Abstract
Worldwide, age-related macular degeneration (AMD) is a multifactorial progressive fundus disorder that can cause vision impairment and severe central blindness in older adults. Currently, there are no approved prevention or treatment strategies for non-exudative AMD. While targeting VEGF is the main therapeutic approach to delay the degeneration process in exudative AMD, a significant number of patients show insensitivity or ineffectiveness to anti-VEGF therapy. Despite years of research, the exact mechanism underlying drusen formation and macular atrophy in AMD remains unknown. In the pathogenesis of AMD, lncRNAs play crucial roles, as discussed in this paper. This review focuses on the function of dysregulated lncRNAs and the mechanisms by which specific molecules target these lncRNAs in AMD. The analysis reveals that lncRNAs primarily regulate the progression of AMD by mediating apoptosis, epithelial-mesenchymal transition (EMT), dedifferentiation, and oxidative stress in choroidal vascular endothelial cells, retinal pigment epithelium (RPE) cells, and photoreceptors. Consequently, the regulation of apoptosis, dedifferentiation, EMT, and other processes by lncRNAs has emerged as a crucial focus in AMD research.These findings contribute to our understanding of the role of lncRNAs in AMD and their potential as valuable biomarkers. Furthermore, they highlight the need for further basic and clinical studies to explore the value of lncRNAs as biomarkers and potential therapeutic targets for AMD.
Keywords: Long non-coding RNA, Age-related macular degeneration, Macular disease, Macular degeneration, Biomarker
SEARCH STRATEGY: The search strategy for this review involved searching the MEDLINE/PubMed, Google Scholar, and CNKI database with specific keywords. The following search terms were used: “long non-coding RNA”, “age-related macular degeneration”, and “AMD pathogenesis”. To further refine the search, the headlines “epithelial-mesenchymal transition in age-related macular degeneration” and “altered gene expression in AMD pathogenesis” were employed. Additionally, a manual search was conducted based on the references of the identified articles and review articles.
1. Introduction
1.1. AMD
Age-related macular degeneration (AMD) is a major public health concern worldwide, leading to irreversible vision loss in older individuals [1,2]. The incidence of AMD is increasing, and it is estimated that by 2040, around 300 million people globally [3,4], including approximately 40 million in China [5], will be diagnosed with AMD due to aging populations.
AMD causes progressive central visual impairment by affecting the photoreceptor-retinal pigment epithelium complex. It can manifest as dry AMD, characterized by drusen, focal detachment of the retinal pigment epithelium (RPE), and atrophy of the outer layer of the retina [6], or as wet AMD, characterized by the development of choroidal neovascularization (CNV) between Bruch's membrane and the retina [7]. Advanced stages of AMD, whether in the form of geographic atrophy (GA) or CNV, lead to permanent vision loss [8].
Currently, there are no proven therapies to prevent the progressive degeneration and atrophy of photoreceptors and RPE cells in dry AMD [9]. Although anti-vascular endothelial growth factor (VEGF) treatments such as bevacizumab, aflibercept, or ranibizumab are effective for most cases of wet AMD [10], continuous treatment poses a significant social and economic burden [11]. Additionally, approximately 10 % of patients do not respond to anti-VEGF therapy, highlighting the need for novel therapeutic targets [12]. Therefore, there is an urgent need to overcome the challenges in AMD treatment and identify potential therapeutic candidates. Emerging research suggests that long non-coding RNAs (lncRNAs) play various roles in AMD pathogenesis and have the potential to serve as diagnostic and treatment targets. These lncRNAs hold promise in advancing our understanding of AMD and may contribute to the development of innovative therapies.
1.2. Long non-coding RNAs
Non-coding RNAs were previously considered as mere by-products of RNA polymerase II without practical function. However, advancements in technology, particularly the application of second and third-generation high-throughput sequencing, have revealed the involvement of non-coding RNAs in gene regulation. Among them, long non-coding RNAs (lncRNAs) represent the largest proportion [13]. LncRNAs are RNA molecules with more than 200 nucleotides and exhibit functional uniqueness through various intracellular localizations. In the nucleus, they can serve as molecular scaffolds, assist in alternative splicing, and regulate chromosome structure [14]. In the cytoplasm, lncRNAs can promote or inhibit mRNA degradation, act as competing endogenous RNAs (ceRNAs) by sequestering microRNAs, and regulate translation [15]. LncRNAs, are expressed in specific patterns in cells or during different developmental stages, making them important regulators in gene expression networks. They participate in various biological processes, including tumorigenesis, cell differentiation, and epithelial-mesenchymal transition (EMT), operating at transcriptional and post-transcriptional levels, as well as through epigenetic modifications [16]. Consequently, lncRNAs have emerged as promising targets for disease treatment. The detection of lncRNA expression in specific cells or disease states is valuable for understanding their function, elucidating their mechanisms of action, and identifying effective biomarkers. Numerous studies in recent years have established a causal relationship between lncRNAs and ocular diseases. These studies have revealed different regulatory mechanisms, involvement of multiple molecules and signaling pathways within cells, and the potential of lncRNAs to serve as diagnostic markers and therapeutic targets [[17], [18], [19]].
2. Roles and mechanisms of lncRNA in AMD
Since the year 2000, advancements in high-throughput sequencing technology have contributed to the identification of lncRNAs involved in the pathogenesis of AMD [20]. LncRNAs play a role in regulating various processes such as apoptosis [21], dedifferentiation [22], oxidative stress [23] and EMT [24] in choroidal vascular endothelial cells, RPE cells and photoreceptors through their influence on the epigenetic profile [25], transcriptional [26] and post-transcriptional regulation [24] (Fig. 1). Consequently, the regulation of apoptosis, dedifferentiation, EMT, and other processes by lncRNAs has become a crucial area of research in AMD. In this review, our aim is to summarize the progress made in understanding the roles of lncRNAs associated with AMD and similar retinal injuries. This will provide novel insights into the pathological processes, molecular diagnosis, and potential therapeutic approaches for AMD.
Fig. 1.
Schematic diagram of AMD pathological mechanism. Healthy photoreceptors, RPE cells and choroids will undergo apoptosis, RPE dedifferentiation, RPE EMT, and CNV in the face of challenges such as oxidative stress, lipid deposits, inflammatory response, immune activation, DNA methylation and increase of VEGF.
2.1. LncRNA and oxidative stress
The retina is a tissue that has a high oxygen consumption rate, and oxidative stress is considered the primary trigger in the pathological process of AMD [27,28]. Factors such as aging, light damage, and air pollution contribute to the attack of oxidative stress on RPE cells. However, the antioxidant and repair abilities of RPE cells decrease significantly with age [29]. The accumulation of lipofuscin in RPE cells leads to the production of excessive reactive oxygen species and oxidized lipoproteins. This, in turn, causes peptide chain breakage, conformational changes, and immunogenicity alterations in proteins, leading to the inhibition of photoreceptor outer segment processing and degeneration of RPE cells, ultimately resulting in the development of AMD. In aging human retinas, oxidative stress also acts as an effective inducer of inflammatory cytokines, promoting inflammation and macrophage infiltration [30].
A recent study identified a unique oxidation-related lncRNA called CYLD-AS1 and observed its up-regulation in RPE cells under oxidative stress [30]. Depletion of CYLD-AS1 was found to protect RPE cells from damage caused by hydrogen peroxide (H2O2) by enhancing cell proliferation and mitochondrial function. Bioinformatics analysis plays a crucial role in the identification of disease biomarkers and therapeutic targets, facilitating the discovery of differentially expressed genes and related signaling pathways. This approach reduces the time and cost associated with blind exploration by providing molecular expression and phenotypic function verification.
A pathway analysis of lncRNA transcriptome in human RPE cells treated with N-retinylidene-N-retinylethanolamine (A2E), an oxidant, revealed a strong advanced association between oxidative stress-related metabolic damage and RPE degeneration [31]. The study identified dysregulated lncRNAs, including lncRNA NEAT1. Bioinformatics analysis indicated that these dysregulated lncRNAs may be associated with impaired oxidative stress response, impaired carbohydrate and lipid metabolism, dysregulated co-factor metabolism, alterations in melanin biosynthesis, and inadequate cellular response to amino acid starvation. The presence of these oxidative damage-related lncRNAs supports the concept that oxidative stress plays a significant role in the pathogenesis and progression of AMD. However, the precise functions of the predicted lncRNAs require further experimental verification.
2.2. LncRNA and cell apoptosis
Apoptosis of RPE cells and photoreceptors is a fundamental pathological change observed in early-stage AMD patients [32,33]. In a study, researchers downloaded expression profiles of RPE/choroid from 9 early AMD cases and 7 controls, and conducted a microarray analysis to investigate the role of lncRNAs in AMD pathogenesis [34]. They identified 64 differentially expressed lncRNAs, which may play important roles in sensory perception of light stimulation, visual perception, cognition, and other processes. The two most affected pathways were found to be phototransduction and purine metabolism. By creating an aging RPE cell model, the downregulation of RP11-234O6.2 was observed, and overexpression of RP11-234O6.2 was found to reduce cell apoptosis and promote cell viability in aging RPE cells [34]. Although this study involved a small number of samples, it holds significant value in exploring diagnostic biomarkers and providing insights into the pathogenesis and related pathways of RPE damage in early AMD. Importantly, the study suggests the need for controlled studies on AMD patients at different stages to identify specific diagnostic markers and treatment targets for patients at different stages of the disease.
Light-induced biochemical reactions can lead to degeneration of cones/rods and apoptosis of RPE cells [35]. Previous studies have shown that the expression of lncRNA MEG3 is upregulated in murine photoreceptor cells (661W) and retinas of C57BL/6J mice after exposure to intense light for different durations [36]. Silencing MEG3 using short hairpin RNA (shRNA) was found to protect the retina from intense light exposure in vivo and reduce photoreceptor apoptosis in vitro. MEG3, acting as a p53 decoy, also participates in the function of photoreceptors.
Another downregulated lncRNA, PWRN2, has been shown to protect human RPE cells from mitochondrial damage and apoptosis induced by various stressors, suggesting that it could serve as a promising therapeutic target in AMD [21]. Further investigations are needed to validate the relationships between downstream signaling pathways and PWRN2, which would enhance our understanding of lncRNA's epigenetic regulation in the process of AMD.
2.3. LncRNA and immune cell activation
Studies have reported that the increased incidence of any form of AMD is associated with an increase in peripheral monocytes [37]. In early-stage AMD, monocytes are recruited from the peripheral blood to the Bruch membrane area to phagocytose debris from RPE and Bruch membrane deposits [[38], [39], [40]]. In the eyes of AMD patients with geographic atrophy, thick basement sediments, and CNV membranes, the size and quantity of CD163+ macrophages are significantly increased in the outer retinal layer, subretinal, and subretinal pigment epithelial tissue areas, whereas in normal eyes, CD163+ macrophages are restricted to the inner retina [41]. Animal models have also revealed that the infiltration of bone marrow-derived macrophages into the retina is associated with AMD-related changes during early retinal degeneration [42]. Macrophages with inflammatory effects may cause non-lethal and lethal damage to photoreceptors and RPE cells, and may directly promote the progress of AMD [43].
In addition to inducing inflammation, macrophages also recruit and activate bone marrow-derived mesenchymal precursor cells from the circulation, leading to the transformation of these cells into myofibroblasts and vascular smooth muscle cells, resulting in perivascular fibrosis of CNV [44]. This is an important reason why some patients with wet AMD show an incomplete response to anti-VEGF therapy.
A transcriptome sequencing study conducted on a laser-induced murine CNV model for AMD identified 716 significantly differentially expressed lncRNAs in the RPE-choroid-sclera complex [45]. Bioinformatics analysis indicated that these altered mRNAs, which interact with lncRNAs, are enriched in the chemokine signaling pathway and immune system processes. These findings suggest that lncRNAs may regulate downstream target genes and affect cytokines, chemokines, and their receptors in the pathogenesis of CNV. Clec4e, which encodes phage-induced C-type lectin [46], was found to be significantly increased and correlated with six lncRNAs, suggesting its involvement in the mRNA-lncRNA network [45].
In the aqueous humor of patients with wet AMD, LncRNA H19 is found to be significantly upregulated [47], suggesting that lncRNAs could be used as diagnostic tools. Additionally, the authors discovered that the expression of M2 macrophage markers and VEGFA significantly decreased after intravitreal injection of an H19 smart silencer in laser-induced CNV mice. This study suggests that H19 could potentially become a new target for AMD prevention and therapy.
These studies expand our understanding of the regulatory roles of lncRNAs in AMD, particularly in the context of immune cells. They provide new insights into the resistance to anti-VEGF therapy and offer a fresh perspective for exploring diagnostic and therapeutic strategies in the search for breakthroughs in AMD research.
2.4. LncRNA and EMT
EMT is a process in which epithelial cells lose their adhesion and polarization characteristics and acquire a fibroblast-like phenotype. It plays a crucial role in tissue interstitial formation, embryonic development, wound repair, and the development of malignant tumors [48,49]. In the context of AMD, RPE cells are key players in disease pathogenesis. Normally, RPE cells maintain a mature epithelial phenotype and remain in a quiescent state [50]. However, in response to the harsh microenvironment associated with AMD progression, some degenerated RPE cells undergo EMT, losing their apical-to-basal polarity and cell-to-cell adhesion, and migrate to the retina and subretinal RPE space [51]. Laboratory studies on RPE cells from patients with non-neovascular AMD have shown increased expression of Vimentin and Snail1, key markers of EMT, along with decreased expression of E-cadherin, indicating the occurrence of EMT [50]. In cases of chronic inflammation or repeated injury, RPE cells can undergo further transdifferentiation into myofibroblasts, leading to fibrotic scarring, which is a hallmark of the late stage of wet AMD [52]. Progressive lesion fibrosis is a significant consequence of persistent disease activity and is associated with resistance to anti-VEGF therapy in AMD [44]. EMT, as a crucial pattern of RPE dysfunction, is also a key factor contributing to anti-VEGF resistance [53]. Therefore,EMT of RPE cells is closely related to the pathogenesis and treatment of AMD [54].
In a 2016 study, researchers found that the lncRNA MALAT1 significantly increased in TGF-β1-treated RPE cells, and knocking down MALAT1 inhibited cell EMT, migration, and proliferation [55]. A similar phenomenon was observed with the lncRNA ERLR, where knocking down ERLR suppressed TGF-β1-induced EMT in RPE cells [24]. Subsequent studies revealed that the transcription factor TCF4 and its target protein MYH9, which are associated with ERLR, also participate in TGF-β1-induced EMT. The authors observed colocalization of ERLR and the RPE marker pan-cytokeratin on subretinal proliferative membranes obtained from patients during surgery. Another upregulated lncRNA, NEAT1, was found to increase SOX4 levels by acting as a molecular sponge for miR-204 in human RPE cells under high glucose conditions, promoting EMT both in vivo and in vitro [56]. These findings indicate that lncRNAs play a significant role in the occurrence of EMT in RPE cells and represent promising targets for regulating AMD (Fig. 2).
Fig. 2.
The possible mechanism of lncRNAs regulating EMT of RPE cells in AMD.
2.5. LncRNA and inflammatory response
Inflammation is an essential cellular response to disrupted homeostasis [57]. However, chronic inflammation can be detrimental and is involved in various age-related chronic diseases [58]. In the context of AMD, inflammatory responses can lead to dysfunction of RPE cells, which in turn contributes to the degeneration of RPE cells and subsequent photoreceptor death, resulting in central vision impairment. RPE cells are particularly sensitive to oxidative stress, which can lead to functional deterioration and inflammation [58,59].
A recent study found that transfection of Alu RNA into RPE cells resulted in NLRP3 inflammation and increased expression of p16INK4a (senescence marker), IL-18 and IL-1β (pro-inflammatory cytokines) [59].
Furthermore, the pro-inflammatory cytokine IFN-γ can modulate the expression of the lncRNA BANCR in RPE cells through the activation of the Janus kinase (JAK)/STAT3 signaling pathway, suggesting a link between IFN-γ-induced RPE cell EMT and upregulation of BANCR [60]. This indicates that BANCR may serve as a link between RPE dysfunction and the inflammatory response by regulating EMT.
2.6. LncRNA and RPE dedifferentiation
Dedifferentiation refers to the process in which differentiated cells regain their proliferative capacity and acquire embryonic cell-like characteristics under certain factors such as trauma or in vitro culture [61]. RPE dedifferentiation is characterized by downregulation of RPE-specific proteins, reduced secretion of VEGFA, decreased mitochondrial reactive oxygen species (ROS) accumulation, and impaired phagocytic capacity [22]. RPE dedifferentiation and dysfunction have been identified as key factors in the development of AMD [62,63]. Inhibiting the dedifferentiation of RPE cells could potentially delay or block the progression of AMD and help maintain visual function in affected patients.
In a study by Chen et al., the researchers identified 217 differentially expressed lncRNAs associated with RPE cell differentiation using human induced pluripotent stem cell (hiPSC)-derived RPEs [64]. Among these lncRNAs, ZNF503-AS1 was found to accumulate in the cytoplasm of RPE cells and was upregulated during RPE differentiation. However, in RPE-choroid samples from patients with dry AMD, ZNF503-AS1 was downregulated. Deleting ZNF503-AS1 inhibited RPE differentiation and promoted the migration and proliferation of hiPSC-derived RPE cells. Another lncRNA, LINC00167, was found to be downregulated in dysfunctional RPE cells and RPE-choroid samples from AMD patients [22]. However, during RPE differentiation, LINC00167 showed a steady upregulation. Inhibiting LINC00167 expression led to RPE dedifferentiation, impaired phagocytosis, and increased production of mitochondrial reactive oxygen species (ROS). Additionally, LINC00167 restored the expression of SOCS3 by acting as a sponge for miR-203a-3p and inhibiting the JAK/STAT pathway.
The lncRNA MEG3 showed upregulation during RPE differentiation but was significantly reduced in RPE cells exposed to H2O2 or TNF-α [25]. Knocking down MEG3 resulted in RPE dedifferentiation, decreased VEGFA secretion, and accumulation of mitochondrial ROS. MEG3 acted as a ceRNA to regulate RPE differentiation and Pax6 expression by sponging miR-7-5p. In conclusion, lncRNAs such as ZNF503-AS1, LINC00167, and MEG3 have the potential to prevent RPE dedifferentiation, thereby contributing to the prevention and delay of AMD progression. These findings highlight the importance of lncRNAs in regulating RPE cell differentiation and their potential as therapeutic targets for AMD.
2.7. LncRNA and DNA methylation
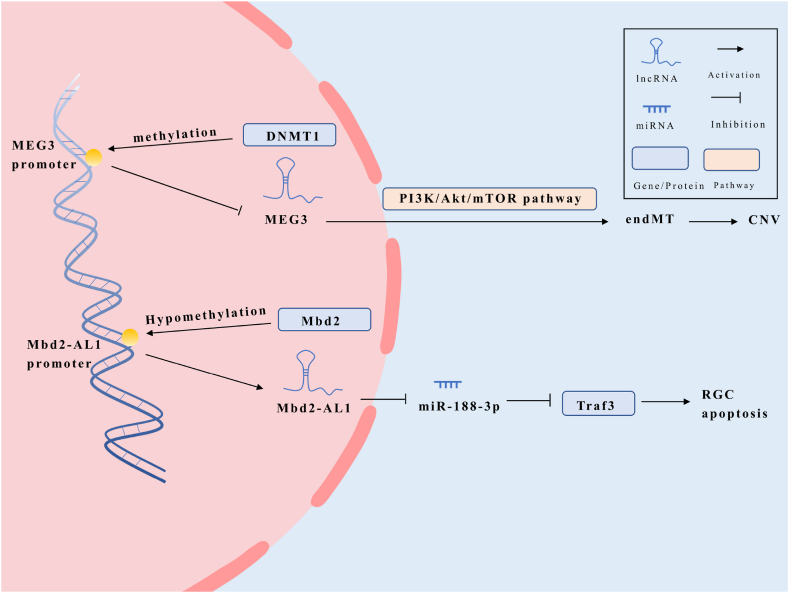
DNA methylation is a crucial epigenetic mechanism that involves the addition of a methyl group to the fifth carbon of the cytosine residue within CpG dinucleotides, catalyzed by DNA methyltransferases (DNMTs) [65,66]. There are three kinds of DNA methyltransferases in mammalian eyes, among which DNA methyltransferase 1 (DNMT1) is mainly expressed in human RPE cells [67]. Studies using retina-specific DNMT1 deletion mutant mice have shown that DNMT1 deficiency significantly impairs photoreceptor differentiation, leading to reduced and mislocalized rhodopsin-expressing cells [68]. The absence of DNMT1 not only affects neuronal differentiation but also causes rapid death of photoreceptors and neurons in the retina after birth, highlighting the essential role of DNMT1-dependent DNA methylation in retinal progenitor pool expansion and the survival and maturation of neurons post-mitosis.
Recent research has demonstrated that DNMT1 may promote methylation of the MEG3 promoter by recruiting methyltransferases, resulting in the inhibition of MEG3 expression [69]. Conversely, overexpression of MEG3 has been shown to inhibit endothelial mesenchymal transition (endMT) by suppressing the PI3K/Akt/mTOR pathway in diabetic retinopathy (DR) models, suggesting that MEG3 methylation is involved in the pathogenesis of DR [25,36,69,70]. Interestingly, the expression of MEG3 may exhibit opposite effects in different cell lines or under different pathological stimuli, either inhibiting or promoting retinal damage.
DNA methylation has also been implicated in retinal cell death. Methyl-CpG binding domain protein 2 (Mbd2) upregulates the expression of Mbd2-associated long noncoding RNA 1 (Mbd2-AL1) by demethylating its promoter [71]. Subsequently, Mbd2-AL1 acts as a sponge for miR-188-3p, preventing the downregulation of TNF receptor-associated factor 3 (Traf3) and inducing apoptosis of retinal ganglion cells (RGCs).
During the progression of AMD, the expression levels of lncRNAs may be regulated by DNA methylation (Fig. 3). Overall, DNA methylation plays a significant role in retinal cell fate and survival. The regulation of lncRNA expression through DNA methylation provides insights into the molecular mechanisms underlying retinal diseases such as DR and AMD.
Fig. 3.
Possible mechanisms by which LncRNAs are regulated by DNA methylation in AMD.
2.8. LncRNA and lipid metabolism
Abnormal lipid metabolism, often accompanied by oxidative stress, is a key process in the pathogenesis of AMD [72]. Dysfunctional RPE cells lead to dysregulation of lipid transport and cholesterol cycling between the RPE and photoreceptors. Excess cholesterol loaded onto ABC transporters is removed from the cells by high-density lipoprotein, while some excess cholesterol is secreted into Bruch's membrane in the form of VLDL-like lipoprotein, contributing to the formation of drusen [73,74]. Lipid-rich deposits and drusen containing lipoproteins have been observed in the macular area of AMD patients [75]. The high levels of oxidative stress in the macular environment promote lipid oxidation, impairing its normal function and leading to the generation of oxidation-specific epitopes (OSE) and detrimental inflammation, which contribute to the progression of AMD [76].
Recent deep sequencing studies by Donato et al. identified 174 lncRNAs involved in fatty acid synthesis and biological metabolism in human RPE cells treated with A2E, a toxic byproduct of the visual cycle [31]. Among these lncRNAs, AC089983.1 was found to be overexpressed, while AC007283.1 and AC012442.2 were underexpressed. These lncRNAs may play a role in regulating lipid metabolism through peroxisome proliferator-activated receptor-alpha (PPAR-α), which is a key regulator of lipid metabolism, glucose homeostasis, and inflammation. Understanding the regulation of lipid metabolism in RPE cells and identifying the connections between impaired lipid metabolism and downstream pathways could provide insights into the development and progression of AMD.
In conclusion, abnormal lipid metabolism and oxidative stress are important factors in AMD. The dysregulation of lipid metabolism in RPE cells, as well as the identification of lncRNAs involved in this process, could contribute to a better understanding of AMD pathogenesis and potentially lead to the development of novel therapeutic strategies.
2.9. LncRNA and CNV
CNV is a common and serious complication of wet AMD, and VEGFA plays a key role in its development [77]. While anti-VEGF therapy has advanced the management of wet AMD, it does not provide a cure or definitive reversal of the disease [78]. Hypoxia-inducible factor 1 (HIF-1) is a crucial transcription factor for VEGF and is secreted by RPE cells under continuous hypoxia conditions. HIF-1 is not only a hallmark of wet AMD but also regulates various growth factors and cytokines involved in inflammation and angiogenesis [79]. In the context of hypoxia, lncRNA HDAC4-AS1 has been found to inhibit HDAC4 expression in ARPE-19 cells [26]. HDAC4-AS1 binds to the HDAC4 promoter in a hypoxic environment, leading to the recruitment of HIF-1. This regulatory axis of HDAC4-AS1/HIF-1α/HDAC4 represents a potential therapeutic target for wet AMD as it may help modulate VEGF expression and angiogenesis.
Age-related lncRNA MALAT1 promotes proliferation, migration, capillary-like tube formation, and vascular permeability of human retinal microvascular endothelial cells (hRMECs) through the regulation of the miR-125b/VE-cadherin axis [80]. Another lncRNA, myocardial infarction-associated transcript (MIAT), is known to regulate vascular disorders, including angiogenesis and vascular permeability [81]. MIAT reduces VEGF expression by sequestering miR-150-5p in retinal endothelial cells through a ceRNA mechanism [82]. These findings suggest that lncRNAs, such as MALAT1 and MIAT, could serve as potential therapeutic targets for suppressing CNV in AMD by modulating angiogenesis and vascular permeability.
In summary, the dysregulation of lncRNAs and their interactions with key molecular pathways involved in angiogenesis and vascular permeability offer potential avenues for therapeutic intervention in CNV associated with wet AMD. Understanding the roles of specific lncRNAs and their interactions could pave the way for developing novel treatment strategies for wet AMD.
3. Conclusions and future insights
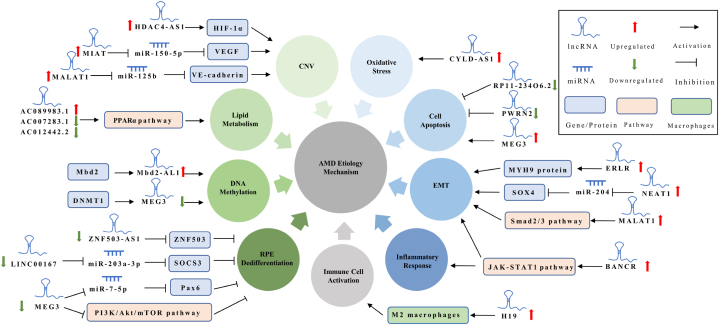
AMD is a prevalent age-related organ degradation disorder with limited treatment options, posing a significant global burden. Recent evidence suggests that lncRNAs play crucial roles in AMD pathogenesis, including LINC00167, ZNF503-AS1, MEG3, and others. These lncRNAs exhibit either a protective effect in AMD progression (Table 1) or contribute to detrimental effects on the retina leading to AMD pathology (Table 2). In either case, these lncRNAs may represent significant targets for the prevention and treatment of AMD. These lncRNAs participate in various regulatory mechanisms and are involved in the occurrence and development of AMD through interactions with downstream molecules and signaling pathways in aging cells. A comprehensive summary of the lncRNAs involved in AMD etiology can be found in Fig. 4. Additionally, lncRNAs can serve as indicators to identify patients who are most likely to benefit from specific treatments. Notably, the activation of immune cells and EMT regulated by lncRNAs shed light on the drug resistance observed in anti-VEGF therapy for AMD, as discussed earlier.
Table 1.
LncRNAs that protect AMD.
| LncRNA | Samples | Mechanism | Effect | Reference |
|---|---|---|---|---|
| RP11-234O6.2 | Microarray data from GSE50195; ARPE-19 cells | Activates phototransduction or purine metabolism | Increases the vitality of aging RPE cells and reduce apoptosis | Zhu et al. [34] |
| PWRN2 | ARPE-19 cells | Regulates cytotoxicity | Reduces RPE cell apoptosis and mitochondrial damage | Yu et al. [21] |
| ZNF503-AS1 | Microarray data from GSE29801; macular RPE-choroid samples; hiPSC-RPE;ARPE-19 cells | Down-regulates ZNF503 expression | Inhibits the dedifferentiation, proliferation and migration of RPE cells | Chen et al. [64] |
| LINC00167 | RPE-choroid samples of AMD patients;hiPSC-RPE;ARPE-19 cells | Targets the miR-203a-3p/SOCS3 axis | Regulates RPE differentiation | Chen et al. [22] |
| MEG3 | hiPSC-RPE;ARPE-19 cells. rat microvascular endothelial cells.Blood samples of patients; Primary human RPE cells; ARPE-19 cells | Targets miR-7-5p/Pax6 axis;Inhibits PI3K/Akt/mTOR signaling pathway;Targets miR-93/Nrf2 axis | Modulates RPE differentiation;Suppresses endMT;Inhibits RPE apoptosis and inflammation | Sun et al. [25] He et al. [69]Luo et al.[70] |
Table 2.
LncRNAs that promote AMD.
| LncRNA | Samples | Mechanism | Effect | Reference |
|---|---|---|---|---|
| CYLD-AS1 | ARPE-19;human primary RPE cell; human eyeball frozen section | Promotes RPE inflammation and oxidative stress | Inhibits cell proliferation and mitochondrial function | Du et al. [83] |
| MEG3 | C57BL/6 mice; murine photoreceptor cells (661W) | Regulates the activity of caspase 3/7 and the expression of Bcl-2 and Bax | Promotes retinal injury and photoreceptor cell apoptosis | Zhu et al. [36] |
| H19 | Laser-induced CNV mouse model; aqueous humor of wet AMD patients | Promotes the expression of M2 macrophages | Promotes immune cell activation | Zhang et al. [47] |
| MALAT1 | ARPE-19 cells;primary human RPE; hRMECs | Partially through activating Smad2/3 signaling; activates the VE-cadherin/β-catenin complex by reducing miR-125b | Promotes EMT, migration and proliferation of RPE cells; promotes proliferation, migration and angiogenesis in hRMECs | Yang et al. [55] Liu et al.[80] |
| ERLR | ARPE-19 cells; primary human RPE; pigmented rabbits | Regulate the stability of MYH9 protein | Promotes EMT, RPE cells migration and proliferation | Yang et al. [24] |
| NEAT1 | C57BL/6 mice; ARPE-19 cells | Sponges miR-204 to increase the level of SOX4 | Promotes EMT of RPE cells | Yang et al. [56] |
| BANCR | ARPE-19 cells | Activates the JAK/STAT1 signaling pathway | Promotes EMT of RPE cells | Kutty et al. [60] |
| Mbd2-AL1 | Mice primary culture RGCs; C57BL/6J mice; Mbd2 knockout mice | Sponges miR-188-3p to increase the level of Traf3 | Induces of RGC apoptosis | Ge et al. [71] |
| HDAC4- AS1 | ARPE-19 cells | Binds to HDAC4 promoter, facilitates HIF-1α recruitment | Induces CNV | Pan et al. [26] |
| MIAT | Diabetic rats' retina; db/db mice's retina;HUVECs; HMVECs;RF/6A cells; RPE; rat Müller cells;EA.hy 926; RGC-5; fibrovascular membranes in patients | Sponges miR-150-5p to increase the level of VEGF | Regulates retinal angiogenesis, corneal angiogenesis and vascular permeability | Yan et al. [82] |
Fig. 4.
Roles and Mechanisms of lncRNAs involved in AMD.
In 2021, Blasiak et al. [20] conducted a comprehensive review on the potential of lncRNAs in AMD, focusing on retinal development, AMD patients, and AMD models. Sharma et al. [84] discussed the impact of lncRNAs on various retinal pathologies, including AMD and other proliferative retinal diseases,such as DR, proliferative vitreoretinopathy (PVR), and retinopathy of prematurity (ROP). Hyttinen et al. [85] delve into the regulation of mitochondrial function and antioxidant stress response in AMD by ncRNAs, including microRNAs, lncRNAs, and cyclic non-coding RNAs. Additionally, Vishwakarma et al. [86] examined the involvement of various non-coding RNAs in regulating genes associated with different pathways in AMD, DR and ROP, with a particular focus on retinal angiogenesis pathogenesis. However, our review focuses specifically on the role of lncRNAs in AMD disease and the pathways they affect, and classifies them according to different pathological mechanisms to provide a more comprehensive overview. We shed light on how these non-coding RNAs regulate critical processes such as apoptosis, dedifferentiation, oxidative stress, EMT, and retinal angiogenesis in choroidal vascular endothelial cells, RPE cells, and photoreceptor cells. Understanding the specific roles of different lncRNAs and their interactions with key pathways in AMD provides valuable insights for the development of novel therapeutic strategies. The findings from our review have significant implications for both researchers and practitioners in the field.
Firstly, this review underscores the need for further translational research to validate the diagnostic and prognostic potential of specific lncRNAs in AMD. While several studies have implicated certain lncRNAs in AMD pathogenesis, their clinical utility and reliability as biomarkers require rigorous validation in large patient cohorts. Future studies should focus on conducting prospective clinical trials to evaluate the diagnostic accuracy and predictive value of these lncRNAs, which can ultimately aid in early detection and personalized treatment strategies for AMD patients.
Secondly, our review highlights the therapeutic potential of targeting dysregulated lncRNAs in AMD. The identification of specific lncRNAs that play crucial roles in AMD pathogenesis opens up new avenues for developing targeted therapies. Further research is needed to investigate the mechanisms of action of these lncRNAs and to develop efficient delivery systems for lncRNA-based therapeutics. Additionally, exploring the interplay between lncRNAs and other molecular pathways implicated in AMD, such as the VEGF signaling pathway, could provide insights into novel combination therapies that can overcome treatment resistance.
Furthermore, this review identifies several gaps in the current understanding of lncRNAs in AMD. For instance, the functional characterization of many dysregulated lncRNAs remains limited, and their precise roles in AMD development and progression are yet to be fully elucidated. Future studies should aim to decipher the molecular mechanisms by which these lncRNAs regulate key cellular processes in the retina and explore their interactions with other non-coding RNAs, transcription factors, and epigenetic modifiers.
In summary, this review underscores the importance of further translational research in the field of lncRNAs and AMD. The validation of lncRNAs as diagnostic and prognostic markers, the development of lncRNA-based therapeutics, and the elucidation of the functional and mechanistic roles of dysregulated lncRNAs are crucial areas that require immediate attention. By addressing these urgent and emerging questions, researchers and practitioners can contribute to the advancement of personalized medicine and improve the management of AMD.
Data Availability Statement
Has data associated with your study been deposited into a publicly available repository?
No. No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Rong Zhang: Conceptualization, Data curation, Funding acquisition, Visualization, Writing – original draft. Lin Wang: Data curation. Yang Li: Investigation. Chenwei Gui: Writing – review & editing. Yajing Pei: Writing – review & editing. Guohong Zhou: Project administration, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Rong Zhang reports financial support was provided by Natural Science Foundation for Young Scientists of Shanxi Province. Rong Zhang reports financial support was provided by Graduate Education Innovation Project of Shanxi Province. Rong Zhang reports was provided by Scientific Research Project of Shanxi Administration of Traditional Chinese Medicine. Rong Zhang reports financial support was provided by Research Foundation of Shanxi Eye Hospital. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Haixia Liang, Ruilin Xiao, Xinxin Li for their detailed review of this article and the constructive comments that they incorporated. This work was supported by Natural Science Foundation for Young Scientists of Shanxi Province (No.202203021212071), Scientific Research Project of Shanxi Administration of Traditional Chinese Medicine (No.2023ZYYC053), Graduate Education Innovation Project of Shanxi Province (No.2022Y356) and Research Foundation of Shanxi Eye Hospital (No. Q202201) from Rong Zhang.
References
- 1.Deemer A.D., Massof R.W., Rovner B.W., et al. Functional outcomes of the Low vision Depression prevention trial in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2017;58:1514–1520. doi: 10.1167/iovs.16-20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P., Liew G., Gopinath B., et al. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindekleiv H., Erke M.G. Projected prevalence of age-related macular degeneration in Scandinavia 2012-2040. Acta Ophthalmol. 2013;91:307–311. doi: 10.1111/j.1755-3768.2012.02399.x. [DOI] [PubMed] [Google Scholar]
- 4.Jonas J.B., Cheung C.M.G., Panda-Jonas S. Updates on the Epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 5.Wong C.W., Yanagi Y., Lee W.K., et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog. Retin. Eye Res. 2016;53:107–139. doi: 10.1016/j.preteyeres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hussaini H., Schneiders M., Lundh P., et al. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp. Eye Res. 2009;88:610–612. doi: 10.1016/j.exer.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 7.van Lookeren Campagne M., LeCouter J., Yaspan B.L., et al. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014;232:151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 8.Miller J.W. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2013;155:1–35 e13. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Cabral de Guimaraes T.A., Daich Varela M., Georgiou M., et al. Treatments for dry age-related macular degeneration: therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022;106:297–304. doi: 10.1136/bjophthalmol-2020-318452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maronas O., Garcia-Quintanilla L., Luaces-Rodriguez A., et al. Anti-VEGF treatment and response in age-related macular degeneration: Disease's Susceptibility, Pharmacogenetics and Pharmacokinetics. Curr. Med. Chem. 2020;27:549–569. doi: 10.2174/0929867326666190711105325. [DOI] [PubMed] [Google Scholar]
- 11.Nashine S. Potential therapeutic Candidates for age-related macular degeneration (AMD) Cells. 2021;10 doi: 10.3390/cells10092483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S., Li T., Jia H., et al. Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abj2177. [DOI] [PubMed] [Google Scholar]
- 13.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridges M.C., Daulagala A.C., Kourtidis A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahariya S., Paddibhatla I., Kumar S., et al. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Yao R.W., Wang Y., Chen L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 17.Wan P., Su W., Zhang Y., et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020;27:176–191. doi: 10.1038/s41418-019-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng M., Zheng Y., Gao M., et al. Expression and clinical value of lncRNA MALAT1 and lncRNA ANRIL in glaucoma patients. Exp. Ther. Med. 2020;19:1329–1335. doi: 10.3892/etm.2019.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X.D., Li K.R., Li X.M., et al. Long non-coding RNAs: new players in ocular neovascularization. Mol. Biol. Rep. 2014;41:4493–4505. doi: 10.1007/s11033-014-3320-5. [DOI] [PubMed] [Google Scholar]
- 20.Blasiak J., Hyttinen J.M.T., Szczepanska J., et al. Potential of long non-coding RNAs in age-related macular degeneration. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22179178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X., Luo Y., Chen G., et al. Long non-coding RNA PWRN2 regulates cytotoxicity in an in vitro model of age-related macular degeneration. Biochem. Biophys. Res. Commun. 2021;535:39–46. doi: 10.1016/j.bbrc.2020.10.104. [DOI] [PubMed] [Google Scholar]
- 22.Chen X., Sun R., Yang D., et al. LINC00167 regulates RPE differentiation by targeting the miR-203a-3p/SOCS3 Axis. Mol. Ther. Nucleic Acids. 2020;19:1015–1026. doi: 10.1016/j.omtn.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczynski T.J., Au E.D., Farkas M.H. Exploring the lncRNA localization landscape within the retinal pigment epithelium under normal and stress conditions. BMC Genom. 2022;23:539. doi: 10.1186/s12864-022-08777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S., Li H., Yao H., et al. Long noncoding RNA ERLR mediates epithelial-mesenchymal transition of retinal pigment epithelial cells and promotes experimental proliferative vitreoretinopathy. Cell Death Differ. 2021;28:2351–2366. doi: 10.1038/s41418-021-00756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H.J., Zhang F.F., Xiao Q., et al. lncRNA MEG3, Acting as a ceRNA, Modulates RPE Differentiation through the miR-7-5p/Pax6 Axis. Biochem. Genet. 2021;59:1617–1630. doi: 10.1007/s10528-021-10072-9. [DOI] [PubMed] [Google Scholar]
- 26.Pan J., Zhao L. Long non-coding RNA histone deacetylase 4 antisense RNA 1 (HDAC4-AS1) inhibits HDAC4 expression in human ARPE-19 cells with hypoxic stress. Bioengineered. 2021;12:2228–2237. doi: 10.1080/21655979.2021.1933821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 2018;9:1280. doi: 10.3389/fphar.2018.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown E.E., DeWeerd A.J., Ildefonso C.J., et al. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang F.-Q., Godley B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 30.Lin R., Yu J. The role of NAD+ metabolism in macrophages in age-related macular degeneration. Mechanisms of ageing and development. 2023;209 doi: 10.1016/j.mad.2022.111755. [DOI] [PubMed] [Google Scholar]
- 31.Donato L., Scimone C., Alibrandi S., et al. Transcriptome Analyses of lncRNAs in A2E-Stressed retinal epithelial cells Unveil advanced links between metabolic impairments related to oxidative stress and retinitis Pigmentosa. Antioxidants. 2020;9 doi: 10.3390/antiox9040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns R.P., Feeney-Burns L. Clinico-morphologic correlations of drusen of Bruch's membrane. Trans. Am. Ophthalmol. Soc. 1980;78:206–225. [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelsalam A., Del Priore L., Zarbin M.A. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 1999;44:1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W., Meng Y.F., Xing Q., et al. Identification of lncRNAs involved in biological regulation in early age-related macular degeneration. Int J Nanomedicine. 2017;12:7589–7602. doi: 10.2147/IJN.S140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung H., Lee H., Lamoke F., et al. Neuroprotective role of erythropoietin by antiapoptosis in the retina. J. Neurosci. Res. 2009;87:2365–2374. doi: 10.1002/jnr.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y.X., Yao J., Liu C., et al. Long non-coding RNA MEG3 silencing protects against light-induced retinal degeneration. Biochem. Biophys. Res. Commun. 2018;496:1236–1242. doi: 10.1016/j.bbrc.2018.01.177. [DOI] [PubMed] [Google Scholar]
- 37.Xue C.C., Cui J., Gao L.Q., et al. Peripheral monocyte Count and age-related macular degeneration. The Tongren health Care study. Am. J. Ophthalmol. 2021;227:143–153. doi: 10.1016/j.ajo.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Killingsworth M.C., Sarks J.P., Sarks S.H. Macrophages related to Bruch's membrane in age-related macular degeneration. Eye (Lond) 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 39.Skeie J.M., Mullins R.F. Macrophages in neovascular age-related macular degeneration: friends or foes? Eye (Lond) 2009;23:747–755. doi: 10.1038/eye.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherepanoff S., McMenamin P., Gillies M.C., et al. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 41.Lad E.M., Cousins S.W., Van Arnam J.S., et al. Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2015;253:1941–1945. doi: 10.1007/s00417-015-3094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy O., Calippe B., Lavalette S., et al. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol. Med. 2015;7:211–226. doi: 10.15252/emmm.201404524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mettu P.S., Wielgus A.R., Ong S.S., et al. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol Aspects Med. 2012;33:376–398. doi: 10.1016/j.mam.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Mettu P.S., Allingham M.J., Cousins S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021;82 doi: 10.1016/j.preteyeres.2020.100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Zeng H., Wang J.H., et al. Altered long non-coding RNAs involved in Immunological regulation and associated with choroidal neovascularization in mice. Int. J. Med. Sci. 2020;17:292–301. doi: 10.7150/ijms.37804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X., Nagata M., Yamasaki S. Mincle: 20 years of a versatile sensor of insults. Int. Immunol. 2018;30:233–239. doi: 10.1093/intimm/dxy028. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P., Lu B., Xu F., et al. Analysis of long noncoding RNAs in choroid neovascularization. Curr. Eye Res. 2020;45:1403–1414. doi: 10.1080/02713683.2020.1748659. [DOI] [PubMed] [Google Scholar]
- 48.Thiery J.P., Acloque H., Huang R.Y., et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Ye X., Tam W.L., Shibue T., et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S., Shang P., Terasaki H., et al. A role for βA3/A1-Crystallin in type 2 EMT of RPE cells occurring in dry age-related macular degeneration. Investigative ophthalmology & visual science. 2018;59:AMD104–AMD113. doi: 10.1167/iovs.18-24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu D.Y., Butcher E., Saint-Geniez M. EMT and EndMT: emerging roles in age-related macular degeneration. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21124271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa K., Kannan R., Hinton D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsusaka S., Zhang W., Cao S., et al. TWIST1 Polymorphisms Predict survival in patients with Metastatic Colorectal Cancer Receiving First-Line bevacizumab plus Oxaliplatin-based Chemotherapy. Mol Cancer Ther. 2016;15:1405–1411. doi: 10.1158/1535-7163.MCT-15-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Llorián-Salvador M., Byrne E.M., Szczepan M., et al. Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells. J. Neuroinflammation. 2022;19:182. doi: 10.1186/s12974-022-02546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S., Yao H., Li M., et al. Long non-coding RNA MALAT1 mediates transforming growth factor Beta1-induced epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y., Zhou J., Li W.H., et al. LncRNA NEAT1 regulated diabetic retinal epithelial-mesenchymal transition through regulating miR-204/SOX4 axis. PeerJ. 2021;9 doi: 10.7717/peerj.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chovatiya R., Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kauppinen A., Paterno J.J., Blasiak J., et al. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada K., Kaneko H., Shimizu H., et al. Lamivudine inhibits Alu RNA-induced retinal pigment epithelium degeneration via anti-inflammatory and anti-senescence Activities. Transl Vis Sci Technol. 2020;9:1. doi: 10.1167/tvst.9.8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kutty R.K., Samuel W., Duncan T., et al. Proinflammatory cytokine interferon-gamma increases the expression of BANCR, a long non-coding RNA, in retinal pigment epithelial cells. Cytokine. 2018;104:147–150. doi: 10.1016/j.cyto.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jopling C., Boue S., Izpisua Belmonte J.C. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 62.Zhao C., Yasumura D., Li X., et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. The Journal of clinical investigation. 2011;121:369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Jiang C., Sun R., et al. Circular noncoding RNA NR3C1 acts as a miR-382-5p sponge to protect RPE functions via regulating PTEN/AKT/mTOR signaling pathway. Mol. Ther. : the journal of the American Society of Gene Therapy. 2020;28:929–945. doi: 10.1016/j.ymthe.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X., Jiang C., Qin B., et al. LncRNA ZNF503-AS1 promotes RPE differentiation by downregulating ZNF503 expression. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bestor T.H., Tycko B. Creation of genomic methylation patterns. Nat. Genet. 1996;12:363–367. doi: 10.1038/ng0496-363. [DOI] [PubMed] [Google Scholar]
- 66.Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 67.Huang P., Sun J., Wang F., et al. DNMT1 and Sp1 competitively regulate the expression of BACE1 in A2E-mediated photo-oxidative damage in RPE cells. Neurochem. Int. 2018;121:59–68. doi: 10.1016/j.neuint.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Rhee K.-D., Yu J., Zhao C.Y., et al. Dnmt1-dependent DNA methylation is essential for photoreceptor terminal differentiation and retinal neuron survival. Cell Death Dis. 2012;3:e427. doi: 10.1038/cddis.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He Y., Dan Y., Gao X., et al. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2021;320:E598–E608. doi: 10.1152/ajpendo.00089.2020. [DOI] [PubMed] [Google Scholar]
- 70.Luo R., Jin H., Li L., et al. Long noncoding RNA MEG3 inhibits apoptosis of retinal pigment epithelium cells induced by high glucose via the miR-93/Nrf2 Axis. Am. J. Pathol. 2020;190:1813–1822. doi: 10.1016/j.ajpath.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Ge Y., Zhang R., Feng Y., et al. Mbd2 mediates retinal cell apoptosis by targeting the lncRNA Mbd2-AL1/miR-188-3p/Traf3 Axis in ischemia/reperfusion injury. Mol. Ther. Nucleic Acids. 2020;19:1250–1265. doi: 10.1016/j.omtn.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung C.M.G., Gan A., Fan Q., et al. Plasma lipoprotein subfraction concentrations are associated with lipid metabolism and age-related macular degeneration. J. Lipid Res. 2017;58:1785–1796. doi: 10.1194/jlr.M073684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Storti F., Raphael G., Griesser V., et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human RPE cells. Exp. Eye Res. 2017;165:65–77. doi: 10.1016/j.exer.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Biswas L., Zhou X., Dhillon B., et al. Retinal pigment epithelium cholesterol efflux mediated by the 18 kDa translocator protein, TSPO, a potential target for treating age-related macular degeneration. Hum. Mol. Genet. 2017;26:4327–4339. doi: 10.1093/hmg/ddx319. [DOI] [PubMed] [Google Scholar]
- 75.Ebrahimi K.B., Handa J.T. Lipids, lipoproteins, and age-related macular degeneration. J Lipids. 2011;2011 doi: 10.1155/2011/802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jun S., Datta S., Wang L., et al. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp. Eye Res. 2019;181:346–355. doi: 10.1016/j.exer.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin K.W., Kim J.H., Park J.Y., et al. Long-term outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-93899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang X., Zhou G., Wu W., et al. Genome editing abrogates angiogenesis in vivo. Nat. Commun. 2017;8:112. doi: 10.1038/s41467-017-00140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mammadzada P., Corredoira P.M., Andre H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: a gene therapy perspective. Cell. Mol. Life Sci. 2020;77:819–833. doi: 10.1007/s00018-019-03422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu P., Jia S.-B., Shi J.-M., et al. LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang Q., Shan K., Qun-Wang X., et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016;7:49688–49698. doi: 10.18632/oncotarget.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan B., Yao J., Liu J.Y., et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 83.Du H., Huang Z., Zhou X., et al. Oxidative stress-induced lncRNA CYLD-AS1 promotes RPE inflammation via Nrf2/miR-134-5p/NF-kappaB signaling pathway. FASEB J. 2022;36 doi: 10.1096/fj.202200887R. [DOI] [PubMed] [Google Scholar]
- 84.Sharma A., Singh N.K. Long non-coding RNAs and proliferative retinal diseases. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15051454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hyttinen J.M.T., Blasiak J., Kaarniranta K. Non-coding RNAs regulating mitochondrial functions and the oxidative stress response as Putative targets against age-related macular degeneration (AMD) Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24032636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vishwakarma S., Kaur I. Molecular Mediators and regulators of retinal angiogenesis. Semin. Ophthalmol. 2023;38:124–133. doi: 10.1080/08820538.2022.2152706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Has data associated with your study been deposited into a publicly available repository?
No. No data was used for the research described in the article.