Abstract
Background
Data are limited on long-term outcomes in patients who have undergone a reoperation following failure of a stentless aortic valve.
Methods
Between 2006 and 2016, a retrospective analysis was performed on 24 patients who underwent open aortic valve replacement surgery for a failed stentless aortic valve prosthesis at Health Sciences North, Sudbury, Ontario, Canada. The primary outcome was a low mortality rate from cardiac-related deaths after 5 years.
Results
All patients underwent insertion of a Medtronic Freestyle bioprosthesis (Minneapolis, MN) implanted using the modified subcoronary technique for their initial operation. The interval from the first operation to the stentless redo surgery ranged from 6 to 13 years. Aortic valve reoperation was performed for structural valve deterioration in 96% (n = 23) of the cases. Reoperations involved a removal of the stented valve leaflets and standard aortic valve replacement within the stentless casing in 20% (n = 5) of the cases, with the remaining cases requiring complete removal of the stentless prosthesis and aortic valve replacement. In those in whom a complete removal of the stentless valve was possible (n = 19), no disruption of the native aortic root occurred, with a 0% rate of conversion to a Bentall procedure. No intraoperative mortality occurred. The 30-day and 10-year operative mortality rates were 4% and 16%, respectively.
Conclusions
Redo surgery for failing stentless valves can be done with relatively low risk and with acceptable long-term outcomes without resorting to root-replacement techniques.
Résumé
Contexte
Il existe peu de données sur les résultats à long terme chez les patients qui ont subi une réintervention chirurgicale après une défaillance d’une valve aortique sans armature (stentless) ayant été implantée.
Méthodologie
Nous avons réalisé une analyse rétrospective, de 2006 à 2016, auprès de 24 patients ayant subi une intervention chirurgicale invasive de remplacement de valve aortique en raison de la défaillance d’une prothèse aortique sans armature à l’hôpital Health Sciences North situé à Sudbury (Ontario), au Canada. Le paramètre principal d’évaluation était un faible taux de mortalité d’origine cardiaque après 5 ans.
Résultats
Tous les patients avaient initialement subi l’implantation d’une bioprothèse Medtronic Freestyle (Minneapolis, Minnesota) par la technique sous-coronaire modifiée. La période écoulée entre la première intervention chirurgicale et la réintervention au niveau de la valve sans armature allait de 6 à 13 ans. Dans 96 % des cas (n = 23), la réintervention était réalisée en raison d’une détérioration de structure de la valve aortique. La réintervention avait consisté en un retrait des cuspides avec armature et un remplacement de valve aortique standard dans la membrane sans armature dans 20 % des cas (n = 5) et un retrait complet de la prothèse sans armature avec remplacement de la valve aortique avait été nécessaire dans les autres cas. Chez les patients pour qui le retrait complet de la valve sans armature a été possible (n = 19), aucune déchirure de la racine aortique native n’est survenue et le taux de passage à une intervention de Bentall était de 0 %. Aucun décès peropératoire n’est survenu. Les taux de mortalité à 30 jours et à 10 ans s’élevaient à 4 % et à 16 %, respectivement.
Conclusions
La réintervention chirurgicale après la défaillance d’une valve aortique sans armature peut être réalisée avec des risques re-lativement faibles et des résultats à long terme acceptables sans avoir recours à des techniques de remplacement de la racine aortique.
The Freestyle Stentless Porcine Valve (Medtronic, Minneapolis, MN) has been used since 19931 as a third-generation porcine valve. Compared to conventional stented bioprostheses, stentless valves provide better hemodynamics, with a larger effective orifice area with respect to their valve size.2 This allows for improved left ventricular function secondary to left ventricular mass regression3, 4, 5 and reduces incidences of patient-prosthesis mismatch. However, despite the advantages, historically, the utilization of stentless valves has been controversial. The benefits in hemodynamics have been influenced negatively by the technical difficulty of implanting the valve, with a longer learning curve and longer ischemic time.6 Multiple methods can be used to implant a Freestyle valve, including the root inclusion technique, an isolated complete subcoronary technique, or a full root replacement.7
As the use of the stentless valve gained popularity, acceptance of the fact that these valves would eventually degenerate was reasonable, leading to stentless valve reoperations. In addition to the technical difficulty of implantation, reintervention also is widely regarded as a greater surgical challenge. Over the course of 2 decades at our centre—Health Sciences North, Sudbury, Ontario—over 300 Medtronic Freestyle stentless valves were inserted. Many patients did return for reoperations. Prior to the broad introduction of transcatheter aortic valve replacement (TAVR), all patients returned to the operating room for reintervention. Subsequent to 2016, with the introduction of catheter-based aortic valve replacement in our centre, valve-in-valve TAVR soon evolved as another treatment option.8 This option, however, also presented technical challenges, with the lack of radio-opaque markers to guide transcatheter valve implantation.
The objective of the current study was to evaluate the short- and long-term outcomes of patients who underwent a stentless valve reoperation at our centre, including their perioperative outcomes, operative mortality, and long-term survival following aortic valve reoperation following an initial implantation of a Medtronic Freestyle stentless valve.
Material and Methods
Study approval and design
This study was approved by the Institutional Review Board at Health Sciences North (Sudbury, Ontario) and was in compliance with Health Insurance Portability and Accountability Act regulations. Operative reports and medical records were reviewed by a single cardiac surgeon (B.B.) to confirm that these patients all had received a Medtronic Freestyle stentless valve for their initial operation and had received a reoperation for a failed stentless valve. Long-term survival was obtained through medical record review and was supplemented with telephone calls with 100% follow-up of all patients. All reoperations for stentless valves were performed by the same team of cardiac surgeons (B.B. and D.J.M.).
Patient selection
From 2006 to 2016, all consecutive patients (N = 24) who underwent open reoperative aortic valve replacement for a failing stentless valve were enrolled in this study.
Operative technique
In all cases, surgery was performed via redo median sternotomy. Prior cannulation of the femoral artery or subclavian artery was used only when the right ventricle was in close proximity to the posterior table of the sternum. Cardiopulmonary bypass, with standard ascending aortic cannulation, the aortic arch or femoral artery and the right atrium or femoral vein, was used with systemic hypothermia of 320C. Myocardial protection was achieved with intermittent antegrade cold blood cardioplegia. All concomitant procedures were performed according to standard techniques.
Intraoperatively, the size of the stentless valve inserted in the first operation often determined whether another stented aortic valve could be sewn in standard fashion with pledgeted sutures to the porcine neo-annulus. We found that larger stentless valves (size 25 or larger) often allowed for another adequately sized aortic valve to be inserted. When the stentless valve was of a smaller size (size 23 or smaller), meticulous removal of the entire valve (porcine leaflet tissue, subvalvular pannus, and stentless casing) was necessary before another aortic valve was inserted, in order to avoid patient-prosthesis mismatch.
In our practice, this decision to proceed with complete removal of the stentless valve, or to insert another aortic valve within the stentless casing, was often made intraoperatively. Factors such as the amount of calcification of the stentless casing—particularly in the area of the noncoronary sinus—the quality of the native tissue, the proximity of the subcoronary suture line to the ostium of the coronaries, and the anmular size of the stentless bioprosthesis played a role in this decision. Additionally, if the patient had significant comorbidities, and the perceived length of time required on cardiopulmonary bypass to completely explant the entire stentless valve was considered too lengthy, we could choose the more conservative, relatively quicker surgical strategy by suturing another aortic valve.
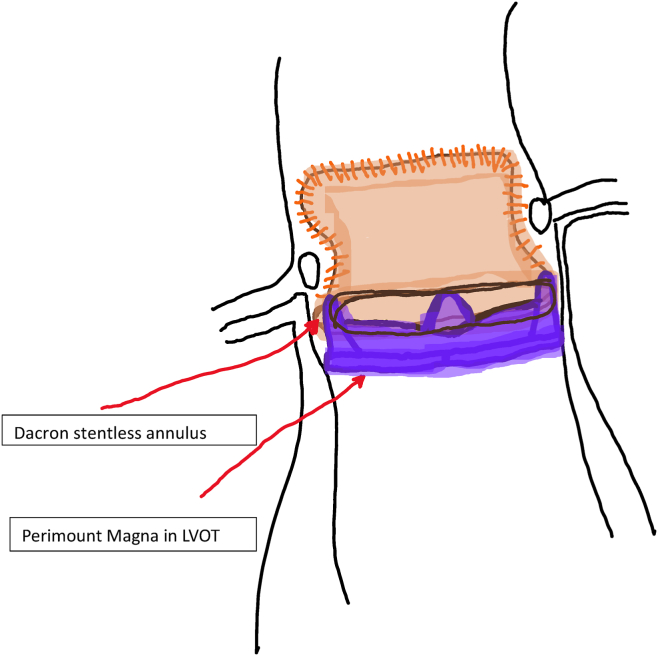
With removal of the torn porcine leaflet tissue and the pannus in the left ventricular outflow tract, only the stentless casing adherent to the native aorta was left. This casing provided a tough fibrous construct to which pledgeted 2-0 Ethibond aortic valve sutures (Ethibond Excel, Ethicon Inc. Raritan, NJ) could be used to anchor another aortic valve in standard fashion. However, the stentless valve, similar to the aortic homograft, often becomes a shell of calcium, which makes suture insertion impossible. The stentless casing is assessed carefully for this, and an attempt is made to insert sutures below the subvalvar Dacron sewing ring. If sutures could be placed with good full thickness bites, then often we would avoid having to remove the entire casing for another aortic valve implant. See Figure 1.
Figure 1.
Stented aortic valve position within stentless subcoronary implant. Manufacturer information: Perimount Magna (Carpentier-Edwards Perimount Magna Ease; Edwards Lifesciences, Irvine, CA). LVOT, left ventricular outflow tract.
Standard 2-0 Ethibond pledgeted sutures were used to secure the aortic valve prosthesis.
Although the use of simple, non-pledgeted sutures may have allowed for up-sizing the valve prosthesis, the risk of paravalvular leak is increased, especially in the redo root. An important point to note is that sutureless valves were not available at our hospital, and TAVR was still at its inception. Although these approaches are quicker, reproducible, and more practical solutions for older patients today, the surgeons had to resort to a classic surgical technique, albeit with some modifications, as the insertion of an aortic valve within a stentless casing had not been described previously.
When the stentless valve was particularly adherent to the native root, we found that starting the removal in the area of the noncoronary sinus was the most forgiving strategy. If the wrong tissue plane between the stentless valve and the native aortic root was entered here, a pericardial or bovine patch repair of the root could be performed more easily here than in other areas of the disrupted root. Stentless extraction was typically a lengthy process, requiring great care and patience to preserve the integrity of the native aortic root with strict adherence to myocardial protection. Fine Penfield dissectors (Medline Industries, Inc, Northfield, IL) were employed for much of this process and were supported with sharp dissection when necessary. Great caution was used in the subcoronary areas where the margin of the stentless bioprosthesis was extremely intimate with the ostium of the coronary arteries. Removal of the stentless valve in this area often left a ridge or flap of tissue near the inferior margin of the main coronary artery, where the native aortic intimal plane was entered. In such instances, this area was reinforced with several 7-0 Prolene sutures in order to prevent progression of the intimal tearing and potential root dissection.
When another aortic valve was inserted, 4-0 Ethibond pldegeted sutures were used, and the fragile tissue of the remaining porcine annulus was avoided. In these instances, the native aortic annulus was covered by the stentless valve’s subvalvar Dacron sewing ring (Medtronic, Minneapolis, MN) and therefore was not visible. Sutures were placed just below the inferior margin of the cuff and exited through the stentless valve sewing ring, ensuring good anchoring of the aortic valve that was being implanted. Maintenance cold blood cardioplegia at 8-120C was given selectively into the coronary ostia at 20-minute intervals for the duration of the procedure.
Statistical analysis
Descriptive statistics were computed for the study cohort. Continuous variables were summarized by reporting the median and ranges. Categorical variables were reported as N (%) in frequency tables.
Results
All patients (n = 24) received a Medtronic Freestyle Aortic Root bioprosthesis for their initial operation due to disease from aortic valve pathology. All patients had their initial stentless surgery performed by the same surgeon at the Sudbury Regional Hospital, Sudbury, Ontario, Canada. All patients had their initial valve inserted with the modified subcoronary implantation technique.
Preoperative status and comorbidities of the patients are outlined in Table 1. The stentless valve required reoperation on average after 9.8 years, with aortic insufficiency as the mode of failure in all cases except one. Intraoperative examination of the stentless valve and the aortic root revealed a linear tear at or near the base of one of the leaflets. The leaflets, although demonstrating remarkably little calcification, were extremely brittle, with minimal traction or manipulation.
Table 1.
Population characteristics, preoperative status, and comorbidities of patients who received stentless valve reoperation (n = 24)
| Characteristic | n (%) or median (range) |
|---|---|
| Sex | |
| Male | 18 (75.0) |
| Female | 6 (25.0) |
| Smoking status | |
| Smoker | 7 (29.2) |
| Nonsmoker | 17 (70.8) |
| Age/Age at death, y | 71.5 (58–92) |
| Age at first operation, y | 53.5 (40–69) |
| Age at second operation, y | 62.5 (47–82) |
| Time between first and second operation, y | 9.5 (6–13) |
| NYHA Functional Classification Scale | |
| I | 0 (0) |
| II | 3 (12.5) |
| III | 14 (58.3) |
| IV | 6 (25.0) |
| n/a | 1 (4.2) |
| LV grade (1–4) | |
| 1 | 18 (75.0) |
| 2 | 6 (25.0) |
| 3 | n/a |
| 4 | n/a |
| Comorbidities | |
| Hypertension | 18 (75.0) |
| Cholesterol | 11 (45.8) |
| Diabetes mellitus | 2 (8.3) |
| CVA/ TIA | 0 (0) |
| Chronic renal failure | 2 (8.3) |
| Coronary artery disease | 6 (25.0) |
| Peripheral vascular disease | 1 (4.2) |
| Atrial fibrillation | 3 (12.5) |
| AI | 23 (95.8) |
| AS | 2 (8.3) |
| Endocarditis | 2 (8.3) |
| Structural valve deterioration | 23 (95.8) |
AI, aortic insufficiency; AS, aortic stenosis; CVA, cerebrovascular accident; LV, left ventricular; n/a, not available; NYHA, New York Heart Association; TIA, transient ischemic attack.
No serious adverse events occurred during re-sternotomy. All 5 patients who underwent the valve-in-valve technique received a biological tissue valve. A total of 37.5% (N = 9) of patients required a concomitant procedure, predominantly coronary artery bypass grafting. Surgical variables and characteristics are outlined in Table 2.
Table 2.
Surgical variables and surgical characteristics of patients who received stentless valve reoperation (n = 24)
| Surgical variables | n (%) or median (range) |
|---|---|
| Urgent surgery | 5 (20.8) |
| Elective surgery | 19 (79.2) |
| Concomitant procedure | 9 (37.5) |
| Mechanical valve | 10 (41.7) |
| Tissue valve | 14 (58.3) |
| Valve-in-valve | 5 (20.8) |
| Intra-op Bentall procedure | 0 (0) |
| Transfusion | 20 (83.3) |
| Ventricular assist device | 0 (0) |
| Post-operative myocardial infarct | 0 (0) |
| Stroke | 0 (0) |
| New-onset renal failure | 1 (4.2) |
| Permanent pacemaker required | 0 (0) |
| Reoperation bleeding | 2 (8.3) |
| Second reoperation | 2 (8.3) |
| Cross-clamp time, min | 146.5 (72–319) |
| Bypass time, min | 194 (117–355) |
In patients for whom the stentless valve was explanted (n = 15), the root was preserved in 100% of cases, with no need for a conversion to a Bentall procedure. Of the 24 patients who underwent this procedure, 10 received mechanical valves, and 14 received a stented aortic prosthesis. In 5 of these patients, the stentless leaflets were removed, and the new valve was sutured to the native annulus (valve-in-valve technique). Urgent surgeries were required in 20.8% (n = 5) of the cases, mostly due to hemodynamic compromise or endocarditis. Mean cross-clamp time was 156 minutes. The 30-day outcomes revealed no postoperative myocardial infarctions, or strokes, with only one patient suffering from new-onset renal failure.
Hospital length of stay averaged 7.5 days. The single in-hospital mortality was an immunocompromised patient presenting with Staphylococcus aureus endocarditis involving the Freestyle valve. Intraoperatively, the valve was successfully explanted, but the patient required a concomitant repair of a ventricular septal defect, reconstruction of the left ventricular outflow tract, and concomitant coronary artery bypass grafting to the left anterior descending artery. The patient had a significantly lengthy cardiopulmonary bypass run, with postoperative coagulopathy and left ventricular dysfunction, necessitating mediastinal re-exploration twice. The patient subsequently died on postoperative day 5, of multi-system organ failure.
Follow-up at 2 months postoperatively and then once annually was performed. All patients had improved functional status, and all were in New York Heart Association functional classes I-II. None of the valve-in-valve patients had a measurable paraprosthetic leak, and 2 of the patients ultimately required reinterventions on their replaced aortic valves. One patient had a TAVR procedure performed 8 years later, and one patient underwent a third sternotomy with a tissue aortic valve replacement. Procedural outcomes are detailed in Table 3.
Table 3.
Procedural outcomes in patients who underwent stentless valve reoperation (n = 24)
| Procedural outcome | n (%) or median (range) |
|---|---|
| Intraoperative mortality | 0 (0) |
| In-hospital mortality | 1 (4.2) |
| 30-d mortality | 1 (4.2) |
| 5-y mortality | 1 (4.2) |
| 10-y mortality | 4 (16.7) |
| 10-y all-cause mortality | 8 (33.3) |
| Hospital length-of-stay, d | 6 (2–20) |
The 5-year mortality rate was 4% (n = 1)—the in-hospitality mortality identified above. The 10-year mortality incidence was 16.7% (n = 4). Causes of death included 2 patients with acute renal failure and encephalopathy, and one patient with Staphylococcus aureus endocarditis and acute renal failure.
The 10-year all-cause mortality rate was 33% (n = 8). Of these 4 deaths, 2 were secondary to metastatic cancers, and 2 were listed as death secondary to multi-system organ failures.
Discussion
In this study, we summarized a retrospective review of a single surgical team’s experience of reoperative surgery for failing, predominantly regurgitant, stentless valves. The primary findings of the study are as follows. The 30-day mortality rate was 4% (1 of 24) for all patients, and the long-term cardiac 10-year mortality rate was 16% (4 of 24) for all patients. The primary operative method involved complete explantation of the stentless xenograft, without incurring any damage to the root necessitating the need for a complete root replacement. In their own retrospective series, Borger and colleagues9 reported an operative mortality rate of 11% with 63% of patients requiring aortic root replacements, despite the specific attempt to preserve the preexisting native aortic root. In a similar study, Boning and colleagues10 reported a 21% (5 of 24) 30-day operative mortality rate following their stentless reoperations, which was higher than the 4% operative mortality rate in their patients who had a stented aortic valve reoperation.
An important point to note is that the TAVR procedure and implantation of current sutureless valves were not options at the time of these operations at our institution. The authors appreciate the fact that current techniques for valve replacement may present a much quicker and reproducible result, especially in elderly patients considered to have a prohibitive risk for reoperation. However, valve-in-valve TAVR for a degenerated stentless valve has its own technical challenges, as noted by certain groups studying this particular subset of patients.11 Positioning of the TAVR valve is complicated by the lack of radiopaque markers on the stentless valve, variations in the implantation technique, proximity of the coronary ostia to the annulus, and a lack of calcium in the aortic leaflets themselves. With patients at our centre still presenting with degenerated stentless valves, we have moved away from offering them a complex reoperation as a first option. They are now discussed at multidisciplinary valve rounds. Those who are considered inoperable, or high-risk surgical candidates, are considered for TAVR. But the experience in the literature is still limited. As Sang and colleagues point out,12 valve-in-valve TAVR for a failing 29-mm Medtronic Freestyle valve was a risk factor for procedural failure at their centre. As experience grows with these patients, who are currently living longer with their degenerated prostheses, the limitations of the TAVR approach also will be interesting in order to refine this approach in the future.
Our only early death was in a patient who had an active infection of their stentless valve, highlighting the further complexity in removing not only the stentless valve, but also adjacent structures that were involved in the infectious process. In our hands, although all attempts were made to completely remove the stentless valve, if annular dimensions were adequate in an otherwise relatively higher-risk patient, another aortic valve was implanted within the stentless casing.
As in other centres,8 our reoperative approach to stentless valves has evolved over the years, with the potential role for valve-in-valve TAVR in this particular setting. However, the following difficulties and potential complications still have to be considered with this approach: the initial implantation technique of the stentless valve, the bulky nature of the leaflet tissue, lack of annular calcification, device malpositioning due to lack of anatomic markers to guide landing zones, and coronary obstruction.13, 14, 15 Future studies will need to analyze the long-term impact of valve-in-valve TAVR prior to committing to this approach for stentless reoperations as a primary approach.
Limitations
The primary limitation of our study is its retrospective nature and small sample size. In combination with the small sample size, the very low event rate of major adverse events, including death, makes statistical analysis difficult. However, few studies report longitudinal and complete follow-up of all patients up to 10 years. Although we acknowledge the limitations of this study, we still feel the results indicate that redo surgery for a failing stentless valve can be considered a reasonable option with good long-term outcomes.
Acknowledgments
Ethics Statement
This study was approved by the Institutional Review Board at Health Sciences North (Sudbury, Ontario) and was in compliance with Health Insurance Portability and Accountability Act regulations.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a retrospective article using de-identified data; therefore the IRB did not require consent from the patient.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 797 for disclosure information.
References
- 1.Yun K.L., Sintek C.F., Fletcher A.D., et al. Aortic valve replacement with the freestyle stentless bioprosthesis: five-year experience. Circulation. 1999;100(19 suppl):II17–II23. doi: 10.1161/01.cir.100.suppl_2.ii-17. [DOI] [PubMed] [Google Scholar]
- 2.Baur L.H., Houdas Y., Peels K.H., et al. Stentless bioprostheses have ideal haemodynamics, even in the small aortic root. Int J Card Imaging. 2000;16:359–364. doi: 10.1023/a:1026521211249. [DOI] [PubMed] [Google Scholar]
- 3.Christ T., Grubitzwch H., Claus B., et al. Hemodynamic behavior of stentless aortic valves in long term follow-up. J Cardiothorac Surg. 2014;9:197. doi: 10.1186/s13019-014-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach D.S., Kon N.D., Dumesnil J.G., Sintek C.F., Doty D.B. Ten-year outcome after aortic valve replacement with the Freestyle stentless bioprosthesis. Ann Thorac Surg. 2005;80:480–486. doi: 10.1016/j.athoracsur.2005.03.027. discussion 486-7. [DOI] [PubMed] [Google Scholar]
- 5.Bach D.S., Kon N.D. Long-term clinical outcomes 15 years after aortic valve replacement with the Freestyle stentless aortic bioprosthesis. Ann Thorac Surg. 2014;97:544–551. doi: 10.1016/j.athoracsur.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Finch J., Roussin I., Pepper J. Failing stentless aortic valves: redo aortic root replacement or valve in a valve? Eur J Cardiothorac Surg. 2013;43:495–504. doi: 10.1093/ejcts/ezs335. [DOI] [PubMed] [Google Scholar]
- 7.Patel P.M., Chen E.P. Case report: challenging surgical management of failed Freestyle stentless porcine valve: two case reports. J Vis Surg. 2022;8:28. [Google Scholar]
- 8.Grubitzsch H., Zobel S., Christ T., et al. Redo procedure for degenerated stentless aortic zenografts and the role of valve-in-valve transcatheter techniques. Eur J Cardiothorac Surg. 2017;51:653–659. doi: 10.1093/ejcts/ezw397. [DOI] [PubMed] [Google Scholar]
- 9.Borger M.A., Prasongsukarn K., Armstrong M.S., et al. Stentless aortic valve reoperations: a surgical challenge. Ann Thorac Surg. 2007;84:737–744. doi: 10.1016/j.athoracsur.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Boning A., Niemann B., Ennker I., et al. Are aortic valve reoperations after primary replacement with stentless heart valve prostheses more demanding than after stented biological prostheses? Thorac Cardiovasc Surg. 2014;62:475–481. doi: 10.1055/s-0034-1371697. [DOI] [PubMed] [Google Scholar]
- 11.Cekmecelioglu D., Preventza O., Dougherty K., et al. Transcatheter valve-in-valve implantation for degenerated stentless aortic bioroots. Ann Cardiothorac Surg. 2021;10:641–650. doi: 10.21037/acs-2021-tviv-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wai S., DeBruine N., Beute T., et al. Midterm outcomes for valve-in-valve transcatheter aortic valve replacement in the failed Freestyle bioprosthesis. Ann Thorac Surg. 2020;110:1951–1958. doi: 10.1016/j.athoracsur.2020.03.116. [DOI] [PubMed] [Google Scholar]
- 13.Webb J.G., Dvir D. Transcatheter aortic valve replacement for bioprosthetic aortic valve failure: the valve-in-valve procedure. Circulation. 2013;127:2542–2550. doi: 10.1161/CIRCULATIONAHA.113.000631. [DOI] [PubMed] [Google Scholar]
- 14.Lange R., Piazza N. Transcatheter aortic valve-in-surgical aortic valve implantation: current status and future perspectives. Eur J Cardiothorac Surg. 2013;44:403–406. doi: 10.1093/ejcts/ezt373. [DOI] [PubMed] [Google Scholar]
- 15.Bapat V., Davies W., Attia R., et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg. 2014;148:917–924. doi: 10.1016/j.jtcvs.2014.05.029. [DOI] [PubMed] [Google Scholar]