Abstract
White rot fungi can oxidize high-molecular-weight polycyclic aromatic hydrocarbons (PAH) rapidly to polar metabolites, but only limited mineralization takes place. The objectives of this study were to determine if the polar metabolites can be readily mineralized by indigenous microflora from several inoculum sources, such as activated sludge, forest soils, and PAH-adapted sediment sludge, and to determine if such metabolites have decreased mutagenicity compared to the mutagenicity of the parent PAH. 14C-radiolabeled benzo[a]pyrene was subjected to oxidation by the white rot fungus Bjerkandera sp. strain BOS55. After 15 days, up to 8.5% of the [14C]benzo[a]pyrene was recovered as 14CO2 in fungal cultures, up to 73% was recovered as water-soluble metabolites, and only 4% remained soluble in dibutyl ether. Thin-layer chromatography analysis revealed that many polar fluorescent metabolites accumulated. Addition of indigenous microflora to fungal cultures with oxidized benzo[a]pyrene on day 15 resulted in an initially rapid increase in the level of 14CO2 recovery to a maximal value of 34% by the end of the experiments (>150 days), and the level of water-soluble label decreased to 16% of the initial level. In fungal cultures not inoculated with microflora, the level of 14CO2 recovery increased to 13.5%, while the level of recovery of water-soluble metabolites remained as high as 61%. No large differences in 14CO2 production were observed with several inocula, showing that some polar metabolites of fungal benzo[a]pyrene oxidation were readily degraded by indigenous microorganisms, while other metabolites were not. Of the inocula tested, only PAH-adapted sediment sludge was capable of directly mineralizing intact benzo[a]pyrene, albeit at a lower rate and to a lesser extent than the mineralization observed after combined treatment with white rot fungi and indigenous microflora. Fungal oxidation of benzo[a]pyrene resulted in rapid and almost complete elimination of its high mutagenic potential, as observed in the Salmonella typhimurium revertant test performed with strains TA100 and TA98. Moreover, no direct mutagenic metabolite could be detected during fungal oxidation. The remaining weak mutagenic activity of fungal cultures containing benzo[a]pyrene metabolites towards strain TA98 was further decreased by subsequent incubations with indigenous microflora.
Bioremediation of polycyclic aromatic hydrocarbon (PAH)-polluted soil is severely hampered by the low rate of degradation of the higher PAH, particularly the four- and five-ring PAH (6, 32). These higher PAH have very low water solubility and are often tightly bound to soil particles. This results in very low bioavailability for bacterial degradation. The observation that white rot fungi can oxidize PAH rapidly with their extracellular ligninolytic enzyme systems has therefore raised interest in the use of these organisms for bioremediation of PAH-polluted soils (3, 9). Although PAHs are extensively oxidized by white rot fungi, the degree of mineralization to CO2 is always limited. In various studies evaluating the degradation of the potent carcinogen benzo[a]pyrene by several white rot fungal species, from 0.17 to 19% of the radiolabeled PAH was recovered as 14CO2 (4, 5, 26). The major products of the oxidation were both nonpolar and polar metabolites. The accumulation of such metabolites could be a reason for concern, since mammalian and fungal monooxygenases can oxidize benzo[a]pyrene to epoxides and dihydrodiols, which are very potent carcinogens (28, 29). However, peroxidase-mediated extracellular oxidation of benzo[a]pyrene in cultures of white rot fungi results initially in benzo[a]pyrenediones, which show weak mutagenic activity (29). These primary metabolites are rapidly oxidized further to unidentified metabolites by Phanerochaete laevis and Phanerochaete chrysosporium (5, 26). Furthermore, the oxidized benzo[a]pyrene metabolites have a higher aqueous solubility. Since the low bioavailability of PAH is a major rate-limiting factor in the degradation of these compounds by bacteria (27, 31), the increased bioavailability of oxidized PAH metabolites suggests that these compounds can be more easily mineralized by bacteria.
The aim of this study was to investigate the degradation and mineralization of the five-ring PAH benzo[a]pyrene by the white rot fungus Bjerkandera sp. strain BOS55 and the subsequent mineralization of the metabolites by natural mixed cultures of microorganisms. During the oxidation and mineralization of benzo[a]pyrene, the decrease in the mutagenicity of the metabolites was monitored. The white rot fungal strain Bjerkandera sp. strain BOS55 was used because of its outstanding ability to rapidly oxidize PAH (8, 19) and because extensive information concerning its physiology is available (7, 18, 20, 22, 23).
MATERIALS AND METHODS
Organisms used.
The white rot fungus Bjerkandera sp. strain BOS55 (ATCC 90940) was maintained on peptone-yeast extract agar slants (17) at 10°C. Malt extract plates were inoculated 5 days prior to the experiments, and fungal cultures were inoculated with an agar plug as described previously (16).
Natural mixed populations of microorganisms were retrieved from different sources. Activated sludge was obtained from a municipal wastewater treatment plant (Bennekom, The Netherlands) (concentration of volatile suspended solids, 5 g liter−1) and was used without further treatment. Samples of two soils were collected from forests from the litter layer (forest 1) (pH 4.5) and from a decomposed beech log (forest 2) (pH 4.8). The forest soils were diluted 1:1 (wt/wt) with water to prepare slurries and were filtered through cheesecloth. A 50-year-old PAH-polluted sediment sludge dredged from Rotterdam Harbor was diluted 1:10 in buffer (pH 7) containing 0.02% yeast extract and incubated for 10 days at 21°C in shake flasks before it was used as an inoculum. An enrichment culture on 2,2′-diphenic acid, an intermediate of phenanthrene oxidation by P. chrysosporium (11), was prepared from activated sludge (from a municipal wastewater treatment plant in Delft, The Netherlands) containing four different bacteria (as determined on the basis of morphology on yeast-glucose agar plates). This enrichment culture was incubated for 10 days in a pH 7 buffer containing 1 g of 2,2′-diphenic acid liter−1 at 21°C before it was used as an inoculum. In all cases, approximately 2 × 108 to 5 × 108 CFU was added to each fungal culture on day 15 in a volume of 5 to 10 ml.
Culture conditions.
Fungal cultures were cultivated on the standard high-nitrogen, manganese-free medium modified from the medium described by Tien and Kirk (30). This medium contained 33 mM N as mycological peptone (5 g liter−1) and 10 g of glucose liter−1 buffered at pH 6 with 40 mM phosphate. Manganese-containing medium was obtained by adding 66 μM Mn (as MnSO4). The media were autoclaved for 30 min at 115°C. After sterilization, 10 ml of a filter-sterilized thiamine solution (200 mg liter−1) was added. Sterile 250-ml serum bottles containing 5 ml of medium were loosely capped to facilitate aeration during incubation for 6 days at 30°C. Preparations containing bacteria were incubated in a shaking water bath (80 rpm) at 21°C.
Additions to fungal cultures.
Benzo[a]pyrene was added on day 6 as a 100×-concentrated stock solution in acetone, which resulted in a final benzo[a]pyrene concentration of 20 mg liter−1 and an acetone concentration of 1%. Glucose (5 g liter−1) and Tween 80 (2.5 g liter−1) were added as 100×-concentrated stock solutions in water. The bottles were then tightly capped, and an oxygen atmosphere was supplied by flushing the bottles for 5 min with pure oxygen. This was repeated once every 2 or 3 days.
The microbial inocula from soils, sludge, or sediments were added 15 days later, and the bottles were placed in a shaking water bath at 21°C. The bottles were tightly capped, and each headspace was flushed once every 2 or 3 days with air. Control experiments indicated that the spent fungal medium was not toxic towards the microorganisms; in fact, the levels of CO2 production and plate counts increased when spent medium was added, indicating that organic matter in the spent fungal culture medium was metabolized.
[14C]benzo[a]pyrene experiments.
In the 14C experiments, 80,000 to 100,000 dpm of [7,10-14C]benzo[a]pyrene (specific activity, 2.24 MBq μmol−1; Amersham, Amersham, United Kingdom) was added to each culture together with 20 mg of benzo[a]pyrene liter−1. Production of 14CO2 was measured by flushing the headspace once every 2 or 3 days for 8 min through a three-stage trap in which each stage contained 6 ml of 2 M NaOH. The 14CO2 production values for triplicate cultures were pooled. Control experiments revealed a CO2-trapping efficiency of more than 99%. For detection of volatile metabolites, a fourth trap containing acetonitrile was added. The total recovery of label remaining in the culture medium was measured by adding 3 volumes of acetone to each bottle; the bottles were then shaken for 1 h. The recovery of benzo[a]pyrene in autoclaved fungal controls, as determined by a high-performance liquid chromatography (HPLC) analysis of unlabeled benzo[a]pyrene, was more than 98% when this method was used. The distribution of label remaining in the culture medium between the water-soluble and organic compound-soluble phases was measured by adding 10 ml of dibutyl ether. After dibutyl ether was added, the cultures were vigorously shaken for 1 h. After phase separation, the amount of label in both the water phase and the solvent phase was measured. The amount of water-soluble label was sometimes also measured quickly by filtering the culture fluids through hydrophilic Schleicher & Schuell type FP 030/3 0.2-μm-pore-size filters, which resulted in levels of recovery that were 5 to 10% higher than those obtained after dibutyl ether extraction. The amount of label associated with fungal biomass was measured by incubating acetone-extracted fungal biomass with Soluene (Packard). One milliliter of the partially homogenized biomass was then transferred to a microvial with 5 ml of scintillation cocktail. Quenching by the biomass was corrected by adding a known amount of label to control vials with and without equal amounts of Soluene-treated biomass. All samples (volume, up to 1 ml) were added to 5 ml of scintillation cocktail (Ultima-gold; Packard) in microvials, and the radioactivity was measured with a liquid scintillation analyzer (model Tri-Carb 1600 TR; Packard) with appropriate controls.
Salmonella typhimurium revertant test (Ames test).
To monitor the mutagenic potential of benzo[a]pyrene metabolites, the Salmonella typhimurium revertant plate test, first described by Ames et al. (1), was used. Experiments were performed basically by using the plate incorporation test described by Maron and Ames (21). Both strain TA98 for point mutations and strain TA100 for frameshift mutations were used. Overnight cultures were inoculated directly from a −80°C stock, and the genotypes were tested after preparation of the −80°C stock. To mimic liver biotransformation, a rat liver homogenate (S9-mix) (Boehringer, Mannheim, Germany) from Araclor-induced rats was used. Benzo[a]pyrene dissolved in acetone was used as a positive control in experiments performed with S-9 mix, and 4-nitroquinoline-N-oxide was used as a positive control in experiments performed without S-9 mix. A preincubation step (10 min, 37°C) included in initial tests resulted in no significant increase in the mutagenic response and was therefore omitted in the subsequent experiments.
The culture fluids were separated from the fungal biomass by filtration through cheesecloth. The biomass in samples containing the indigenous microflora (which contained no insoluble benzo[a]pyrene) was removed by filtration through a Schleicher & Schuell type FP 030/2 0.45-μm-pore-size filter. All samples were treated aseptically and were boiled for 5 min before use. Both 100-μl samples and 400-μl samples were used, and the data shown below were obtained with 400-μl samples. Inoculated plates were incubated at 37°C for 48 h. The samples taken at the start of the benzo[a]pyrene incubation had relatively low mutagenic activities compared to the mutagenic activities of the benzo[a]pyrene standards (they had 40 to 50% of the expected response). This was due to retention of large benzo[a]pyrene precipitates in the filtration step and was not due to adsorption of benzo[a]pyrene to the fungal biomass. This was of no concern, since we were mainly interested in the mutagenic activity of the free available water-soluble benzo[a]pyrene metabolites in the extracellular culture fluids, which were not retained by the filtration step.
Analytical methods.
For the analysis of residual unlabeled benzo[a]pyrene, triplicate cultures were sacrificed by adding 3 volumes of acetone to each bottle. The bottles were then sealed with Teflon liners, and shaken for 1 h, and samples were centrifuged in an Eppendorf centrifuge (10 min, 13,000 × g). The analysis was conducted with a HPLC equipped with a diode array detector as described previously (8). The range of benzo[a]pyrene concentrations was 0 to 5 mg liter−1, the detection limit was around 0.05 mg liter−1, and the reproducibility of the HPLC was more than 99.5%. Fungal cultures were used to monitor abiotic losses of benzo[a]pyrene. The abiotic losses never exceeded 2% of the benzo[a]pyrene added.
For extraction of the polar benzo[a]pyrene metabolites from the water phase, culture fluid was first separated from biomass by filtration through cheesecloth. The filtrate was then acidified with HCl, saturated with NaCl, and extracted six times with a 0.5 volume of ethyl acetate. The ethyl acetate was evaporated at 40°C under a nitrogen atmosphere, and the residue was dissolved in acetone. The extracts were analyzed on thin-layer chromatography (TLC) plates (Silica Gel F254; Merck) developed with chloroform-methanol (97:3, 75:25, and 65:35), petroleum ether 40-60–ethyl acetate (2:1), and ethyl acetate-methanol (65:35). The metabolites were detected by UV illumination and by autoradiography on Kodak X-Omat film.
The numbers of CFU were determined on yeast-glucose plates.
Chemicals.
All of the chemicals used were commercially available and were analytical grade or higher. The solvents used for TLC were chromatography grade. All chemicals were used without further treatment.
Statistical procedures.
Unless indicated otherwise, the data shown are the means and standard deviations of values obtained from triplicate cultures.
RESULTS
Degradation of benzo[a]pyrene by the white rot fungus Bjerkandera sp. strain BOS55.
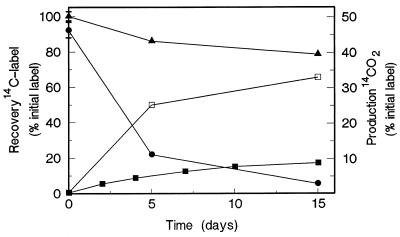
In the first experiments, degradation of benzo[a]pyrene was monitored in both Mn-sufficient and Mn-deficient cultures in the presence of the surfactant Tween 80, since the presence of Mn has been shown to affect PAH oxidation by Bjerkandera sp. strain BOS55 (20). For Mn-sufficient cultures (Fig. 1), the level of recovery of 14CO2 after 15 days was 8.4% of the initial amount of label added; in manganese-deficient cultures a slightly lower level of recovery of 14CO2 (7.9%) was observed (results not shown). The distribution of the 14C label in the culture fluid in the hydrophobic solvent and water phases was monitored in Mn-sufficient cultures (Fig. 1). The amount of 14C label extractable with dibutyl ether decreased very rapidly, while water-soluble polar metabolites quickly accumulated. After 15 days of incubation, the major products of fungal benzo[a]pyrene oxidation were water-soluble products, which accounted for 68% of the initial amount of label, as shown in Fig. 1. In separate experiments, the water-soluble products at day 15 accounted for 67 to 73% of the initial amount of label. HPLC analysis of simultaneously grown cultures containing unlabeled benzo[a]pyrene showed that benzo[a]pyrene (20 mg liter−1) was eliminated for 91, 96, and 100% after 1, 5, and 15 days, respectively. The total amount of 14C label recovered in the gas and water phases decreased slightly with time, whereas the amount of label associated with the fungal biomass increased from zero at the start of the experiment to around 8% at day 15. Volatile compounds other than CO2 were not observed. In the Mn-deficient cultures, polar water-soluble products were also found to be the major products, accounting for 70% of the label (results not shown). After 15 days, no 14CO2 production was detected in the autoclaved fungal controls; the level of recovery of label with acetone was 100%, and only 0.1% of the label was recovered from the water phase.
FIG. 1.
Oxidation of [14C]benzo[a]pyrene by Mn-sufficient fungal cultures. Zero time was the time when benzo[a]pyrene (20 mg liter−1) was added to 6-day-old fungal cultures. Symbols: • and □, distribution of 14C label from benzo[a]pyrene in the dibutyl ether and water phases, respectively; ▴, total recovery of label in the culture medium after addition of acetone; ■, mineralization to 14CO2.
Characterization benzo[a]pyrene metabolites.
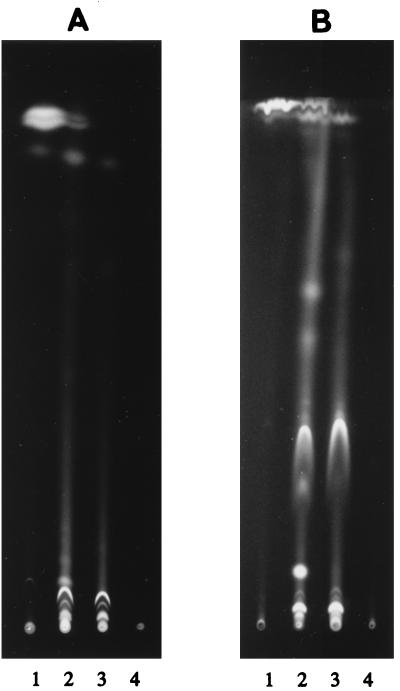
The spectrum of oxidized benzo[a]pyrene products produced by Mn-sufficient cultures of Bjerkandera sp. strain BOS55 was investigated further. When the ethyl acetate-extracted metabolites were examined by using TLC with nonpolar eluents, such as petroleum ether 40-60–ethyl acetate (3:1) (results not shown) and chloroform-methanol (97:3), most of the metabolites remained in the origin, while benzo[a]pyrene exhibited significant migration (Fig. 2A). A more polar eluent, such as chloroform-methanol (75:25), was required for any significant migration of the polar metabolites (Fig. 2B). Use of the eluents chloroform-methanol (65:35) and ethyl acetate-methanol (65:35) resulted in migration of all of the metabolites, but the resolution was very poor (results not shown). Clearly, extensive oxidation of benzo[a]pyrene to polar metabolites took place during the first day of incubation. After 15 days no intact benzo[a]pyrene was detected, and some metabolites present on day 1 were apparently oxidized further. Ethyl acetate extracts of 15-day-old control fungal cultures (without added benzo[a]pyrene) produced only two faint blue spots under UV light (not visible in Fig. 2), which were tentatively identified as the secondary metabolites veratryl alcohol and veratraldehyde. Identical benzo[a]pyrene metabolite profiles were observed after TLC with an autoradiogram of 14C-labeled benzo[a]pyrene metabolites (results not shown). Acidification of the culture fluids with HCl was necessary for a high level of recovery of the metabolites by ethyl acetate extraction. Still, the extraction efficiency decreased during the incubation period; around 15% of the water-soluble label was not extractable after 15 days. Extensive degradation of benzo[a]pyrene and accumulation of polar metabolites were also observed when benzo[a]pyrene was incubated for 1 day in extracellular culture fluids from 6-day-old fungal cultures (results not shown).
FIG. 2.
TLC profiles of benzo[a]pyrene metabolites during incubation in fungal cultures, as visualized by UV illumination. (A) Preparation developed twice with chloroform-methanol (97:3). (B) Metabolites which were retained near the origin in panel A after an additional run with chloroform-methanol (75:25). Lane 1, zero time; lanes 2 and 3, 1 and 15 days after addition of benzo[a]pyrene, respectively; lane 4, control fungal culture that did not receive benzo[a]pyrene.
Mineralization of benzo[a]pyrene by Bjerkandera sp. strain BOS55 and indigenous microflora.
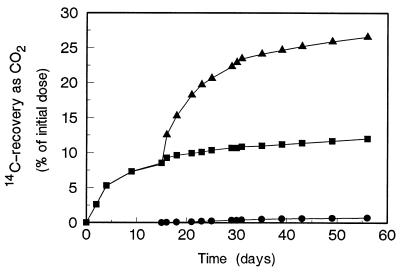
Mineralization of benzo[a]pyrene metabolites by natural mixed cultures of microorganisms not previously adapted to PAH from four different inoculum sources was examined. The inocula included activated sludge, two acidic forest soils, and an enrichment culture grown on 2,2′-diphenic acid. The results of this kind of experiment are shown in Fig. 3; in this experiment [14C]benzo[a]pyrene was oxidized for 15 days by Mn-sufficient cultures of Bjerkandera sp. strain BOS55 before activated sludge was added to the fungal cultures. Just before the activated sludge was added, the fungal cultures had already mineralized 8% of the benzo[a]pyrene. Just after the activated sludge was added, the level of mineralization rapidly increased to 20% in a few days, and thereafter it increased slowly to 27% by day 56 of the experiment. Adding 5 ml of fresh activated sludge and 0.02% yeast extract at this point had no effect on 14CO2 production. In the control fungal cultures that did not receive activated sludge, the level of mineralization was only 12% on day 56. Adding autoclaved sludge to the fungal cultures had no effect on 14CO2 production (data not shown).
FIG. 3.
Mineralization of [14C]benzo[a]pyrene by fungal cultures, by activated sludge, and by both. Zero time was the time when benzo[a]pyrene was added to the 6-day-old fungal cultures; at day 15 activated sludge was added. Symbols: ■, fungus; ▴, fungus plus activated sludge; •, dead fungus plus activated sludge plus intact [14C]benzo[a]pyrene.
Similar results were obtained with the other natural inocula, as shown in Table 1. The maximum extent of benzo[a]pyrene mineralization was somewhat lower when the 2,2′-diphenic acid enrichment culture was used.
TABLE 1.
Effect of adding mixed cultures to fungal cultures with oxidized [14C]benzo[a]pyrene at day 15 on the amount of label recovered from [14C]benzo[a]pyrene as 14CO2 and watersoluble metabolites and the total amount of label recovered in culture medium with acetone
| Treatment | % of label recovered in 14CO2
|
% of label recovered on day 215
|
||
|---|---|---|---|---|
| Day 56 | Day 215 | With acetone | In water phasea | |
| Fungus | 12.0 | 13.5 | 66 | 61 |
| Fungus + activated sludge | 26.6 | 28.7 | 39 | 33 |
| Fungus + forest 1 soil | 25.8 | 32.0 | 25 | 18 |
| Fungus + forest 2 soil | 26.9 | 34.0 | 24 | 16 |
| Fungus + 2,2′-diphenic acid enrichment culture | 20.4 | 21.7 | 45 | 40 |
As determined by filtration through a hydrophilic filter. Duplicate measurements were obtained.
In control cultures in which the inocula were directly incubated with intact benzo[a]pyrene in the presence or absence of an autoclaved fungal culture, very low levels (only 0.4 to 1%) of benzo[a]pyrene mineralization were observed at the end of the experiment, as shown in Fig. 3 for activated sludge in the presence of an autoclaved fungal culture. Also, incubation of benzo[a]pyrene with the various inocula resulted in no significant decreases in the benzo[a]pyrene concentration.
The levels of recovery of water-soluble label in the culture fluids were also monitored. On day 15 of the experiment, 72% of the 14C label was present in water-soluble metabolites in the fungal cultures. In the cultures not receiving indigenous microflora, 69% of the label was still recovered in water-soluble metabolites 29 days later. On the other hand, only 48 and 37% of the 14C-labeled water-soluble metabolites remained in the cultures supplied with activated sludge and forest soils, respectively. As shown in Table 1, the experiment was extended to day 215, and at that time the 14CO2 production rate was virtually zero. The levels of recovery in water-soluble metabolites in the fungal cultures were still as high as 61%, whereas in the indigenous microflora-inoculated cultures the levels of recovery were much lower. In the culture with the highest level of recovery of 14CO2, 34% (forest 2), only 16% of the label was recovered in water-soluble metabolites at the end of the experiment. In all cultures the total levels of recovery of label in the culture fluids by acetone extraction were slightly higher than the levels of recovery in water-soluble metabolites alone. Between 1 and 2% of the label was extractable with dibutyl ether (data not shown). The amount of label associated with the biomass was not determined in this experiment; by assuming a normal conversion of the metabolites to CO2 (60%) and biomass (40%), mass balances ranging from 80 to 90% were obtained.
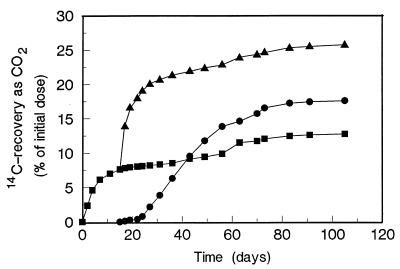
Mineralization of benzo[a]pyrene metabolites by a natural mixed culture previously adapted to PAH pollution was also examined in a similar experiment. As shown in Fig. 4, addition of this PAH-adapted culture had an effect on 14CO2 production similar to the effect of the addition of nonadapted microorganisms (Fig. 3). A rapid increase in 14CO2 production was observed during the first few days, and then the rate slowed down, resulting in a total level of mineralization of 26% by day 105. In this experiment, the level of recovery in water-soluble metabolites was 46%.
FIG. 4.
Mineralization of [14C]benzo[a]pyrene by fungal cultures, by PAH adapted sludge, and by both. Zero time was the time when benzo[a]pyrene was added to the 6-day-old fungal cultures; at day 15 activated sludge was added. Symbols: ■, fungus; ▴, fungus plus PAH-adapted sludge; •, dead fungus plus PAH-adapted sludge plus intact [14C]benzo[a]pyrene.
In contrast to the results obtained with the nonadapted mixed cultures, incubation of intact benzo[a]pyrene with the PAH-adapted sludge resulted in fast mineralization after a lag period of 5 days. However, mineralization by cultures stopped at a lower level (17%) than mineralization of benzo[a]pyrene by the fungus and adapted microflora combined. On day 105, 3% of the label was recovered in water-soluble metabolites in these cultures, and in parallel experiments without label HPLC analysis showed that 60% of the initial amount of benzo[a]pyrene was still present in these cultures.
Mutagenicity studies.
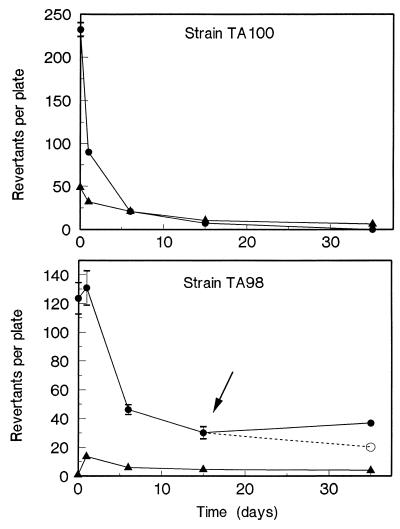
Ames tests were performed to monitor the changes in the mutagenic potential of benzo[a]pyrene during its oxidation and mineralization. In the experiments performed with S. typhimurium TA100, no increase in the number of revertants was observed in the fungal culture broth itself in the absence of the rat liver activation mixture (S-9 mix) compared to the spontaneous number of revertants (65 revertants per plate). However, the fungal culture broth did cause a small increase (10 to 20 revertants) in the presence of S-9 mix throughout the experiment compared to the number of spontaneous revertants in the presence of S-9 mix (72 revertants per plate). Addition of benzo[a]pyrene (20 mg liter−1) to the fungal cultures resulted in high mutagenic activity only when S-9 mix was added, and this mutagenic activity was reduced to background levels within 15 days when cultures were incubated with Bjerkandera sp. strain BOS55 (Fig. 5). No decrease in the mutagenic potential of benzo[a]pyrene was caused by autoclaved fungal cultures (results not shown).
FIG. 5.
Mutagenic activity of benzo[a]pyrene and benzo[a]pyrene metabolites towards S. typhimurium TA100 and TA98. At zero time benzo[a]pyrene (20 mg liter−1) was added to 6-day-old Mn-sufficient fungal cultures. At different times samples were taken and tested. The number of spontaneous revertants in the controls was subtracted from the values shown. The arrow indicates when activated sludge was added. Symbols: •, fungal cultures plus benzo[a]pyrene plus S-9 mix; ▴, fungal cultures plus benzo[a]pyrene; ○, effect of activated sludge addition.
In the case of strain TA98, no increase in the number of revertants was observed in the fungal culture broth compared to the average number of spontaneous revertants (14 revertants per plate). In the presence of S-9 mix, the fungal culture broth caused an increase of 15 to 20 revertants per plate throughout the experiment compared to the number of spontaneous revertants in the presence of S-9 mix (28 revertants per plate). Again, benzo[a]pyrene-containing cultures displayed high mutagenic activity only in the presence of S-9 mix. Although this high mutagenic activity was quickly reduced by fungal activity, it remained slightly higher than the mutagenic activity of the fungal culture itself. Addition of activated sludge on day 15 to the cultures containing oxidized benzo[a]pyrene metabolites further reduced the number of revertants in the presence of S-9 mix. The addition of activated sludge reduced the number of revertants in the fungal culture broth in the presence of S-9 mix to background levels (results not shown).
DISCUSSION
In this study, mineralization of the recalcitrant pollutant benzo[a]pyrene was investigated by successively incubating [14C]benzo[a]pyrene with the white rot fungus Bjerkandera sp. strain BOS55 and natural mixed cultures of microorganisms from soil, sediment, and sludge. Bjerkandera sp. strain BOS55 can oxidize PAH very rapidly in high-nitrogen cultures supplied with adequate H2O2 for maximal peroxidase activity and the surfactant Tween 80 to improve PAH bioavailability. Under such conditions, benzo[a]pyrene supplied at a concentration of 50 mg liter−1 was oxidized at rates of up to 450 mg liter−1 day−1 (19). In the experiments performed here with 14C-labeled benzo[a]pyrene, we found that with a level of recovery of 8% 14CO2 at an initial mineralization rate of 0.3 mg liter−1 day−1, water-soluble metabolites accounting for up to 73% of the label accumulated after 15 days. These results correlate well with the results of other studies in which oxidation of PAH was examined, although the results depend strongly on the white rot species and strain used. For [14C]benzo[a]pyrene, levels of 14CO2 recovery between 0.17 and 19% have been reported in studies with different species (4, 5, 26). The initial level of accumulation of water-soluble metabolites observed in this study is high compared to the levels found in other studies. This difference can be attributed to both the species used and the definition of the term water soluble.
The metabolites of peroxidase-mediated benzo[a]pyrene oxidation have not been identified yet. The initial oxidation products have been identified as benzo[a]pyrenequinones (10), but these quinones are rapidly further oxidized to more water-soluble products with unknown structures by cultures of P. laevis and P. chrysosporium (4, 5, 26). Peroxidase-mediated oxidation of the low-molecular-weight PAH, such as anthracene and phenanthrene, also results in quinones. Depending on the strain used, anthraquinones are either accumulated as metabolites (2, 8) or are further oxidized to unidentified products (2, 8) or phthalate (12). Oxidation of phenanthrenequinone to 2,2′-diphenic acid by ligninolytic cultures of P. chrysosporium has been reported (11). The observation in this study that all benzo[a]pyrene metabolites detected by autoradiography also were highly fluorescent under UV light indicates that the polyaromatic structure of benzo[a]pyrene was not completely destroyed. The high water solubility of these metabolites can presumably be attributed to the presence of carboxyl and/or hydroxyl groups. The presence of carboxyl groups is suggested because the acidification of the culture media greatly increased the efficiency of extraction of these metabolites with ethyl acetate.
The interest in white rot fungi for PAH degradation is mainly fueled by the slow breakdown of PAH by bacteria. It has been reported many times that low bioavailability of PAH is the main factor limiting bacterial PAH degradation (27, 31), and until now, the attempts to improve PAH bioavailability and degradability by using surfactants have not been very successful (25). Oxidation of PAH by white rot fungi to more water-soluble products with greater bioavailability could therefore result in rates of mineralization of these metabolites by bacteria higher than the rates of mineralization of the parent PAH compounds. A study performed with the three-ring PAH anthracene has confirmed that all known oxidation products of this compound are mineralized by activated sludge more rapidly than anthracene itself is mineralized (24).
Addition of undefined microbial inocula, irrespective of the source, to oxidized benzo[a]pyrene metabolites clearly resulted in initially rapid mineralization rates, comparable to 1.0 mg of benzo[a]pyrene liter−1 day−1. Benzo[a]pyrene not previously subjected to fungal oxidation was not mineralized at all by the inocula not adapted to PAH, whereas PAH-adapted sludge mineralized intact benzo[a]pyrene at a rate of only 0.1 mg of benzo[a]pyrene liter−1 day−1. This confirms that fungal preoxidation of PAH increases the rate of mineralization by bacteria. A similar synergistic effect of a combination of white rot fungi and soil microorganisms has also been observed for pyrene mineralization by Pleurotus sp. and Dichomitus squalens (14). Andersson and Henrysson (2) showed that the dead-end metabolites of both anthracene oxidation and benzo[a]anthracene oxidation by P. chrysosporium were slowly further degraded in the presence of nonadapted soil microorganisms.
By the end of the successive mineralization experiments, the maximum yields of 14CO2 were between 39 and 47%, and at least 16% of the initial label remained in water-soluble metabolites. The lack of mineralization of this fraction could not be attributed to toxicity of the metabolites or a lack of trace elements or vitamins. This suggests that not all of the fungus-oxidized metabolites were easily mineralized by indigenous microflora. If these metabolites are cometabolically degraded by bacteria, as has been described for higher PAH, such as benzo[a]pyrene and dibenzo[a,h]anthracene (13, 15), the absence of a suitable cosubstrate could explain the lack of complete mineralization. However, addition of glucose, autoclaved spent fungal medium, or activated sludge as a cosubstrate did not have any significant effect. This suggests that the remaining metabolites have structures that are poorly degradable.
Degradation of PAH like benzo[a]pyrene is desired since oxidation by eukaryotic monooxygenases can result in metabolites with high carcinogenic activity (28). The lack of complete mineralization of benzo[a]pyrene after sequential treatment by fungi and bacteria made it necessary to monitor the mutagenic activity during this treatment. The high mutagenic activity of S-9 mix-activated benzo[a]pyrene towards S. typhimurium TA100 and TA98 rapidly decreased during fungal incubation without any significant accumulation of direct or indirect mutagens. This suggests that either the intracellular monooxygenases were not involved in the oxidation of benzo[a]pyrene or their oxidation products did not accumulate. In any case, involvement of the extracellular peroxidases, which previously have been shown to rapidly oxidize anthracene and benzo[a]pyrene (19), was in this study demonstrated by the extensive oxidation of benzo[a]pyrene in the extracellular culture fluids of 6-day-old cultures of Bjerkandera sp. strain BOS55.
This research showed that benzo[a]pyrene is quickly oxidized into polar, water-soluble compounds by the white rot fungus Bjerkandera sp. strain BOS55. Most of these metabolites could be mineralized by non-PAH-adapted microbial indigenous communities under aerobic conditions. The lack of complete mineralization is not a serious setback for the use of white rot fungal techniques in PAH bioremediation, since the highly mutagenic potential of the parent compound was eliminated.
ACKNOWLEDGMENT
This work was funded by an IOP project from Senter, an agency of the Dutch Ministry of Economics.
REFERENCES
- 1.Ames B A, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 2.Andersson B E, Henrysson T. Accumulation and degradation of dead-end metabolites during treatment of soil contaminated with polycyclic aromatic hydrocarbons with five strains of white-rot fungi. Appl Microbiol Biotechnol. 1996;46:647–652. [Google Scholar]
- 3.Aust S D. Degradation of environmental pollutants by Phanerochaete chrysosporium. Microbiol Ecol. 1990;20:197–209. doi: 10.1007/BF02543877. [DOI] [PubMed] [Google Scholar]
- 4.Bezalel L, Hadar Y, Cerniglia C E. Mineralization of polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:292–295. doi: 10.1128/aem.62.1.292-295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogan B W, Lamar R T. Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol. 1996;62:1597–1603. doi: 10.1128/aem.62.5.1597-1603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossert I D, Bartha R. Structure-biodegradability relationships of polycyclic aromatic hydrocarbons in soil. Bull Environ Contam Toxicol. 1986;37:490–495. doi: 10.1007/BF01607793. [DOI] [PubMed] [Google Scholar]
- 7.De Jong E, Field J A, de Bont J A M. Evidence for a new extracellular peroxidase: manganese inhibited peroxidase from the white-rot fungus Bjerkandera sp. strain BOS55. FEBS Lett. 1992;299:107–110. doi: 10.1016/0014-5793(92)80111-s. [DOI] [PubMed] [Google Scholar]
- 8.Field J A, de Jong E, Feijoo-Costa G, de Bont J A M. Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white-rot fungi. Appl Environ Microbiol. 1992;58:2219–2226. doi: 10.1128/aem.58.7.2219-2226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field J A, de Jong E, Feijoo Costa G, de Bont J A M. Screening for ligninolytic fungi applicable to the biodegradation of xenobiotics. Trends Biotechnol. 1993;11:44–49. [Google Scholar]
- 10.Haemmerli S D. Lignin peroxidase and the ligninolytic system of Phanerochaete chrysosporium. Ph.D. dissertation. Zurich, Switzerland: Swiss Federal Institute of Technology; 1988. [Google Scholar]
- 11.Hammel K E, Gai W Z, Green B, Moen M A. Oxidative degradation of phenanthrene by the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:1832–1838. doi: 10.1128/aem.58.6.1832-1838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammel K E, Green B, Gai W Z. Ring fission of anthracene by a eukaryote. Proc Natl Acad Sci USA. 1991;88:10605–10608. doi: 10.1073/pnas.88.23.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitkamp M A, Cerniglia C E. Effects of chemical structure and exposure on the microbial degradation of polycyclic aromatic hydrocarbons in freshwater and estuarine ecosystems. Environ Toxicol Chem. 1987;6:535–546. [Google Scholar]
- 14.In der Wiesche C, Martens R, Zadrazil F. Two-step degradation of pyrene by white-rot fungi and soil microorganisms. Appl Microbiol Biotechnol. 1996;46:653–659. doi: 10.1007/s002530050876. [DOI] [PubMed] [Google Scholar]
- 15.Juhasz A L, Britz M L, Stanley G A. Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a,h]anthracene by Burkholderia cepacia. J Appl Microbiol. 1997;83:189–198. [Google Scholar]
- 16.Kaal E E J, de Jong E, Field J A. Stimulation of ligninolytic peroxidase activity by nitrogen nutrients in the white rot fungus Bjerkandera sp. strain BOS55. Appl Environ Microbiol. 1993;59:4031–4036. doi: 10.1128/aem.59.12.4031-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura Y, Asada Y, Kuwahara M. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl Microbiol Biotechnol. 1990;32:436–442. doi: 10.1007/BF00903779. [DOI] [PubMed] [Google Scholar]
- 18.Kotterman M J J, Heessels E, de Jong E, Field J A. The physiology of anthracene biodegradation by the white rot fungus Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol. 1994;42:179–186. [Google Scholar]
- 19.Kotterman M J J, Rietberg H-J, Hage A, Field J A. Polycyclic aromatic hydrocarbon oxidation by the white-rot fungus Bjerkandera sp. strain BOS55 in the presence of nonionic surfactants. Biotechnol Bioeng. 1998;57:220–227. [PubMed] [Google Scholar]
- 20.Kotterman M J J, Wasseveld R A, Field J A. Hydrogen peroxide as a limiting factor in xenobiotic compound oxidation by nitrogen-sufficient cultures of Bjerkandera sp. strain BOS55 overproducing peroxidases. Appl Environ Microbiol. 1996;62:880–885. doi: 10.1128/aem.62.3.880-885.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron D M, Ames B R. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 22.Mester T, de Jong E, Field J A. Manganese regulation of veratryl alcohol in white rot fungi and its indirect effect on lignin peroxidase. Appl Environ Microbiol. 1995;61:1881–1887. doi: 10.1128/aem.61.5.1881-1887.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mester T, Pena M, Field J A. Nutrient regulation of extracellular peroxidases in the white rot fungus Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol. 1996;44:778–784. [Google Scholar]
- 24.Meulenberg R, Rijnaarts H H M, Doddema H J, Field J A. Partially oxidized polycyclic aromatic hydrocarbons show an increased bioavailability and biodegradability. FEMS Microbiol Lett. 1997;152:45–49. doi: 10.1111/j.1574-6968.1997.tb10407.x. [DOI] [PubMed] [Google Scholar]
- 25.Rouse J D, Sabatini D A, Suflita J M, Harwell J H. Influence of surfactants on microbial degradation of organic compounds. Crit Rev Environ Sci Technol. 1994;24:325–370. [Google Scholar]
- 26.Sanglard D, Leisola S A, Fiechter A. Role of extracellular ligninases in biodegradation of benzo[a]pyrene by Phanerochaete chrysosporium. Enzyme Microb Technol. 1986;8:209–212. [Google Scholar]
- 27.Stucki G, Alexander M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl Environ Microbiol. 1987;53:292–297. doi: 10.1128/aem.53.2.292-297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland J B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992;9:53–62. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- 29.Thakker D R, Yagi H, Levin W, Wood A W, Conney A H, Jerina D M. Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogens. In: Anders M W, editor. Bioactivation of foreign compounds. Orlando, Fla: Academic Press; 1985. pp. 177–242. [Google Scholar]
- 30.Tien M, Kirk T K. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161B:238–248. [Google Scholar]
- 31.Volkering F, Breure A M, Sterkenburg A, van Andel J G. Microbial degradation of polycyclic aromatic hydrocarbons: effect of substrate availability on bacterial growth kinetics. Appl Microbiol Biotechnol. 1992;36:548–552. [Google Scholar]
- 32.Wilson S C, Jones K C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut. 1993;81:229–249. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]