Abstract
Ehrlichia DNA was identified by nested PCR in operculate snails (Pleuroceridae: Juga spp.) collected from stream water in a northern California pasture in which Potomac horse fever (PHF) is enzootic. Sequencing of PCR-amplified DNA from a suite of genes (the 16S rRNA, groESL heat shock operon, 51-kDa major antigen genes) indicated that the source organism closely resembled Ehrlichia risticii, the causative agent of PHF. The minimum percentage of Juga spp. harboring the organism in the population studied was 3.5% (2 of 57 snails). No ehrlichia DNA was found in tissues of 123 lymnaeid, physid, and planorbid snails collected at the same site. These data suggest that pleurocerid stream snails may play a role in the life cycle of E. risticii in northern California.
Potomac horse fever (PHF), also called equine monocytic ehrlichiosis, is an important disease of horses caused by a monocytotropic rickettsia, Ehrlichia risticii (19–23, 27, 34). PHF was first observed in 1979 in pastures along the Potomac River in Maryland (20). Soon thereafter it was reported in Virginia and Pennsylvania, and it has since been identified in a number of other states and in Europe (27). Clinical signs include anorexia, lethargy, variable fever, and diarrhea. Laminitis is a complication in a significant percentage of cases. Fatalities may result if severely affected horses are not treated promptly with fluids, electrolytes, and antibiotics.
The means by which horses become exposed to E. risticii has remained mysterious. The disease is infectious but not contagious; this, combined with its seasonality, geographical distribution, and ease of transmission by intradermal inoculation, has been thought to reflect spread by an arthropod vector, possibly a tick, but direct evidence of tick transmission is lacking (3, 18, 27, 29, 30, 37). The disease can be transmitted by oral inoculation, however, and the causative agent has been identified in feces (25, 28, 35).
Since the earliest reports of PHF, an association of cases with riverine and other aquatic habitats has been noted. Research has revealed a close phylogenetic relationship between E. risticii and the following three rickettsiae associated with aquatic environments: Neorickettsia helminthoeca, the agent of “salmon poisoning,” a frequently fatal systemic, metacercaria-borne disease of canids; the SF agent, isolated in Japan from trematode metacercariae (Stellantchasmus falcatus) parasitic on gray mullet fish; and Ehrlichia sennetsu, the agent of human sennetsu ehrlichiosis in Japan and Malaysia (15, 16, 31, 33, 43). Based on 16S rRNA gene sequences, antigenic cross-reactivity, and the proven or suspected mechanism of transmission, these four agents form a distinct cluster or genogroup distinct from other rickettsiae (11, 33, 36, 43).
These data suggest that the vector of E. risticii may not be an arthropod but instead an organism closely associated in some way with river and/or irrigation water. Accordingly, we have directed our efforts recently toward identifying a potential aquatic reservoir for E. risticii. In this report we describe the detection of ehrlichia DNA in freshwater operculate snails (Pleuroceridae: Juga spp.) collected from stream water in a pasture in northern California in which PHF is enzootic. Analysis of PCR-amplified DNA indicated that the ehrlichia detected is clearly related to the type strain of E. risticii and virtually identical to a new strain (the strain responsible for Shasta River crud [SRC]) identified in the blood buffy-coat cells of an affected horse a few miles from the pasture which we studied. We hypothesize that operculate snails of the genus Juga may play a role in the life cycle of E. risticii in northern California.
MATERIALS AND METHODS
Snail collection.
Freshwater snails were collected in August 1996 from a pasture in Weed, Calif. (Siskiyou County), with a history of PHF in some horses allowed to graze in it. No case had been observed for at least several months prior to sampling. Snails were collected by hand or with a net from the clear, shallow margins of a stream that flows through the pasture and is accessible to horses for drinking. The stream is fed by the nearby Shasta River. A total of 180 snails were collected, including 55 lymnaeids, 24 physids, 44 planorbids, and 57 pleurocerids (Table 1). The pleurocerid snails (Fig. 1) were identified as members of the genus Juga (5). Based on current nomenclature derived from classical shell characteristics, the majority of the individuals resembled the subspecies Juga hemphilli hemphilli, in which ribs (costae) are for the most part limited to the apical whorl of the shell (5). However, because the systematics of the Pleuroceridae is in need of thorough revision (5, 10), identification of the snails to the species level remains presumptive.
TABLE 1.
E. risticii nested PCR results (16S rRNA gene fragment) for freshwater stream snails collected at a site in northern California in which PHF is enzootic
| Family | No. of PCR-positive pools/no. of snails tested |
|---|---|
| Lymnaeidae | 0/55 |
| Physidae | 0/24 |
| Planorbidae | 0/44 |
| Pleuroceridae | 2/57 |
| Total | 2/180 |
FIG. 1.
Pleurocerid snails collected from stream water fed by the Shasta River in Weed, Calif. (Siskiyou County). Bar = 1 cm. (Photograph courtesy of Jerry Fields.)
Processing of snails.
The snails collected were frozen (−70°C), thawed, segregated by family into pools of 2 to 11 snails depending on size, and processed for PCR by a modification of previous methods (2, 3). Briefly, snails were washed in sterile distilled water, dissected from their shells with sterile scissors, and placed into 2-ml microtubes. The tissues were mechanically disrupted for 1 min with a Mini-BeadBeater (Biospec Products, Bartlesville, Okla.) by using the high setting. The tubes were centrifuged at the maximum speed in a microcentrifuge for 1 min. Excess crude extract was removed until approximately 1 ml remained in each microtube. The tubes were filled nearly to the top with DNA extraction buffer (10 mM Tris [pH 8.0], 2 mM EDTA, 0.1% sodium dodecyl sulfate, 500 μg of proteinase K per ml), vortexed, placed in a heating block (57°C) for 3 h, vortexed again, and then heated at 97°C for 15 min to inactivate the proteinase K.
After thorough vortexing, 500-μl portions of each extract were transferred to sterile 1.7-μl Eppendorf tubes, and DNA extraction was performed by using a phenol-chloroform-isoamyl alcohol (25:24:1) mixture. Each sample was extracted twice. Portions (500 μl) of the final aqueous layer were transferred to fresh microtubes. The DNA was precipitated by adding 3 to 4 volumes of absolute ethanol and 100 μl of 3 M sodium acetate (pH 5.2) to each tube and incubating the tubes at −20°C for 24 to 48 h. The DNA was pelleted by centrifugation at the maximum speed in a microcentrifuge for 15 min at 4°C and washed twice with 70% ethanol. After the DNA was dried, it was resuspended in 100 μl of Tris-EDTA buffer (pH 8.0). The DNA content was checked with a UV spectrophotometer, and the DNA concentration of each sample was adjusted to approximately 300 ng per μl. One microliter was used in each PCR mixture.
Nested PCR assays.
A nested PCR that amplifies a 5′ segment (529 bp) of the 16S rRNA gene of E. risticii was used as an initial screening procedure for infected snail pools. The components and conditions of this PCR procedure have been described in detail elsewhere (3). The cycling parameters used in this study were as follows: preheating at 94°C for 5 min and then 35 cycles consisting of denaturation at 94°C for 1 min, annealing at 60°C for 2 min, and extension at 72°C for 1.5 min, followed by a final extension step at 72°C for 7 min. The PCR products were visualized in ethidium bromide-stained 1.5% agarose minigels.
Segments of two additional ehrlichia genes were amplified by PCR. Sets of nested primers were designed to detect portions of the E. risticii homolog of the Escherichia coli groESL heat shock operon (39) and the E. risticii 51-kDa major antigen gene (12, 13, 41). The following primer sequences were used to amplify the heat shock operon: 5′-ACCAGGCTACCTCACAGGC-3′ and 5′-TTGACCCTCGCATCAATG-3′ (outer primers); and 5′-CACAAGTTGGTTCAATTTCTGC-3′ and 5′-CCGAGATCTTCAACAGTAAGGC-3′ (inner primers). The amplified sequence was entirely contained within the groEL portion of the operon. The following primer sequences were used to amplify the 51-kDa major antigen gene: 5′-GGATCGATAACTGCGATGCT-3′ and 5′-ACCGGCCTGACCACTAAAG-3′ (outer primers); and 5′-TCCTATAATGGCACCACTAGCG-3′ and 5′-CCATCCGCAGTAGAGTTTGAG-3′ (inner primers).
The predicted molecular sizes for the groESL fragment were 823 bp (first-round product) and 526 bp (nested product). The nested product comprised nucleotides 500 through 1025 of the operon (numbering based on the sequence deposited under GenBank accession no. U96732). The predicted sizes for the 51-kDa major antigen gene fragment were 818 bp (first-round product) and 569 bp (nested product). The nested product comprised nucleotides 1303 through 1871 of the gene (numbering based on the sequence deposited under GenBank accession no. U85784). The components and conditions of the PCR assays used for groESL and the 51-kDa major antigen gene were similar to the components and conditions used for standard 16S rRNA gene amplification, except that the annealing temperatures were 45°C (for the 51-kDa major antigen gene) and 55°C (for groESL).
Cloning and sequencing of amplified PCR products.
A nearly complete sequence for the 16S rRNA gene was obtained by amplifying and cloning the gene in three overlapping fragments. The majority of the gene (ca. 1,440 bases) was amplified in the first round by using primers ER-3 (5′-ATTTGAGAGTTTGATCCTGG-3′) (3, 8) and PC5 (5′-TACCTTGTTACGACTT-3′) (44). In the nested round the 5′ segment of the gene was amplified with primers ER-3 and ER-2 (5′-GTTTTAAATGCAGTTCTTGG-3′) (3); the middle segment was amplified with primers ER-2a(R) (5′-CCCGTAAGTTAGGTGTG-3′) and ER-X (5′-CATCTCACGACACGAGC-3′); and the 3′ segment was amplified with primers ER-Y (5′-CCAACACAGGTGTTGC-3′) and ER-Z2 (5′-ACCCCAGTCACCCACCCC-3′). The cycling conditions used were the same as those described above for the standard nested PCR except that annealing was performed at 52°C and each 72°C extension step was 2 min long.
Nested PCR products of the 16S rRNA, groESL, and 51-kDa major antigen genes were purified by spin chromatography (PCR SELECT-II spin columns; 5 Prime → 3 Prime, Inc., Boulder, Colo.) and were cloned by using the pNoTA/T7 shuttle vector and competent E. coli (Prime PCR Cloner cloning system; 5 Prime → 3 Prime). Double-stranded maxiprep DNA was isolated with a PERFECTprep plasmid DNA preparation kit (5 Prime → 3 Prime). One microgram of maxiprep DNA was used for restriction endonuclease digestion with BamHI (New England Biolabs, Beverly, Mass.) to verify the insert sizes. The inserts were sequenced by using the universal M13 sequencing primers present in the vector. Sequencing was performed with a fluorescence-based automated sequencing system (Applied Biosystems, Foster City, Calif.). The sequences in both directions were obtained for all inserts. Three cycles of cloning and sequencing were performed to verify the sequence and the repeatability of the procedure.
Nucleotide sequence accession numbers.
Most of the 16S rRNA gene sequences used were obtained from the GenBank database, and the accession numbers were as follows: E. risticii Illinois (type strain), M21290; SF agent, U34280; E. sennetsu, M73225; N. helminthoeca, U12457; Ehrlichia canis, M73221; Ehrlichia chaffeensis, U23503; Ehrlichia equi, M73223; Ehrlichia ewingii, M73227; HGE agent, U02521; Anaplasma marginale, M60313; Cowdria ruminantium, U03776; Rickettsia rickettsii, U11021; and Rickettsia prowazekii, M21789. The sequences of the Kentucky, Ohio 081, and SRC strains of E. risticii were obtained from previously published sources (21, 42). The GenBank accession numbers for the groESL and 51-kDa major antigen gene sequences of E. risticii were U96732 and U85784, respectively. Other relevant groESL sequences used were the groESL sequences of E. sennetsu (GenBank accession no. U88092), E. chaffeensis (L10917), E. canis U96731 and C. ruminantium (U13638). The groESL and 51-kDa major antigen gene sequences of the SRC agent were determined in our laboratory at the University of California, Davis.
Phylogenetic analysis of sequence data.
The sequences were subjected to a BLAST analysis (1) performed with GenBank nucleic acid sequences to determine similarity ranks, levels of identity, and deduced amino acid sequences (the latter for the groESL protein and the 51-kDa major antigen only). Multiple-sequence alignments of the 16S rRNA genes were constructed with CLUSTAL W (40). A phylogenetic analysis was performed by using the DNAML maximum-likelihood method of PHYLIP (14).
RESULTS
Initial PCR screening.
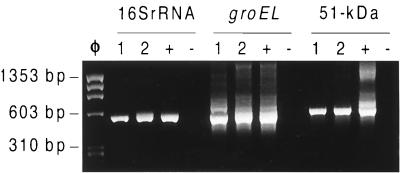
During the initial screening by nested PCR (5′ segment of the 16S rRNA gene), two positive pools, which consisted of 8 and 10 pleurocerid snails of the genus Juga, were identified (Fig. 2). The positive pools were designated Shasta snail-1 and Shasta snail-2. The minimum percentage of Juga spp. harboring the microorganism in the population studied was 3.5% (2 of 57 snails). Pools derived from lymnaeid, physid, and planorbid snails produced negative PCR results.
FIG. 2.
E. risticii nested PCRs performed for the 16S rRNA, groEL (of groESL), and 51-kDa antigen genes by using DNA from the Shasta snail-1 (lanes 1) and Shasta snail-2 (lanes 2) pools and positive (lanes +) and negative (lanes −) E. risticii DNA controls. Lane φ, φX174 replicative-form DNA HaeIII digest (molecular size marker).
16S rRNA gene.
Nearly complete 16S rRNA genes (length, ca. 1,440 bp) from the two positive snail pools were cloned, sequenced, and compared to previously described sequences of E. risticii strains (Table 2). Both Shasta snail-1 and Shasta snail-2 sequences were closely related to the sequence of the SRC agent, a strain of E. risticii identified in May 1996 in blood buffy-coat cells of an affected horse at Mt. Shasta City, Calif., a few miles from the pasture where the snails were collected (21). The Shasta snail-2 sequence differed from the E. risticii sequence by a single nucleotide (position 828), while the Shasta snail-1 sequence differed by two nucleotides (positions 36 and 309). The next most closely related sequence was that of E. risticii Kentucky. As expected, E. sennetsu and the SF agent, both of which are closely related to E. risticii, exhibited similarity to the snail ehrlichiae (Table 3). N. helminthoeca, the agent of salmon poisoning, has been found in snails of the genus Juga but was clearly different from the snail ehrlichiae (94 to 95% identity). The most closely related arthropod-borne agent was A. marginale (level of identity, ca. 85%).
TABLE 2.
Nucleotide differences in the 16S rRNA genes of the Shasta snail-1 and Shasta snail-2 ehrlichiae and four strains of E. risticii (Illinois, SRC agent, Kentucky, Ohio 081)
| Strain or pool | Nucleotide at positiona:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 | 76 | 77 | 90 | 92 | 97 | 131 | 309 | 619 | 828 | 956 | 971 | 1221 | 1231 | 1246 | |
| Illinois | G | G | G | C | T | C | G | A | G | A | T | G | C | C | G |
| SRC agentb | • | • | • | • | • | • | • | • | • | • | C | • | T | T | A |
| Shasta snail-1 | A | • | • | • | • | • | • | G | • | • | C | • | T | T | A |
| Shasta snail-2 | • | • | • | • | • | • | • | • | • | G | C | • | T | T | A |
| Kentuckyc | • | • | • | • | • | A | A | • | • | • | C | • | T | • | A |
| Ohio 081c | • | A | A | T | C | • | • | • | T | • | C | A | T | T | A |
TABLE 3.
Comparison of the Shasta snail-1 and Shasta snail-2 16S rRNA gene sequences with the 16S rRNA gene sequences of other rickettsiae
| Strain or agent | % Identity toa:
|
|
|---|---|---|
| Shasta snail-1 sequence | Shasta snail-2 sequence | |
| SRC agent | 99.86 | 99.93 |
| E. risticii Kentucky | 99.65 | 99.72 |
| E. risticii Illinois | 99.58 | 99.65 |
| E. risticii Ohio O81 | 99.44 | 99.51 |
| SF agentb | 99.02 | 99.09 |
| E. sennetsu | 99.02 | 99.09 |
| N. helminthoeca | 95.23 | 94.43 |
| A. marginale | 85.93 | 85.94 |
Values were derived from a BLAST search.
Ehrlichia isolated from the fluke Stellantchasmus falcatus (43).
groESL heat shock operon.
The gene fragments amplified from both snail pools (Fig. 2) were most similar to groESL of the SRC agent and E. risticii (Table 4). The sequences of Shasta snail-1 and the SRC agent were, in fact, identical; the sequence of Shasta snail-2 differed by a single nucleotide. The next most similar sequences, those of E. sennetsu, E. chaffeensis, E. canis, and C. ruminantium, differed more. The deduced amino acid sequences of the GroEL fragments from the SRC agent and Shasta snail-1 were ientical to each other and to the GroEL fragment of E. risticii, indicating that the few nucleotide changes were silent (Fig. 3). For Shasta snail-2, the single added nucleotide resulted in a conservative amino acid substitution (Fig. 3).
TABLE 4.
Comparison of the Shasta snail-1 and Shasta snail-2 partial groESL gene sequences with the partial groESL gene sequences of other rickettsiae
| Organism | % Identity toa:
|
|
|---|---|---|
| Shasta snail-1 sequence | Shasta snail-2 sequence | |
| SRC agent | 100.00 | 99.81 |
| E. risticii | 98.48 | 98.28 |
| E. sennetsu | 96.39 | 96.20 |
| E. chaffeensis | 65.59 | 65.77 |
| E. canis | 64.83 | 64.64 |
| C. ruminantium | 64.26 | 64.07 |
Values were derived from a BLAST search.
FIG. 3.
Deduced amino acid sequences of GroEL segments. Periods indicate conserved positions relative to the E. risticii sequence.
51-kDa major antigen gene.
As expected, the sequences obtained from the snail pools showed greater variation from E. risticii in the 51-kDa major antigen gene sequence than in the more conserved 16S rRNA and groESL sequences. The nucleotide sequences amplified from Shasta snail-1, Shasta snail-2, and the SRC agent were identical, and all three exhibited 91.74% identity to the E. risticii gene sequence deposited in the GenBank database (derived from a Maryland strain). An analysis of the deduced amino acid sequences for Shasta snail-1, Shasta snail-2, and the SRC agent revealed 12 amino acid changes compared to E. risticii, and 6 of the 12 changes were conservative in nature (Fig. 4).
FIG. 4.
Deduced amino acid sequences of E. risticii 51-kDa major surface antigen segments. Periods indicate conserved positions relative to the E. risticii sequence.
Phylogenetic analysis of sequence data.
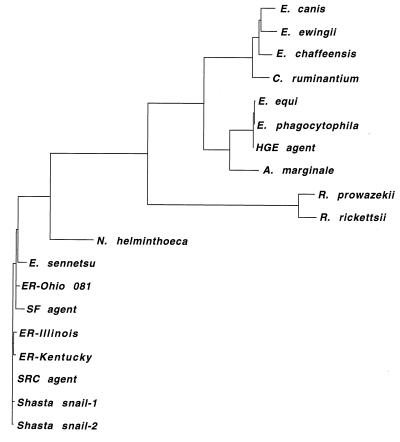
A phylogenetic tree generated by TreeView PPC from the DNAML analysis of the 16S rRNA gene sequences is shown in Fig. 5. The sequences of the snail ehrlichiae were found to cluster with the sequence of the SRC agent and were most closely associated with the Kentucky and Illinois strains of E. risticii.
FIG. 5.
Phylogenetic tree generated by GrowTree from a DNAML-based alignment of 16S rRNA gene sequences (length, ca. 1,400 bp), showing the relationship of the Shasta snail-1 and Shasta snail-2 ehrlichiae to other rickettsiae. ER, Ehrlichia risticii.
DISCUSSION
Here we describe identification of ehrlichia DNA in two pools of pleurocerid snails of the genus Juga collected from stream water in a pasture in Siskiyou County, Calif., in which PHF is enzootic. Determination of the 16S rRNA gene sequences ruled out the possibility that the organism detected was N. helminthoeca, the agent of salmon poisoning, which is related to E. risticii and occurs in snails belonging to the family Pleuroceridae (24, 31). The 16S rRNA gene sequences of both Shasta snail-1 and Shasta snail-2 were virtually identical to the 16S rRNA gene sequence of the SRC agent and were clearly similar to the 16S rRNA gene sequences of other variants of E. risticii reported previously (42). This result was mirrored by the results obtained for the DNA sequences and deduced amino acid sequences of the less conserved groESL heat shock operon and the E. risticii 51-kDa major antigen gene. These data provide evidence that pleurocerid stream snails may play a role in the life cycle of E. risticii and the transmission of PHF in northern California.
Sequences of 16S rRNA genes are known to vary in an orderly fashion throughout the phylogenetic tree and thus are desirable targets for PCR amplification and relatedness testing (26, 44, 45). The variation observed in the 16S rRNA gene sequences obtained from the two snail pools was equal to or less than the variation observed in the sequences of other strains of E. risticii associated with disease in horses (8, 21, 42). This information by itself normally would be sufficient to relate the snail ehrlichiae to E. risticii.
To investigate this relationship further, we sequenced portions of two additional genes, the groESL heat shock operon and the 51-kDa major antigen gene; the latter represents a DNA sequence more specific for E. risticii. Like the 16S rRNA gene, groESL is considered to be a useful molecular clock for phylogenetic studies (17). We found that the sequences of amplified groESL fragments from the snail ehrlichiae and the SRC agent differed by either eight or nine nucleotides from the homologous sequences of E. risticii. The deduced amino acid sequences, however, were identical or nearly identical to the amino acid sequence of E. risticii, indicating that the nucleotide changes in the snail ehrlichiae were for the most part silent. Thus, the GroEL segments of E. risticii, the snail ehrlichiae, and the SRC agent were virtually indistinguishable, providing further evidence of relatedness.
Although 16S rRNA gene and groESL sequences are valuable in studies of phylogenetic relationships, the variation inherent in other genes, particularly the genes coding for antigenic proteins, is more likely to affect such important properties as host range, virulence, and vector competence. Accordingly, we investigated the 51-kDa major antigen gene of E. risticii, whose sequence is not significantly related to any other sequence deposited in GenBank, which makes it, to the best of knowledge, specific for E. risticii. We were able to amplify this gene from both positive snail pools and from the SRC agent; as expected, we found more variation from the E. risticii sequence than we found in either the 16S rRNA gene sequence or the groESL sequence. The deduced amino acid sequences of the snail ehrlichiae and the SRC agent were identical but differed from the E. risticii deduced amino acid sequence at 12 positions, and 6 of the 12 changes were conservative (Fig. 4). Variation in antigenic proteins is a widespread and common characteristic of many bacteria, including E. risticii (8, 12, 13, 41). For example, there are strains of E. risticii which have been described that differ in their antigenic components but exhibit identity at the 16S rRNA gene level (41). Such findings are in accord with the findings of the present study, particularly in light of the sequences obtained from the SRC agent and the known association of the SRC agent with equine diarrheal disease (21).
Whether the pleurocerid snail itself or another agent harbored by the snail is the actual vector of the snail ehrlichiae remains unclear. Certainly, a trematode vector is a likely possibility, considering the heavy trematode burden of snails and the historical precedent in the region concerning Nanophyetus salmincola, the vector which transmits the salmon poisoning rickettsia to canids (4, 9, 24, 38). In addition to snails, intermediate hosts of N. salmincola include salmonid fish and the Pacific giant salamander; definitive hosts are represented by mammals and certain fish-eating birds (24, 38). Areas where fluke infestation and hence salmon poisoning are enzootic are determined by the geographical distribution of the host snail. A similar situation occurs with the SF agent, which was isolated from a trematode of gray mullet fish (15, 16, 43). Human disease caused by E. sennetsu has been associated with the consumption of gray mullet (15, 16). The SF agent causes mild clinical signs of fever in dogs (16), while E. sennetsu can establish infections in horses but evidently is not pathogenic in this species (36).
Several trematodes that occur in western pleurocerids and could potentially serve as vectors for ehrlichiae have been described. The trematode Acanthatrium oregonense, for example, produces virgulate cercariae that penetrate the gills of caddisfly larvae (order Trichoptera) (6, 7). The insects are eaten by bats, the definitive hosts, in which the adult flukes develop in the intestine. Many caddisfly larvae were observed in close association with Juga spp. in the stream water described here, and bats are known to be common in the area. In our laboratory at the University of California, Davis, we have observed many virgulate and rare furcocercous cercariae in secretions of Juga specimens from the Siskiyou County site that have been maintained in aquarium culture (32). Cercariae characteristic of N. salmincola were not observed in these secretions. Horses could conceivably be exposed to E. risticii by consuming infected cercariae (which are released from the snails after a period of environmental warming and so are available to horses in drinking water at certain times of the year), either free in the water or encysted on vegetation or in a second intermediate host, such as an insect. The seasonality of cercaria release from Juga spp. could explain the widespread seasonal occurrence of PHF only in late spring, summer, and early fall.
The known geographical distribution of Juga spp. encompasses northern California, northern Nevada, Oregon, and Washington (5), an area similar to the geographical distribution of the pleurocerid host of N. salmincola. If snails play a role in the life cycle of a trematode vector of E. risticii in other areas of the United States, different snails certainly must be involved. Pleurocerid genera in the midwestern and eastern states that might be examined for E. risticii include the genera Elimia, Pleurocera, Lithasia, and Gyrotoma (5). We have recently identified a putative E. risticii strain, resembling Ohio 081 in its 16S rRNA gene sequence, in a pool of lymnaeid snails (Stagnicola spp.) collected from an irrigation canal at Klamath Falls, Oreg., 70 miles north of the Siskiyou County site (32). The distribution of the genus Stagnicola is particularly wide, so members of this genus could provide a separate line of investigation in other areas of the United States where PHF occurs. It is possible that strains of ehrlichiae that cause PHF occur in association with a variety of snail genera, a host range that may be reflected in the genetic and antigenic variation commonly observed among E. risticii isolates. Thus, the “agent” of PHF may actually be an array of closely related ehrlichia strains that differ in their vectors and snail hosts and, perhaps, in their pathogenicities for horses as well.
ACKNOWLEDGMENTS
We thank Stacia Hoover, Eric Bowman, and Elfriede DeRock for laboratory assistance; Norm Anderson for help with snail identification; Jerry Fields for help with molluscan photography; Jerry Theis, Steve Dumler, Ron Hedrick, Janet Foley, and Walter Boyce for useful discussions; and Tom Sampson, Mike Ronne, Jon Goodell, Carie Owings, Bob Cook, Wayne Merhoff, John Ballestin, Harry Sumner, Inderpal K. W. Singh, and Tim and Christi Saltonstall for valuable contributions to our studies of SRC and PHF.
This work was supported by grants from the Center for Equine Health, University of California, Davis, with funds provided by the Oak Tree Racing Association, by the State of California satellite wagering fund, and by contributions from private donors; and by discretionary funds from the Department of Medicine and Epidemiology, School of Veterinary Medicine, University of California, Davis.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 3.Barlough J E, Rikihisa Y, Madigan J E. Nested polymerase chain reaction for detection of Ehrlichia risticii genomic DNA in infected horses. Vet Parasitol. 1997;68:367–373. doi: 10.1016/s0304-4017(96)01083-7. [DOI] [PubMed] [Google Scholar]
- 4.Bennington E, Pratt I. The life history of the salmon-poisoning fluke, Nanophyetus salmincola (Chapin) J Parasitol. 1960;46:91–100. [PubMed] [Google Scholar]
- 5.Burch J B. North American freshwater snails. Hamburg, Mich: Malacological Publications; 1989. [Google Scholar]
- 6.Burns W C. Six virgulate xiphidiocercariae from Oregon, including redescriptions of Allassogonoporus vespertilionis and Acanthatrium oregonense. J Parasitol. 1961;47:919–925. [PubMed] [Google Scholar]
- 7.Burns W C. Penetration and development of Allassogonoporus vespertilionis and Acanthatrium oregonense (Trematoda: Lecithodendriidae) cercariae in caddis fly larvae. J Parasitol. 1961;47:927–932. [PubMed] [Google Scholar]
- 8.Chaichanasiriwithaya W, Rikihisa Y, Yamamoto S, Reed S, Crawford T B, Perryman L E, Palmer G E. Antigenic, morphologic, and molecular characterization of new Ehrlichia risticii isolates. J Clin Microbiol. 1994;38:3026–3033. doi: 10.1128/jcm.32.12.3026-3033.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordy D R, Gorham J R. The pathology and etiology of salmon disease in the dog and fox. Am J Pathol. 1950;26:617–637. [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon R T. Karyotypic evolution in pleurocerid snails. II. Pleurocera, Goniobasis, and Juga. Malacologia. 1991;33:339–344. [Google Scholar]
- 11.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S K, Shankarappa B, Mattingly-Napier B L. Molecular cloning and analysis of recombinant major antigens of Ehrlichia risticii. Infect Immun. 1991;59:1162–1169. doi: 10.1128/iai.59.3.1162-1169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta S K, Shankarappa B, Thaker S R, Mattingly-Napier B L. DNA restriction endonuclease cleavage pattern and protein antigen profile of Ehrlichia risticii. Vet Microbiol. 1990;25:29–38. doi: 10.1016/0378-1135(90)90090-i. [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein J. PHYLIP—phylogeny inference package. Cladistics. 1989;5:164–166. [Google Scholar]
- 15.Fukuda T, Sasahara T, Kitao T. Studies on the causative agent of “Hyuganetsu” disease. X. Vector. J Jpn Assoc Infect Dis. 1972;36:235–241. . (In Japanese.) [Google Scholar]
- 16.Fukuda T, Yamamoto S. Neorickettsia-like organism isolated from metacercaria of a fluke, Stellantchasmus falcatus. Jpn J Med Sci Biol. 1981;34:103–107. doi: 10.7883/yoken1952.34.103. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R S. Evolution of the chaperonin families (Hsp60, Hsp10 and Tcp-1) of proteins and the origin of eukaryotic cells. Mol Microbiol. 1995;15:1–11. doi: 10.1111/j.1365-2958.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 18.Hahn N E, Fletcher M, Rice R M, Kocan K M, Hansen J W, Hair J A, Barker R W, Perry B D. Attempted transmission of Ehrlichia risticii, causative agent of Potomac horse fever, by the ticks, Dermacentor variabilis, Rhipicephalus sanguineus, Ixodes scapularis, and Amblyomma americanum. Exp Appl Acarol. 1990;8:41–50. doi: 10.1007/BF01193380. [DOI] [PubMed] [Google Scholar]
- 19.Kahler S. Transmission is unsolved mystery of equine monocytic ehrlichiosis. J Am Vet Med Assoc. 1989;194:1681–1687. [PubMed] [Google Scholar]
- 20.Knowles R C, Anderson C W, Shipley W D, Whitlock R H, Perry B D, Davidson J P. Proceedings of the 29th Annual Meeting of the American Association of Equine Practitioners, Lexington, Ky. 1983. Acute equine diarrhea syndrome (AEDS): a preliminary report; pp. 353–357. [Google Scholar]
- 21.Madigan J E, Barlough J E, Rikihisa Y, Wen B, Miller P E, Sampson T J. Identification of an enzootic diarrhea (“Shasta River crud”) in northern California as Potomac horse fever. J Equine Vet Sci. 1997;17:270–272. [Google Scholar]
- 22.Madigan J E, Cohen M, Stabbe M T, True R G, Wohlford L A. Serologic evidence of Potomac horse fever (Ehrlichia risticii) in three California horses with enterocolitis and fever. Calif Vet. 1987;41:8–10. [Google Scholar]
- 23.Madigan J E, Rikihisa Y, Palmer J E, DeRock E, Mott J. Evidence for a high rate of false-positive results with the indirect fluorescent antibody test for Ehrlichia risticii antibody in horses. J Am Vet Med Assoc. 1995;207:1448–1453. [PubMed] [Google Scholar]
- 24.Millemann R E, Knapp S E. Biology of Nanophyetus salmincola and “salmon poisoning” disease. Adv Parasitol. 1970;8:1–41. [PubMed] [Google Scholar]
- 25.Mott J, Rikihisa Y. Polymerase chain reaction and Southern blot analysis of Ehrlichia risticii in the blood and feces of horses. J Clin Microbiol. 1997;35:2215–2219. [Google Scholar]
- 26.Olson G J, Woese C R. Ribosomal RNA: a key to phylogeny. FASEB J. 1993;7:113–123. doi: 10.1096/fasebj.7.1.8422957. [DOI] [PubMed] [Google Scholar]
- 27.Palmer J E. Potomac horse fever. Vet Clin North Am Equine Pract. 1993;9:399–410. doi: 10.1016/s0749-0739(17)30406-6. [DOI] [PubMed] [Google Scholar]
- 28.Palmer J E, Benson C E. Studies on oral transmission of Potomac horse fever. J Vet Inter Med. 1994;8:87–92. doi: 10.1111/j.1939-1676.1994.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 29.Perry B D, Rikihisa Y, Saunders G. Transmission of Potomac horse fever by intradermal route. Vet Rec. 1985;116:246–247. doi: 10.1136/vr.116.9.246. [DOI] [PubMed] [Google Scholar]
- 30.Perry B D, Schmidtmann E T, Rice R M, Hansen J W, Fletcher M, Turner E C, Robl M G, Hahn N E. Epidemiology of Potomac horse fever: an investigation into the possible role of non-equine mammals. Vet Rec. 1989;125:83–86. doi: 10.1136/vr.125.4.83. [DOI] [PubMed] [Google Scholar]
- 31.Pretzman C, Ralph D, Stothard D R, Fuerst P A, Rikihisa Y. 16S rRNA gene sequence of Neorickettsia helminthoeca and its phylogenetic alignment with members of the genus Ehrlichia. Int J Syst Bacteriol. 1995;45:207–211. doi: 10.1099/00207713-45-2-207. [DOI] [PubMed] [Google Scholar]
- 32.Reubel G H, Barlough J E, Madigan J E. Production and characterization of Ehrlichia risticii, the agent of Potomac horse fever, from snails (Pleuroceridae: Juga spp.) in aquarium culture and genetic comparison to equine strains. J Clin Microbiol. 1998;36:1501–1511. doi: 10.1128/jcm.36.6.1501-1511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rikihisa Y. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J Clin Microbiol. 1991;29:2024–2029. doi: 10.1128/jcm.29.9.2024-2029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rikihisa Y, Perry B D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985;49:513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikihisa Y, Perry B D, Cordes D O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985;49:505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikihisa Y, Pretzman C I, Johnson G C, Reed S M, Yamamoto S, Andrews F. Clinical and immunological responses of ponies to Ehrlichia sennetsu and subsequent Ehrlichia risticii challenge. Infect Immun. 1988;56:2960–2966. doi: 10.1128/iai.56.11.2960-2966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidtmann E T, Robl M G, Carroll J F. Attempted transmission of Ehrlichia risticii by field-captured Dermacentor variabilis (Acari: Ixodidae) Am J Vet Res. 1986;47:2393–2395. [PubMed] [Google Scholar]
- 38.Simms B T, Donham C R, Shaw J N, McCapes A M. Salmon poisoning. J Am Vet Med Assoc. 1931;78:181–195. [Google Scholar]
- 39.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improved sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemulapalli R, Biswas B, Dutta S K. Pathogenic, immunologic, and molecular differences between two Ehrlichia risticii strains. J Clin Microbiol. 1995;33:2987–2993. doi: 10.1128/jcm.33.11.2987-2993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen B, Rikihisa Y, Fuerst P A, Chaichanasiriwithaya W. Diversity of 16S rRNA genes of new Ehrlichia strains isolated from horses with clinical signs of Potomac horse fever. Int J Syst Bacteriol. 1995;45:315–318. doi: 10.1099/00207713-45-2-315. [DOI] [PubMed] [Google Scholar]
- 43.Wen B, Rikihisa Y, Yamamoto S, Fuerst P. Characterization of the SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol. 1996;46:149–154. doi: 10.1099/00207713-46-1-149. [DOI] [PubMed] [Google Scholar]
- 44.Wilson K H, Blitchington R B, Greene R C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 1990;28:1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]