Abstract
Many wood-rotting fungi, including Phellinus pomaceus, produce chloromethane (CH3Cl). P. pomaceus can be cultured in undisturbed glucose mycological peptone liquid medium to produce high amounts of CH3Cl. The biosynthesis of CH3Cl is catalyzed by a methyl chloride transferase (MCT), which appears to be membrane bound. The enzyme is labile upon removal from its natural location and upon storage at low temperature in its bound state. Various detergents failed to solubilize the enzyme in active form, and hence it was characterized by using a membrane fraction. The enzyme had a sharp pH optimum between 7 and 7.2. Its apparent Km for Cl− (ca. 300 mM) was much higher than that for I− (250 μM) or Br− (11 mM). A comparison of these Km values to the relative in vivo methylation rates for different halides suggests that the real Km for Cl− may be much lower, but the calculated value is high because the CH3Cl produced is used immediately in a coupled reaction. Among various methyl donors tested, S-adenosyl-l-methionine (SAM) was the only one that supported significant methylation by MCT. The reaction was inhibited by S-adenosyl-l-homocysteine, an inhibitor of SAM-dependent methylation, suggesting that SAM is the natural methyl donor. These findings advance our comprehension of a poorly understood metabolic sector at the origin of biogenic emissions of halomethanes, which play an important role in atmospheric chemistry.
Halogenated organic compounds are ubiquitous in nature (29). They participate in the depletion of stratospheric ozone and have a profound impact on atmospheric chemistry (4, 18, 24). Although the dominant sources of these compounds are biogenic emissions (12, 25, 26, 28), their significance to the emitter organisms is rather poorly understood, with only a few indications of the roles they might play. In fungi, halomethanes serve as methyl group donors for the biosynthesis of esters, anisoles, and veratryl alcohol (9, 11). In algae, halomethanes are by-products of reactions in which scavenging of H2O2 releases HOBr, which is presumed to be a defense molecule against bacteria, fungi, and herbivores (23, 27). A recent report (28) that a marine alga, Endocladia muricata, and a salt-tolerant plant, Mesembryanthemum crystallinum, could methylate Cl− ions to chloromethane (CH3Cl) triggered speculation that this may be a mechanism for Cl− detoxification and salt tolerance. The S-adenosyl-l-methionine (SAM)-dependent methyl chloride transferase (MCT) that catalyzes this reaction was partially purified from E. muricata (28). The enzyme can also use I− and Br− as substrates.
These results suggest possibilities for engineering a Cl− detoxification capability into crop plants, many of which are sensitive to Cl− (6, 17). Wood-rotting fungi of the family Hymenochaetaceae are the most efficient producers of CH3Cl (5, 7, 13). Phellinus pomaceus converts Cl− to CH3Cl with over 90% efficiency, even at extremely low concentrations of the ion (7). A low MCT activity was detected in cell extracts of this fungus (28).
Halomethanes are the primary carriers of halogens between the biosphere and the atmosphere (4, 18) and therefore play pivotal roles in the effect of halogens on atmospheric chemistry and the integrity of the ozone layer (24). Since biogenic sources are major contributors of atmospheric halomethanes (7, 12, 18, 25, 28), attempts to understand atmospheric composition must include an understanding of the metabolic processes underlying the generation of these gases. In addition, engineering a Cl− detoxification capability into plants depends on the identification of novel metabolic pathways and an understanding of their regulation. Within this dual context, our objective was to determine the biochemical nature of the CH3Cl-evolving system of P. pomaceus.
MATERIALS AND METHODS
Organism and maintenance.
P. pomaceus (ATCC 62800) was obtained from the American Type Culture Collection (Rockville, Md.). The culture was maintained by a slightly modified method of Harper and Kennedy (12), on 5% (wt/vol) malt extract agar (MAA) supplemented with 10 mM KCl and 25 μg of chloramphenicol ml−1. After 10 to 15 days at 25°C, the cultures were stored in a refrigerator and used for further inoculation within 20 days.
Culture conditions and in vivo CH3Cl emission.
The fungus was grown in 250-ml Erlenmeyer flasks on a glucose mycological peptone (GMP) medium containing 30 g of glucose liter−1, 5 g of peptone liter−1, 25 μg of chloramphenicol ml−1, and the applicable concentration of KCl. The pH of the medium was adjusted to 6.8 with 5 N NaOH. For solid cultures, 7 g of agarose was added to 70 ml of the medium, which was inoculated with 1 ml of mycelial suspension from MAA plates, and the mixture was incubated at 25°C in the dark. For the liquid cultures, 25 ml of GMP medium was inoculated with 2- to 3-mm2 pieces of mycelial mat from MAA plates. The flasks were closed with rubber stoppers and incubated without agitation as described above. Coating stoppers with tetrafluoroethylene (12) did not change the amount of CH3Cl measured; hence, uncoated stoppers were used. The amount of accumulated CH3Cl was determined periodically over the next 15 days by gas chromatography.
Preparation of crude extracts of P. pomaceus.
Mycelia from 15- to 20-day-old liquid cultures were harvested by draining the medium through a strainer (1- by 1-mm-opening mesh), dried between folds of filter paper, and frozen in liquid N2. The frozen mycelia were ground to a fine powder with a pestle and mortar and suspended in 100 mM phosphate buffer (2 ml/g of mycelium) containing 1 mM dithiothreitol, 1 mM EDTA, 10% (vol/vol) glycerol, 3 μg of pepstatin ml−1, and 2 μg of leupeptin ml−1. The suspension was thawed on ice, and the resulting homogenate was centrifuged at 1,000 × g for 20 min at 4°C. The supernatant was desalted by passage through a PD-10 column (Pharmacia, Uppsala, Sweden), and the eluate was used as the crude enzyme preparation.
Effect of centrifugation speed on MCT activity.
The homogenate was centrifuged at 1,000 × g, 3,000 × g, and 10,000 × g for 20 min at 4°C. The three supernatants were then centrifuged at 100,000 × g for 1 h at 4°C. The enzyme activity in the supernatant and pellet from each centrifugation was determined.
Enzyme and protein assays.
MCT activity was measured by assaying the production of CH3Cl, CH3Br, and CH3I by using KCl (100 mM), KBr (25 mM), and KI (10 mM), respectively. The activity was assayed in 500 μl of reaction mixture containing the extraction buffer, 0.5 mM SAM, the substrate, and 100 μl of enzyme preparation (50 to 150 μg of protein). The mixture was contained in a 5-ml glass vial sealed with a screw-cap fitted with a Teflon-lined septum (Supelco, Oakville, Ontario, Canada) and incubated for 30 min on an orbital shaker (150 rpm) at room temperature. Total soluble proteins were determined by the method of Bradford (3) with Bio-Rad (Hercules, Calif.) protein reagent and the microassay procedure, with bovine serum albumin as the standard.
Gas chromatography.
In vivo CH3Cl emissions and other gaseous reaction products were analyzed as described by Attieh et al. (2) with a Hewlett-Packard (Avondale, Pa.) 5890 Series II gas chromatograph equipped with a flame ionization detector. One-milliliter headspace samples were removed through the septa with a syringe and injected in a 210- by 0.3-cm stainless-steel column packed with 80/100 mesh Porapak Q (Supelco). Column temperatures were 140°C for CH3I and CH3Br and 130°C for CH3Cl. Products were quantified by peak area and identified by comparison of their retention times with those of the authentic methyl halides used to calibrate the instrument.
Chemicals.
SAM was from Boehringer Mannheim Canada (Laval, Quebec, Canada), peptone was from Becton Dickinson (Cockeysville, Md.), and CH3Cl, CH3Br, and CH3I were from Aldrich (Milwaukee, Wis.). All other chemicals and media compounds were from Sigma (St. Louis, Mo.). All chemicals were of analytical grade or better.
Replication.
All experiments were repeated at least once. Analyses within an experiment were done in three or more replicates, except that in vivo CH3Cl emissions from single flasks were determined in six separate experiments. Results of one representative experiment are presented.
RESULTS
In vivo CH3Cl production by P. pomaceus.
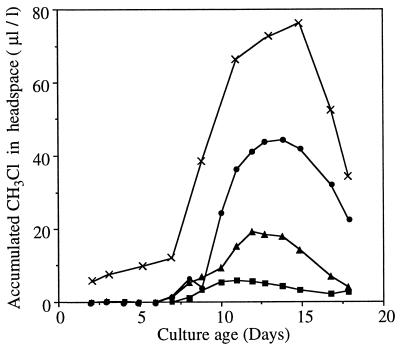
CH3Cl production by P. pomaceus on solid GMP medium increased with Cl− concentration up to 18 mM (Fig. 1). The small quantity of CH3Cl produced in the controls could be attributed to the traces of Cl− in peptone. The temporal patterns of CH3Cl accumulation were identical for all the concentrations. The fungus was cultured in liquid GMP medium to facilitate harvest of mycelia for biochemical studies. Inoculation of the medium with macerated mycelial suspension gave extremely poor growth in a continuously shaking system (data not shown). However, inoculation of nonshaking liquid medium with 2- to 3-mm2 mycelial pieces yielded vigorous cultures. CH3Cl production on the liquid medium containing 18 mM KCl began 5 days earlier than on the solid medium but peaked at the same time in both cases (Fig. 1). Maximum CH3Cl accumulation on the liquid medium was approximately 1.5-fold higher than that on the solid medium.
FIG. 1.
Time course of CH3Cl accumulation in flasks containing P. pomaceus on solid and liquid GMP media supplemented with KCl at different concentrations. Solid medium was supplemented with 0 (■), 1 mM (▴), and 18 mM (•) KCl, and liquid medium was supplemented with 18 mM KCl (×). Each data set represents a series of measurements for one flask; six repeats of this experiment gave similar patterns of CH3Cl emission.
Extraction of the MCT.
Published information (20) and a serious decline in the enzyme activity upon even the mildest cellular disruption indicated that the enzyme was probably membrane bound and highly labile. Hence, with a view to obtain a reasonably active enzyme preparation, we subjected mycelial homogenate to different centrifugation speeds. With increasing centrifugation speed, more MCT activity settled into the pellet, and ultracentrifugation of the supernatants removed nearly all of the remaining activity (Table 1). In addition, rehomogenization of the pellet from the ultracentrifugation step reduced the activity by half.
TABLE 1.
Relative distribution of MCT activity in the supernatant and pellet on subjecting the homogenate from P. pomaceus to different centrifugation speeds
| Fraction | Mean activitya ± SE (nmol min−1) after centrifugation at:
|
||
|---|---|---|---|
| 1,000 × g | 3,000 × g | 10,000 × g | |
| Before ultracentrifugation | |||
| Supernatant | 7.2 ± 0.3 | 5.2 ± 0.2 | 3.2 ± 0.1 |
| Pellet | 11 ± 0.2 | 15 ± 0.3 | 17 ± 0.1 |
| After ultracentrifugationb | |||
| Supernatant | 1.1 ± 0 | 0.8 ± 0 | 0.8 ± 0.2 |
| Pellet | 11 ± 0.1 | 6.0 ± 0.1 | 5.5 ± 0.1 |
| Pellet (rehomogenized) | 4.7 ± 0.1 | 2.7 ± 0.1 | 3.3 ± 0.1 |
Activity was measured in the presence of 100 mM KCl.
These fractions resulted from ultracentrifugation (100,000 × g) of the supernatant listed above.
We attempted to solubilize this enzyme from the 10,000 × g pellet by using standard protocols (22) and various concentrations of common detergents such as Triton X-100, octylglucoside, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), sodium cholate, sarcosyl, sodium dodecyl sulfate, and cetyltrimethylammonium bromide (CTAB). In all cases, the activity stayed in the pellet until the detergent concentration was high enough to solubilize the membrane proteins; at this point, the concentration of soluble proteins increased but all the MCT activity was lost (data not shown).
Therefore, all further experiments were done with an enzyme extract prepared by centrifuging the crude extract at 1,000 × g for 20 min at 4°C and desalting the supernatant on a PD-10 column. We assume that this preparation was a suspension of the enzyme still attached to its native niche. The reaction was linear up to 1 h and within the range of 0 to 300 μg of protein in the assay. The activity was completely lost upon boiling the preparation (Table 2).
TABLE 2.
Effects of different methyl donors and of an inhibitor of SAM-dependent methylation on the methylation of Cl− (100 mM KCl) by crude extracts of P. pomaceus
| Methyl donor(s) or inhibitor | Concn(s) (mM) | Sp act ± SE (pmol min−1 mg of protein−1) | Activity (% of SAM) |
|---|---|---|---|
| None | 1.2 ± 0.05 | 10.2 | |
| SAM | 0.25 | 12 ± 0.24 | 100 |
| 0.5 | 12 ± 0.03 | 100 | |
| SMM | 0.25 | 0.9 ± 0.09 | 7.6 |
| 0.5 | 0.8 ± 0.07 | 6.8 | |
| 1.0 | 0.8 ± 1.00 | 6.8 | |
| 2.0 | 0.5 ± 0.07 | 4.2 | |
| l-Methionine | 0.25 | 0.8 ± 0.04 | 6.8 |
| 0.5 | 0.8 ± 0.05 | 6.8 | |
| 1.0 | 0.7 ± 0.01 | 5.9 | |
| 2.0 | 0.8 ± 0.00 | 6.8 | |
| SAH | 0.25 | NDa | |
| 0.5 | ND | ||
| 1.0 | ND | ||
| 2.0 | ND | ||
| SAM + SAH | 0.0 + 0.25 | ND | |
| 0.5 + 0.25 | 7.0 + 0.08 | 59.3 | |
| 0.5 + 0.50 | 6.0 ± 0.28 | 50.8 | |
| 0.5 + 1.0 | 4.0 ± 0.10 | 36.3 | |
| SAM + boiled enzyme | 0.5 | ND |
ND, not detected.
Stability and pH optimum of the MCT.
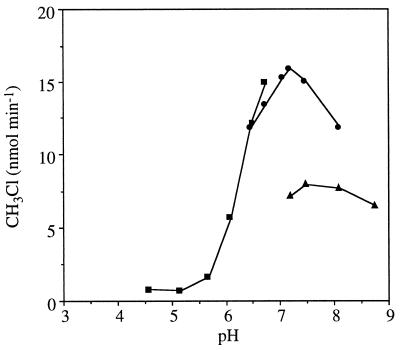
The enzyme was highly unstable and lost all activity when stored overnight at 4°C or −20°C, even in the presence of protease inhibitors (data not shown). The enzyme had a pH optimum between 7 and 7.2 (Fig. 2). The activity was lower in Tris-acetate buffer, although the optimum pH was in the same range as that with other buffers.
FIG. 2.
Effect of pH on MCT activity in crude extracts of P. pomaceus. The pH dependence was determined by using 100 mM citrate-phosphate (■), phosphate (•), and Tris-acetate (▴) buffers. The substrate was 100 mM KCl. The standard error values were smaller than the data points.
Determination of methyl donor and kinetic properties of the MCT.
The MCT activity in the presence of SAM was approximately 12 pmol min−1 mg of protein−1 (Table 2). The CH3Cl levels produced in the presence of methionine or S-methylmethionine (SMM), even at 2 mM, were not significantly different from those in the absence of any methyl donor. S-Adenosyl-l-homocysteine (SAH) eliminated the CH3Cl production in controls and strongly inhibited the SAM-dependent CH3Cl formation (Table 2). The inhibition was approximately 50% at equimolar concentrations of SAH and SAM.
The Vmax and Km values of MCT for various halides and SAM, calculated from Lineweaver-Burk plots, are shown in Table 3.
TABLE 3.
Kinetic parameters of MCT from P. pomaceus
| Substrate | Km | Vmax (pmol min−1 mg of protein−1) |
|---|---|---|
| KCl | 300 mM | 166.6 |
| KBr | 10 mM | 208.3 |
| KI | 250 μM | 90.9 |
| SAM | 4.5 μM | 200.0 |
DISCUSSION
We characterized an MCT from P. pomaceus that is involved in the biosynthesis of CH3Cl, large quantities of which are emitted by this fungus (8, 10). This membrane-bound and highly labile enzyme uses SAM as the methyl donor and has a sharp near-neutral pH optimum and an unexpectedly high calculated Km for Cl−. It can also methylate Br− and I−, for which its Km values are considerably lower.
Our initial attempts to obtain extracts of mycelia grown on previously used solid media (12) were frustrated by difficulties in separating the mycelia from the medium, the impossibility of obtaining accurate fresh weights, and a precipitous drop in CH3Cl production upon separating the fungus from the medium. These problems were overcome by producing a stationary liquid culture of the fungus in GMP medium. The amount of CH3Cl produced by this culture (Fig. 1) was among the largest recorded for this fungus under artificial conditions (12). The peak CH3Cl emission rate on the liquid medium was 325 nmol day−1 g (fresh weight)−1. This was 46,000 times greater than that from the only liquid culture previously reported for this fungus (28). The CH3Cl production rate from our liquid culture of P. pomaceus also exceeded the highest reported rates for nonfungal organisms—a plant, Brassica oleracea, and an alga, Macrocystis pyrifera—by approximately 740- and 850-fold, respectively (19, 25). Thus, P. pomaceus contains by far the most efficient biological system known for the conversion of Cl− to CH3Cl.
An increasing proportion of the MCT activity settled into the pellets with successive increases in centrifugal force, and only a negligible activity remained in the supernatant after ultracentrifugation (Table 1). These observations support the hypothesis that the enzyme is membrane bound (8, 20). In contrast, Wuosmaa and Hager (28) reported that most of the activity was in the soluble fraction of the homogenate. However, as pointed out by Harper (8), their extremely low reported CH3Cl production rates would be very difficult to measure accurately with the gas chromatographic technique used. Thus, their conclusion about the localization of the enzyme is questionable. Unlike the P. pomaceus MCT, the MCT from E. muricata and the comparable halide or bisulfide methyltransferase (H/BMT) from cabbage are soluble cytosolic enzymes (1, 28).
A precipitous decline in MCT activity upon disruption of cultures or pellet and the failure of detergents to solubilize it in an active state show that the enzyme is highly susceptible to mechanical damage. The enzyme was also very unstable during low-temperature storage. By comparison, halide methyl transferases from the algae Papenfusiella kuromo, Sargassum hornei, and Pavlova gyrans are less fragile (14). The H/BMT from B. oleracea was very stable at −20°C in its crude form but became increasingly labile upon purification (2).
The pH optimum (7 to 7.2) of P. pomaceus MCT is similar to those of the halide methyltransferases from marine algae (14, 28) but is higher than that for halide methylation (5.5 to 7) by the cabbage H/BMT (2).
The most efficient methylation of Cl− was observed when the methyl donor was SAM (Table 2). The methylation was strongly inhibited by SAH, a well-known inhibitor of SAM-dependent reactions (2, 15, 16). The background levels of CH3Cl emission in controls, with SMM, or with methionine were probably due to the presence of some endogenous SAM, because this emission was completely eliminated by the addition of SAH. Together, these results strongly suggest that SAM is the methyl donor for MCT-catalyzed CH3Cl formation in P. pomaceus. A similar dependence on SAM was also reported for the halide methyltransferases from the algae Pavlova gyrans, Papenfusiella kuromo, and S. hornei, the MCT from E. muricata, and the H/BMT from cabbage (2, 14, 28).
The calculated Km value of MCT for Cl− (Table 3) was surprisingly high in view of the high efficiency of in vivo CH3Cl production from low concentrations of Cl− (Fig. 1). This high Km value is particularly intriguing when compared with the much lower Km values for Br− and I− (11 mM and 250 μm), for which in vivo methylation rates are lower than that for Cl− (12). Even the H/BMT from cabbage has a lower Km for Cl− (85 mM), despite the fact that the in vivo CH3Cl formation rate in cabbage is nearly 3 orders of magnitude lower than that in P. pomaceus (2) (Fig. 1). Moreover, as expected from the relative in vivo methylation rates, the Km values of the P. pomaceus MCT for Br− and I− were markedly lower than those for the comparable enzymes from B. oleracea and several marine algae (2, 14, 28). These results, together, suggest that the real Km of the P. pomaceus MCT for Cl− is probably also lower than that observed, but the experimental determination gives a higher Km because CH3Cl formed in the reaction is probably removed for a coupled sequential reaction, such as the well-documented methylation of carboxylic acids or phenols (20, 21). In contrast, CH3I and CH3Br are much less efficient as methyl donors for coupled reactions (11), and hence the Km values for these are affected to a much lesser extent. Since the fungus does not accumulate chloride (7), the Km value for Cl− would in fact have to be very low for the MCT to methylate low concentrations of this ion as efficiently as the in vivo emissions suggest. Development of technical strategies to overcome these difficulties in determining the Km of MCT is, therefore, critical to assessing the utility of this enzyme for metabolic engineering to improve Cl− tolerance of plants. The Km value for SAM was 4.5 μM (Fig. 3), significantly lower than those reported for other halide methyltransferases (2, 14, 28).
Except for a report of a rather low MCT activity in cell extracts of P. pomaceus (28), this is the first attempt to characterize the enzyme responsible for the massive amounts of CH3Cl produced by this fungus. The results show that the fungus contains a SAM-dependent MCT that is responsible for the biosynthesis of CH3Cl. Careful extraction of mycelia can yield an enzyme preparation, probably a suspension of minute membrane fragments, that is around 1,000-fold more active (Table 2) than that previously reported (28). Moreover, even this preparation represents less than half of the total detectable activity of this enzyme (Table 1). The difficulty of solubilization of this membrane-bound labile enzyme is presently the greatest obstacle to its purification and to cloning the corresponding gene. These studies, aside from contributing to our understanding of the metabolic origin of the environmentally important CH3Cl gas (8, 18, 24), could permit the transfer of the MCT gene to a higher plant to study its role in Cl− detoxification. The latter biotechnological application has been suggested for some time (7), but its realization depends not only on the isolation and transfer of the gene but also on our ability to accurately predict the impact of this manipulation on the atmospheric budget of CH3Cl. This impact can be best assessed when estimates of CH3Cl emission per unit biomass of the transgenic plants expressing the MCT gene are available.
ACKNOWLEDGMENTS
This work was supported by research grants to H. S. Saini from the Natural Sciences and Engineering Research Council of Canada and Fonds pour la Formation de Chercheurs et l’Aide à la Recherche, Quebec. S. Aouad received a graduate scholarship from the Canadian International Development Agency.
D. Saxena and S. Aouad contributed equally to this work.
REFERENCES
- 1.Attieh, J., K. Kleppinger-Sparace, C. Nunes, S. Sparace, and H. S. Saini. Unpublished data.
- 2.Attieh J M, Hanson A D, Saini H S. Purification and characterization of a novel methyltransferase responsible for biosynthesis of halomethanes and methanethiol in Brassica oleracea. J Biol Chem. 1995;270:9250–9257. doi: 10.1074/jbc.270.16.9250. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Chameides W L, Davis D D. Iodine: its possible role in tropospheric photochemistry. J Geophys Res. 1980;85:7383–7398. [Google Scholar]
- 5.Cowan M I, Glen A T, Hutchinson S A, MacCartney M E, Mackintosh J M, Moss A M. Production of volatile metabolites by species of Fomes. Trans Br Mycol Soc. 1973;60:347–351. [Google Scholar]
- 6.Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol. 1980;31:149–190. [Google Scholar]
- 7.Harper D B. Halomethanes from halide ion—a highly efficient fungal conversion of environmental significance. Nature. 1985;315:55–57. [Google Scholar]
- 8.Harper D B. Biosynthesis and metabolic role of halomethanes in fungi and plants. In: Sigel H, Sigel A, editors. Metal ions in biological systems. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 345–388. [Google Scholar]
- 9.Harper D B, Buswell J A, Kennedy J T, Hamilton T G. Chloromethane, methyl donor in veratryl alcohol biosynthesis in Phanerochaete chrysosporium and other lignin-degrading fungi. Appl Environ Microbiol. 1990;56:3450–3457. doi: 10.1128/aem.56.11.3450-3457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper D B, Hamilton J T G. Biosynthesis of chloromethane in Phellinus pomaceus. J Gen Microbiol. 1988;134:2831–2839. [Google Scholar]
- 11.Harper D B, Hamilton J T G, Kennedy J T, McNally K J. Chloromethane, a novel methyl donor for biosynthesis of esters and anisoles in Phellinus pomaceus. Appl Environ Microbiol. 1989;55:1981–1989. doi: 10.1128/aem.55.8.1981-1989.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper D B, Kennedy J T. Effect of growth conditions on halomethane production by Phellinus species: biological and environmental implications. J Gen Microbiol. 1986;132:1231–1246. [Google Scholar]
- 13.Hutchinson S A. Biological activity of volatile fungal metabolites. Trans Br Mycol Soc. 1971;57:185–200. doi: 10.1038/211868a0. [DOI] [PubMed] [Google Scholar]
- 14.Itoh N, Tsujita M, Ando T, Hisatomi G, Higashi T. Formation and emission of monohalomethanes from marine algae. Phytochemistry. 1997;45:67–73. [Google Scholar]
- 15.James F, Nolte K D, Hanson A D. Purification and properties of S-adenosyl-l-methionine:l-methionine S-methyltransferase from Wollastonia biflora leaves. J Biol Chem. 1995;270:22344–22350. doi: 10.1074/jbc.270.38.22344. [DOI] [PubMed] [Google Scholar]
- 16.Khouri H E, De Luca V, Ibrahim R K. Enzymatic synthesis of polymethylated flavonols in Chrysosplenium americanum. Arch Biochem Biophys. 1988;265:1–7. doi: 10.1016/0003-9861(88)90364-5. [DOI] [PubMed] [Google Scholar]
- 17.Levitt J. Plant responses to environmental stress. II. New York, N.Y: Academic Press, Inc.; 1980. pp. 365–488. [Google Scholar]
- 18.Lovelock J E. Natural halocarbons in the air and in the sea. Nature. 1975;256:193–194. doi: 10.1038/256193a0. [DOI] [PubMed] [Google Scholar]
- 19.Manley S L, Dastoor M N. Methyl halide (CH3X) production from the giant kelp, Macrocystis, and estimates of global CH3X production by kelp. Limnol Oceanogr. 1987;32:709–715. [Google Scholar]
- 20.McNally K J, Hamilton T G, Harper D B. The methylation of benzoic and n-butyric acids by chloromethane in Phellinus pomaceus. J Gen Microbiol. 1990;136:1509–1515. [Google Scholar]
- 21.McNally K J, Harper D B. Methylation of phenols by chloromethane in the fungus Phellinus pomaceus. J Gen Microbiol. 1991;137:1029–1032. [Google Scholar]
- 22.Neugebauer J. Detergents: an overview. In: Deutscher M P, editor. Guide to protein purification. New York, N.Y: Academic Press, Inc.; 1990. pp. 239–277. [Google Scholar]
- 23.Pedersén M, Collen J, Abrahamsson K, Ekdahl A. Production of halocarbon from seaweeds: an oxidative stress reaction. Sci Mar. 1996;60:257–263. [Google Scholar]
- 24.Prather M J, Watson R T. Stratospheric ozone depletion and future levels of atmospheric chlorine and bromine. Nature. 1990;344:729–734. [Google Scholar]
- 25.Saini H S, Attieh J M, Hanson A D. Biosynthesis of halomethanes and methanethiol by higher plants via a novel methyltransferase reaction. Plant Cell Environ. 1995;18:1027–1033. [Google Scholar]
- 26.Varns J L. The release of methyl chloride from potato tubers. Am Potato J. 1982;59:593–604. [Google Scholar]
- 27.Wever R, Tromp M G M, Krenn B E, Marjani A, Van Tol M. Brominating activity of the seaweed Ascophyllum nodosum: impact on the biosphere. Environ Sci Technol. 1991;25:446–449. [Google Scholar]
- 28.Wuosmaa A M, Hager L P. Methyl chloride transferase: a carbocation route for biosynthesis of halometabolites. Science. 1990;249:160–162. doi: 10.1126/science.2371563. [DOI] [PubMed] [Google Scholar]
- 29.Zafiriou O C. Reaction of methyl halides with sea water and marine aerosols. J Mar Res. 1975;33:75–81. [Google Scholar]