Abstract
Biochemical controls that regulate the biosynthesis of poly-3-hydroxybutyrate (PHB) were investigated in Rhizobium (Cicer) sp. strain CC 1192. This species is of interest for studying PHB synthesis because the polymer accumulates to a large extent in free-living cells but not in bacteroids during nitrogen-fixing symbiosis with chickpea (Cicer arietinum L.) plants. Evidence is presented that indicates that CC 1192 cells retain the enzymic capacity to synthesize PHB when they differentiate from the free-living state to the bacteroid state. This evidence includes the incorporation by CC 1192 bacteroids of radiolabel from [14C]malate into 3-hydroxybutyrate which was derived by chemically degrading insoluble material from bacteroid pellets. Furthermore, the presence of an NADPH-dependent acetoacetyl coenzyme A (CoA) reductase, which was specific for R-(−)-3-hydroxybutyryl-CoA and NADP+ in the oxidative direction, was demonstrated in extracts from free-living and bacteroid cells of CC 1192. Activity of this enzyme in the reductive direction appeared to be regulated at the biochemical level mainly by the availability of substrates. The CC 1192 cells also contained an NADH-specific acetoacetyl-CoA reductase which oxidized S-(+)-3-hydroxybutyryl-CoA. A membrane preparation from CC 1192 bacteroids readily oxidized NADH but not NADPH, which is suggested to be a major source of reductant for nitrogenase. Thus, a high ratio of NADPH to NADP+, which could enhance delivery of reductant to nitrogenase, could also favor the reduction of acetoacetyl-CoA for PHB synthesis. This would mean that fine controls that regulate the partitioning of acetyl-CoA between citrate synthase and 3-ketothiolase are important in determining whether PHB accumulates.
The exchange of metabolites between the partners in nitrogen-fixing legume-Rhizobium symbioses results in the bacteroid microsymbiont receiving a supply of carbon in return for providing the host legume with reduced nitrogen. The preferred substrate taken up by the bacteroids from the host is malate, which may be oxidized to oxaloacetate by malate dehydrogenase (DH) or may be converted to acetyl coenzyme A (CoA) by the concerted action of malic enzyme and pyruvate DH. Further oxidation of acetyl-CoA and oxaloacetate in the tricarboxylic acid (TCA) cycle can generate the large amount of energy and reductant required by the nitrogenase reaction (7, 17, 27, 31). However, in many types of symbioses, substantial amounts of poly-R-3-hydroxybutyrate (PHB) accumulate in bacteroids, which indicates that the bacteroids take up more carbon than can be immediately utilized. The role of PHB in symbiotic nitrogen fixation is unclear. It has been suggested that this reserve of carbon and reductant is important in maintaining respiratory activities that protect nitrogenase from damage by O2 and in extending nitrogen fixation into the pod-filling stage (3, 4, 12, 22). On the other hand, bacteroids in some symbioses do not accumulate PHB, and nodules formed with Rhizobium mutants unable to synthesize PHB exhibit enhanced nitrogen-fixing activity (5, 19). Indeed, seeds from Phaseolus vulgaris plants nodulated by one such Rhizobium etli mutant contained significantly more nitrogen than seeds from plants nodulated by the wild-type strain (5).
The biosynthesis of PHB in most bacteria is initiated by the condensation of two molecules of acetyl-CoA by 3-ketothiolase to form acetoacetyl-CoA, which is reduced in an enantiomerically selective reaction by acetoacetyl-CoA reductase to R-(−)-3-hydroxybutyryl-CoA and is incorporated into PHB by PHB synthase (2). The acetoacetyl-CoA reductase involved in PHB synthesis is generally considered to be specific for NADPH (2, 8, 16, 23, 24, 30), although in some bacteria the enzyme has been reported to have activity with NADH as well as NADPH (1, 15, 21, 33). Our understanding of the biochemical controls that regulate the partitioning of acetyl-CoA between the TCA cycle and this pathway in Rhizobium bacteroids is limited. To increase our knowledge of these controls, we are studying PHB biosynthesis in Rhizobium (Cicer) sp. strain CC 1192, which forms a symbiosis with chickpea (Cicer arietinum L.) plants. An interesting characteristic of this symbiosis is that CC 1192 bacteroids do not contain PHB, whereas the corresponding free-living cells can accumulate substantial amounts of this reserve (10, 13). 3-Ketothiolase has recently been characterized from CC 1192 (12), and acetoacetyl-CoA reductase activity has been shown to be present in extracts of free-living and bacteroid cells of CC 1192 (10). However, since most bacteria contain an acetoacetyl-CoA reductase which forms S-(+)-3-hydroxybutyryl-CoA from the oxidation of fatty acids, it was not possible to conclude in our earlier study that the acetoacetyl-CoA reductase activity of CC 1192 bacteroids was related to PHB synthesis. In this report we present evidence that CC 1192 bacteroids can incorporate 14C label from malate into PHB and that these cells contain an acetoacetyl-CoA reductase which is specific for R-(−)-3-hydroxybutyryl-CoA and NADP+ in the oxidative direction and utilizes only NADPH for the reduction of acetoacetyl-CoA. We propose that CC 1192 cells retain the capacity for PHB synthesis when they differentiate from free-living cells into the bacteroid form.
MATERIALS AND METHODS
Materials.
Nodulated chickpea (C. arietinum L. cv. Amethyst) plants and cultures of Rhizobium sp. (Cicer) strain CC 1192 were grown as described previously (10). Unless indicated otherwise, all enzymes and biochemicals were purchased from Boehringer Mannheim GmbH (Mannheim, Germany) or Sigma Chemical Co. (St. Louis, Mo.).
Preparation of bacteroid and bacterial extracts.
All procedures were performed at 0 to 4°C. Bacteroids were isolated by homogenizing nodules (2 to 6 g) from 38- to 43-day-old chickpea plants with a mortar and pestle in 30 ml of ice-cold 50 mM Tris-HCl (pH 7.5)–50 mM KCl. The homogenate was filtered through four layers of Miracloth (Calbiochem, San Diego, Calif.), and the filtrate was centrifuged at 4,000 × g for 5 min. Free-living cells of CC 1192 were harvested from liquid cultures by centrifugation at 20,000 × g for 15 min, and the pellets washed by resuspension in 10 ml of 50 mM Tri-HCl (pH 7.5)–50 mM KCl. Bacteroid and bacterial pellets were resuspended in approximately 5 ml of a solution containing 50 mM Tris-HCl (pH 7.5), 50 mM KCl, and 5 mM 1,4-dithiothreitol (DTT), sonicated three times (45 s each) at 80% of the maximum energy setting (1 kW), and centrifuged at 20,000 × g for 20 min. The supernatant was used to isolate acetoacetyl-CoA reductase.
Bacteroids used to measure O2 uptake were partially purified in a Percoll gradient as follows. The pellet obtained by centrifugation at 4,000 × g was resuspended in 50 mM Tris-HCl (pH 7.5)–50 mM KCl, layered over 30 ml of 55% (vol/vol) Percoll in 50 mM Tris-HCl (pH 7.5)–50 mM KCl, and centrifuged at 40,000 × g for 30 min in a Sorvall SS-34 rotor. The bacteroids were isolated from a band near the bottom of the gradient, diluted fivefold with 50 mM Tris-HCl (pH 7.5)–50 mM KCl, and pelleted by centrifugation at 10,000 × g for 15 min. The bacteroids were resuspended in 20 mM Pi (pH 7)–10 mM KCl, sonicated, and centrifuged at 14,000 × g for 2 min. The supernatant, which contained a suspension of membrane fragments, was used to measure O2 uptake.
Synthesis of R-(−)- and S-(+)-3-hydroxybutyryl-CoA.
The two enantiomers of 3-hydroxybutyryl-CoA were synthesized via the respective mixed anhydrides by using the method of Stadtman (25), as follows. R-(−)- or S-(+)-3-hydroxybutyric acid (104 mg; Sigma Chemical Co.) was dissolved in 2 ml of dry diethyl ether at 4°C. Ice-cold, dry pyridine (80 μl) was added, and then 94 μl of ice-cold ethyl chloroformate was added dropwise with continuous stirring. The mixture was left in an ice bath for 60 min, and the supernatant, which contained the mixed anhydride, was decanted from precipitated pyridine hydrochloride. A volume of the solution containing one equivalent of the mixed anhydride was added dropwise with shaking to an ice-cold solution of CoA (Na salt; 50 mg) in 2 ml of 0.2 M KHCO3 which had been adjusted to pH 7.5 with 1 M HCl. One drop from the mixture was tested for free SH groups with nitroprusside reagent. Additional mixed-anhydride solution was added if the test gave a positive reaction. When no free SH groups were detected by the nitroprusside test, the pH was adjusted to 6 with 1 M HCl, and the reaction mixture was extracted three times with 2 ml of diethyl ether. Nitrogen gas was bubbled through the aqueous phase to remove traces of ether. Unreacted 3-hydroxybutyric acid was removed from the 3-hydroxybutyryl-CoA preparations by using a Sephadex G-10 column in water. Fractions which contained 3-hydroxybutyryl-CoA were pooled, 3 M sodium acetate solution (pH 6.6) was added until the final sodium acetate concentration was 10 mM, and the preparation was stored at −20°C.
To estimate the concentration of R-(−)-3-hydroxybutyryl-CoA, an aliquot of the preparation was hydrolyzed with an equal volume of 0.6 M NaOH for 10 min in a boiling water bath. After cooling and after the pH was adjusted to 8.5, the concentration of 3-hydroxybutyrate was estimated from the change in absorbance at 340 nm in 1-ml reaction mixtures which contained 50 mM Tris-HCl (pH 8.5), 15 mM MgCl2, 5 mM NAD+, 80 mM hydrazine hydrate, and 0.15 U of 3-hydroxybutyrate DH. The concentrations of solutions of R-(−)-3-hydroxybutyryl-CoA after Sephadex G-10 chromatography were 4 to 5 mM, which corresponded to yields of up to 60%. The concentrations of S-(+)-3-hydroxybutyryl-CoA solutions were assumed to be similar.
Incorporation of [14C]malate into PHB by chickpea bacteroids.
Crude chickpea bacteroids in the pellet obtained by centrifugation 4,000 × g were resuspended in a microcentrifuge tube in a mixture which contained, in a final volume of 0.5 ml, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-NaOH (pH 8.5), 2.5 mM Pi, 10 mM MgCl2, 20 mM KCl, and 10 mM l-[2,314C]malate (1 μCi). The microcentrifuge tube was flushed for 2 min with N2, sealed, and incubated at 30°C. A mixture which contained bacteroids that had been heated in a boiling water bath for 5 min was used as a control. After incubation, reaction mixtures were centrifuged at 12,000 × g for 5 min, and each pellet washed twice by resuspension in 0.5 ml of the reaction solution which contained 10 mM unlabelled l-malate.
The washed pellet was transferred to a glass vial with 0.5 ml of water, dried at 80°C, and subjected to acidic propanolysis by the methods of Riis and Mai (20), as follows. The dried pellet was heated for 2 h at 100°C in the tightly sealed glass vial with a mixture which contained 0.5 mg of Alcaligenes eutrophus PHB (Sigma) in a solution containing 2 ml of 1,2-dichloroethane and 2 ml of propan-1-ol–10 M HCl (4:1). After cooling, the reaction mixture was partitioned twice with 4 ml of water, and the lower (organic) phase was heated at 80°C for 1 h with 0.2 M KOH in propan-1-ol. The reaction mixture was acidified with 2 ml of 4 M H2SO4 and partitioned twice with 1 ml of water. The aqueous phase was partitioned six times with 2 ml of diethyl ether, the ether extracts were combined, and the solvent was removed. The recovery of 3-hydroxybutyrate in the ether extract was only 30 to 40% because of the high solubility of this compound in both water and diethyl ether. The residue was redissolved in ethanol and chromatographed by using Whatman no. 1 paper in ethanol-NH4OH-H2O (80:5:15) and ethyl acetate-acetic acid-H2O (3:1:1); standard 3-hydroxybutyrate, as detected with 0.05% (wt/vol) methyl red, had relative mobilities of 0.7 and 0.85, respectively, in these two solvent systems, whereas the corresponding relative mobilities of malate were 0.25 and 0.56, respectively. Chromatograms were cut into segments which were counted by liquid scintillation spectrometry.
Isolation of R-(−)-3-hydroxybutyryl-CoA DH.
Crude bacteroid or bacterial extracts were chromatographed at room temperature (20 to 22°C) through two 5-ml Econo Q columns (Bio-Rad, Richmond, Calif.) which were joined in series and had been equilibrated with 50 mM Tris-HCl (pH 7.5)–50 mM KCl–5 mM DTT. The unbound fraction, which contained both R-(−)- and S-(+)-3-hydroxybutyryl-CoA DH activities, was applied to a Red Sepharose CL-6B column (1 ml; Pharmacia, Uppsala, Sweden) which had been equilibrated previously with 50 mM Tris-HCl (pH 7.5)–5 mM DTT–50 mM KCl. Most of the S-(+)-3-hydroxybutyryl-CoA DH activity passed through the column unbound. The small amount of this activity which did bind and R-(−)-3-hydroxybutyrate DH activity were eluted with 20 ml of 50 mM Tris-HCl (pH 7.5)–5 mM DTT–0.1 M KCl. R-(−)-3-Hydroxybutyryl-CoA DH activity was eluted with 50 mM Tris-HCl (pH 7.5)–5 mM DTT–0.5 M KCl. Active fractions were pooled and stored at −80°C after glycerol was added to a concentration of 30% (vol/vol).
Enzyme assays.
Enzyme activities were assayed at 30°C by monitoring the change in absorbance at 340 nm due to oxidation of NAD(P)H or reduction of NAD(P)+ in reaction mixtures which had a final volume of 1 ml. Standard reaction mixtures for NADH- and NADPH-dependent acetoacetyl-CoA reductase activity experiments contained 50 mM Tris-HCl (pH 8.5), 15 mM MgCl2, 250 μM NAD(P)H, and 100 μM acetoacetyl-CoA. 3-Hydroxybutyryl-CoA and 3-hydroxybutyrate DH activities were measured in reaction mixtures which contained 50 mM Tris-HCl (pH 8.5), 15 mM MgCl2, 1 mM NAD(P)+, and 100 μM R-(−)- or S-(+)-3-hydroxybutyryl CoA or 100 μM R-(−)-3-hydroxybutyrate. Activities were calculated from initial reaction rates which were linear for at least 2 to 4 min. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1 μmol of product min−1. Protein contents were determined with Coomassie blue reagent (Bio-Rad) by using the supplier’s instructions and bovine serum albumin as the standard.
Determination of native molecular mass.
Native molecular mass was determined by size exclusion chromatography at room temperature (20 to 22°C) in a Superose 6 column (Pharmacia) with 50 mM Tris-HCl (pH 7.5)–0.5 M KCl–5 mM DTT by using a flow rate of 0.5 ml min−1. Ferritin (450 kDa), catalase (240 kDa), aldolase (158 kDa), bovine serum albumin (68 kDa), chick albumin (45 kDa), chymotrypsinogen A (25 kDa), and cytochrome c (12.5 kDa) were used to calibrate the column. The void volume of the column was measured with Blue Dextran 2000 (Pharmacia).
Uptake of oxygen.
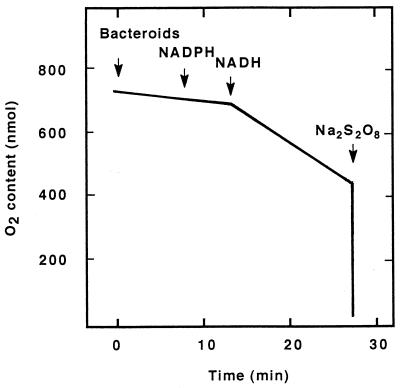
Uptake of O2 was measured polarographically at 30°C with a Clark type of electrode at pH 7.2 in reaction mixtures which contained, in a volume of 3 ml, 10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES)–KOH, 10 mM Pi, 5 mM MgCl2, 0.1% (wt/vol) bovine serum albumin, and substrate (see Fig. 2). The concentration of oxygen in water saturated with air was taken to be 250 μM.
FIG. 2.
Consumption of O2 by an extract obtained from chickpea bacteroids. Bacteroids were isolated from 38- to 43-day-old chickpea nodules, centrifuged in a Percoll gradient, and sonicated as described in the text. The line represents a recorder trace in which the rates of O2 consumption in the absence of added substrate and after addition to reaction mixtures of 1 mM NADPH and 1 mM NADH were 6.3 ± 0.7, 6.3 ± 0.7, and 24.8 ± 2.0 nmol min−1 mg of protein−1, respectively (means ± standard deviations of values from three determinations). An Na2S2O8 solution was added for calibration. The experiment was performed in triplicate.
RESULTS
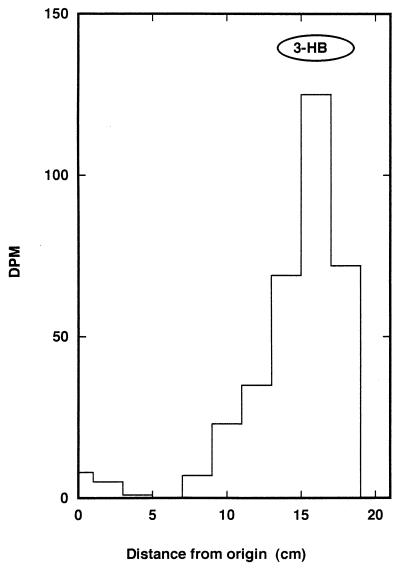
Isolated CC 1192 bacteroids were incubated with [14C]malate, and the resulting pellets were washed, dried, and heated with n-propanol and HCl to convert any labelled PHB that had been formed, together with the PHB added as carrier, to 3-propyl 3-hydroxybutyrate. The propanolysis product was then hydrolyzed with KOH, acidified, and partitioned, in turn, with H2O and diethyl ether to separate acids that are freely soluble in H2O and ether (e.g., 3-hydroxybutyrate) from acids with low solubility in ether (e.g., malate) and H2O-insoluble acids (e.g., fatty acids) (Table 1). Paper chromatography in ethanol-NH4OH-H2O (80:5:15) (Fig. 1) and ethyl acetate-acetic acid-H2O (3:1:1) (results not shown) indicated that essentially all of the radiolabel in the water- and ether-soluble acids was in a single spot which corresponded to standard 3-hydroxybutyrate (Fig. 1). No radiolabel corresponded to malate in the chromatograms. The small amount of radioactivity in the control samples, which was recovered mostly in the fraction that contained the water-soluble, either-insoluble acids, may have represented residual malate that was not removed by washing and was subsequently esterified. It is difficult to compare the efficiency of conversion of malate to PHB by isolated CC 1192 bacteroids with the efficiency of PHB synthesis in free-living cells since conditions for the incubations were probably very different from the in situ conditions.
TABLE 1.
Incorporation of [14C]malate into PHB by chickpea bacteroidsa
| Fraction | Radioactivity (dpm)b
|
||
|---|---|---|---|
| Controlc | After 2 h | After 4 h | |
| Esterified lipids | 1,750 ± 438 | 12,420 ± 566 | 14,410 ± 14 |
| Water-soluble acids | 1,313 ± 297 | 8,829 ± 62 | 7,394 ± 309 |
| Water- and ether-soluble acids | 625 ± 84 | 1,891 ± 855 | 2,479 ± 245 |
Bacteroids (3.6 mg of protein) were incubated for 2 and 4 h with 1 μCi of l-[2,3-14C]malate as described in the text.
Means ± standard deviations of values from duplicate incubations.
The preparation was heated in a boiling water bath for 5 min.
FIG. 1.
Paper chromatography of water- and ether-soluble acids isolated from chickpea bacteroids that were incubated with [14C]malate. Bacteroids (3.6 mg [wet weight]) were incubated, and the water- and ether-soluble acids which were obtained from insoluble material as described in the text were chromatographed on Whatman no. 1 paper in ethanol-NH4OH-H2O (80:5:15). The chromatogram is one of duplicate chromatograms obtained from duplicate incubations. The position of 3-hydroxybutyrate (3-HB) is indicated at the top.
Using the procedure summarized in Table 2, we obtained an enzyme preparation which specifically oxidized R-(−)-3-hydroxybutyryl-CoA from CC 1192 bacteroids. This enzyme was specific for NADP+ and had no activity with NAD+, S-(+)-3-hydroxybutyryl-CoA, and R-(−)-3-hydroxybutyrate. Only NADPH was utilized in the reductive direction. The preparative procedure shown in Table 2 also led to the separation of NAD-specific DH which oxidized S-(+)-3-hydroxybutyryl-CoA and R-(−)-3-hydroxybutyrate (Table 2). A similar distribution of R-(−)- and S-(+)-3-hydroxybutyryl-CoA and R-(−)-3-hydroxybutyrate DH activities was observed with extracts from free-living CC 1192 cells (data not shown).
TABLE 2.
Separation of R-(−)- and S-(+)-3-hydroxybutyryl-CoA and R-(−)-3-hydroxybutyrate DH from Rhizobium sp. (Cicer) CC 1192 bacteroidsa
| Fraction | Rate (nmol min−1)
|
||
|---|---|---|---|
| R-(−)-3-hydroxybutyryl-CoA DHb | S-(+)-3-hydroxybutyryl-CoA DHc | 3-Hydroxybutyrate DHc | |
| Crude extract | 121 | 508 | 695 |
| Econo Q unbound | 121 | 427 | 306 |
| Red Sepharose | |||
| Unbound | 0 | 120 | 157 |
| 0.1 M KCl | 0 | 106 | 52 |
| 0.2 M KCl | 113 | 0 | 0 |
| 0.5 M KCl | 87 | 0 | 0 |
Bacteroid cells (approximately 1.5 g [wet weight]) were extracted from 43-day-old nodules as described in the text. The data are typical of data obtained in five replicate experiments.
Active with NADP+ but no activity with NAD+.
Active with NAD+ but no activity with NADP+.
NADPH-dependent acetoacetyl-CoA reduction was optimal at pH 8.5 and when the concentration of MgCl2 in reaction mixtures was between 10 and 15 mM. The relationship between activity and substrate concentration was hyperbolic, and the apparent Km values for acetoacetyl-CoA and NADPH were 22 ± 10 and 44 ± 24 μM, respectively (means ± standard deviations of values from five determinations). These values were obtained by varying the concentration of acetoacetyl-CoA between 0 and 100 μM at a fixed NADPH concentration of 200 μM and by varying the concentration of NADPH between 0 and 200 μM at a fixed acetoacetyl-CoA concentration of 100 μM. Under the conditions used in these experiments, substrate inhibition was noted at acetoacetyl-CoA concentrations above 100 μM. The enzyme had no activity with acetoacetate. NADPH-dependent reduction of acetoacetyl-CoA was inhibited 35 and 50% by 0.2 and 0.4 mM NADP+, respectively, and 20 to 30% by 0.2 mM R-(−)-3-hydroxybutyryl-CoA, 0.05 mM S-(+)-3-hydroxybutyryl-CoA, and 0.1 mM acetyl-CoA. There was no inhibition by NAD+ (0.1 mM), acetoacetate (0.2 mM), and R-(−)-3-hydroxybutyrate (0.2 mM).
In the oxidation direction, the substrate kinetics were hyperbolic, and the apparent Km values for R-(−)-3-hydroxybutyryl-CoA and NADP+ were 120 ± 29 and 38 ± 16 μM, respectively (means ± standard deviations of values from four determinations). The concentrations of R-(−)-3-hydroxybutyryl-CoA and NADP+ were varied between 0 and 200 μM in these experiments. Activity was inhibited approximately 30 to 40% by NADPH (0.1 mM), S-(+)-3-hydroxybutyryl-CoA (0.05 mM), and acetoacetyl-CoA (0.05 mM). The oxidation reaction was not inhibited by 0.25 mM NADH.
The native molecular mass of the NADPH-dependent acetoacetyl-CoA reductase in extracts from free-living and bacteroid cells of CC 1192 was approximately 90 to 95 kDa, as determined by size exclusion chromatography.
Consumption of O2 by a membrane preparation from CC 1192 bacteroids was measured with NADH and NADPH as the substrates. When NADPH was added to a bacteroid extract, the rate of O2 uptake did not increase above the background rate (Fig. 2). However, when NADH was added subsequently to the reaction mixture, the rate of O2 consumption increased approximately fourfold (Fig. 2).
DISCUSSION
The incorporation by CC 1192 bacteroids of radiolabel from [14C]malate into 3-hydroxybutyrate which had been isolated from the breakdown of insoluble material provides evidence that these cells retained the enzymic capacity to synthesize PHB, even though they did not accumulate this reserve. This means that in these cells biochemical controls are likely to operate which direct acetyl-CoA into the TCA cycle rather than towards PHB synthesis. The greater potential in CC 1192 bacteroids than in free-living cells for oxidizing malate to oxaloacetate, as opposed to decarboxylating it to pyruvate, and potent inhibition of 3-ketothiolase by CoA may be significant elements of these controls (10, 11).
Since the reaction catalyzed by acetoacetyl-CoA reductases is readily reversible, we adopted a strategy of seeking a DH which specifically oxidized R-(−)-3-hydroxybutyryl-CoA to demonstrate that CC 1192 bacteroids contain a reductase which can participate in PHB biosynthesis. Accordingly, we isolated from CC 1192 bacteroids an acetoacetyl-CoA reductase which had no activity in the oxidative direction with S-(+)-3-hydroxybutyryl-CoA and R-(−)-3-hydroxybutyrate and was active only with NADPH and NADP+. The reductive reaction was inhibited by NADP+ but not by NAD+, whereas NADPH but not NADH inhibited the oxidation reaction. The presence of this enzyme in extracts of CC 1192 bacteroids and the earlier demonstration of 3-ketothiolase in these cells (11) provide further evidence that CC 1192 bacteroids retain the capacity for PHB synthesis. The genes which encode the enzymes of the PHB biosynthetic pathway (i.e., 3-kethiolase, acetoacetyl-CoA reductase, and PHB synthase) have been shown to be arranged as an operon in several bacterial genomes (26, 29).
The NADPH-dependent acetoacetyl-CoA reductase from free-living and bacteroid cells of CC 1192 had a native molecular mass of approximately 90 to 95 kDa. The estimated native molecular masses of NADP-dependent acetoacetyl-CoA reductases from other bacterial sources are mostly between 80 and 90 kDa (8, 9, 15, 30), and a subunit molecular mass of approximately 26 kDa has been determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and from the nucleotide sequence of the gene (18, 27, 33). The substrate affinities of the NADPH-dependent acetoacetyl-CoA reductase from CC 1192 were somewhat lower than those of the enzyme from other sources, for which the reported apparent Km values for acetoacetyl-CoA and NADPH are near 10 and 20 μM, respectively (8, 9, 15, 30). However, the differences between the results of this study and the results of other studies may not be significant, if differences in experimental conditions are considered. The kinetic properties indicate that activity of the CC 1192 acetoacetyl-CoA reductase is likely to be regulated mainly by the availability of acetoacetyl-CoA and the ratio of NADPH to NADP+.
The nitrogenase reaction has high demands for low-potential reductant and ATP, equivalent to eight electrons and 16 molecules of ATP per molecule of N2 fixed, which need to be generated in a microaerobic environment that is characteristic of bacteroids in legume-Rhizobium symbioses. These demands could be met by partitioning reductant generated in the TCA cycle and related oxidations between the electron transport reactions of the respiratory pathway and the nitrogenase complex (4, 7, 17). How this occurs is not clear, and indeed, the source of low-potential electrons for nitrogenase remains an important unanswered question. It has been suggested that NADPH is the main source of reductant for nitrogenase on the basis that it is a poor respiratory substrate for soybean bacteroids (4, 6, 7). The inability of NADPH to stimulate O2 uptake by a membrane preparation from CC 1192 bacteroids in chickpea root nodules lends further support to this hypothesis. In contrast, NADH is oxidized readily by membrane preparations from soybean and chickpea bacteroids (6; this study) and may be the main substrate for ATP production.
In the microaerobic environment of bacteroids, rapid cycling of reductant through the pyridine nucleotide pools is essential for sustained operation of the TCA cycle and for maintaining the supply of reductant and energy for nitrogen fixation. If NADH is reoxidized mainly in association with ATP generation by the respiratory pathway and NADPH is reoxidized by the nitrogenase complex, the pathways that feed reductant into the respective pyridine nucleotide pools would need to be coordinated for optimum nitrogen fixation. An excess of NADPH could be absorbed by the NADPH-dependent acetoacetyl-CoA reductase, in which case PHB synthesis could serve as an overflow pathway (32). The NADP+/NADPH ratio was found to be highly reduced in bacteroids isolated from soybean nodules (28), and although this value needs to be interpreted cautiously because of the metabolic lability of the pyridine nucleotide pools, it is consistent with the accumulation of PHB in this symbiosis. The accumulation of PHB by Escherichia coli harboring a plasmid containing the pha operon from A. eutrophus is influenced most strongly by the concentration of NADPH (14). According to this interpretation, PHB should not accumulate in bacteroids when reductant is provided to nitrogenase at a rate which closely balances the availability of energy. On the other hand, it is possible that accumulation of PHB is not simply a means of absorbing excess reductant. The increased rate of nitrogen fixation exhibited by Rhizobium mutants which are unable to synthesize PHB suggests that carbon and reductant are allocated to PHB synthesis at the expense of nitrogenase in bacteroids formed with the corresponding wild-type cells (5). Since acetoacetyl-CoA reductase activity appears to be regulated at the biochemical level mainly by the availability of substrates, a high ratio of NADPH to NADP+, which could favor the provision of low-potential reductant to nitrogenase, could also stimulate reduction of acetoacetyl-CoA. In this case, it is more likely that controls which regulate the partitioning of acetyl-CoA between citrate synthase and 3-ketothiolase determine whether PHB accumulates.
ACKNOWLEDGMENT
This research was supported in part by funds from the Australian Research Council.
REFERENCES
- 1.Amos D A, McInerny M J. Formation of d-3-hydroxybutyryl-coenzyme A reductase in Syntrophomonas wolfei subsp. wolfei. Arch Microbiol. 1993;159:16–20. [Google Scholar]
- 2.Anderson A, Dawes E. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergersen F J, Turner G L. Supply of O2 regulates O2 demand during utilization of reserves of poly-β-hydroxybutyrate in N2-fixing soybean bacteroids. Proc R Soc Lond B Biol Sci. 1992;249:143–148. doi: 10.1098/rspb.1992.0096. [DOI] [PubMed] [Google Scholar]
- 4.Bergersen F J, Peoples M B, Turner G L. A role for poly-β-hydroxybutyrate in bacteroids of soybean root nodules. Proc R Soc Lond B Biol Sci. 1991;245:59–64. [Google Scholar]
- 5.Cevallos M A, Encarnación S, Leija A, Mora Y, Mora J. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-β-hydroxybutyrate. J Bacteriol. 1996;178:1646–1654. doi: 10.1128/jb.178.6.1646-1654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland L, Quinnell R G, Day D A. Malic enzyme in bacteroids from soybean nodules. J Gen Microbiol. 1989;135:2005–2011. [Google Scholar]
- 7.Day D A, Copeland L. Carbon metabolism and compartmentation in nitrogen-fixing legume nodules. Plant Physiol Biochem. 1991;29:185–201. [Google Scholar]
- 8.Fukui T, Ito M, Saito T, Tomita K. Purification and characterization of NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Biochim Biophys Acta. 1987;917:365–371. [PubMed] [Google Scholar]
- 9.Haywood G W, Anderson A J, Chu L, Dawes E A. The role of NADH- and NADPH-dependent acetoacetyl-CoA reductases in the poly-3-hydroxybutyrate synthesising organism Alcaligenes eutrophus. FEMS Microbiol Lett. 1988;52:259–264. [Google Scholar]
- 10.Kim S A, Copeland L. Enzymes of poly-β-hydroxybutyrate metabolism in soybean and chickpea bacteroids. Appl Environ Microbiol. 1996;62:4186–4190. doi: 10.1128/aem.62.11.4186-4190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S A, Copeland L. Acetyl coenzyme A acetyltransferase of Rhizobium sp. (Cicer) strain CC 1192. Appl Environ Microbiol. 1997;63:3432–3437. doi: 10.1128/aem.63.9.3432-3437.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretovich W L, Romanov V I, Yushkova L A, Shramko V I, Fedulova N G. Nitrogen fixation and poly-β-hydroxybutyric acid content in bacteroids of Rhizobium lupini and Rhizobium leguminosarum. Plant Soil. 1977;48:291–302. [Google Scholar]
- 13.Lee H-S, Copeland L. Ultrastructure of chickpea nodules. Protoplasma. 1994;182:32–38. [Google Scholar]
- 14.Lee I Y, Kim M K, Park Y H, Lee S Y. Regulatory effects of cellular nicotinamide nucleotides and enzyme activities on poly(3-hydroxybutyrate) synthesis in recombinant Escherichia coli. Biotechnol Bioeng. 1996;52:707–712. doi: 10.1002/(SICI)1097-0290(19961220)52:6<707::AID-BIT8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Manchak J, Page W J. Control of polyhydroxyalkanoate biosynthesis in Azotobacter vinelandii strain UWD. Microbiology. 1994;140:953–963. [Google Scholar]
- 16.Mansfield D A, Anderson A J, Naylor L A. Regulation of PHB metabolism in Alcaligenes eutrophus. Can J Microbiol. 1995;41:44–49. [Google Scholar]
- 17.McDermott T R, Griffith S M, Vance C P, Graham P H. Carbon metabolism in Bradyrhizobium japonicum bacteroids. FEMS Microbiol Rev. 1989;63:327–340. [Google Scholar]
- 18.Peoples O P, Sinskey A J. Poly-β-hydroxybutyrate synthesis in Alcaligenes eutrophus H 16. J Biol Chem. 1989;264:15293–15297. [PubMed] [Google Scholar]
- 19.Povolo S, Tombolini R, Morea A, Anderson A, Casella S, Nuti M. Isolation and characterization of mutants of Rhizobium meliloti unable to synthesize poly-β-hydroxybutyrate. Can J Microbiol. 1994;40:823–829. [Google Scholar]
- 20.Riis V, Mai W. Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J Chromatogr. 1988;445:285–289. [Google Scholar]
- 21.Ritchie G A F, Senior P J, Dawes E A. The purification and characterisation of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J. 1971;121:309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romanov V I, Fedulova N G, Tchermenskaya I E, Shramko V I, Molchanov M I, Kretovich W L. Metabolism of poly-β-hydroxybutyrate in bacteroids of Rhizobium lupini in connection with nitrogen fixation and photosynthesis. Plant Soil. 1980;56:379–390. [Google Scholar]
- 23.Saito T, Fukui T, Ikedo F, Tanaka Y, Tomita K. An NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera. Arch Microbiol. 1977;114:211–217. doi: 10.1007/BF00446864. [DOI] [PubMed] [Google Scholar]
- 24.Shuto H, Fukui T, Saito T, Shirakura Y, Tomita K. An NAD-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Eur J Biochem. 1981;118:53–59. doi: 10.1111/j.1432-1033.1981.tb05485.x. [DOI] [PubMed] [Google Scholar]
- 25.Stadtman E R. Preparation and assay of acyl coenzyme A and other thioesters; use of hydroxylamine. Methods Enzymol. 1957;3:931–944. [Google Scholar]
- 26.Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 27.Streeter J G. Recent developments in carbon transport and metabolism in symbiotic systems. Symbiosis. 1995;19:175–196. [Google Scholar]
- 28.Tajima S, Kouzai K. Nucleotide pools in soybean nodule tissues, a survey of NAD(P)/NAD(P)H ratios and energy charge. Plant Cell Physiol. 1989;30:589–593. [Google Scholar]
- 29.Tombolini R, Povolo S, Buson A, Squartini A, Nuti M. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology. 1995;141:2553–2559. doi: 10.1099/13500872-141-10-2553. [DOI] [PubMed] [Google Scholar]
- 30.Tomita K, Saito T, Fukui T. Bacterial metabolism of poly-β-hydroxybutyrate. In: Lennon D L F, Stratman F W, Zahlten R N, editors. Biochemistry of metabolic processes. Amsterdam, The Netherlands: Elsevier Science Publishing Co., Inc.; 1983. pp. 353–366. [Google Scholar]
- 31.Udvardi M K, Day D A. Metabolite transport across symbiotic membranes of legume nodules. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:493–523. doi: 10.1146/annurev.arplant.48.1.493. [DOI] [PubMed] [Google Scholar]
- 32.Walshaw D L, Wilkinson A, Mundy M, Smith M, Poole P S. Regulation of the TCA cycle and the general amino acid permease by overflow metabolism in Rhizobium leguminosarum. Microbiology. 1997;143:2209–2221. doi: 10.1099/00221287-143-7-2209. [DOI] [PubMed] [Google Scholar]
- 33.Yabutani T, Maehara A, Yamane T. Analysis of β-ketothiolase and acetoacetyl-CoA reductase genes of a methylotrophic bacterium, Paracoccus denitrificans, and their expression in Escherichia coli. FEMS Microbiol Lett. 1995;133:85–90. doi: 10.1111/j.1574-6968.1995.tb07865.x. [DOI] [PubMed] [Google Scholar]