Abstract
The aim of the current study was to test the technical and clinical feasibility of a robotic system and investigate its potential in the surgical repair of perforated Schneiderian membranes using an ex-vivo porcine model. Eight pig heads were operated conventionally via a surgical loop and eight pig heads with the surgical robot “Symani® Surgical System” (Medical Microinstruments, Inc., Pisa, Italy). On each specimen, the Schneiderian membrane was incised over a length of 0.7 mm resembling a perforation. Operation time, the maximum sinusoidal pressure, the course of the pressure and the filling volume were measured. Additionally, adaptation of the wound edges has been detected via scanning electron microscopy. There were no significant differences for the pressure maximum (p = 0.528), for the time until the pressure maximum was reached (p = 0.528), or for the maximum filling volume (p = 0.674). The time needed for the suturing of the membrane via robotic surgery was significantly longer (p < 0.001). However, the scanning electron microscope revealed a better adaptation of the wound edges with robotic surgery. The technical feasibility of robot-assisted suturing of Schneiderian membrane laceration using the robotic system has been confirmed for the first time. No differences considering the pressure resistance compared to the conventional repair could be observed, but advantages in wound adaptation could be found with an electron microscope. Regarding the material and training costs and limited indications spectrum, robotic surgery systems still might not present financially feasible options in the daily dental practice yet.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11701-023-01721-9.
Keywords: Dentistry, Implantology, Robotic surgery, Schneiderian membrane
Introduction
Due to the lack of sufficient bone volume secondary to the atrophy of alveolar bone and sinus pneumatization, rehabilitation of the posterior maxilla with dental implants poses a special challenge for dental professionals [1]. A well-established technique with several modifications to overcome this problem is the grafting of the maxillary sinus after the elevation of the Schneiderian membrane, which is called as sinus lift surgery. The procedure is mainly classified into crestal (internal sinus lift) and lateral (lateral sinus lift) approaches regarding the residual bone height. In cases where the residual bone height is 5 mm or less, the lateral approach, which is performed with exploration and elevation of the sinus membrane throughout a bony window, is recommended.
Despite high success rates, intraoperative complications could jeopardize the wound healing at the grafted maxillary sinus. A normal sinus membrane thickness is only 0.3–0.8 mm, and the most common intraoperative complication is the laceration of the membrane, which could negatively affect the graft's stabilization, lead to displacement and infection of the bone graft [2]. The incidence of perforations has been stated to occur with a range from 22 to 50% [3, 4]. Additionally, Nolan et al. have stated that perforated sinuses presented three times the risk of bone graft failure and six times the incidence of maxillary sinusitis compared with non-perforated sinuses [5]. Therefore, it is necessary to be aware of the various methods for avoiding or closing perforations.
In the literature, various methods have been proposed for the intraoperative management of membrane repair including suturing with resorbable materials, sealing with fibrin glue [6], the use of collagen membrane with or without combination with platelet-rich-fibrin [7] and augmentations with a buccal fat pad or laminar bone block [3]. When perforations are smaller than 5 mm, the most widely recommended treatment is the repair with collagen membrane [8], whereas moderate perforations (5–10 mm) should be managed with resorbable sutures before the collagen membrane has been placed [6]. However, the strength of the suture and the sufficient adaptation of the rupture edges play a key role in the prognosis, thus, the closure should withstand the intra-sinusoidal pressure and prevent graft particles from escaping into the sinus during the healing phase.
The technical and clinical feasibility of robot-assisted suturing in different disciplines has become the main subject of recent studies [9]. The “Symani® Surgical System” (Medical Microinstruments, Pisa, Italy) is a surgical robot, designed for microsurgery as free flap reconstructions, lymphatic surgery, nerve reconstruction or replantation. The system consists of two robotic arms on which microinstruments of a few millimetres in size can be placed. A 7–20 × motion scaling is intended to enable freedom from tremors in microsurgery, the instruments guarantee seven degrees of freedom. The surgeon operates from a console consisting of a chair, two joysticks and a foot switch. The use of the system in the maxillary sinus has not been presented until now. The aim of the current study was to test the technical feasibility of the Symani Surgical System in surgical repair of perforated Schneiderian membranes using an ex-vivo porcine model and investigate its potential advantages.
Material and methods
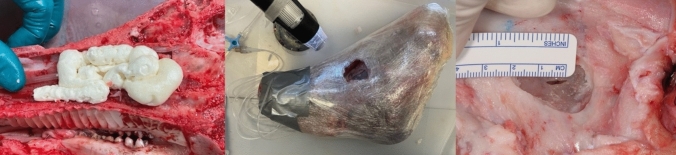
A total of 16 pig head halves were obtained commercially from a slaughterhouse in Kiel (age 16–18 months). Eight head halves were operated conventionally (free hand via a surgical loop) whereas a surgical robot “Symani® Surgical System” (Medical Microinstruments, Inc., Pisa, Italy) was used in eight specimens. In both groups, a bone window of approximately 2.5 × 1.5 × 3 cm was created on each pig head in the area of the sinus maxillaris. The Schneiderian membrane was mobilized as described by Tatum [10]. After the preparation was completed, a control measurement of the maxillary sinus pressure was taken to ensure the integrity of the mucosa. At least a pressure of 3.4 kPa was built up on the Schneiderian membrane via a perfusor and the continuity of the membrane has been approved. For this purpose, each head half was wrapped with plastic wrap for an additional seal. The bone hole of the sinus maxillaris with the Schneider's membrane was cut free for surgery and measurement. For insufflation and pressure measurement, a tube was inserted over the concha nasalis media and sealed airtight with construction foam. After that, the Schneiderian membrane was incised over a length of 0.7 mm resembling a membrane laceration. This perforation had to be closed again surgically with one of the surgical procedures (Fig. 1). To minimize the decomposition of the material during the sample preparation process for SEM and to reduce the friction when passing through the fragile membrane, a none-resorbable monofilament with very smooth surface characteristics (9–0 Prolene, Ethicon, Johnson & Johnson Medical GmbH, Norderstedt, Germany) has been selected for the repair.
Fig. 1.
Illustration of the airtight seal with construction foam as well as plastic wrap for measuring the pressure without any leakage and the perforation of 0.7 mm
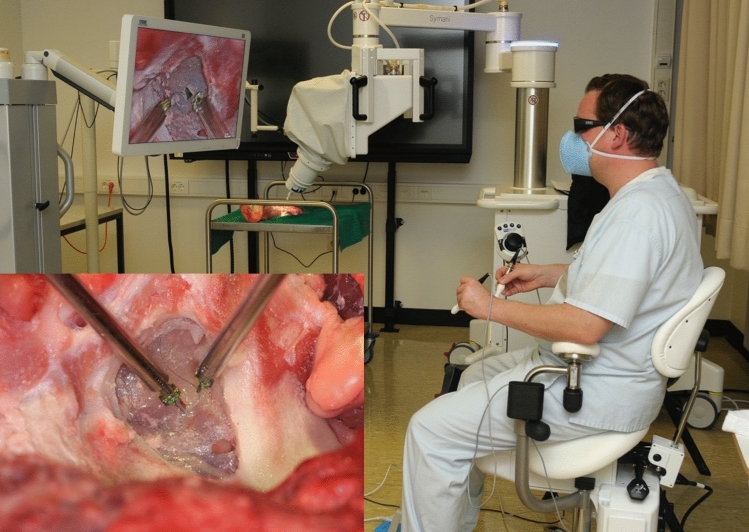
The surgical closure of the perforation was achieved in both surgical procedures using 9–0 Prolene (Ethicon, Johnson & Johnson Medical GmbH, Norderstedt, Germany). All operations were performed by a single experienced surgeon for comparability. The surgeon had experience in both operation procedures. A needle holder, surgical forceps, and magnifying glasses with × 2.5 magnification were available for conventional surgery. For robotic-assisted surgery, the NanoWrist Needle Holder and Dilator microinstruments with 3 mm wrists were used (Fig. 2).
Fig. 2.
For robotic-assisted surgery, the NanoWrist Needle Holder and Dilator microinstruments with 3 mm wrists were used
For better comparability of the two surgical procedures, a continuous suture was preferred, which had also less tendency for punctual leakages during pressure measurement. For the measurement, the tube was connected via a three-way valve to the Syramed µSP6000 perfusor (Arcomed AG Medical Systems, Zurich, Switzerland) and to the GM520 manometer (Bestone Industrial Ltd., Shenzhen, China), which is connected to a laptop for recording the pressure course For each specimen, pressure curve in the maxillary sinus was documented by the manometer, which was connected to the measuring system via the three-way valve, and the maximum pressure was determined before the suture of the Schneiderian membrane ruptured (Video 1).
In addition, the sutured laceration was examined with the scanning electron microscope XL30CP (Philips Electron Optics GmbH, Kassel, Germany). For this purpose, the membrane was first washed with phosphate-buffered saline and then dehydrated in an ascending alcohol series (2 × 10 min. 50%, 1 × 10 min. 60%, 2 × 10 min. 70%, 2 × 10 min. 90%, 3 × 10 min. 100%). After that, hexamethyldisilazane was applied for a few seconds. The membranes were post-dried in a desiccator overnight and then vaporized with 10 nm gold during sputtering (SCD 500, Bal-Tec, Balzers, Lichtenstein). The imaging was performed at a voltage of 10 kV under the scanning electron microscope.
Statistical analysis
Statistical analyses were performed using SPSS (IBM®, Ehningen, Germany). The average values for the pressure maximum, the time until the pressure maximum was reached, the maximum filling volume, and the operation time were calculated (mean, standard deviation). The relation between variables was evaluated by Mann–Whitney-U Test. Associations were considered significant when the p value was < 0.05.
Results
Pressure measurement
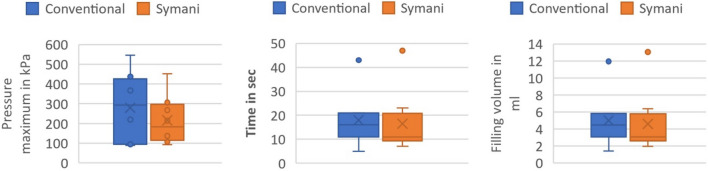
The mean values and the standard deviations for the pressure maximum, the time until the pressure maximum was reached, the filling volume, and the operation time are shown in Table 1 as well as in Fig. 3. No statistical significance was present among both groups regarding the above-mentioned pressure parameters.
Table 1.
Imaged are the mean values and the standard deviations (SD) for both operation procedures
| Pressure maximum (Mean ± SD) |
Time until pressure maximum was reached (Mean ± SD) | Filling volume (Mean ± SD) |
Operation time (Mean ± SD) |
|
|---|---|---|---|---|
| Conventional | 279 ± 159 kPa | 18 ± 11 s | 4.995 ± 3.002 ml | 3:31 ± 1:32 min |
| Symani | 215 ± 114 kPa | 16.5 ± 12 s | 4.579 ± 3.441 ml | 7:39 ± 2:32 min |
Fig. 3.
Box plots of the pressure maximum in kPa, the time in seconds until the pressure maximum was reached and the maximum filling volume in ml
Table 1 Imaged are the mean values and the standard deviations (SD) for both operation procedures. The pressure maximum, the time until the pressure maximum was reached the filling volume as well as the operation time were calculated.
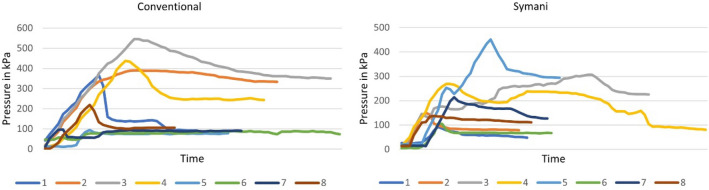
There were no significant differences for the pressure maximum (p = 0.528), for the time until the pressure maximum was reached (p = 0.528) or for the maximum filling volume (p = 0.674) (Fig. 3). The time needed for the suturing of the membrane was significantly longer in the robotic surgery group (p < 0.001). The pressure curve for each measurement has been shown in Fig. 4.
Fig. 4.
The pressure curve for all eight measurements using the conventional surgical method as well as the Symani
SEM evaluation
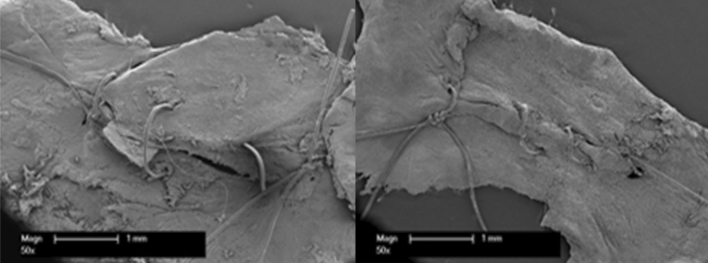
The quantification via scanning electron microscope (SEM) revealed a relatively better adaptation of the wound edges in the study group (Fig. 5).
Fig. 5.
The scanning electron microscope revealed a better occlusion of the wound edges with robotic surgery (right) compared to the conventional technique
Discussion
Robotic surgery is a modern minimally invasive procedure that allows the reduction of morbidity through the minimally invasive approach with excellent optical representation of the surgical field [11]. In terms of functional outcomes, robotic surgery may show advantages in reducing morbidity in patients with cancer of the upper digestive and respiratory tracts [10]. It was originally developed for the disciplines such as general surgery and urology; however, it has gained popularity also in different surgical fields. Transoral robotic surgery (TORS) has been an established procedure since 2009 and the indication for robotic surgery with minimally invasive technique is increasing in the field of oral and maxillofacial surgery [9]. Moreover, TORS has advantages, such as that the robotic arms can be moved to various angles even within confined spaces of the maxillofacial region, as well as smaller and smaller operation procedures could be performed and operative complications could be prevented [12]. Initial concerns about robotic surgery for oral and maxillofacial surgery were about visualization, possible damage to vital structures, and the limited availability of effective instrumentation [6]. However, the system used for the study was developed specifically for the smallest anatomical structures in oral and maxillofacial surgery and plastic surgery, such as small anatomizes, to overcome the limits of manual precision and physiological tremor [7]. Despite the high fragility of the Schneiderian membrane, robotic surgery-assisted repair resulted in qualitatively better results compared to the conventional technique.
The management of intraoperative complications via using robotic systems has also become the subject of experimental studies. Kupferman et al. have used a robotic system for the repair of dura defects and reported successfully results [13]. Veleur et al. have reported the use of robot-assisted surgery for several categories of middle ear procedures including the repair of the tympanic membrane ruptures [14]. Perforation of the Schneiderian membrane is a common complication of sinus floor augmentation, with an incidence of 10.0–35.9% [15] and poses a great challenge for both dental and ENT clinicians and the efficiency of perforation’s closure plays a great role in the prognosis of the procedure, especially after grafting of the maxillary sinus [16]. Covering small perforations with resorbable membranes or fibrin glue and suturing are the main intraoperative solutions [17–19]. The results of the current study showed that robotic technology can be successfully implemented in Schneiderian membrane repair with efficient occlusion of the wound edges.
It is well known that the injuries or surgical interventions of the maxillary sinus could jeopardize the integrity of the Schneiderian membrane and air could penetrate the subcutaneous tissues. In some cases, if the air pressure in the maxillary sinus increases with sneezing, coughing or nose blowing, a traumatic subcutaneous emphysema could also occur [20]. Therefore, it is important to advise patients to avoid increasing the intraoral pressure after sinus-lift procedure [21]. Wu et al. have quantified sinus pressures along the skull base during sneezing and stated that the anterior nasal pressure was 3153.22 ± 957.36 Pa [22]. The results of the current study confirmed that the leakage after achieving the sinusoidal peak pressure was similar in both groups. Due to the limitations of the experimental model described herein, it should be mentioned that the pressure after reconstruction with both techniques remained significantly under the nasal pressure reached by sneezing, however, it is not possible to speculate on the efficiency of the performed reconstruction regarding the physiological limits.
Robotic surgery has been criticized for its expense (e.g., an estimated increase of $1500 to $2000 per patient) [23], however, the global surgical robots market size was valued at USD 4.4 billion in 2022 and is expected to expand at a compound annual growth rate of 18.0% from 2023 to 2030 [24]. MMI's complete Symani Surgical System including cart, console and NanoWrist instruments requires an investment of approximately 900,000 euro [25]. Due to their high costs and limited indication spectrum in dentistry, the use of robotic systems in daily dental practice is financially not feasible. Besides that, extensive training and intensive education are necessary to achieve optimal surgical results [26]. It also takes a long time to establish robotic surgery, although this procedure shows a steep learning curve for both young and experienced surgeons in many operation cases [27]. Barbon et al. already described the steep learning curve for anastomotic reconstructions of the Symani and stated that the system enables promising potential for limits in reconstructive microsurgery, such as reliably performing anastomoses on small blood and lymph vessels or on structures deeper in the body cavities [28]. Therefore, the feasibility regarding both the material costs and the steep learning curve should be taken into consideration before the deployment of the system in the field of dentistry.
Time pressure is the most common stressor on dental professionals’ performance [29]. Due to the patients’ demands regarding re-establishment of function, phonation and aesthetics within the shortest possible time, rapid rational concepts are increasingly becoming the preferred treatment option in the dental implantology practice [30]. It has been previously also demonstrated that the feasibility of performing precise micro sutures and anastomoses robotic procedures were longer in time using the Symani system, but showed greater precision compared to standard manual techniques [31]. Our results have also shown that, despite the high experience of the surgeon, the time needed for the suturing with robotic surgery was significantly longer compared to the conventional technique and could not offer an advantage for the dental clinicians, who are already working under time pressure.
Conclusion
To the best of our knowledge, the technical feasibility of robot-assisted suturing of Schneiderian membrane laceration using the Symani Surgical System has been performed for the first time in the literature. Robotic suturing required a longer time to complete but was superior to the manual procedure regarding the SEM data collected. However, no differences considering the pressure resistance compared to the conventional repair could be observed. Regarding the material and training costs and limited indications spectrum, robotic surgery systems might not be a feasible option in daily dental practice yet.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the financial support of the Land Schleswig-Holstein within the funding program Open Access Publication Fonds.
Author contributions
A.G has designed the study. H.W. and M.L. have operated on the specimens. The experimental design has been developed by A.G, C.H and M.R. A.G. and J.H.S. have prepared the manuscript. J.W. and Y.A. have assessed the accuracy of the data. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author (A.G.).
Declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethical approval
The study did not require ethics approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Emmert M, Spille J, Behrens E, Ayna M, Karayurek F, Wiltfang J, et al. comparative assessment of the primary stability of straumann BLX implant design using an in vitro sinus lift-simultaneous implant insertion model. J Oral Implantol. 2022;48(4):269–275. doi: 10.1563/aaid-joi-D-20-00411. [DOI] [PubMed] [Google Scholar]
- 2.Kim YK, Ku JK. Sinus membrane elevation and implant placement. J Korean Assoc Oral Maxillofac Surg. 2020;46(4):292–298. doi: 10.5125/jkaoms.2020.46.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Alfaro F, Torradeflot MM, Marti C. Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin Oral Implants Res. 2008;19(1):91–98. doi: 10.1111/j.1600-0501.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 4.Oncu E, Kaymaz E. Assessment of the effectiveness of platelet rich fibrin in the treatment of Schneiderian membrane perforation. Clin Implant Dent Relat Res. 2017;19(6):1009–1014. doi: 10.1111/cid.12528. [DOI] [PubMed] [Google Scholar]
- 5.Nolan PJ, Freeman K, Kraut RA. Correlation between Schneiderian membrane perforation and sinus lift graft outcome: a retrospective evaluation of 359 augmented sinus. J Oral Maxillofac Surg. 2014;72(1):47–52. doi: 10.1016/j.joms.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Beck-Broichsitter BE, Westhoff D, Behrens E, Wiltfang J, Becker ST. Impact of surgical management in cases of intraoperative membrane perforation during a sinus lift procedure: a follow-up on bone graft stability and implant success. Int J Implant Dent. 2018;4(1):6. doi: 10.1186/s40729-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koleilat A, Mansour A, Alkassimi FM, Aguirre A, Almaghrabi B. A combination of platelet-rich fibrin and collagen membranes for sinus membrane repair: a case report (repair of sinus membrane perforation) Dent J (Basel). 2023;11(3):84. doi: 10.3390/dj11030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Olivares LA, Cortes-Breton Brinkmann J, Martinez-Rodriguez N, Martinez-Gonzalez JM, Lopez-Quiles J, Leco-Berrocal I, et al. Management of Schneiderian membrane perforations during maxillary sinus floor augmentation with lateral approach in relation to subsequent implant survival rates: a systematic review and meta-analysis. Int J Implant Dent. 2021;7(1):91. doi: 10.1186/s40729-021-00346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savastano A, Rizzo S. A novel microsurgical robot: preliminary feasibility test in ophthalmic field. Transl Vis Sci Technol. 2022;11(8):13. doi: 10.1167/tvst.11.8.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatum H., Jr Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30(2):207–229. doi: 10.1016/S0011-8532(22)02107-3. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan O, Ozkan O, Cinpolat A, Arici C, Bektas G, Can UM. Robotic harvesting of the omental flap: a case report and mini-review of the use of robots in reconstructive surgery. J Robot Surg. 2019;13(4):539–543. doi: 10.1007/s11701-019-00949-8. [DOI] [PubMed] [Google Scholar]
- 12.Park YM, Choi EC, Kim SH, Koh YW. Recent progress of robotic head and neck surgery using a flexible single port robotic system. J Robot Surg. 2022;16(2):353–360. doi: 10.1007/s11701-021-01221-8. [DOI] [PubMed] [Google Scholar]
- 13.Kupferman ME, Demonte F, Levine N, Hanna E. Feasibility of a robotic surgical approach to reconstruct the skull base. Skull Base. 2011;21(2):79–82. doi: 10.1055/s-0030-1261258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veleur M, Lahlou G, Torres R, Daoudi H, Mosnier I, Ferrary E, et al. Robot-assisted middle ear endoscopic surgery: preliminary results on 37 patients. Front Surg. 2021;8:740935. doi: 10.3389/fsurg.2021.740935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbu HM, Iancu SA, Jarjour Mirea I, Mignogna MD, Samet N, Calvo-Guirado JL. Management of Schneiderian membrane perforations during sinus augmentation procedures: a preliminary comparison of two different approaches. J Clin Med. 2019;8(9):1491. doi: 10.3390/jcm8091491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck-Broichsitter BE, Gerle M, Wiltfang J, Becker ST. Perforation of the Schneiderian membrane during sinus floor elevation: a risk factor for long-term success of dental implants? Oral Maxillofac Surg. 2020;24(2):151–156. doi: 10.1007/s10006-020-00829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz-Arad D, Herzberg R, Dolev E. The prevalence of surgical complications of the sinus graft procedure and their impact on implant survival. J Periodontol. 2004;75(4):511–516. doi: 10.1902/jop.2004.75.4.511. [DOI] [PubMed] [Google Scholar]
- 18.van den Bergh JP, ten Bruggenkate CM, Disch FJ, Tuinzing DB. Anatomical aspects of sinus floor elevations. Clin Oral Implants Res. 2000;11(3):256–265. doi: 10.1034/j.1600-0501.2000.011003256.x. [DOI] [PubMed] [Google Scholar]
- 19.van den Bergh JP, ten Bruggenkate CM, Krekeler G, Tuinzing DB. Maxillary sinusfloor elevation and grafting with human demineralized freeze dried bone. Clin Oral Implants Res. 2000;11(5):487–493. doi: 10.1034/j.1600-0501.2000.011005487.x. [DOI] [PubMed] [Google Scholar]
- 20.Brasileiro BF, Cortez AL, Asprino L, Passeri LA, De Moraes M, Mazzonetto R, et al. Traumatic subcutaneous emphysema of the face associated with paranasal sinus fractures: a prospective study. J Oral Maxillofac Surg. 2005;63(8):1080–1087. doi: 10.1016/j.joms.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Sakakibara A, Suzuki H, Yamashita A, Hasegawa T, Minamikawa T, Furudoi S, et al. Facial emphysema after sinus lift. J Surg Case Rep. 2015;2015(6):067. doi: 10.1093/jscr/rjv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Craig JR, Maza G, Li C, Otto BA, Farag AA, et al. Peak sinus pressures during sneezing in healthy controls and post-skull base surgery patients. Laryngoscope. 2020;130(9):2138–2143. doi: 10.1002/lary.28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun JE, Lee NR, Kwak C, Rha KH, Seo SI, Hong SH, et al. Clinical outcomes and costs of robotic surgery in prostate cancer: a multiinstitutional study in Korea. Prostate Int. 2019;7(1):19–24. doi: 10.1016/j.prnil.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Market Analysis Report. (978-1-68038-811-4):118. https://www.grandviewresearch.com/industry-analysis/surgical-robot-market. Accessed 30 May 2023
- 25.https://www.mmimicrocom/symani-system-overview. Accessed 30 May 2023
- 26.Soomro NA, Hashimoto DA, Porteous AJ, Ridley CJA, Marsh WJ, Ditto R, et al. Systematic review of learning curves in robot-assisted surgery. BJS Open. 2020;4(1):27–44. doi: 10.1002/bjs5.50235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennington Z, Judy BF, Zakaria HM, Lakomkin N, Mikula AL, Elder BD, et al. Learning curves in robot-assisted spine surgery: a systematic review and proposal of application to residency curricula. Neurosurg Focus. 2022;52(1):E3. doi: 10.3171/2021.10.FOCUS21496. [DOI] [PubMed] [Google Scholar]
- 28.Barbon C, Grunherz L, Uyulmaz S, Giovanoli P, Lindenblatt N. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. 2022;34:126–133. doi: 10.1016/j.jpra.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plessas A, Nasser M, Hanoch Y, O'Brien T, Bernardes Delgado M, Moles D. Impact of time pressure on dentists' diagnostic performance. J Dent. 2019;82:38–44. doi: 10.1016/j.jdent.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Ayna M, Karayurek F, Jepsen S, Emmert M, Acil Y, Wiltfang J, et al. Six-year clinical outcomes of implant-supported acrylic vs ceramic superstructures according to the all-on-4 treatment concept for the rehabilitation of the edentulous maxilla. Odontology. 2021;109(4):930–940. doi: 10.1007/s10266-021-00605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballestin A, Malzone G, Menichini G, Lucattelli E, Innocenti M. New robotic system with wristed microinstruments allows precise reconstructive microsurgery: preclinical study. Ann Surg Oncol. 2022;29(12):7859–7867. doi: 10.1245/s10434-022-12033-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (A.G.).