Abstract
Hyphomonas strain VP-6 is a prosthecate bacterium isolated from the Guayamas vent region and is a member of a genus of primary and common colonizers of marine surfaces. It adheres to solid substrata as a first step in biofilm formation. Fine-structure microscopy and the use of specific stains and lectins reveal that it synthesizes two different extracellular polymeric substances (EPS). One is a temporally synthesized, polar holdfast EPS, and the other is a capsular EPS that is present during the complete life cycle and surrounds the entire cell, including the prosthecum. The timing and location of Hyphomonas strain VP-6 EPS elaboration correlate with adhesion to surfaces, suggesting that the EPS serves not only as the biofilm matrix but also as a primary adhesin. The temporality and polarity of VP-6 EPS expression substantially differ from those properties of Hyphomonas strain MHS-3 EPS expression.
This paper reports on a relationship between adhesion and capsule synthesis in the prosthecate, budding, marine bacterium Hyphomonas strain VP-6. There are two separate, but equally important, steps in the adhesion of bacteria at liquid-solid interfaces (15). The first is primary, reversible adhesion that occurs when the cell initially approaches a surface (15, 20). Bacteria have evolved structures designed to span the electrical repulsion barrier between them and a surface, including fimbriae, pili, flagella, and surface polysaccharides. The lengths and diameters of these appendages dictate that they are less susceptible to charge repulsion than are whole cells (14). Adhesion at this stage occurs through van der Waals forces, hydrophobic bonds, and electrostatic interactions (6). After primary adhesion, a cell displays Brownian and/or flagellum-mediated motion but can become easily detached from the surface (14). It is believed that chemotactic sensors are employed by the cells to determine if the attachment site is suitable for growth (10). Should the organism colonize, primary reversible attachment becomes secondary, permanent adhesion. This step is time dependent and may involve the production of additional extracellular polymers (6). At this point, Brownian motion ceases and cells cannot be removed from the surface without the application of significant shear force (14).
Some studies have shown that surface polysaccharides are responsible for the primary adhesion of cells to surfaces (4, 18, 23). However, most report that other structures are involved in primary adhesion. The identity of the polymer responsible for permanent adhesion of cells to surfaces is also a major topic of debate (8). However, it is generally accepted that capsular extracellular polymeric substances (EPS; polysaccharide-based polymers, possibly associated with proteins or lipids) are the main components of biofilm matrixes, serving as the glue that holds the conglomerates of cells together (5, 14). In this context, it is important to understand where and when biofouling bacteria produce surface polysaccharides as well as how many and what types of surface polysaccharides they produce.
Bacteria have evolved various types of surface polysaccharides that mediate adhesion to surfaces (7, 16, 18, 28). The holdfast is an extracellular structure that is usually spatially confined to a single pole of the cell (21). It is typically composed of heteropolysaccharide (18) and has been reported to be a tenacious adhesive (18). While the holdfast does not function as a complete EPS capsule (which serves as a nutrient sink and protects from predation and desiccation), its synthesis does not require nearly as much energy as that required by a full capsule, which needs as much as 62% of that used by the cell at any moment (6).
Electron microscopy has proven to be a powerful tool for investigating surface polysaccharide-mediated adhesion by microorganisms. A study by Marshall and Cruickshank (16) showed that species of Hyphomicrobium and Flexibacter use holdfasts to mediate adhesion to surfaces. Likewise, Fletcher and Floodgate (7) showed that Pseudomonas strain NCMB 2021 produces two separate types of EPS during the adhesion process. One is a hydrophilic polymer involved in primary adhesion, while the other is a hydrophobic EPS produced subsequent to the first polymer, believed to solidify the organism’s attachment to a surface. Thus, scanning and transmission electron microscopy (SEM and TEM, respectively) may be used to elucidate the number of adhesive polymers, their locations on a cell, and their function in the adhesion process.
Plant lectins are also effective tools for investigating surface polysaccharide production. Soybean and wheat germ agglutinins were used to probe the locations of EPS on Rhizobium japonicum (29) and Seliberia stellata (9), respectively. Merker and Smit (18) used wheat germ agglutinin to label the holdfast of Caulobacter crescentus, and workers in our laboratory have used Bauhinia purpuria lectin to label the EPS of another strain of Hyphomonas (17), designated MHS-3.
Hyphomonas spp. have a relatively complex biphasic life cycle. A swarm cell is planktonic and specialized for survival in the pelagic zone, while a prosthecate cell is adherent and adapted for survival in more nutrient-rich biofilms. In a prior study of Hyphomonas strain MHS-3, we showed that it has a single EPS holdfast that is less localized than other holdfasts in that it covers the entire main body of the reproductive cell (23). The MHS-3 holdfast is expressed coincidentally with formation of prosthecal outgrowth (23, 24). Treatment with specific lectins inhibited adhesion. Here we report on the spatial and temporal production of surface polysaccharides by Hyphomonas strain VP-6 and show that its synthesis and spatial arrangement are very different than those of MHS-3. We show that VP-6 synthesizes two EPS structures. One, a holdfast, is expressed during a limited stage of growth and only at one pole. The other, a capsular EPS, is constitutively expressed and surrounds the entire cell.
(A portion of these results was presented at the 96th General Meeting of the American Society for Microbiology (12) and were used by S. Langille in partial fulfillment of the Ph.D. requirements of the University of Maryland, College Park.)
MATERIALS AND METHODS
Strains, culture conditions, and chemicals.
Hyphomonas strain VP-6 was isolated from the Guayamas Basin hydrothermal vent region by H. Jannasch (Woods Hole Oceanographic Institute). VP-6 was stored and enumerated on marine agar (55.1 g/liter) and cultivated aerobically in marine broth 2216 (MB; 37.4 g/liter; Difco Laboratories, Detroit, Mich.) at 25°C. All chemicals were purchased from Sigma Chemical Company (St. Louis, Mo.) or Fisher Biologicals (Melvin, Pa.), unless otherwise noted.
TEM.
TEM was done on a JEM 100CX transmission electron microscope operating at 80 kV with collodion-coated 200-mesh copper grids (EY Labs, San Mateo, Calif.). Middle-logarithmic-phase broth cultures were used for all experiments. Cells used for rosette and holdfast visualization were layered on grids for 1 min and then passed through 3 drops of distilled water (dH2O), blotted dry, and stained for 15 s in a drop of 1% uranyl acetate. EPS were stained with cationic ferritin. The cells were centrifuged and washed once with sterile phosphate-buffered saline (PBS; 8.0 g of NaCl, 2.0 g of KCl, 1.2 g of Na2HPO4, 0.2 g of KH2PO4/liter of dH2O [pH 7.2]). Cells were resuspended in PBS, incubated for 1 h with 1.0 mg of cationic ferritin per ml, washed twice with PBS, and layered on collodion-coated copper grids. Ruthenium red staining was done essentially according to the method of Luft (13). Briefly, the culture was harvested in mid-logarithmic phase and resuspended in a 0.5-mg/ml solution of ruthenium red dissolved in PBS containing 0.9% glutaraldehyde. After 1 h, cells were washed twice with PBS, layered onto collodion-coated grids, and stained for 15 s with 1% uranyl acetate.
SEM.
SEM was done on an Amray 1820D scanning electron microscope operating at 20 kV. Cells attached to surfaces were visualized by coating glass coverslips with MB for 5 min and then layering mid-logarithmic-phase cultures on the coverslips for 60 min. Attached cells were then dehydrated in a graded series of ethanol solutions (75, 90 and 100, 100 and 100%) for 5 min each and dried in a Denton DCP-1 critical-point drying apparatus. Each coverslip was glued to an aluminum stub (EY Labs) with silver paint (Ted Pella Inc., Redding, Calif.) and evaporation coated with gold-palladium.
FITC-conjugated lectin EPS probes.
Cells were harvested during mid-logarithmic phase, washed once in sterile PBS, and resuspended in PBS along with 50 μg of fluorescein isothiocyanate (FITC)-labeled sweet pea lectin (SPL), coral tree lectin (CTL), or Griffonia simplicifolia (GSII) lectin for 1 h. Cells were then washed three times in sterile PBS and photographed with a Zeiss Axiophot microscope by incident light fluorescence microscopy.
Orientation of adhesion.
One hundred-microliter samples of a mid-logarithmic-phase culture were layered onto glass coverslips precoated with MB. Coverslips were incubated in a humid chamber at 25°C and then washed after 5, 15, 30, 60, 120, or 240 min with 10 ml of artificial seawater (23.0 g of NaCl, 0.24 g of Na2CO3, 0.33 g of KCl, 4.0 g of MgCl2 · 6H2O, 0.66 g of CaCl2 · H2O/liter of dH2O). The number and orientation of cells attached to 80-μm2 areas of the coverslip were determined at each time point with a Zeiss Axiophot light microscope (objective, numerical aperture 1.32) and an ocular micrometer. We used 30 samples, from which the mean and standard deviation were calculated.
Culture synchronization.
A modification of the size-sorting procedure of Wali et al. (32) was used to synchronize VP-6 cultures. One liter of early- to mid-logarithmic-phase culture was centrifuged at 13,000 × g for 5 min. The supernatant was passed through a 0.6-μm-pore-size membrane (Poretics Corp., Livermore, Calif.) and then centrifuged at 16,000 × g for 30 min. The pellet, consisting of swarm cells, was then resuspended in 30 ml of MB and shaken at 100 rpm at 25°C. Aliquots (1.5 ml) were taken every 20 min for 6 h. Twenty microliters of culture from each time point was layered directly onto a collodion-coated copper grid and stained with 1% uranyl acetate for 15 s. The remaining culture sample from each time point was frozen at −80°C. Aliquots from each time point were thawed and labeled with cationic ferritin.
RESULTS
VP-6 produces a polar holdfast.
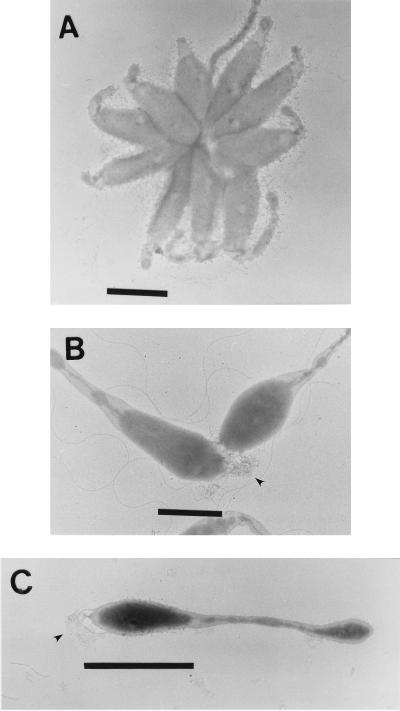
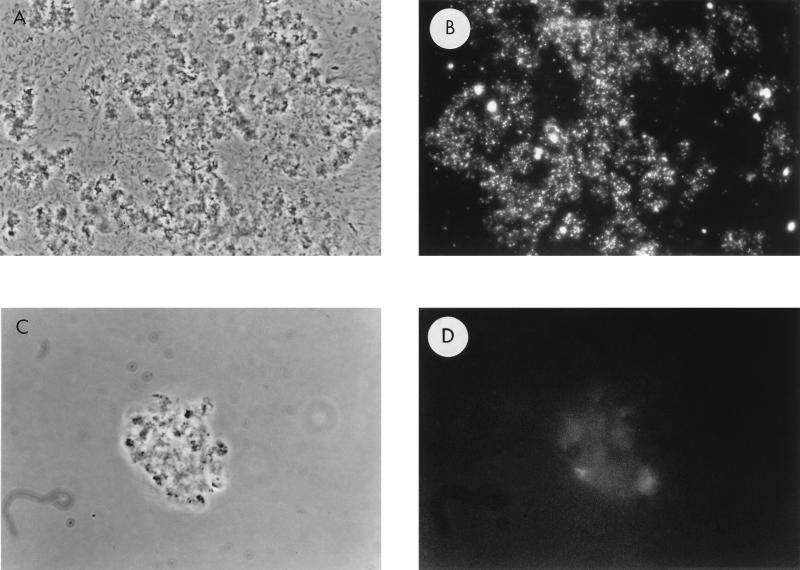
The first indication that VP-6 produces holdfasts came from its ability to form rosettes consisting of three or more cells attached at a common pole, with prostheca radiating outward (Fig. 1A). Closer inspection revealed fibrous material at the tip of the mother cell, distal to the prosthecum, which mediated intercellular adhesion (Fig. 1B and C). Holdfasts were approximately 30 nm wide at the base of the cell, fanning out to a width of approximately 200 nm. The holdfast enlarges with cell maturation, reaching a length of up to 300 nm. The CTL, specific for galactose-β-1,4-N-acetylglucosamine linkages, bound holdfasts, as determined by immunofluorescence microscopy (Fig. 2). Note the pinpoint labeling pattern of CTL-treated cells (Fig. 2B) compared to that of control cells (Fig. 2D).
FIG. 1.
Holdfast production. Middle-logarithmic-phase VP-6 cells were layered on copper grids and stained with uranyl acetate. Shown are a rosette (A), two cells attached by the fibrous holdfast (arrowhead) (B), and a single cell with a holdfast (arrowhead) (C). Bar = 1 μm.
FIG. 2.
VP-6 holdfasts labeled with FITC-conjugated CTL. Logarithmically growing cells were washed and resuspended in PBS with 50 μg of FITC-labeled CTL or G. simplicifolia lectin per ml. A phase-contrast image of VP-6 cells labeled with FITC-conjugated CTL (A) and the same preparation viewed with incident light fluorescence wavelengths (B) are shown. Notice the pinpoint fluorescence indicating the labeling of holdfast EPS. Also shown are control cells labeled with FITC-conjugated G. simplicifolia lectin (C) and the same cells viewed by incident light fluorescence (negative control) (D). Magnification, ×863.
Evidence for a VP-6 capsular EPS.
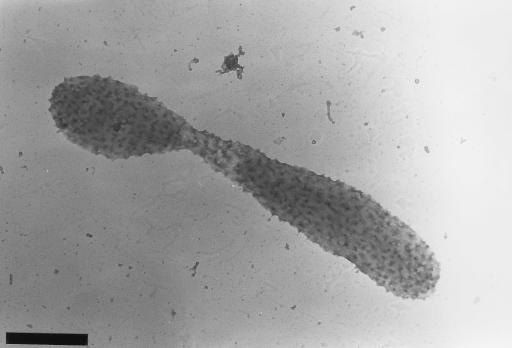
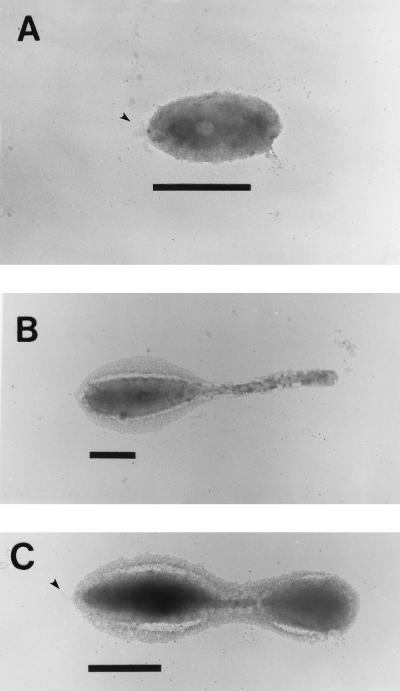
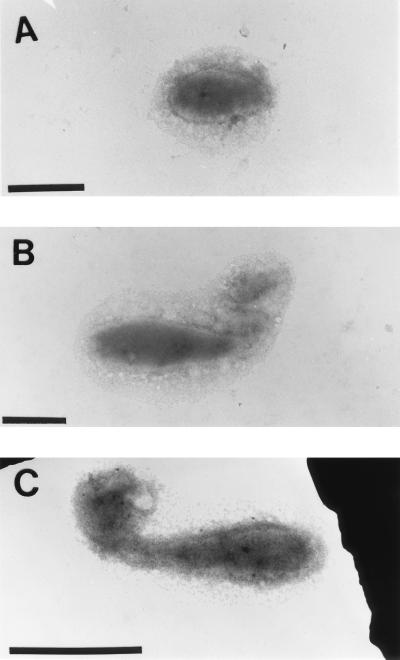
Ruthenium red staining, specific for acidic polysaccharides (1), revealed that VP-6 produces a rough acidic capsular EPS that surrounds the entire budding reproductive cell (Fig. 3). Cationic-ferritin-stained cells (Fig. 4) and specific lectin immunoprobes corroborated these results. EPS were viewed on swarm, reproductive, and budding reproductive cells, which demonstrated constitutive production throughout the life cycle (Fig. 4). The thickness of capsular EPS ranged from approximately 100 nm on swarm cells and buds to 300 nm on the prostheca and the main bodies of reproductive cells. Capsular EPS appeared to be tightly bound to cells and was not easily sheared during the multiple centrifugation and resuspension steps involved in the cationic-ferritin labeling process (Fig. 4). The holdfasts of VP-6 cells were not bound by cationic ferritin (Fig. 4). Holdfasts were not obvious on cationic-ferritin-labeled cells because they could not be stained with uranyl acetate.
FIG. 3.
Budding, prosthecate VP-6 cell stained with Ruthenium Red. Logarithmically growing cells were suspended in a 0.5-mg/ml solution of ruthenium red dissolved in PBS containing 0.9% glutaraldehyde. After 1 h, cells were washed twice with PBS, layered on collodion-coated copper grids, and stained with uranyl acetate. Notice the rough capsular EPS covering the entire cell. Bar = 1 μm.
FIG. 4.
Cationic-ferritin-labeled capsules. Cells were incubated in 1.0 mg of cationic ferritin/ml of dH2O solution, washed, and layered on collodion-coated copper grids. Swarm (A), prosthecate reproductive (B), and budding reproductive (C) cells are shown. Notice that in all cases the entire cell is surrounded by capsular EPS. Arrowheads point to unlabeled holdfasts. Bar = 1 μm.
Timing of EPS and holdfast production by VP-6.
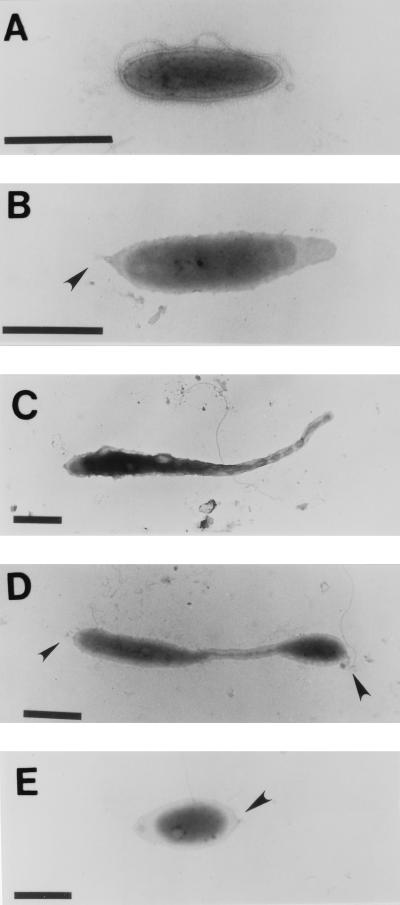
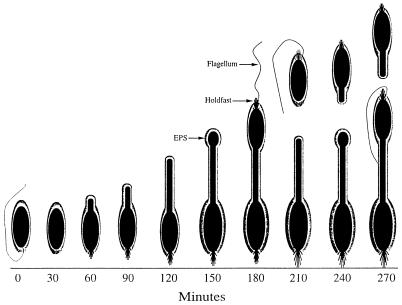
The timing of both EPS and holdfast expression was determined in synchronously growing populations. Figure 5 shows the stages in the morphogenesis of Hyphomonas strain VP-6. Swarm cells required 60 ± 10 min for maturation and the initiation of prosthecal outgrowth. Prosthecate cells formed buds at 150 ± 10 min. The reproductive cells shed buds at the distal tips of their prostheca after 210 ± 10 min. The holdfast and flagellum were synthesized at the same pole 180 ± 10 min into the cycle (Fig. 5D), indicating that individual swarm cells have both a flagellum and a holdfast when they are released from the reproductive cell. Synchronized populations expressed capsular EPS at every stage of the developmental cycle, as was revealed by TEM of cells labeled with cationic ferritin (Fig. 6).
FIG. 5.
Uranyl acetate-stained synchronized cultures. VP-6 was synchronized by using differential centrifugation and size sorting. Twenty microliters of culture from each time point was directly layered onto collodion-coated copper grids and stained with uranyl acetate. Shown are cells at 0 min (A), 60 min (B), 120 min (C), 180 min (D), and 240 min (E). Arrowheads point to holdfasts. Bar = 1 μm.
FIG. 6.
Synchronized VP-6 cells labeled with cationic ferritin. Cells were removed from a synchronous culture, treated with a 1.0-mg/ml solution of cationic ferritin, washed, and layered onto collodion-coated copper electron micrograph grids. Cells shown in these photomicrographs were removed from the synchronous culture at 0 min (A), 180 min (B), and 360 min (C). Notice that VP-6 produced capsular EPS at each of the time points. Bar = 1 μm.
Correlation of surface polysaccharide production and adhesion.
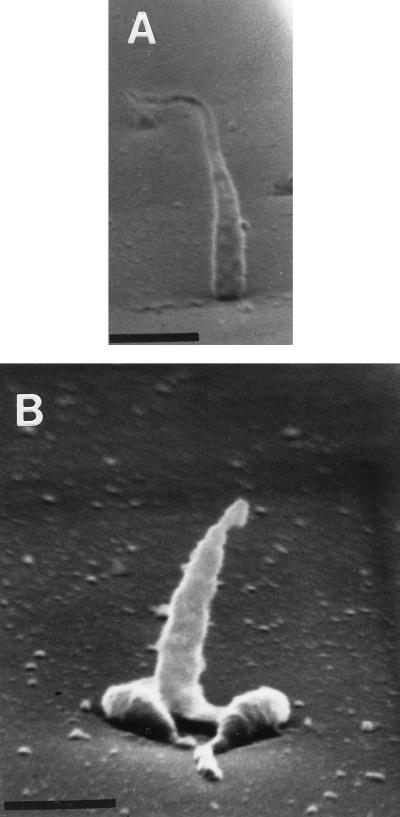
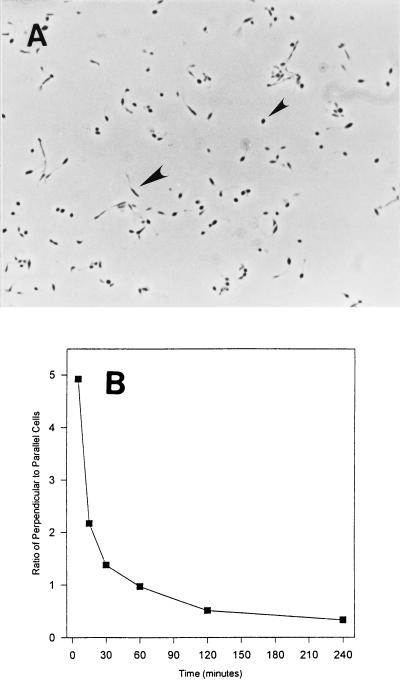
The orientations of cells of Hyphomonas strain VP-6 during surface attachment and whether attachment varies with the location of surface polysaccharide were examined by SEM of critical-point dried cells attached to glass coverslips. VP-6 cells affixed themselves either perpendicularly or parallel to the surface (Fig. 7). Cells affixed perpendicularly to the surface appeared spherical when they were viewed by phase-contrast microscopy (Fig. 8). More of each cell that affixed itself parallel to the surface was visible in the plane of focus (Fig. 8A). The holdfasts, being polar, adhered cells at one pole so that they stood perpendicularly; the capsule, which surrounds the entire cell, adhered it parallel to the surface. As shown in Fig. 8B, the ratio of perpendicular to parallel cells decreased with time, suggesting that cells adhered initially via the holdfast and later predominately via the capsule.
FIG. 7.
Orientation of VP-6 cell attachment. Glass coverslips, coated with MB, were layered with logarithmically growing cells and viewed with an Amray 1820D scanning electron microscope. Notice that some VP-6 cells stand perpendicular to the surface (panel A and middle cell in panel B) but that others lie parallel to it (flanking cells in panel B). Bar = 1 μm.
FIG. 8.
Perpendicular versus parallel adhesion. (A) Phase-contrast image of VP-6 cells attached to a glass coverslip. Perpendicularly attached cells appear spherical (small arrowhead) and can be easily differentiated from those lying parallel to the surface (large arrowhead). Magnification, ×925. (B) Ratio of perpendicular to parallel cells relative to time of adhesion. The ratio of cells exhibiting perpendicular adhesion to those exhibiting parallel adhesion decreases with time.
DISCUSSION
An investigation of the spatial and temporal production of surface polysaccharides by Hyphomonas strain VP-6 shows that it produces two separate adhesive surface polysaccharides, both of which mediate cellular adhesion to surfaces. These polymers differ in their charges, structures, locations, and temporal levels of production. A holdfast is produced polarly and at a specific time during the life cycle, while a capsular EPS surrounds the entire cell during all morphogenic stages. Cells with holdfasts form rosettes by binding to one another by each holdfast (19, 31). The production of surface polysaccharide in VP-6 is very different from that in MHS-3, which produces a single EPS that surrounds the main body of the prosthecate cell and is synthesized only at reproductive-cell stages (24).
Electron microscopy is a useful tool for studying the locations and fine structures of surface polysaccharides on microbial cells. However, because surface polysaccharides are highly hydrated polymers (up to 99% water [26]), artifact formation during sample preparation is a legitimate concern. The major effect of dehydration is shrinking of the surface polysaccharides and loss of fine structure (26). However, negatively charged surface polysaccharides remain preserved, close to their original state, when they are treated with cationic ferritin or ruthenium red before the dehydration steps (1, 26). In addition, critical-point drying and chemical fixation, although not able to prevent shrinkage and artifact formation completely, have been used successfully in the observation of capsular material (26).
A number of aquatic bacteria have been shown to produce holdfasts. These include Asticacaulis biprosthecum, C. crescentus, Flexibacter aurantiacus, Hyphomicrobium vulgarae, S. stellata, species of Thiothrix, and Hyphomonas strains MHS-3 (9, 17, 18, 23, 31, 33) and VP-6 (this study). All of these organisms are believed to employ the holdfast for adhesion, and with the exception of MHS-3, which produces a less localized holdfast, all form rosettes in liquid culture. Unlike the holdfasts of R. japonicum, A. biprosthecum, Thiothrix species, C. crescentus, and Hyphomonas strain MHS-3, the VP-6 holdfast does not appear to be negatively charged, as was demonstrated by its inability to bind cationic ferritin (Fig. 4). This is surprising, because most adhesive surface polysaccharides carry a net negative charge (3). However, a neutral or positively charged holdfast may have the advantage of experiencing decreased repulsion by the negatively charged surface.
The VP-6 capsular EPS is a 550-kDa negatively charged polymer able to bind certain cationic metals and the carbohydrate-specific dye toluidine blue (11). The capsular EPS may measure up to 300 nm in thickness and is produced constitutively throughout the life cycle of the organism. Data presented both here and elsewhere (11) confirm that the EPS is used for adhesion and also probably serves as the biofilm matrix.
C. crescentus produces small amounts of a high-molecular-weight EPS believed to cover the entire cell; however, its function has not yet been determined (25). S. stellata also produces a second EPS in late growth stages (9) which contains an N-acetylated amino sugar. VP-6 EPS is produced constitutively during the entire life cycle and functions in cell adhesion (11). Thus, to the best of our knowledge, VP-6 is the first organism found to produce an adhesive holdfast and a second adhesive EPS concurrently. The reason for this apparent redundancy is not entirely clear but might be explained in the following way. The holdfast, either through its location on the cell or its chemical composition, is more specialized to make the initial adhesive bond. With time, the EPS exchanges hydrogen bonds with water for hydrogen bonds with the surface, thus slowly pulling the cell parallel to the surface. The selective advantage for this mechanism may be that cells lying parallel to the surface are more resistant to shear forces than cells attached perpendicularly, offering an advantage in turbulent environments. It is also possible that the EPS capsule may function primarily for protection, as a nutrient sink, or as the biofilm matrix and secondarily as an adhesin.
Staphylococcus epidermidis RP62A produces two extracellular polysaccharides known as capsular polysaccharide adhesin (CPA) and slime-associated antigen (SAA). Mutants for either CPA or SAA production showed that the CPA was used in the early stages of adhesion but that the SAA was involved in bacterial accumulation (27), a mechanism which might be mimicked by the holdfast and EPS of VP-6.
Capsular EPS surrounded the entire cell throughout the entire life cycle. However, SPL bound very weakly to the EPS of synchronized cells compared to its extent of binding to the EPS of a mid-logarithmic-phase culture. Thus, VP-6 may alter the composition of its capsular EPS as the culture ages. The exopolysaccharides of Acinetobacter calcoaceticus and Klebsiella sp. strain K32 varied in composition depending upon the available carbon source in the growth media (2). Similarly, Pseudomonas atlantica decreased the proportion of galactose and increased the proportion of uronic acids in its glycocalyx as the growth cycle progressed (30). VP-6 may similarly alter the composition of its capsular EPS, with one type being produced during lag and early-logarithmic growth phases (as would be the case in a synchronized culture) and another being produced as nutrients become depleted from the media in middle- to late-logarithmic phase (two-day culture). VP-6 also synthesizes a capsular EPS-associated 64-kDa protein (11). Perhaps that protein is synthesized only in older cultures, by modifying the orientation of EPS and making it a more suitable SPL ligand.
Figure 9 outlines the morphological development of VP-6 in a synchronous culture. An encapsulated and flagellated swarm cell sheds its flagellum after 30 ± 10 min and begins to produce a prosthecum and holdfast at 60 ± 10 min. Prosthecum elongation continues for the next 60 ± 10 min, with bud formation taking place at 150 ± 10 min. The holdfast and flagellum form on the bud after 180 ± 10 min, and separation of the swarm cell from the reproductive cell occurs at 210 ± 10 min. Reproductive cells continue to produce buds during the time that swarm cells begin the process of prosthecum elongation and, eventually, bud formation. Thus, VP-6 required 210 ± 10 min from the swarm cell stage to the release of the first progeny. Swarm cell maturation required 29%, prosthecal elongation required 43%, and swarm cell maturation required 29% of the developmental cycle. These values were 165 ± 15 min, 21%, 58%, and 21%, respectively, for MHS-3 (24).
FIG. 9.
Morphogenesis of VP-6 cells. Culture synchronization, electron microscopy, and incident light fluorescence microscopy were used to delineate morphogenesis. Prosthecum and holdfast production begins at 60 ± 10 min. The holdfast and flagellum are produced on a developing swarm cell after 180 ± 10 min. The swarm cell separates from the bud after 210 ± 10 min.
The synchronized synthesis of holdfast by VP-6 and its use as an adhesin most closely resembles those properties of C. crescentus. Both produce holdfasts at the pole of the cell where the flagellum forms, and both organisms use holdfasts as a mechanism of swarm cell adhesion. The difference is that C. crescentus forms a prosthecum at the pole where the holdfast forms (22) while VP-6 forms a prosthecum at the opposite pole. In addition, C. crescentus does not produce a second adhesive EPS and therefore remains perpendicular to the surface while VP-6 initially binds perpendicularly but eventually lies parallel to the surface.
MHS-3 produces polar fimbriae at the tip of the reproductive cell distal to the prosthecum (22). Since the VP-6 holdfast and MHS-3 fimbriae are found on the same pole of the reproductive cell, parallels can be drawn between the two. Like fimbriae, the holdfast fibers may be of sufficient length (up to 300 nm) and diameter to breach the electrostatic repulsion barrier that exists between the cells and the surface. However, SEM of cells, attached to glass coverslips, shows that after moderate washing of the surfaces to remove loosely bound cells, remaining MHS-3 cells are attached via the holdfast, not the fimbriae. In contrast, VP-6 may use either the holdfast or the cell-surrounding EPS capsule to adhere strongly enough so that they are not removed by moderate shear forces. Thus, we postulate that the holdfast of VP-6 is primarily used in initial cell attachment to surfaces and that it can also function as a permanent adhesin. With Hyphomonas, Caulobacter, and a few other genera, temporal and spatial expression of capsule is a resource-conserving measure.
ACKNOWLEDGMENTS
We thank G. Geesey and E. Quintero for helpful insight and discussion and A. Snyder for technical assistance.
This work was supported in part by a subcontract from G. Geesey from the Office of Naval Research, Arlington, Va.
REFERENCES
- 1.Bayer M E. Visualization of the bacterial polysaccharide capsule. Curr Top Microbiol Immunol. 1990;150:129–157. doi: 10.1007/978-3-642-74694-9_7. [DOI] [PubMed] [Google Scholar]
- 2.Bryan B A, Linhardt R J, Daniels L. Variation in composition and yield of exopolysaccharides produced by Klebsiella sp. strain K32 and Acinetobacter calcoaceticus BD4. Appl Environ Microbiol. 1986;51:1304–1308. doi: 10.1128/aem.51.6.1304-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen B E. The role of extracellular polysaccharides in biofilms. J Biotechnol. 1989;10:181–202. [Google Scholar]
- 4.Christensen B E, Kjosbakken J, Smidsrod O. Partial physical and chemical characterization of two extracellular polysaccharides produced by marine, periphytic Pseudomonas sp. strain NCMB 2012. Appl Environ Microbiol. 1985;50:837–845. doi: 10.1128/aem.50.4.837-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton J W, Geesey G G, Cheng K J. How bacteria stick. Sci Am. 1978;120:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 6.Decho A W. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev. 1990;28:73–153. [Google Scholar]
- 7.Fletcher M, Floodgate G D. An electron-microscopic demonstration of an acidic polysaccharide involved in the adhesion of a marine bacterium to solid surfaces. J Gen Microbiol. 1973;74:325–334. [Google Scholar]
- 8.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood M A, Schmidt J M. The examination of Seliberia stellata exopolymers using lectin assays. Microb Ecol. 1996;31:281–290. doi: 10.1007/BF00171572. [DOI] [PubMed] [Google Scholar]
- 10.Korber D R, Lawrence J R, Lappin-Scott H M, Costerton J W. Growth of microorganisms on surfaces. In: Costerton J W, Lappin-Scott H M, editors. Microbial biofilms. London, United Kingdom: Cambridge University Press; 1995. pp. 15–45. [Google Scholar]
- 11.Langille S L. Capsular and holdfast extracellular polymeric substances of Hyphomonas strain VP-6 mediate adhesion to solid substrata. Ph.D. dissertation. College Park: University of Maryland; 1996. [Google Scholar]
- 12.Langille S L, Weiner R M. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Adhesive exopolysaccharide of Hyphomonas VP-6, abstr. I83; p. 160. [Google Scholar]
- 13.Luft J H. Ruthenium Red and Violet: chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971;171:374–378. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- 14.Marshall K C. Biofilms: an overview of bacterial adhesion, activity, and control at surfaces. ASM News. 1992;58:202–207. [Google Scholar]
- 15.Marshall K C. Interfaces in microbial ecology. Boston, Mass: Harvard University Press; 1976. [Google Scholar]
- 16.Marshall K C, Cruickshank R H. Cell surface hydrophobicity and the orientation of certain bacteria at interfaces. Arch Mikrobiol. 1973;91:29–40. doi: 10.1007/BF00409536. [DOI] [PubMed] [Google Scholar]
- 17.Melick, M., and R. M. Weiner. Unpublished data.
- 18.Merker R I, Smit J. Characterization of the adhesive holdfast of marine and freshwater caulobacters. Appl Environ Microbiol. 1988;54:2078–2085. doi: 10.1128/aem.54.8.2078-2085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell D, Smit J. Identification of genes affecting production of the adhesion organelle of Caulobacter crescentus CB2. J Bacteriol. 1990;172:5425–5431. doi: 10.1128/jb.172.9.5425-5431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira D R. Physicochemical aspects of adhesion. In: Melo L F, et al., editors. Biofilms—science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 45–58. [Google Scholar]
- 21.Ong C J, Wong M L Y, Smit J. Attachment of the adhesive holdfast organelle to the cellular stalk of Caulobacter crescentus. J Bacteriol. 1990;172:1448–1456. doi: 10.1128/jb.172.3.1448-1456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintero E J. Ph.D. dissertation. College Park: University of Maryland; 1994. Characterization of adhesion and biofilm formation by the marine prokaryote Hyphomonas MHS-3; pp. 114–115. [Google Scholar]
- 23.Quintero E J, Weiner R M. Evidence for the adhesive function of the exopolysaccharide of Hyphomonas strain MHS-3 in its attachment to surfaces. Appl Environ Microbiol. 1995;61:1897–1903. doi: 10.1128/aem.61.5.1897-1903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintero E J, Busch K, Weiner R M. Spatial and temporal deposition of adhesive extracellular polysaccharide capsule and fimbriae by Hyphomonas strain MHS-3. Appl Environ Microbiol. 1998;64:1246–1255. doi: 10.1128/aem.64.4.1246-1255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravenscroft N, Walker S G, Dutton G G S, Smit J. Identification, isolation, and structural studies of extracellular polysaccharides produced by Caulobacter crescentus. J Bacteriol. 1991;173:5677–5684. doi: 10.1128/jb.173.18.5677-5684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth I L. Physical structure of surface carbohydrates. In: Sutherland I W, editor. Surface carbohydrates of the prokaryotic cell. London, United Kingdom: Academic Press; 1977. pp. 5–26. [Google Scholar]
- 27.Schumacher-Perdreau F, Heilman C, Peters G, Gotz F, Pulver G. Comparative analysis of a biofilm forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol Lett. 1994;117:71–78. doi: 10.1111/j.1574-6968.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland I W. Microbial exopolysaccharides—their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10:173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- 29.Tsein H C, Schmidt E L. Localization and partial characterization of soybean lectin binding polysaccharide of Rhizobium japonicum. J Bacteriol. 1981;145:1063–1064. doi: 10.1128/jb.145.2.1063-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlinger D J, White D C. Relationship between physiological status and formation of extracellular polysaccharide glycocalyx in Pseudomonas atlantica. Appl Environ Microbiol. 1983;45:64–70. doi: 10.1128/aem.45.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umbreit T H, Pate J L. Characterization of the holdfast region of wild-type cells and holdfast mutants of Asticacaulis biprosthecum. Arch Microbiol. 1978;118:157–168. [Google Scholar]
- 32.Wali T M, Hudson G R, Danald D A, Weiner R M. Timing of swarmer cell cycle morphogenesis and macromolecular synthesis by Hyphomicrobium neptunium in synchronous culture. J Bacteriol. 1980;144:406–412. doi: 10.1128/jb.144.1.406-412.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams T M, Unz R F, Domain J T. Ultrastructure of Thiothrix spp. and “type 021N” bacteria. Appl Environ Microbiol. 1987;53:1560–1570. doi: 10.1128/aem.53.7.1560-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]