Abstract
Activated sludge not containing significant numbers of denitrifying, polyphosphate [poly(P)]-accumulating bacteria was grown in a fill-and-draw system and exposed to alternating anaerobic and aerobic periods. During the aerobic period, poly(P) accumulated up to 100 mg of P · g of (dry) weight. When portions of the sludge were incubated anaerobically in the presence of acetate, 80 to 90% of the intracellular poly(P) was degraded and released as orthophosphate. Degradation of poly(P) was mainly catalyzed by the concerted action of polyphosphate:AMP phosphotransferase and adenylate kinase, resulting in ATP formation. In the presence of 0.3 mM nitric oxide (NO) in the liquid-phase release of phosphate, uptake of acetate, formation of poly-β-hydroxybutyrate, utilization of glycogen, and formation of ATP were severely inhibited or completely abolished. In cell extracts of the sludge, adenylate kinase activity was completely inhibited by 0.15 mM NO. The nature of this inhibition was probably noncompetitive, similar to that with hog adenylate kinase. Activated sludge polyphosphate glucokinase was also completely inhibited by 0.15 mM NO. It is concluded that the inhibitory effect of NO on acetate-mediated phosphate release by the sludge used in this study is due to the inhibition of adenylate kinase in the phosphate-releasing organisms. The inhibitory effect of nitrate and nitrite on phosphate release is probably due to their conversion to NO. The lack of any inhibitory effect of NO on adenylate kinase of the poly(P)-accumulating Acinetobacter johnsonii 210A suggests that this type of organism is not involved in the enhanced biological phosphate removal by the sludges used.
Biological phosphorus removal from wastewater has become an interesting process because of its low operational costs and efficiency. This process is currently perceived to hinge on the introduction of an anaerobic period preceding the aerobic period in the conventional activated-sludge process. In the anaerobic period, influent and return sludge come together and phosphate is released. This release is stimulated by fermentation products, in particular by acetate and propionate. These fatty acids are converted into poly-β-hydroxyalkanoates (PHAs) (13), and the required reducing equivalents are produced by the oxidation of glycogen via glycolysis (20). In the subsequent aerobic phase, no easily degradable extracellular carbon compounds are available any more, intracellular PHAs are used as a source of carbon and as energy for growth, and phosphate is taken up in amounts that are much greater than biosynthetic needs.
Phosphate is frequently removed from wastewater simultaneously with nitrogen compounds (for a review, see reference 14). The removal of nitrogen compounds is achieved by nitrification, which is strictly an aerobic process, and by denitrification, which occurs anaerobically. It has been found repeatedly that phosphate release was reduced when the influent contained large quantities of nitrate, and in the long run biological phosphorus removal was completely abolished (16). Five hypotheses have been tested to explain the inhibitory effect of nitrate on phosphate release, and only one was found to be valid for the sludge grown in the fill-and-draw (F&D) system (2–4) and for Renpho sludge (22). An intermediate in denitrification, nitric oxide (NO), was found to be a potent inhibitor of phosphate release (2). NO inhibited phosphate release maximally at 40 μM when it was continuously flushed through the F&D sludge suspension. In batch experiments, the maximum inhibitory effect occurred at initial concentrations in the liquid phase of 0.3 mM NO and higher.
This study describes the inhibition of phosphate release by nitrate in activated sludge at the molecular level. NO appeared to inhibit the activity of adenylate kinase and polyphosphate glucokinase.
MATERIALS AND METHODS
Sludges.
Renpho sludge originated at a pilot plant built for enhanced biological phosphate removal. The pilot plant was fed with settled domestic wastewater from the village of Bennekom, in The Netherlands (22). F&D system sludge was grown in the lab on synthetic medium without nitrate (900 ml) in a one-reactor vessel of 2 liters as described by Appeldoorn et al. (3). The sludge was exposed to cycles with three distinct, consecutive periods; first an anaerobic period of 75 min, then an aerobic period of 165 min, and finally a settlement period of 120 min. In the period of settlement, one-third of the liquid was replaced with fresh medium. To keep the sludge concentration at about 3.5 g per liter, 27 ml of mixed liquor (medium plus sludge) was removed after the aerobic period. During this study, 90 to 100% of the bacteria in F&D sludge contained poly(P). Neither sludge contained significant numbers of denitrifying poly(P) bacteria (2), and the F&D sludge had lost its ability to nitrify after about 30 days (3).
Organisms.
Acinetobacter johnsonii 210 was grown in butyrate-limited continuous culture at a dilution rate of 0.1 h−1 according to Bonting et al. (10) or in batch culture (9). The unidentified strain 2.8 and the other strains were isolated from activated sludge grown according to the Renpho system (22) and were grown in batch culture with acetate as the carbon source.
Medium.
The phosphate-release medium (pH 7.0) contained (grams per liter) NH4Cl, 0.32; MgSO4 · 7H2O, 0.6; CaCl2 · 2H2O, 0.07; EDTA, 0.1; and piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES–KOH, 3.02; and 2 ml of a trace-elements solution (3).
Phosphate-release experiments.
Renpho sludge was harvested at the end of the aerobic period by centrifugation at 10,000 × g. The sludge was washed once with demineralized water and resuspended in demineralized water up to a concentration of about 15 g of (dry) weight liter−1. Suspension and headspace were made anaerobic by vigorously gassing for 5 min with a mixture of 99.5% N2 and 0.5% CO2. Five milliliters of the anaerobic suspension was added with a syringe to sealed 39-ml serum bottles containing 20 ml of 1.2-fold-concentrated release medium and a headspace of 99.5% N2 and 0.5% CO2. After the sludge was added, the bottles were incubated in a thermostated shaker at 25°C. The reaction was started by injecting acetate to a final concentration of 5 mM.
Fresh F&D sludge (100 ml) was harvested at the end of the aerobic period and had a P content of 8 to 10% of the (dry) weight. This was usually the case when the system was run for 40 to 50 days and Renpho sludge was used as the inoculum. The sludge was centrifuged at 10,000 × g and washed once with 10 mM PIPES–KOH (pH 7.0). The pellet was resuspended in phosphate-release medium to a final volume of 100 ml. Serum vials (39 ml each) were filled with 20 ml of the sludge suspension, and suspension and headspace were made anaerobic by gassing for 5 min with the above-mentioned gas mixture. The vials were sealed with butyl rubber stoppers.
NO was added to a concentration of 0.3 mM in the liquid phase with gas-tight syringes. Sludge suspensions with NO were then allowed to equilibrate for 5 min. The experiment was started by injecting acetate to a final concentration of 5 mM. The incubation mixture was kept at 25°C and stirred continuously with a magnetic stirrer. Phosphate release was followed by analysis of samples taken at intervals.
Cell extract preparation.
At the end of the anaerobic period, 100 ml of F&D sludge was taken from the system and centrifuged (19,000 × g for 10 min at 4°C), washed with PIPES–KOH buffer (10 mM; pH 7.0), and centrifuged again. The pellet was resuspended in the same buffer and then sonified (40 W at 0°C) with an ultrasonic disintegrator (12 times for 30 s each at 30-s intervals with model B12; Branson Sonic Power Company). Cell debris was removed by centrifugation (19,000 × g, 10 min, 4°C). The supernatant was collected and centrifuged again. The supernatant contained 0.3 to 2.1 mg of protein ml−1. Cell extracts from A. johnsonii 210A were prepared in a similar way.
Enzyme assays.
All assays were carried out anaerobically at 30°C and pH 7.0. The reaction mixture and headspace were gassed with 99.5% N2 plus 0.5% CO2 for 2 to 3 min, or the individual solutions and empty cuvettes were gassed. The reaction mixture contained 0.01 to 0.2 ml of cell extract per ml of assay, resulting in 60 to 600 μg of protein per ml. When NO was added (0.2 ml per cuvette = 0.3 mM in liquid phase), the gas was added about 5 min prior to the start of the reaction.
Polyphosphate:AMP phosphotransferase.
The activity was measured photometrically at 340 nm by following the oxidation of NADH by lactate dehydrogenase (9) and not by an assay involving adenylate kinase (25). The reaction was started by the addition of phosphoenolpyruvate (5 mM).
Adenylate kinase.
The activity was measured by monitoring the reduction of NADP+. The reaction mixture contained 100 mM Tris-HCl (pH 8.5); 8 mM MgCl2; 0.4 mM NADP+; 5 mM glucose; hexokinase, 2 U; glucose-6-phosphate dehydrogenase, 1 U. The reaction was started by the addition of ADP (2 mM). This assay was also used to determine the activity of commercial adenylate kinase isolated from hogs.
Polyphosphate glucokinase.
The activity was measured by monitoring the reduction of NADP+ by glucose-6-phosphate dehydrogenase (25). The reaction was started by the addition of 50 mM glucose.
Polyphosphatase.
This activity was determined by following the formation of phosphate from poly(P) (10). The reaction was started by adding cell extract.
Acetate kinase.
This activity was determined by monitoring the formation of acetyl-phosphate (7). The reaction was started by adding cell extract. Samples were taken at intervals and mixed with 1 volume of a freshly made mixture of trichloroacetic acid (24.5 mM) and FeCl3 (0.1 mM) in 1 N HCl. Absorption was measured spectrophotometrically at 540 nm.
Analytical methods.
For the determination of intracellular ATP concentrations, cells were extracted with perchloric acid by the method of Otto et al. (21). ATP in neutralized extracts was determined with a firefly bioluminescence assay (LUMIT; Lumac, Breda, The Netherlands). For (dry) weight determinations, 10 ml of the sludge suspension was centrifuged, washed once with demineralized water, and dried at 100°C for 24 h. Protein was measured by the method of Bradford (11). Orthophosphate (ascorbic acid method) was quantified according to the American Public Health Association (1). Acetate and butyrate were determined by high-pressure liquid chromatography with a Chrompack organic acids column (30- by 6.5-mm inner diameter) and a differential refractometer (model LKB 2142). The mobile phase was 0.01 N H2SO4, with a flow rate of 0.6 ml min−1. The working temperature was 60°C. Samples (20 μl each) were injected with a Spectra Physics autosampler (model SP8775).
RESULTS
Effect of NOs on phosphate release.
The intermediates in denitrification were examined for their potential to inhibit phosphate release in Renpho sludge. This type of activated sludge was not previously adapted to nitrate. The highest phosphate release was observed in the absence of nitrogen oxides (Table 1). In the presence of nitrate, nitrite and, in particular, NO, this release was severely reduced. However, nitrous oxide did not affect the release of phosphate. Hydroxylamine, an intermediate in the assimilatory reduction of nitrate, reduced phosphate release only slightly.
TABLE 1.
Phosphate release by Renpho sludge, determined after an incubation period of 1 h in the presence of several nitrogen oxidesa
| Addition | Concn (mM) | Mean ± SD of phosphate release (mg of P/g of [dry] weight−1) |
|---|---|---|
| None (control) | 7.7 ± 0.0 | |
| Nitrate | 3.6 | 4.8 ± 0.8 |
| Nitrite | 3.6 | 4.1 ± 0.2 |
| NO | 3.6b | 3.4 ± 1.3 |
| Nitrous oxide | 3.6b | 7.7 ± 0.1 |
| Hydroxylamine | 3.6 | 6.5 ± 0.2 |
Data are the averages of two independent incubations.
Concentration in the liquid phase.
Effect of NO on anaerobic phosphate release.
The effect of NO was further examined in sludge samples derived from the F&D system. The initial phosphate-release rates with this sludge type in the presence of 5 mM acetate varied between 0.8 and 1.4 mg of P min−1 g of (dry) weight−1. In the presence of NO, at a final concentration of 0.3 mM in the liquid phase, the acceleration of phosphate release by acetate was totally absent, the phosphate-release rate being similar to the endogenous release rate (Fig. 1). The latter was measured in the absence of acetate and NO.
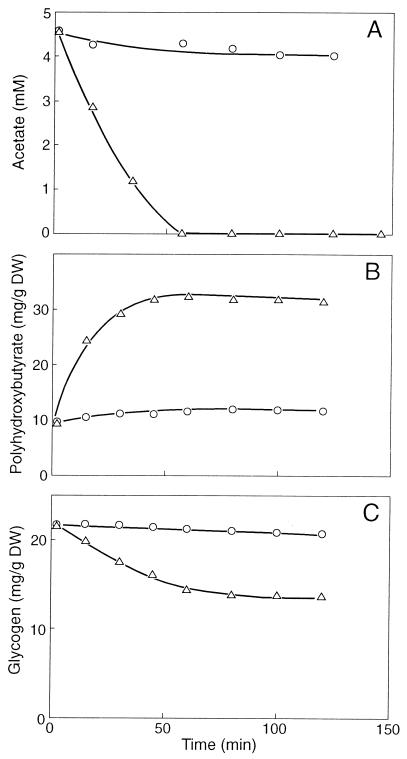
FIG. 1.
Phosphate release by F&D sludge under anaerobic conditions with acetate (▵), acetate plus 0.3 mM NO (○), NO alone, and in the absence of both acetate and NO (endogenous phosphate release) (□). DW, dry weight.
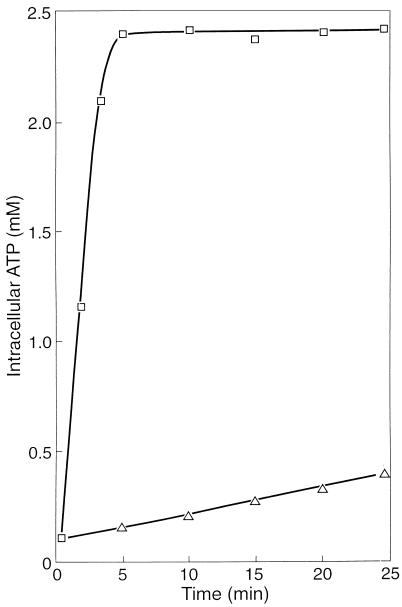
It appeared that the uptake of acetate also ceased almost completely in the presence of NO (Fig. 2A). NO also prevented poly-β-hydroxybutyrate (PHB) formation from acetate (Fig. 2B) and glycogen consumption (Fig. 2C). The conversion of acetate into PHB includes (i) the uptake of acetate, (ii) the activation of acetate, and (iii) the conversion of activated acetate into PHB. The activation of acetate requires ATP. Figure 3 shows that after the addition of acetate, ATP accumulated rapidly until a final cellular concentration of about 2.4 mM was reached. However, when the sludge was incubated with 0.3 mM NO, the ATP concentration increased slowly. After an incubation period of 30 min, the cellular ATP concentration was only about 0.5 mM. These results clearly show that NO markedly inhibited the anaerobic energy conservation in the sludge and that one or more enzymes involved in ATP formation from poly(P) must be inhibited directly or indirectly by NO.
FIG. 2.
Time course experiment with F&D sludge in the absence (▵) and presence (○) of 0.3 mM NO. (A) Uptake of acetate. (B) Formation of PHB. (C) Consumption of glycogen. DW, dry weight.
FIG. 3.
Generation of ATP in F&D sludge under anaerobic conditions in the absence (□) and presence (▵) of 0.3 mM NO. The experiment was started by injecting acetate to a final concentration of 5 mM.
Effect of NO on enzymes involved in poly(P) degradation.
Adenylate kinase appeared to be severely inhibited by 0.3 mM NO (Table 2). Polyphosphate glucokinase activity was also severely reduced by NO. This inhibition was not further examined because the enzyme showed a low specific activity compared to polyphosphate:AMP phosphotransferase and adenylate kinase activities. Polyphosphate:AMP phosphotransferase, polyphosphatase, and acetate kinase activities were not affected by NO or were affected only slightly. The commercial enzymes used in the assays (hexokinase, glucose-6P-dehydrogenase, lactate dehydrogenase, and pyruvate kinase) were not sensitive to NO concentrations up to 1 mM in the liquid phase (data not shown).
TABLE 2.
Specific activities of and inhibitory effect of 0.3 mM NO on several enzymes involved in polyphosphate degradation and acetate metabolism in a cell extract of fresh F&D sludge
| Enzyme | Sp act (nmol min−1 mg of protein−1) | Inhibition by NO (%) |
|---|---|---|
| Polyphosphate:AMP phosphotransferase | 730 | 0 |
| Adenylate kinase | 2,380 | 90–100 |
| Polyphosphate glucokinase | 61 | 100 |
| Polyphosphatase | 210 | 0–10 |
| Acetate kinase | 59 | 0 |
Inhibition kinetics of adenylate kinase.
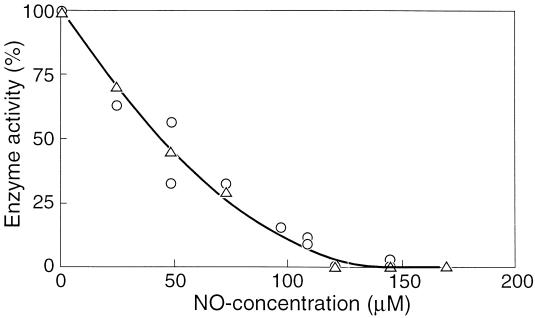
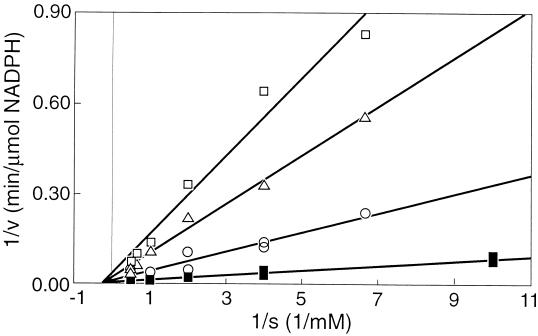
The initial specific activity of adenylate kinase in cell extracts of F&D sludge was measured in the presence of various concentrations of NO, ranging from 0 to 0.17 mM. It appeared that the enzyme was completely inhibited by 0.15 mM NO (Fig. 4). Commercial adenylate kinase from hogs was also tested for inhibition by NO and showed a decline in activity with increasing concentrations of NO, similar to the adenylate kinase activity of the sludge (Fig. 4). It was found that the nature of the inhibition of the hog enzyme by NO was noncompetitive (Fig. 5). Hence, it follows that the maximum velocity is affected by the inhibitor, but the affinity constant (Ks) remains the same. The Michaelis-Menten expression modified for this kind of inhibition is then (15) v = [Vmax × Cs/(Ks + Cs)] × (1 − CI/CI*)n where Cs is substrate concentration, CI is inhibitor concentration, and CI* is the critical inhibitor concentration above which the reaction stops, and n is a constant. This equation is fitted through the data shown in Fig. 4 and yields a CI* value of 0.15 mM NO and an n of 2. The enzyme is already inhibited by 50% at 0.044 mM NO.
FIG. 4.
Adenylate kinase activity as a function of the NO concentration in the liquid phase. ▵, adenylate kinase of F&D sludge; ○, hog adenylate kinase.
FIG. 5.
Lineweaver-Burk plot of hog adenylate kinase at different inhibitor concentrations. □, 0.135 mM NO; ▵, 0.12 mM NO; ○, 0.05 mM NO; and ■, 0 mM NO.
Effect of NO on pure cultures of poly(P)-accumulating organisms.
Anaerobic phosphate-release experiments with pure cultures of the poly(P)-accumulating Acinetobacter johnsonii 210A and the unidentified strain 2.8 showed no influence of NO (data not shown). With cell extracts of A. johnsonii 210A, adenylate kinase activity was not affected by NO. No enzyme inhibition was found at concentrations of NO up to 1 mM in the liquid phase. This was also true of the adenylate kinase activity in extracts from a number of other, unidentified Acinetobacter cultures (data not shown).
DISCUSSION
The acetate-mediated phosphate release by suspensions of Renpho sludge was severely reduced in the presence of nitrate, nitrite, and NO, but not by nitrous oxide or hydroxylamine. Renpho sludge and F&D sludge adenylate kinase activities were severely inhibited by NO, but not by nitrate or nitrite. These results suggest that NO, an intermediate in denitrification, is the cause of the reduced phosphate release by nitrate and nitrite. They support the observation of Appeldoorn (2) that no reduction in phosphate release by nitrate occurred upon inhibition of denitrification of nitrate by cyanide and azide. The accumulation of nitrite and of small amounts of NO and nitrous oxide during denitrification has been observed frequently in a wide variety of bacteria. A kinetic explanation has been provided for the accumulation of these intermediates (8). Indeed, with all activated sludges studied, NO formation from nitrate or nitrite could be shown by passing nitrogen gas through the sludge suspensions (2). The rate of NO formation depended on the sludge type, the NO precursor, the carbon source, and the pH. Usually the concentrations of NO and N2O as a result of denitrification in activated sludges are low (26). However, besides biological reduction of nitrate and nitrite, NO can also be formed by chemodenitrification (12, 24), a spontaneous reduction of nitrite to NO with electrons from ferrous iron or other reduced-metal ions (12, 27). The presence of these ions in sludge might be at least partially responsible for abiotic NO formation from nitrite.
In F&D sludge, the anaerobic acetate-mediated phosphate release was almost completely absent in the presence of 0.3 mM NO. Also, uptake of acetate, formation of PHB, and consumption of glycogen were almost completely impaired by NO. These results indicate that the degradation of poly(P) is closely related to the anaerobic carbon metabolism in F&D sludge poly(P) bacteria and most likely occurs according to the model proposed by Mino et al. (20). In addition, simultaneous inhibition of poly(P) degradation, phosphate release, acetate uptake, PHB formation, and glycogen consumption and the presence of poly(P), PHAs, and glycogen in almost all cells of the F&D sludge (data not shown, but see reference 19) suggest that poly(P) degradation, PHB formation, and glycogen consumption occur in almost each single cell. The great morphological similarity of the cells suggests that only a limited number of different bacterial types are involved. The study of regulatory mechanisms involved in the fine-tuning of the enzymes involved in poly(P) metabolism with those involved in carbon metabolism must wait until the principal poly(P) organism in the sludge has been obtained in pure culture.
In cell extracts of the F&D sludge, adenylate kinase activity was inhibited by 50 and 100% in the liquid phase at NO concentrations of 0.044 and 0.15 mM, respectively. With whole cells from the same sludge, somewhat higher NO concentrations were observed: 50 and 100% inhibition at 0.14 and 0.3 mM NO, respectively (2). With whole cells, NO has to penetrate the cell via the cell membrane containing iron proteins before it can reach the cytoplasmic adenylate kinase, and it is known that NO can easily form complexes with these proteins (12, 28). The F&D sludge adenylate kinase activity is presently being purified to learn more about the mode of action of NO on these kinases. Inhibition of some adenylate kinases and of F&D sludge polyphosphate glucokinase by NO is a new property of this reactive nitrogen oxide.
Although the commercial adenylate kinase from hogs and the adenylate kinase activities of Renpho and F&D sludges were similarly affected by NO, the adenylate kinases of Acinetobacter johnsonii 210A and other Acinetobacter strains were not sensitive to NO, even at concentrations up to the mM level. Activated sludges with a profound capacity to remove phosphate biologically (2) contain hardly any bacteria belonging to the genus Acinetobacter (5, 17). The insensitivity of the adenylate kinase activities of many Acinetobacter strains also indicates that representatives of this genus are most likely not involved in enhanced biological phosphorus removal. Inhibition of phosphate release by NO might be an elegant tool to isolate the bacterium (or bacteria) from F&D sludge, which is predominantly responsible for poly(P) accumulation by this sludge.
Two accepted explanations for inhibition of phosphate release by nitrate in activated sludges are as follows: (i) competition for the same substrate between denitrifying bacteria and poly(P)-accumulating bacteria and (ii) accumulation of poly(P) by poly(P) bacteria which are able to denitrify (6, 13, 14, 16, 18, 19, 22, 23). This study shows that nitrate, through its conversion to NO, may suppress the growth of poly(P)-accumulating bacteria by inhibiting their carbon metabolism and suggests once again that bacteria belonging to the genus Acinetobacter are not responsible for poly(P) accumulation in activated sludges.
ACKNOWLEDGMENTS
We are grateful to Wim Roelofsen for technical assistance with the analytical determinations and to Nees Slotboom for drawing the figures.
This study was supported by a grant from the Dutch Organisation for Applied Research on Wastewater Treatment (STORA).
REFERENCES
- 1.American Public Health Association. Standard methods for examination of water and wastewater. 14th ed. Washington, D.C: American Public Health Association; 1976. [Google Scholar]
- 2.Appeldoorn K J. Ecological aspects of the biological phosphate removal from waste waters. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1993. [Google Scholar]
- 3.Appeldoorn K J, Kortstee G J J, Zehnder A J B. Biological phosphate removal by activated sludge under defined conditions. Water Res. 1992;26:453–460. [Google Scholar]
- 4.Appeldoorn K J, Boom A J, Kortstee G J J, Zehnder A J B. Contribution of precipitated phosphates and acid-soluble polyphosphate to enhanced biological phosphate removal. Water Res. 1992;26:937–943. [Google Scholar]
- 5.Auling G, Pilz F, Busse H-J, Karrasch S, Streichan M, Schön G. Analysis of the polyphosphate-accumulating microflora in phosphorus-eliminating anaerobic-aerobic activated sludge systems by using diaminopropane as a biomarker for rapid estimation of Acinetobacter spp. Appl Environ Microbiol. 1991;57:3585–3592. doi: 10.1128/aem.57.12.3585-3592.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker P S, Dold P L. Denitrification behaviour in biological excess phosphorus removal activated sludge systems. Water Res. 1996;30:769–780. [Google Scholar]
- 7.Barman ThE. Acetate kinase. Enzyme handbook. Vol. 1. Berlin, Germany: Springer-Verlag; 1969. p. 428. [Google Scholar]
- 8.Betlach M R, Tiedje J M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981;42:1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonting C F C, Kortstee G J J, Zehnder A J B. Properties of polyphosphate:AMP phosphotransferase of Acinetobacter strain 210A. J Bacteriol. 1991;173:6484–6488. doi: 10.1128/jb.173.20.6484-6488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonting C F C, van Veen H W, Taverne A, Kortstee G J J, Zehnder A J B. Regulation of polyphosphate metabolism in Acinetobacter strain 210A grown in carbon- and phosphate-limited continuous culture. Arch Microbiol. 1992;158:139–144. [Google Scholar]
- 11.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Brons H J, Hagen W R, Zehnder A J B. Ferrous iron dependent nitric oxide production in nitrate reduction cultures of Escherichia coli. Arch Microbiol. 1991;155:341–348. doi: 10.1007/BF00243453. [DOI] [PubMed] [Google Scholar]
- 13.Comeau Y, Oldham W K, Hall K J. Dynamics of carbon reserves in biological dephosphatation of wastewater. In: Ramadori R, editor. Biological phosphate removal from wastewaters. Proceedings of an International Association on Water Pollution Research and Control Special Conference, Rome. Oxford, England: Pergamon Press; 1987. pp. 39–55. [Google Scholar]
- 14.Egli T, Zehnder A J B. Phosphate and nitrate removal. Curr Opin Biotechnol. 1994;5:275–284. [Google Scholar]
- 15.Han K, Levenspiel O. Extended Monod kinetics for substrate, product, and cell inhibition. Biotechnol Bioeng. 1988;32:430–437. doi: 10.1002/bit.260320404. [DOI] [PubMed] [Google Scholar]
- 16.Hascoet M C, Florentz M. Influence of nitrate on biological phosphorus removal from wastewaters. Water S A (Pretoria) 1985;11:1–8. [Google Scholar]
- 17.Hiraishi A, Masamune K, Kitamura H. Characterization of the bacterial population structure in an anaerobic-aerobic activated sludge system on the basis of respiratory quinone profiles. Appl Environ Microbiol. 1989;55:897–901. doi: 10.1128/aem.55.4.897-901.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerrn-Jespersen J P, Henze M. Biological phosphorus uptake under anoxic and aerobic conditions. Water Res. 1993;27:617–624. [Google Scholar]
- 19.Kuba T, Smolders G, van Loosdrecht M C M, Heijnen J J. Biological phosphorus removal from waste water by anaerobic-anoxic sequencing batch reactor. Water Sci Technol. 1993;27:241–252. [Google Scholar]
- 20.Mino T, Arun V, Tsuzuki Y, Matsuo T. Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process. In: Ramadori R, editor. Biological phosphate removal from wastewaters. Proceedings of an International Association on Water Pollution Research and Control Special Conference, Rome. Oxford, England: Pergamon Press; 1987. pp. 27–38. [Google Scholar]
- 21.Otto R, Klont B, ten Brink B, Konings W N. The phosphate potential, adenylate energy charge and proton motive force in growing cells of Streptococcus cremoris. Arch Microbiol. 1984;139:338–343. [Google Scholar]
- 22.Rensink J H, Donker H J G W, Anink D M. Weitgehende P- und N-Elimination aus kommunalen Abwasser mit Verbesserung der P-Rücklösung. gwf-Wasser/Abwasser. 1989;130:86–89. [Google Scholar]
- 23.Schön G. Biologische Phosphorentfernung bei der Abwasserreinigung im Belebensverfahren. Bioengineering. 1994;4:23–32. [Google Scholar]
- 24.Van Cleemput O, Baert L. Nitrite: a key compound in N loss processes under acid conditions? Plant Soil. 1984;86:233–241. [Google Scholar]
- 25.Van Groenestijn J W, Deinema M H, Zehnder A J B. ATP production from polyphosphate in Acinetobacter strain 210A. Arch Microbiol. 1987;148:14–19. [Google Scholar]
- 26.Von Schulthess R, Wild D, Gujer W. Nitric and nitrous oxides from denitrifying activated sludge at low oxygen concentration. Water Sci Technol. 1994;30:123–132. [Google Scholar]
- 27.Wullstein L H, Gilmour C M. Non-enzymatic gaseous loss of nitrite from clay and soil systems. Soil Sci. 1964;98:428–430. [Google Scholar]
- 28.Zumft W G, Frunzke K. Discrimination of ascorbate-dependent nonenzymatic and enzymatic membrane-bound reduction of nitric oxide in denitrifying Pseudomonas perfectomarinus. Biochim Biophys Acta. 1982;681:459–468. doi: 10.1016/0005-2728(82)90188-8. [DOI] [PubMed] [Google Scholar]