Abstract
We examined growth of mixed microbial cultures (13 fungal species and one actinomycete species) and production of volatile compounds (VOCs) in typical building materials in outside walls, separating walls, and bathroom floors at various relative humidities (RHs) of air. Air samples from incubation chambers were adsorbed on Tenax TA and dinitrophenylhydrazine cartridges and were analyzed by thermal desorption-gas chromatography and high-performance liquid chromatography, respectively. Metabolic activity was measured by determining CO2 production, and microbial concentrations were determined by a dilution plate method. At 80 to 82% RH, CO2 production did not indicate that microbial activity occurred, and only 10% of the spores germinated, while slight increases in the concentrations of some VOCs were detected. All of the parameters showed that microbial activity occurred at 90 to 99% RH. The microbiological analyses revealed weak microbial growth even under drying conditions (32 to 33% RH). The main VOCs produced on the building materials studied were 3-methyl-1-butanol, 1-pentanol, 1-hexanol, and 1-octen-3-ol. In some cases fungal growth decreased aldehyde emissions. We found that various VOCs accompany microbial activity but that no single VOC is a reliable indicator of biocontamination in building materials.
Excessive moisture in building materials supports microbial growth. The absence of visible signs of dampness and low airborne fungal levels are usually considered indications that there is little, if any, biocontamination in a building (21, 23). Although fungal or bacterial cells may not be detected in indoor air, a wide range of microbially produced volatile organic compounds (MVOCs) can permeate building materials and diffuse into the surrounding air (28). Thus, identification of MVOCs may indicate microbial contamination when other signs of microbial growth cannot be detected.
Both the microbial species and the growth substrate affect the MVOC profile (3, 29, 31, 35). The most commonly identified volatile microbial metabolites include 3-methyl-1-butanol, 1-hexanol, 1-octen-3-ol, 2-heptanone, and 3-octanone (4, 11, 14, 15, 25, 26, 31). Before MVOC analysis can be used as a reliable indicator of microbial contamination in buildings, however, it is important to identify the volatile organic compounds (VOCs) expected from growth of microbial species frequently found in moisture-damaged buildings and to verify that these VOCs have no other major sources in buildings.
The MVOC profiles of single microbial species cultured in different building materials have been examined in several studies (2, 5, 22, 31, 32), but little is known about MVOC production by mixed microbial cultures (16). Microbial colonization of building materials is a dynamic process in which population composition changes in response to the equilibrium relative humidity (ERH) of the materials. Primary colonizers (Penicillium, Eurotium, and Aspergillus species) begin to grow when the ERH of the substrate exceeds 75 to 80%, secondary colonizers (e.g., Cladosporium species) appear at an ERH of 80 to 90%, and tertiary colonizers (such as Fusarium and Stachybotrys species, actinomycetes, and yeasts) appear at an ERH above 90% (9, 13, 24, 27).
We measured the concentrations of various VOCs emitted from sterile building materials and from building materials contaminated by mixed microbial cultures at various relative humidities (RHs) of air. VOCs regarded as MVOCs (4, 11, 14, 15, 25, 26, 31) were chosen for analysis, and microbial species frequently recovered from moist building materials (9, 12, 24, 27, 33) were used as test organisms. RHs were selected based on the RHs of construction materials in moisture-damaged buildings (8, 24). Our objectives in this study were (i) to identify VOCs that were differentially present in contaminated and sterile building materials and (ii) to determine if any particular VOC can be used as a reliable indicator of microbial contamination in moisture-damaged buildings.
MATERIALS AND METHODS
Building materials studied.
Typical Finnish building material combinations were used in this study. One combination represented the room side of an outside wall with gypsum board covered with wallpaper on one side and cardboard on the back. Half of the gypsum board was enveloped in a plastic film on the back to represent a vapor diffusion retarder. Another combination represented a floor and separating wall (chipboard and glass wool), and a third combination represented a bathroom floor (ceramic tiles attached to lightweight aggregate block with cement grout). Pieces of the building materials (area, 25 cm2) were stabilized at 75 to 76% RH for not less than 2 weeks in order to adjust the ERH of the materials to the same level as the RH of the air. Pieces were sterilized with gamma radiation (minimum dose, 25 kGy) and were stabilized again at 75 to 76% RH for 1 week before inoculation with microorganisms to ensure that the ERHs of the construction materials were stable.
Inoculation of building materials with microorganisms.
The microbial strains used are listed in Table 1. The microbial species used were selected based on results obtained previously for microorganisms isolated from moisture-damaged buildings and from the building materials used in this study (8, 9, 12, 24, 27). The strains were cultivated at 20 to 23°C for 2 weeks (10 agar plates/strain). Cultures were suspended in 4 to 5 ml of dilution water (42.5 mg of KH2PO4, 250 mg of MgSO4 · 7H2O, and 8 mg of NaOH in 1 liter of deionized water) by flooding a plate, gently stirring the solution with a sterile glass rod, and transferring the suspension to test tubes. The number of microbial propagules was determined with a light microscope by using a Fuchs-Rosendahl counting chamber. Building construction inocula were prepared by pooling the appropriate strains (Table 1) and then spraying 80 μl of the resulting suspension onto both sides of the gypsum board and the ceramaic tile floor, onto one side of the chipboard, and onto one side of the glass wool. Control pieces were not sprayed.
TABLE 1.
Mixed microbial cultures, growth media, building materials, and amounts of inoculates used in this study
| Inoculated microbial strain | Sourcea | Type of colonizerb | Growth mediumc | No. of inoculated propagules (107) per construction block
|
||
|---|---|---|---|---|---|---|
| Gypsum board, wallpaper, and plastic film | Chipboard and glass wool | Ceramic tile | ||||
| Acremonium furcatum UKU 1 | UKU | 3 | 2% MEA | 10 | 0.22 | |
| Aspergillus fumigatus UKU 2 | UKU | 3 | 2% MEA | 7.4 | ||
| Aspergillus versicolor UKU 3 | UKU | 1 | DG18 | 11 | ||
| Aureobasidium pullulans DSM 62074 | DSM | 2 | 2% MEA | 5.7 | ||
| Chaetomium globosum UKU 4 | UKU | 3 | DG18 | 0.014 | ||
| Eurotium herbariorum KTL 538 | KTL | 1 | DG18 | 17 | ||
| Exophiala dermatitidis KTL 461 | KTL | 3 | 2% MEA | 71 | ||
| Fusarium culmorum MTTK 72315/20 | MTTK | 3 | 2% MEA | 1.1 | ||
| Paecilomyces variotii UKU 12 | UKU | 2 | 2% MEA | 9.0 | ||
| Penicillium brevicompactum ATCC 58606 | ATCC | 1 | 2% MEA | 5.4 | ||
| Scopulariopsis brumptii KTL 441 | KTL | 2 | 2% MEA | 6.8 | ||
| Sporobolomyces roseus TTL 146 | TTL | 3 | DG18 | 110 | ||
| Stachybotrys chartarum nontoxigenic strain UKU 10 | UKU | 3 | 2% MEA | 0.63 | ||
| Streptomyces californicus KTL 12 | KTL | 3 | TYGA | 0.0018 | ||
ATCC, American Type Culture Collection, Rockville, Md.; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunshweig, Germany; KTL, National Health Institute, Kuopio, Finland; MTTK, Agricultural Research Centre of Finland, Jokioinen, Finland; TTL, Kuopio Regional Institute of Occupational Health, Kuopio, Finland; UKU, University of Kuopio, Kuopio, Finland.
1, primary colonizer (water activity, <0.80); 2, secondary colonizer (water activity, 0.80 to 0.90); 3, tertiary colonizer (water activity, >0.90) (6, 8, 9, 27).
DG18, dichloran–18% glycerol agar (Lab M, Bury, England); 2% MEA, 2% malt extract agar (Biokar Diagnostics, Beauvais, France); TYGA, tryptone-yeast extract agar (Oxoid Unipath Ltd., Hampshire, England).
Incubation conditions.
Twenty pieces of each building material combination were placed in a sterile, air-tight glass chamber (volume, 24 liters) and incubated at room temperature (20 to 23°C) as shown in Table 2. The same construction pieces were transferred from one RH level to the next. The high RH levels were chosen based on RH values obtained for construction materials in moisture-damaged buildings (8, 24); 32 to 33% RH is a typical RH for dry materials (8). Two construction pieces were removed for microbiological analysis at the end of each incubation period. For each building material combination there were four chambers, two for inoculated building material and two for corresponding sterile material. The RHs in the chambers were adjusted with 750 or 1,000 ml of a saturated aqueous solution containing NaCl (400 g/liter; 75 to 76% RH), (NH4)2SO4 (900 g/liter; 80 to 82% RH), ZnSO4 · 7H2O (1,500 to 1,900 g/liter; 90 to 92% RH), K2SO4 (150 g/liter; 97 to 99% RH), and MgCl2 · 6H2O (3,000 g/liter; 32 to 33% RH). To determine the moisture contents (MCs) of the building materials, we used three additional chambers in which the RH was controlled, one for each set of building material combinations. Two building material pieces were removed from each chamber at the end of each incubation period, and the MC was calculated by determining the weight loss after the pieces were dried at 105°C for 20 h (Table 2).
TABLE 2.
Incubation conditions and MCs of materials
| Incubation time (weeks) | RH of air (%) | MC (%) of:
|
|||
|---|---|---|---|---|---|
| Gypsum board | Chipboard | Glass wool | Ceramic tile | ||
| 1 (1)a | 75–76 | 18 | 10 | 1 | 3 |
| 2–5 | 80–82 | 19 | 11 | 1 | |
| 6–8 (2–5) | 90–92 | 19 | 16 | 2 | 3 |
| 9–11 (6–9) | 97–99 | 20 | 20 | 7 | 5 |
| 12–13 (10–11) | 32–33 | 19 | 9 | <1 | 2 |
The numbers in parentheses are the incubation times (in weeks) for ceramic tile attached to aggregate block.
CO2 monitoring and sampling of volatile compounds.
The CO2 concentrations in the incubation chambers were measured twice a week with an infrared analyzer (ADC infrared gas analysis instrument; The Analytical Development Co. Ltd., Hoddesdon, England). The CO2 concentrations were measured in one chamber containing inoculated building materials and in one chamber containing sterile building materials. The building material pieces used in the microbiological analysis were also taken from these chambers. From the two other chambers (one containing inoculated materials and the other containing sterile materials) only samples for VOC analyses were taken.
Air samples for VOC analyses were collected twice a week. Air samples were taken from each chamber with a needle inserted through a rubber septum on top of the chamber (17). Replacement air, moistened to the same RH, was filtered through activated carbon and a polycarbonate filter (pore size, 0.2 μm; Nuclepore, Cambridge, Mass.) and pumped into the chamber. Two sampling methods were used, one for carbonyl compounds and the other for VOC in general.
Carbonyl compounds.
Using flow rates ranging from 200 to 380 ml/min (sampling time, 5 to 10 min), we collected 2-liter air samples from the chambers with 2,4-dinitrophenylhydrazine (DNPH)-silica Sep-Pak cartridges (type WAT037500; Waters Chromatography Div., Millipore Corp., Milford, Mass.). Aldehydes and ketones react with DNPH to form hydrazone derivatives. Both samples from the same week were collected in the same cartridge. After sampling, the cartridges were stored in an airtight jar at 4°C in the dark and analyzed within 5 weeks. Prior to analysis, hydrazone derivatives were eluted with 3 ml of acetonitrile (Rathburn Chemicals Ltd.), which was then analyzed by high-pressure liquid chromatography (HPLC).
Hydrazone derivative standards were prepared by using the procedures described by Korpi et al. (16) for the following carbonyl compounds: formaldehyde (Merck) (purity, 37%), acetaldehyde (Merck) (>99.5%), acrolein (Merck-Schuchardt) (about 95%), propanal (Merck-Schuchardt) (>98%), butanal (Merck-Schuchardt) (>99%), pentanal (Merck) (>98%), hexanal (Merck) (>98%), heptanal (Merck) (>97%), octanal (Merck) (>98%), nonanal (Merck-Schuchardt) (98%), decanal (Merck) (>97%), acetone (Merck) (>98%), butanone (Riedel-de Haën) (99.7%), 2-pentanone (Fluka) (>99%), 3-methyl-2-pentanone (Riedel-de Haën) (95%), and 2-hexanone (Merck) (>98%). Standard solutions were prepared by dissolving the derivatives in acetonitrile (at concentrations ranging from 1.7 to 8.6 μg of carbonyl compound/ml) and were analyzed at the same time as the samples.
Samples (in acetonitrile) were analyzed by HPLC by using a Hewlett-Packard model 1050 chromatograph, a Hypersil PDS C18 column (100 by 4 mm; particle size, 3 μm), and an eluent consisting of deionized water, acetonitrile, and tetrahydrofuran (Merck) (>99.8%) at a flow rate of 1.3 ml/min. The injection volume was 15 μl. The chromatographic program was as follows: water-acetonitrile-tetrahydrofuran (isocratic, 65:30:5) for the first 2 min, which was changed gradually to water-acetonitrile-tetrahydrofuran (27:73:0) at 15 min and to water-acetonitrile-tetrahydrofuran (10:90:0) at 20 min. The DNPH derivatives were detected by using a UV detector at 360 nm.
Other VOC.
Air samples (1.5 liters) were passed through adsorbent glass tubes (length, 160 mm; inside diameter, 3 mm) containing 150 mg of Tenax TA resin (60-80 mesh; Chrompack) at a flow rate of 150 to 240 ml/min (sampling time, 6 to 10 min). Prior to sampling, the tubes were purged with helium at 270°C for 2 h, and 87 ng of the internal standard used, 1-chlorooctane (Fluka AG) (>98%) in methanol, was injected into each tube. Two samples were collected each time from each chamber; one sample was used for analysis by gas chromatography-mass spectrometry (GC-MS) in SCAN mode, and the other was used for analysis in selected ion monitoring (SIM) mode. Both samples from the same week were collected in the same tube. After sampling, the tubes were stored at 4°C and analyzed within 1 week.
The following compounds were used as representative MVOC in the GC-MS (SIM mode) analyses: the alcohols 1-octanol (Merck) (>99%), 3-octanol (Merck) (>97%), 3-methyl-2-butanol (Aldrich) (98%), 3-methyl-1-butanol (J. T. Baker Chemicals B.V.) (>98%), 1-octen-3-ol (Merck) (>97%), 2-methyl-1-propanol (Aldrich) (99.5%), 1-pentanol (Merck) (>99%), and 1-hexanol (Riedel-de Haën) (98%); the ketones 2-heptanone (Merck) (>98%) and 3-octanone (Fluka AG) (>97%); and the terpenes alpha-pinene (Fluka AG) (>97%), β-pinene (Fluka AG) (80 to 90%), and limonene (Fluka AG) (97%). In addition, geosmin (Sigma) (>98%) and 3-methylanisole (Fluka) (>98%) were analyzed in the ceramic tile experiment. A solution containing approximately 80 ng of reference compound per μl in methanol was prepared for each reference compound. One microliter of this solution and 87 ng of the internal standard were injected into a Tenax TA sampling tube, and 1 liter of VOC-free air was drawn through the tube. Reference tubes were analyzed at the same time as the air sample tubes. Prior to analysis, the sample tubes were purged with helium (40 ml/min) for 2 min to desorb water possibly adsorbed to the resin.
VOCs were thermally desorbed from the adsorbent tubes by using a thermal desorption cold trap injector (Chrompack). Desorption was performed at 250°C for 12 min, and the cold trap was maintained at a temperature below −50°C with liquid nitrogen. Helium was used as the carrier gas. The sample was injected onto the column by heating the cold trap at 200°C for 1 min. The analysis was carried out with a Hewlett-Packard model 5890 gas chromatograph equipped with a fused-silica capillary column (type DB1701; 30 m by 0.25 mm; film thickness, 0.25 μm; J & W Scientific, Folsom, Calif.) and a model 5970 mass selective detector (Hewlett-Packard) by using the following program: 40°C hold for 2 min, ramp at a rate of 5°C/min to 160°C, ramp at a rate of 20°C/min to 200°C, and hold for 2 min. The transfer line was maintained at 280°C. Samples were analyzed by using both SCAN mode (m/z 40 to 260) and SIM mode. A NIST library database (NBS revision F and NBS75k) was used for compound identification.
The ratio of the peak area of the internal standard to the peak area of each VOC was multiplied by the corresponding mass to obtain a response factor that was used to quantify the VOC in each sample. This calibration was repeated daily, and the relative standard deviation of the internal standard peak area was 14% (n = 86). Since no temporal trends in the emission of any single VOC were observed, the results are given below as weekly average yields for each RH. The differences between the average yields obtained from the contaminated samples and the corresponding sterile materials throughout the whole experiment were compared with a one-sided Wilcoxon paired-sample test (SPSS for Windows, release 6.0.1; SPSS, Chicago, Ill.) (20), which is suitable for testing small populations with values that are not normally distributed if the two values are independent.
The results were interpreted by using the following criteria. A VOC was regarded as a MVOC if the average yield obtained from the contaminated chamber was statistically significantly higher than the average yield obtained from the control chamber for all RH levels. Also, if a compound was released from contaminated building materials and not at all from sterile materials at at least one RH level, the compound was considered a MVOC at this RH level. In the latter case, the statistical test would have failed to indicate significance because the number of samples (n ≤ 4) at one RH level was too small to be tested separately.
Microbiological analysis.
After inoculation and each incubation period, microbial concentrations were determined by dilution plating (24) on dichloran–18% glycerol agar, 2% malt extract agar, and tryptone-yeast extract agar. Microbial concentrations were determined for two samples each of wallpaper, cardboard from gypsum board, plastic film, chipboard, glass wool, and ceramic tile floor. The average for each building material combination was calculated from the concentrations obtained for the component materials. To determine average concentrations for building materials, the detection limit divided by two was used when no microorganisms were detected in any of the component construction materials. The dilution plates were incubated at 25°C for 6 days. The concentration of each fungal species was determined on the media indicated in Table 1. The detection limits ranged from 8 to 15,000 CFU/cm2. Because of the large variation in the masses of the materials, microbial concentrations were based on surface area instead of mass.
RESULTS
Outside wall: gypsum board covered with wallpaper and plastic film.
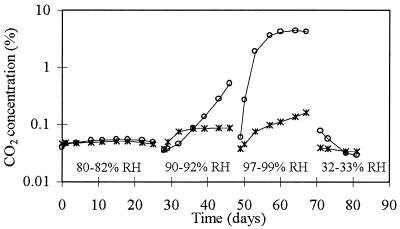
Based on CO2 measurements (Fig. 1), microbial activity occurred only at 90 to 92% RH and at 97 to 99% RH. The CO2 concentration remained below 0.05% in the control chamber. At the beginning of the experiment, the fungal levels in wallpaper, cardboard, and plastic film were the same magnitude. During the incubations at 97 to 99% RH and at 32 to 33% RH, the fungal levels in the plastic were 10 to 100 times lower than those in the other materials. The average fungal levels on the outside wall (Table 3) decreased 90% during incubation at 80 to 82% RH. Slight recovery occurred at 90 to 92% RH, and more rapid growth followed at 97 to 99% RH. The concentration of Acremonium furcatum increased 70-fold during incubation at 97 to 99% RH. Under drying conditions, a sevenfold increase in the level of Penicillium brevicompactum was detected. The control material pieces remained sterile.
FIG. 1.
Microbial activity expressed as CO2 production in floor and separating wall construction materials (○) and outside wall construction materials (∗). The breaks in the lines are due to changes in incubation conditions.
TABLE 3.
Average fungal concentrations in outside wall construction materials (wallpaper, cardboard, and plastic film) after inoculation and at the end of incubation
| Microorganism | Avg concn (103 CFU/cm2) in outside wall construction materials
|
||||
|---|---|---|---|---|---|
| After inoculation | After incubation at:
|
||||
| 80–82% RH | 90–92% RH | 97–99% RH | 32–33% RH | ||
| P. brevicompactum | 7.0 (1.2–14)a | 1.1 (0.77–1.5) | 2.4 (0.54–4.8) | 4.2 (<2.3–9.8) | 30 (3.5–65) |
| A. furcatum | 24 (8.5–48) | 0.42 (<0.024–1.2) | <0.28b | 7.0 (0.082–20) | 1.6 (<0.045–4.6) |
| A. fumigatus | 13 (6.8–21) | 1.5 (0.57–2.8) | 1.6 (0.54–2.3) | 6.1 (0.57–13) | 7.5 (0.59–13) |
| E. herbariorum | 9.4 (6.6–11) | 3.0 (1.1–6.4) | 5.7 (2.9–8.1) | 8.8 (<2.3–23) | 15 (6.5–23) |
The values in parentheses are ranges (minimum and maximum concentrations). The concentration of S. chartarum remained below the detection limit (24 to 3,300 CFU/cm2) throughout the experiment.
The concentration of A. furcatum was below the detection limit for all outside wall construction materials at 90 to 92% RH; thus, the value is the average of the detection limits for the component materials.
The MVOCs detected were 3-methyl-1-butanol, limonene, and acetone (Table 4). In addition, at 80 to 82% RH 3-methyl-2-butanol and 1-octen-3-ol and at 90 to 92% RH 1-hexanol were detected as microbial metabolites, since sterile materials did not emit these compounds. The levels of several aldehydes, particularly acetaldehyde, pentanal, hexanal, heptanal, octanal, and decanal, and the level of 3-octanone emitted from the sterile construction pieces were significantly higher than the levels of those compounds emitted from the inoculated construction pieces. 3-Octanol was not detected. Analysis of 1-hexanol, 1-octanol, and β-pinene was complicated by chromatographic coelution of other lightweight compounds. The retention times and the presence of specific ions (m/z) in the mass spectra verified that these compounds were present.
TABLE 4.
Weekly geometric mean emissions of VOCs during 10 to 12 weeks of incubation of contaminated building materials at three or four different RH levels and corresponding emissions from sterile building materials, as determined by GC-MS and HPLC
| VOCa | Weekly geometric mean yield (ng/liter)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gypsum board, wallpaper, and plastic film
|
Chipboard and glass wool
|
Ceramic tile
|
|||||||
| Contaminated (n = 12) | Sterile (n = 12) | Pb | Contaminated (n = 12) | Sterile (n = 12) | Pb | Contaminated (n = 10) | Sterile (n = 10) | Pb | |
| 3-Methyl-1-butanol | ND–3c | ND | ∗ | ND–35 | ND | † | ND–10 | ND–8 | † |
| 1-Hexanol | ND–24 | ND–9 | † | ND–470 | ND–8 | † | ND–78 | ND | ∗∗ |
| Limonene | 10–44 | 7–44 | ∗∗ | 54–550 | 54–940 | † | 8–41 | 7–65 | † |
| Acetone | 18–520 | 19–490 | ∗ | ND–8,100 | 190–9,100 | † | 17–290 | ND–300 | † |
| Butanone | ND–500 | ND–160 | † | ND–940 | ND–920 | † | ND–310 | ND–230 | ∗∗ |
| 2-Pentanone | ND–30 | ND–110 | † | ND–1,700 | ND–530 | † | ND–220 | ND–160 | ∗ |
| 3-Methyl-2-pentanone | ND–50 | ND–49 | † | ND–300 | ND–370 | † | ND–95 | ND–71 | ∗ |
| 2-Hexanone | ND–31 | ND–280 | † | ND–460 | ND–620 | † | ND–290 | ND–99 | ∗ |
| 3-Octanone | ND–15 | ND–22 | † | ND–40 | ND–27 | ∗ | ND–17 | ND–14 | † |
Only those VOCs regarded as MVOCs with at least one building material are included.
Statistical significance as determined by the Wilcoxon test. †, the yield from the contaminated construction material was not statistically significantly higher than the yield from the sterile construction material; ∗, 0.01 ≤ P ≤ 0.05; ∗∗, P < 0.01.
ND, not detected.
Floor and separating wall: chipboard and glass wool.
Fungal activity as measured by CO2 production started rapidly after 1-day of incubation at 90 to 92% RH and accelerated rapidly at 97 to 99% RH. At 32 to 33% RH, CO2 production dropped rapidly, and the CO2 concentration decreased to the same level as the level in the control chamber (<0.05%) (Fig. 1). Fungal growth on chipboard was 1 to 3 orders of magnitude greater than fungal growth on glass wool. During incubation at 80 to 82% RH, microbial viability decreased several orders of magnitude (Table 5). At 90 to 92% RH, the level of Aspergillus versicolor increased 360-fold, while 97 to 99% RH favored the growth of Paecilomyces variotii, Fusarium culmorum, and Chaetomium globosum. Surprisingly, under drying conditions, the levels of both A. versicolor and C. globosum increased 20-fold compared with the levels at the beginning of the incubation period at this RH. The control construction pieces remained sterile.
TABLE 5.
Average fungal concentrations in floor and separating wall construction materials (chipboard and glass wool) after inoculation and at the end of incubation
| Microorganism | Avg concn (103 CFU/cm2) in floor and separating wall construction materials
|
||||
|---|---|---|---|---|---|
| After inoculation | After incubation at:
|
||||
| 80–82% RH | 90–92% RH | 97–99% RH | 32–33% RH | ||
| S. brumptii | 3.6 (<1.3–6.5)a | <0.071b | <0.73b | <7.6b | <0.69b |
| P. variotii | 8.5 (3.7–13) | 2.1 (0.017–4.2) | 2.8 (<0.075–5.6) | 110 (0.61–230) | 48 (0.56–96) |
| F. culmorum | 1.6 (<0.92–2.7) | <0.071b | <0.73b | 15 (0.15–30) | <0.69b |
| A. versicolor | 21 (20–21) | 0.070 (<0.008–0.14) | 25 (3.4–46) | 16 (2.6–30) | 380 (19–750) |
| C. globosum | <1.1b | <0.071b | <0.73b | 7.7 (0.15–15) | 150 (9.3–290) |
The values in parentheses are ranges (minimum and maximum concentrations).
The concentration of the microorganism was below the detection limit in both materials; thus, the value is the average of the detection limits for the two materials.
The only MVOC emitted in significant amounts throughout the whole experiment was 3-octanone (Table 4). However, at 90 to 92% RH, 3-methyl-1-butanol, 1-pentanol, and 1-hexanol were emitted from the inoculated materials but not from the sterile materials. Thus, these compounds were MVOCs at one RH level but not during the entire experimental period. The sterile construction pieces generally emitted significantly higher amounts of aldehydes, particularly formaldehyde, acetaldehyde, propanal, butanal, hexanal, heptanal, octanal, and nonanal than did the inoculated construction pieces, as indicated by the Wilcoxon test. Also, the amount of acetone emitted was higher from the sterile construction pieces than from the inoculated construction pieces. 2-Methyl-1-propanol, 3-methyl-2-butanol, and 3-octanol were not detected during the experiment.
Bathroom floor: ceramic tile attached to aggregate block.
When bathroom floor materials were examined, microbial concentrations decreased 1 to 2 orders of magnitude during incubation at 90 to 92% RH (Table 6). The predominant fungi were Sporobolomyces roseus and Exophiala dermatitidis. At 97 to 99% RH, the level of A. furcatum increased 660-fold. Streptomyces californicus and Aureobasidium pullulans died during incubation at 90 to 92% RH. The three other fungal species survived better during the drying period. In spite of slight growth of A. furcatum, the CO2 concentrations remained below 0.01% in the chambers containing the inoculated and sterile construction pieces during the entire experiment and therefore are not included in Fig. 1. We think that the lack of detectable CO2 was the result of a carbonalization process, in which Ca(OH)2 in the aggregate block reacted with CO2 to form CaCO3 and H2O.
TABLE 6.
Microbial concentrations in ceramic tile bathroom floor construction after inoculation and at the end of incubation
| Microorganism | Concn (103 CFU/cm2) in bathroom floor
|
|||
|---|---|---|---|---|
| After inoculation | After incubation at:
|
|||
| 90–92% RH | 97–99% RH | 32–33% RH | ||
| A. pullulans | 4.3 | 0.023 | <0.11 | <0.11 |
| E. dermatitidis | 130 | 10 | 2.2 | 1.7 |
| A. furcatum | 2.0 | 0.11 | 73 | 51 |
| S. roseus | 360 | 28 | 20 | 11 |
| S. californicus | 0.26 | 0.012 | <0.11 | <0.11 |
Microorganisms produced 1-hexanol, butanone, 2-pentanone, 3-methyl-2-pentanone, and 2-hexanone on these construction materials (Table 4). Also, 1-pentanol was emitted only from contaminated materials at 90 to 99% RH, and 1-octen-3-ol was emitted at 90 to 92% RH; thus, these compounds can be regarded as MVOCs. Higher levels of aldehydes (acetaldehyde, propanal, and butanal) and limonene were emitted from the sterile construction pieces than from the contaminated construction materials. 3-Octanol was not detected during the experiment.
DISCUSSION
Humidity and the available nutrients affected microbial growth on building materials. Microorganisms, especially fungi, are capable of growing on almost any substrate when the ERH is more than 75 to 80% (9, 13, 24). We found that approximately 10% of spores could survive and germinate following the stress resulting from adaptation from 100% RH (suspension) to 80 to 82% RH. A very low yield of some MVOCs might indicate that there was drastic killing of inoculated spores. At 90 to 99% RH, two to four of the five species inoculated were viable and could grow on the various building materials. Scopulariopsis brumptii, Stachybotrys chartarum, A. pullulans, and S. californicus did not colonize building materials, even though the humidity conditions were favorable for growth. The failure of these strains to colonize the materials may reflect competition by other species (e.g., Penicillium species [10]) or the lack of one or more essential nutrients. In buildings, surfaces are normally covered with a thin layer of dust and organic debris which contains nutrients suitable for microorganisms (16). The materials used in this study were new, however, which may have affected fungal growth, especially on the ceramic tile floor, which contained few nutrients for microorganisms.
The effect of reducing RH on fungal viability which we observed was not as dramatic as the effect observed previously by Abe (1). Her results indicated that Eurotium herbariorum died within 1 h when active hyphae were transferred from 94% RH to 32% RH. Hyphal viability is not necessarily the same as spore viability because spores may be more resistant to environmental stress than the parental hyphae (19). This difference might explain the high viable spore concentrations at 32 to 33% RH in our experiment. The increase in the P. brevicompactum level on gypsum board-wallpaper and the increase in the A. versicolor and C. globosum levels on chipboard-glass wool at 32 to 33% RH could have been due to slow decreases in the MCs of the building materials during the 2-week incubation period. The MCs of materials at the same RH are higher after desorption of water when the material is drying than after absorption of water when the material gets damp (8). This phenomenon, called hysteresis, is sufficient to explain the similar MCs of the building materials after incubation at 80 to 82% RH and at 32 to 33% RH.
Microbial growth in building materials produces volatile metabolites. However, the effect of higher CO2 production at 97 to 99% RH than at other RHs on the corresponding levels of MVOC emission could not be determined because the VOC yields at different RH levels were not compared in this study. This is because the recovery of VOCs in samples at high RH levels is reduced due to water uptake by the Tenax TA adsorbent (16, 30). For example, the amount of 1-octen-3-ol recovered has been observed to be 16% higher at 20% RH than at 85% RH at a concentration of 50 μg/m3 (30). This problem does not invalidate the results of comparisons of VOC levels emitted from contaminated and sterile materials at the same RH.
Production of microbial metabolites is affected by the species and the media (3, 29, 31, 32). We did not clearly determine the effect of fungal species and building materials on MVOC production because various building materials were contaminated by different mixtures of microbial species. 3-Methyl-1-butanol and 3-methyl-2-butanol were produced by P. brevicompactum, Aspergillus fumigatus, A. furcatum, and E. herbariorum in the gypsum board-wallpaper-plastic film combination. Growth of P. variotii, F. culmorum, A. versicolor, and C. globosum produced 3-methyl-1-butanol in the chipboard-glass wool combination, and 1-pentanol and 1-hexanol were metabolic products of E. dermatitidis, A. furcatum, and S. roseus in the ceramic tile-aggregate block combination. These compounds were not emitted from corresponding sterile materials. Fungal growth appeared to increase emission of 1-octen-3-ol, limonene, acetone, butanone, 2-pentanone, 3-methyl-2-pentanone, 2-hexanone, and 3-octanone from at least one of the contaminated materials. These VOCs should not be regarded as reliable MVOCs because they also were emitted from the sterile materials, even though in previous studies it has been suggested that these VOCs are MVOCs (7, 15, 16, 18, 25, 29, 31, 32, 34).
In addition to increasing emissions, microbial growth may also suppress certain volatile emissions from building materials. The decrease in total aldehyde emission during microbial growth was significant. This finding is not new (16, 26) and has yet to be explained.
In this study, no single VOC that was specific to all of the mixed fungal cultures in all of the building materials studied was found. Although some VOCs seemed to be indicators of fungal growth under certain conditions, no single VOC is universally reliable as a MVOC. Thus, in field studies, the significance of MVOCs for detection of biocontamination in buildings should not be overemphasized. In previous studies, the VOC emissions of control materials were not examined, yet as seen in our results, sterile and contaminated materials may emit the same VOCs. Therefore, the background levels of these VOCs in buildings free of biocontamination must be determined if MVOC analysis is to be used as an indicator of microbial growth in buildings.
ACKNOWLEDGMENTS
We thank Hannu Viitanen, Hannu Kääriäinen, and Jouko Rantamäki of the Technical Research Centre of Finland for supplying the building materials.
This research was supported by grants from the Academy of Finland, by grant 33404 from the Research Programme of Ecological Construction 1995-1998, and by grant 33033 from the Health Research Council.
REFERENCES
- 1.Abe K. Proceedings of Indoor Air ’96. Vol. 3. Tokyo, Japan: Seec Ishibashi Inc.; 1996. Effects of reducing relative humidity on fungal viability; pp. 209–214. [Google Scholar]
- 2.Bjurman J, Kristensson J. Production of volatile metabolites by the soft rot fungus Chaetomium globosum on building materials and defined media. Microbios. 1992;72:47–54. [Google Scholar]
- 3.Börjesson T, Stöllman U, Schnürer J. Volatile metabolites and other indicators of Penicillium aurantiogriseum growth on different substrates. Appl Environ Microbiol. 1990;56:3705–3710. doi: 10.1128/aem.56.12.3705-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Börjesson T S, Stöllman U M, Schnürer J L. Off-odorous compounds produced by molds on oatmeal agar: identification and relation to other growth characteristics. J Agric Food Chem. 1993;41:2104–2111. [Google Scholar]
- 5.Chang J C S, Foarde K K, Vanosdell D W. Growth evaluation of fungi (Penicillium and Aspergillus spp.) on ceiling tiles. Atmos Environ. 1995;29:2331–2337. [Google Scholar]
- 6.Domsch K H, Gams W. Fungi in agricultural soils. Edinburgh, United Kingdom: Longman Group Ltd.; 1972. [Google Scholar]
- 7.Ezeonu I M, Price D L, Simmons R B, Crow S A, Ahearn D G. Fungal production of volatiles during growth on fiberglass. Appl Environ Microbiol. 1994;60:4172–4173. doi: 10.1128/aem.60.11.4172-4173.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flannigan B, Morey P R. Control of moisture problems affecting biological indoor air quality. ISIAQ guideline: Task Force 1. Ottawa, Ontario, Canada: International Society of Indoor Air Quality and Climate; 1996. [Google Scholar]
- 9.Grant C, Hunter C A, Flannigan B, Bravery A F. The moisture requirements of moulds isolated from domestic dwellings. Int Biodeterior. 1989;25:259–284. [Google Scholar]
- 10.Gravesen S, Frisvad J, Samson R. Microfungi. Copenhagen, Denmark: Munksgaard; 1994. pp. 144–147. [Google Scholar]
- 11.Harris N D, Karahadian C, Lindsay R C. Musty aroma compounds produced by selected molds and actinomycetes on agar and whole wheat bread. J Food Prot. 1986;49:964–970. doi: 10.4315/0362-028X-49.12.964. [DOI] [PubMed] [Google Scholar]
- 12.Hunter C A, Grant C, Flannigan B, Bravery A F. Mould in buildings: the air spora of domestic dwellings. Int Biodeterior. 1988;24:81–101. [Google Scholar]
- 13.Kalliokoski P, Pasanen A-L, Korpi A, Pasanen P. Proceedings of Indoor Air ’96. Vol. 3. Tokyo, Japan: Seec Ishibashi Inc.; 1996. House dust as a growth medium for microorganisms; pp. 131–135. [Google Scholar]
- 14.Kaminski E, Libbey L M, Stawicki S, Wasowicz E. Identification of the predominant volatile compounds produced by Aspergillus flavus. Appl Microbiol. 1972;24:721–726. doi: 10.1128/am.24.5.721-726.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminski E, Stawicki S, Wasowicz E. Volatile flavor compounds produced by molds of Aspergillus, Penicillium and Fungi imperfecti. Appl Microbiol. 1974;27:1001–1004. doi: 10.1128/am.27.6.1001-1004.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpi A, Pasanen A-L, Pasanen P, Kalliokoski P. Microbial growth and metabolism in house dust. Int Biodeterior Biodegrad. 1997;40:19–27. [Google Scholar]
- 17.Korpi, A., A.-L. Pasanen, and H. Viitanen. Volatile metabolites of Serpula lacrymans, Coniophora puteana, Poria placenta, Stachybotrys chartarum and Chaetomium globosum. Build. Environ., in press.
- 18.Larsen T O, Frisvad J C. Production of volatiles and presence of mycotoxins in conidia of common indoor penicillia and aspergillii. In: Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, Adan O C G, Hoekstra E S, editors. Health implications of fungi in indoor environments. Air quality monographs. Vol. 2. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 251–279. [Google Scholar]
- 19.Levetin E. Fungi. In: Burge H A, editor. Bioaerosols. Boca Raton, Fla: Lewis Publishers; 1995. pp. 87–112. [Google Scholar]
- 20.Neave H R, Worthington P L. Distribution-free tests. London, United Kingdom: Unwin Hyman Ltd.; 1988. pp. 160–166. [Google Scholar]
- 21.Nevalainen A, Pasanen A-L, Niininen M, Reponen T, Kalliokoski P, Jantunen M J. The indoor air quality in Finnish homes with mold problems. Environ Int. 1991;17:299–302. [Google Scholar]
- 22.Nikulin M, Pasanen A-L, Berg S, Hintikka E-L. Stachybotrys atra growth and toxin production in some building materials and fodder under different relative humidities. Appl Environ Microbiol. 1994;60:3421–3424. doi: 10.1128/aem.60.9.3421-3424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasanen A-L, Niininen M, Kalliokoski P, Nevalainen A, Jantunen M J. Airborne Cladosporium and other fungi in damp versus reference residences. Atmos Environ. 1992;26B:121–124. [Google Scholar]
- 24.Pasanen A-L, Juutinen T, Jantunen M J, Kalliokoski P. Occurrence and moisture requirements of microbial growth in building materials. Int Biodeterior Biodegrad. 1992;30:273–283. [Google Scholar]
- 25.Pasanen A-L, Lappalainen S, Pasanen P. Volatile organic metabolites associated with some toxic fungi and their mycotoxins. Analyst. 1996;121:1949–1953. [Google Scholar]
- 26.Pasanen P, Korpi A, Kalliokoski P, Pasanen A-L. Growth and volatile metabolite production of Aspergillus versicolor in house dust. Environ Int. 1997;23:425–432. [Google Scholar]
- 27.Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, Adan O C G, Hoekstra E S, editors. Health implications of fungi in indoor environments. Air quality monographs. Vol. 2. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 531–538. [Google Scholar]
- 28.Ström G, West J, Wessén B, Palmgren U. Quantitative analysis of microbial volatiles in damp Swedish houses. In: Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, Adan O C G, Hoekstra E S, editors. Health implications of fungi in indoor environments. Air quality monographs. Vol. 2. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 291–305. [Google Scholar]
- 29.Sunesson A-L, Vaes W H J, Nilsson C-A, Blomquist G, Andersson B, Carlson F. Identification of volatile metabolites from five fungal species cultivated on two media. Appl Environ Microbiol. 1995;61:2911–2918. doi: 10.1128/aem.61.8.2911-2918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunesson A-L, Nilsson C-A, Andersson B. Evaluation of adsorbents for sampling and quantitative analysis of microbial volatiles using thermal desorption-gas chromatography. J Chromatogr A. 1995;699:203–214. [Google Scholar]
- 31.Sunesson A-L, Nilsson C-A, Andersson B, Blomquist G. Volatile metabolites produced by two fungal species cultivated on building materials. Ann Occup Hyg. 1996;40:397–410. doi: 10.1016/0003-4878(96)00002-6. [DOI] [PubMed] [Google Scholar]
- 32.Sunesson A-L, Nilsson C-A, Carlson R, Blomquist G, Andersson B. Production of volatile metabolites from Streptomyces albidoflavus cultivated on gypsum board and tryptone glucose extract agar—influence of temperature, oxygen and carbon dioxide levels. Ann Occup Hyg. 1997;41:393–413. [Google Scholar]
- 33.Wessén B, Ström G, Schoeps K-O. MVOC profiles—a tool for indoor-air quality assessment. In: Morawska L, Bofinger N D, Maroni M, editors. Indoor air: an integrated approach. Oxford, United Kingdom: Elsevier Science Ltd.; 1995. pp. 67–70. [Google Scholar]
- 34.Wessén B, Schoeps K-O. Proceedings of Indoor Air ’96. Vol. 3. Tokyo, Japan: Seec Ishibashi Inc.; 1996. MVOC ratios—an aid for remediation of sick buildings; pp. 557–561. [Google Scholar]
- 35.Whillans F D, Lamont G S. Fungal volatile metabolites released into indoor air environments: variation with fungal species and growth media. In: Morawska L, Bofinger N D, Maroni M, editors. Indoor air: an integrated approach. Oxford, United Kingdom: Elsevier Science Ltd.; 1995. pp. 47–50. [Google Scholar]