV LA. As of the data cut-off for this analysis (Jan 2023), 248 PWH had reached M6 of which 25 were reported as having discontinued CAB+RPV LA. The most common HCP reported primary reason for initiating CAB+RPV LA was patient request (41%). At M6, of the 803 injections given after the first injections, 667 (83%) occurred within +/-7 days of the target treatment date and 136 (17%) were outside the target treatment window (median 4 days outside). Of 1087 total injections expected, 44 (4%) were missed; of these, 3 (7%) used oral CAB+RPV and 19 (44%) used other oral regimens to cover missed injections. Of 189 PWH with viral load data available at both baseline and M6 (Table 2), 181 (96%) had viral loads of < 50 copies/mL. Confirmed virologic failure (CVF) occurred in 4 (1.6%), including 1 who had missed injections. Resistance was reported in 2 PWH. Discontinuations due to drug intolerance/injection site reactions were reported in 6 PWH.
Conclusion
The M6 results from real world initiation of CAB+RPV LA in the US are consistent with the Phase 3/3b clinical trials with high rates of virologic suppression, low rates of CVFs and treatment emergent resistance, and low rates of discontinuation due to drug intolerance.
Disclosures
Gary I. Sinclair, MD, Abbvie: Grant/Research Support|Gilead: Advisor/Consultant|Gilead: Grant/Research Support|Janssen: Advisor/Consultant|Janssen: Grant/Research Support|Janssen: Honoraria|Merck: Advisor/Consultant|Merck: Grant/Research Support|Merck: Honoraria|Thera: Advisor/Consultant|Thera: Grant/Research Support|Thera: Honoraria|ViiV: Advisor/Consultant|ViiV: Grant/Research Support|ViiV: Honoraria Michael Sension, MD, Gilead: Advisor/Consultant|Gilead: Honoraria|Viiv: Advisor/Consultant|Viiv: Grant/Research Support|Viiv: Honoraria Alexandra Dretler, MD, Gilead: Stocks/Bonds|Johnson and Johnson: Stocks/Bonds|Pfizer: Stocks/Bonds Stefan Schneider, MD, ViiV Healthcare: Grant/Research Support Catherine K. Schubert, PharmD, GSK: Stocks/Bonds|ViiV Healthcare: Employee of ViiV Healthcare Deanna Merrill, PharmD, MBA, AAHIVP, ViiV Healthcare: Employment|ViiV Healthcare: Stocks/Bonds Cindy Garris, MS, GSK: Stocks/Bonds|ViiV Healthcare: Employee