Abstract
Background
Cabotegravir + rilpivirine (CAB+RPV) injections, the first complete long-acting (LA) antiretroviral therapy (ART) regimen, was approved by the FDA in January 2021 for ART-experienced people with HIV (PWH) who are virologically suppressed (viral load [VL] < 50 copies/mL). We assessed the virologic effectiveness of CAB+RPV LA among ART-experienced individuals with VL < 50 copies/mL at initiation in the first 2 years of use in the OPERA® Cohort, stratified by body mass index (BMI).
Methods
All ART-experienced adults with VL< 50 copies/mL at initiation who received ≥ 1 CAB+RPV LA injection for the first time between 21Jan2021 and 28Feb2023 were followed until 25Mar2023. Individuals on either monthly or every 2-month injection schedules were included. Discontinuation was defined as a regimen switch or 2 consecutive missed injections. VLs were monitored from first injection until CAB+RPV discontinuation, death, or study end. Confirmed virologic failure (CVF) was defined as 2 consecutive VLs ≥ 200 copies/mL or 1 VL ≥ 200 copies/mL followed by discontinuation. Results were stratified by BMI at first injection (< 30 vs. ≥ 30 kg/m2).
Results
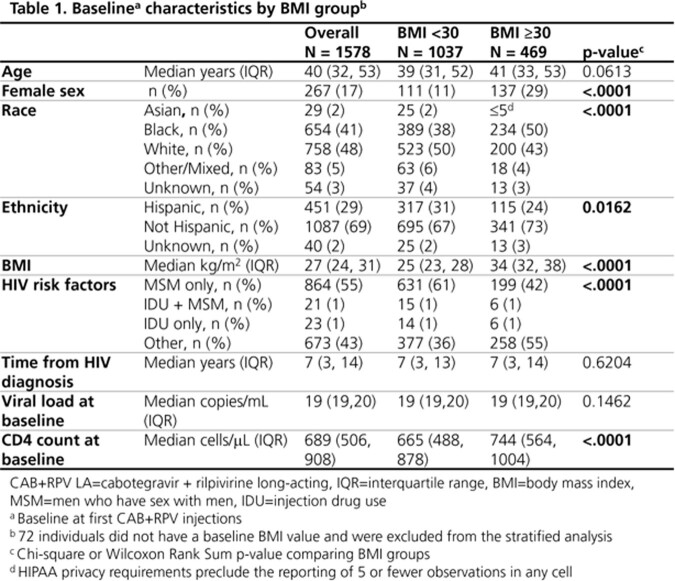
All 1843 PWH who received CAB+RPV injections were ART-experienced at start and 1578 (86%) had VL < 50 copies/mL at initiation. Of the 1578 suppressed, 267 (17%) were women, 654 (41%) were Black, 451 (29%) were Hispanic, and 469 (30%) had a BMI of ≥ 30; median age was 40 (IQR: 32, 53) years (Table 1). Among those with CAB+RPV dose available, 1297 (84%) remained on CAB+RPV LA over a median follow-up of 7.4 (IQR: 3.9, 10.9) months at study end. Among 1323 with VLs after first injection, the last VL measured was < 200 copies/mL in 99% (n=1305) and < 50 copies/mL in 94% (n=1237) (Table 2); all follow-up VLs were < 200 copies/mL in 96% (n=1272), and < 50 copies/mL in 84% (n=1115). Regardless of BMI, virologic suppression was maintained by ≥ 98% of individuals and only 1% experienced CVF over follow-up (Table 2).
Conclusion
In this real-world cohort of PWH in the United States who received CAB+RPV LA injections, observations from the first 2 years suggest that this regimen is effective among individuals virologically suppressed at initiation. Furthermore, the regimen is consistently effective among those with high BMI.
Disclosures
Michael Sension, MD, Gilead: Advisor/Consultant|Gilead: Honoraria|Viiv: Advisor/Consultant|Viiv: Grant/Research Support|Viiv: Honoraria Ricky K. Hsu, MD, Gilead Sciences: Advisor/Consultant|Gilead Sciences: Grant/Research Support|Gilead Sciences: Honoraria|Janssen: Advisor/Consultant|Janssen: Grant/Research Support|Janssen: Honoraria|Merck: Advisor/Consultant|Merck: Honoraria|ViiV Healthcare: Advisor/Consultant|ViiV Healthcare: Honoraria Jennifer S. Fusco, BS, Epividian, Inc.: Salary|Epividian, Inc.: Ownership Interest|Epividian, Inc.: Stocks/Bonds Laurence Brunet, PhD, Epividian, Inc.: Salary|Epividian, Inc.: Stocks/Bonds Gayathri Sridhar, MBBS, MPH, PhD, GlaxoSmithKline: Stocks/Bonds|ViiV Healthcare: Full Time Employee Vani Vannappagari, MBBS, MPH, PhD, GlaxoSmithKline: Stocks/Bonds|ViiV Healthcare: Employee Jean A. van Wyk, MBChB, MFPM, ViiV Healthcare Ltd: Stocks/Bonds Michael B. Wohlfeiler, JD, MD, AAHIVS, ViiV Healthcare: Serves as a PI on clinical trials, but does not receive personal compensation for this work (it goes directly to AIDS Healthcare Foundation) Gregory P. Fusco, MD, MPH, Epividian, Inc.: Board Member|Epividian, Inc.: Ownership Interest|Epividian, Inc.: Stocks/Bonds