Abstract
AIM:
The principal objective of this study was to carry out a comprehensive and thorough analysis to compare the safety and effectiveness of the Arctic Sun, a servo-controlled surface cooling device, with conventional cooling techniques for providing therapeutic hypothermia in adult patients who had experienced hypoxic-ischemic brain injury following cardiopulmonary resuscitation.
METHODS:
In order to achieve our goal, we conducted an extensive search of multiple databases including PubMed, Embase, Cochrane, and ClinicalTrials.gov up to the date of July 30, 2021. We only included studies that compared the safety and efficacy of the Arctic Sun surface cooling equipment with standard cooling approaches such as cooling blankets, ice packs, and intravenous cold saline for treating comatose adult patients who had recovered after experiencing cardiac arrest. We evaluated various outcomes, including all-cause mortality, good neurological outcome at 1 month, and the occurrence of adverse effects such as infections, shock, and bleeding. We employed a random-effects meta-analysis to estimate the odds ratio (OR) with 95% confidence intervals (CIs) for dichotomous outcomes.
RESULTS:
One hundred and fourteen records were identified through our search; however, only three studies met our eligibility criteria, resulting in overall 187 patients incorporated in the meta-analysis. The findings indicated no significant difference in mortality rates among the Arctic Sun device and conventional cooling techniques (OR: 0.64; 95% CI: 0.34–1.19; P = 0.16; I2 = 0%). In addition, we found no significant difference in occurrence of good neurological outcomes (OR: 1.74; 95% CI: 0.94–3.25; P = 0.08; I2 = 0%) between the two cooling methods. However, the application of the Arctic Sun device was associated with increased incidence of infections compared to standard cooling methods (OR: 2.46; 95% CI: 1.18–5.11; P = 0.02; I2 = 0%). While no significant difference occurred in the incidence of shock (OR: 0.29; 95% CI: 0.07–1.18; P = 0.08; I2 = 40%), the use of the Arctic Sun device was linked to significantly fewer bleeding complications compared to standard cooling methods (OR: 0.11; 95% CI: 0.02–0.79; P = 0.03; I2 = 0%).
CONCLUSIONS:
After analyzing the results of our meta-analysis, we concluded that the use of the Arctic Sun device for targeted temperature management following cardiopulmonary resuscitation did not result in significant differences in mortality rates or improve neurological outcomes when compared to standard cooling techniques.
Keywords: Arctic Sun, hypoxic-ischemic encephalopathy, postcardiac arrest syndrome, targeted temperature management, therapeutic hypothermia
Introduction
Hypoxic-ischemic brain injury is a condition that can cause severe neurological damage, leading to increased risk of death and disability in those who have experienced cardiac arrest. Therefore, it is essential to prioritize interventions that aim to minimize secondary brain injury immediately after cardiopulmonary resuscitation. Mild therapeutic hypothermia is one such intervention that can improve the likelihood of neurological recovery and survival upon discharge.[1,2] Consensus guidelines endorse the usage of mild therapeutic hypothermia in adult patients achieving return of spontaneous circulation (ROSC) after advanced cardiac life support interventions for cardiac arrest, with a goal temperature from 32°C to 36°C sustained for a minimum of 1 day.[3] Although the precise mechanism by which hypothermia provides neuroprotection remains uncertain, proposed mechanisms include its influence on cerebral metabolism, modulation of excitotoxic neurotransmitter release, reduction of inflammation, and alteration of gene expression and protein synthesis.[4]
While cooling blankets, ice packs, and cold saline infusion are commonly used to initiate hypothermia, they have limitations when it comes to precise temperature control. The Medivance Arctic Sun temperature control device is a noninvasive alternative that provides more accurate temperature regulation (Medivance Inc., Louisville, CO, USA). The device comprises a control module and conductive surface gel pads that are placed on the patient’s skin to allow for heat exchange between circulating temperature-controlled water and the patient. The device can monitor and control the patient’s temperature within a range of 33°C–37°C, with the temperature of the water adjusted to achieve the desired temperature. Compared to standard cooling methods, this approach allows for more precise temperature regulation during hypothermia induction, maintenance, and rewarming.[5] The purpose of this study was to assess the effectiveness and safety of the Arctic Sun device in targeted temperature management (TTM) for patients who have achieved ROSC after undergoing cardiopulmonary resuscitation. The study aimed to determine whether the use of the Arctic Sun device is associated with a reduction in hypoxic-ischemic brain injury compared to standard cooling techniques. The study evaluated clinical endpoints such as mortality rates, survival rates with favorable neurological outcomes, and potential complications associated with therapeutic hypothermia.
Objectives
The current investigation aimed to juxtapose the effectiveness of two hypothermia induction methods in adult patients who have encountered cardiac arrest: the noninvasive servo-controlled surface cooling technique known as the Arctic Sun device and the conventional cooling practices of cooling blankets, ice packs, or intravenous infusion of ice-cold saline. The principal aim was to scrutinize the influence of both techniques on mortality rates and neurological outcomes that were advantageous. The secondary objective was to delve into the safety of utilizing the Arctic Sun device versus standard cooling techniques, with a particular emphasis on probable complications such as infections, shock, and bleeding.
Methods
Ethics Committee approval and Declaration of Helsinki
As this systematic review and meta-analysis involved the synthesis and analysis of previously published data and did not directly involve human participants, ethical clearance or IRB approval was not required. This study adheres to the principles outlined in the Declaration of Helsinki concerning ethical conduct in research involving human participants, however, human participants were not directly involved in the current analysis.
Eligibility criteria
The current study entailed a systematic literature search for articles that compared the effectiveness and safety of Arctic Sun versus conventional cooling techniques aimed at therapeutic hypothermia in adult patients (>18 years) who had not recovered consciousness after being resuscitated from either out-of-hospital cardiac arrest or inhospital cardiac arrest. The eligibility criteria necessitated that the studies define clinical outcomes concerning the effectiveness (mortality and favorable neurological outcome) and safety (complications such as infections, shock, and bleeding) of TTM with both techniques. The primary outcome measures comprised all-cause mortality and survival with favorable neurological status at more than 1 month in comatose survivors who were revived after cardiac arrest. The secondary outcomes comprised the incidence of complications, including severe infections such as pneumonia, urinary tract infections, and sepsis, shock necessitating vasopressor administration, and significant bleeding due to coagulopathy after therapeutic hypothermia induction with both techniques. The type of study design was not restricted. However, studies that compared endovascular cooling devices with surface cooling strategies designed for TTM were excluded from this study.
Information sources
To ensure a thorough and exhaustive search, one researcher, SCS, combed through multiple databases, including PubMed, Embase, Cochrane, and ClinicalTrials.gov, until July 30, 2021. The search utilized a combination of controlled search terms, comprising Medical Subject Headings in PubMed, such as “Cardiopulmonary Resuscitation,” “Hypothermia, Induced,” “Heart Arrest,” and “Post-Cardiac Arrest Syndrome” along with the text word “Arctic Sun.” In addition, Embase was searched using a combination of search terms, including “induced hypothermia,” “heart arrest,” “resuscitation,” “post-cardiac arrest syndrome,” and “Arctic Sun.” Cochrane was examined using the term “Arctic Sun” in title, abstract, and keywords. Finally, completed studies featuring “Arctic Sun” were searched in ClinicalTrials.gov. Language restrictions were not imposed, and all relevant publications were recognized by studying the references of eligible articles. The investigators conducted a two-phase screening process, ensuring the inclusion of all relevant studies in the analysis. In the first phase, studies were screened by titles and abstracts for eligibility by SCS, MSB, RRJ, AB, AS, AKP, and MKH. In the second phase, the full text of potentially relevant records was examined for eligibility by SCS and MSB. To minimize potential bias, the eligibility of each study was assessed based on predetermined inclusion and exclusion criteria.
Data analysis
To ensure a comprehensive and meticulous analysis of the data, relevant citation details, participant source, study design, treatment allocation process, intervention data, and outcomes were gathered by the investigators. The information was carefully entered into the Review Manager program by a single researcher (SCS) and checked for accuracy by another investigator (MSB). In order to determine the potential for bias in the studies involved in the meta-analysis, a thorough assessment was conducted on several critical areas. The areas analyzed included the random allocation process, concealed allocation of study participants and staff, blind outcome assessment, missing outcomes, and selective reporting.[6] The Oxford Centre for Evidence-Based Medicine guidance was utilized for categorizing the strength of evidence based on the study design and methodology.[7] The strength of evidence ranged from Level 1, representing high-quality evidence obtained from well-conducted randomized controlled trials, to Level 5, representing low-quality evidence from expert opinion or case reports.
Eligible outcomes
The primary outcome measures were all-cause mortality and survival with good neurologic status at >1 month in patients who had been resuscitated after cardiac arrest and underwent TTM using either Arctic Sun or standard cooling techniques such as cooling blankets, ice packs, or infusion of cold saline. In addition, the study sought to identify the development of complications such as infections (a combination of pneumonia, urinary tract infections, and sepsis), shock that required vasopressor administration, and major bleeding (requiring crystalloid infusions, blood transfusions, or vasopressor administration) from any site due to coagulopathy. While evaluating the neurological status, we considered all relevant measurements. However, we gave priority to the specific scale used when interpreting outcomes and selecting those for analysis. For instance, if a study used the Cerebral Performance Category (CPC) Scale, where 1 indicates good neurological functioning and 5 indicates brain death, we regarded values of 1 and 2 as favorable neurological outcomes whereas 3–5 denoted poor outcomes.[8] When multiple outcomes were reported, we gave preference to the most relevant outcome for analysis and presentation of the primary results. For example, if a study reported both CPC scores at 1 week and CPC scores beyond 1 month, we selected the latter outcome as it offered a more comprehensive understanding of the patients’ long-term neurological status.
Statistical analysis
In order to compare outcomes between the Arctic Sun machine and traditional cooling techniques, we analyzed the pooled data of all the resuscitated cardiac arrest patients who were treated with TTM. Our statistical analysis involved conducting meta-analyses using the Review Manager program (The Cochrane Collaboration, RevMan 5.4, 2020), and calculating odds ratio (OR) and 95% confidence intervals (CIs) by means of Mantel–Haenszel random-effects analyses. We considered P < 0.05 as statistically significant. We also visually assessed heterogeneity using forest plots besides statistical assessments used for heterogeneity variance (τ2) and inconsistency (I2). We determined heterogeneity to be significant when I2 values exceeded 50%.
Results
Upon conducting a systematic search, 114 records were initially retrieved, which were then screened for duplicates and relevance to the research question [Figure 1]. After a thorough assessment of 21 abstracts, 9 studies were selected for full-text evaluation, from which 3 studies were ultimately included in the review.[9-11] The selected studies, which included two comparative studies and one randomized controlled trial, reported data on the outcomes of mortality, survival with good neurologic status, and adverse effects of therapeutic hypothermia (such as infections and shock) in patients treated by means of either Arctic Sun or standard cooling methods [Table 1]. Two of the studies also reported data on bleeding as an adverse event. However, six studies were excluded from the review for defined reasons – Geocadin, 2005;[4] Haugk., 2007;[12] Jarrah., 2011;[13] Tømte., 2011;[14] Khan., 2019;[15] and Pérez, 2020.[16] We excluded Geocadin, 2005; Haugk, 2007; and Jarrah, 2011 because they did not evaluate the outcomes under consideration such as mortality, neurological status, or complications in patients undergoing TTM. Khan, 2019, did not compare Arctic Sun with standard cooling methods for therapeutic hypothermia. Tømte, 2011, was excluded because it compared an endovascular core cooling device with a surface cooling device, and Pérez, 2020, studied cooling methods in critically ill children rather than adult postcardiac arrest patients. The included studies provided valuable insights into the outcomes of TTM for hypoxic-ischemic encephalopathy, allowing for a comprehensive analysis of the effectiveness of Arctic Sun compared to standard cooling techniques.
Figure 1.
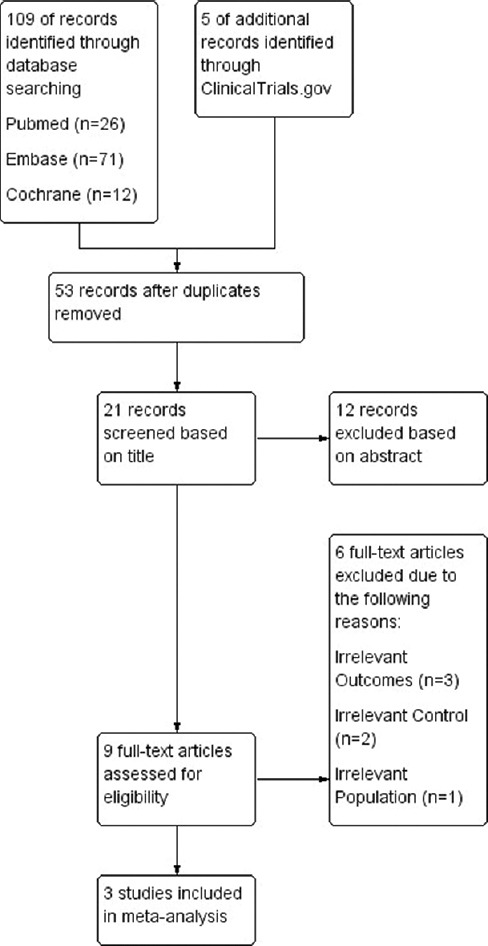
Flow diagram for database search
Table 1.
Details of the included studies
| Author/year | Study design | Country | Number of patients | Intervention | Outcome | Level of evidence* | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Arctic Sun | Conventional cooling methods | Mortality | Good neurologic outcome | Complications | |||||
| Heard et al., 2010[9] | Randomized controlled trial | USA | 64 | ✓ | ✓ | ✓ | ✓ | ✓ | 2 |
| Shinada et al., 2014[10] | Retrospective comparative | Japan | 51 | ✓ | ✓ | ✓ | ✓ | ✓ | 3 |
| Sonder et al., 2018[11] | Prospective comparative | USA | 129 | ✓ | ✓ | ✓ | ✓ | ✓ | 3 |
*The level of evidence was evaluated according to the Oxford Centre for Evidence-Based Medicine guidance with Level 1 signifying high quality of evidence to Level 5 signifying low quality of evidence[7]
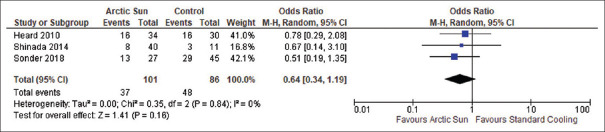
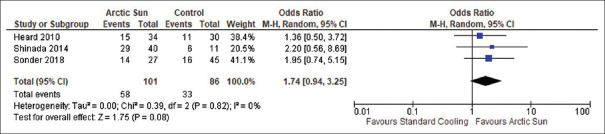
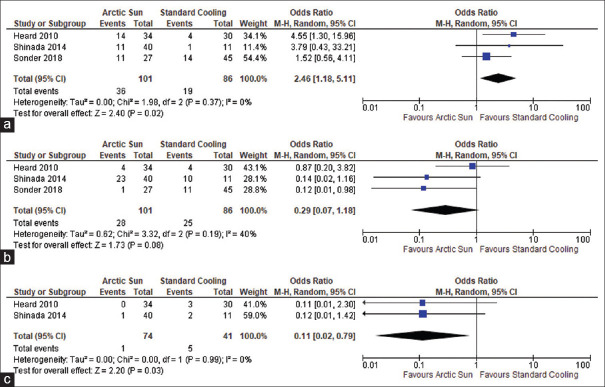
In a series of three studies, a total of 187 resuscitated cardiac arrest patients were analyzed to directly compare the mortality rates due to any cause between those treated with Arctic Sun and those treated with standard cooling techniques. The results were pooled together for a meta-analysis, which found that there was no statistically significant difference in mortality among the two groups (OR: 0.64; 95% CI: 0.34–1.19; P = 0.16), and there was no heterogeneity observed (I2 = 0%), as depicted in Figure 2. All three studies included outcome data for all-cause mortality in adult comatose survivors resuscitated from cardiac arrest who were managed with either Arctic Sun (n = 101) or standard cooling techniques (n = 86). Furthermore, the meta-analysis also investigated the proportion of good neurologic outcomes after cardiopulmonary resuscitation in patients treated with Arctic Sun versus standard cooling techniques. The results of this analysis, which were also based on data from 187 patients across the three studies, did not achieve a significant difference in the rate of good neurologic outcome among the two groups (OR: 1.74; 95% CI: 0.94–3.25; P = 0.08), and no heterogeneity was observed (I2 = 0%), as shown in Figure 3. The studies also reported the rate of adverse events, such as infections and shock, associated with therapeutic hypothermia in the 187 patients. Meta-analysis of these adverse event outcomes suggested an increased incidence of infections with the use of Arctic Sun device compared to standard cooling techniques (OR: 2.46; 95% CI: 1.18–5.11; P = 0.02), without any heterogeneity (I2 = 0%), as presented in Figure 4a. However, as shown in Figure 4b, no significant difference in the incidence of shock was detected among the Arctic Sun and conventional cooling groups (OR: 0.29; 95% CI: 0.07–1.18; P = 0.08), and there was no significant heterogeneity (I2 = 40%). Furthermore, two of the studies that were encompassed in the analysis, involving a total of 115 patients, compared the incidence of bleeding as an adverse event during TTM among the Arctic Sun and conventional cooling groups. The use of Arctic Sun (n = 74) device was associated with significantly decreased bleeding complications compared to the use of standard cooling methods (n = 41) (OR: 0.11; 95% CI: 0.02–0.79; P = 0.03); without any heterogeneity (I2 = 0%), as shown in Figure 4c. Finally, all three studies were evaluated for a range of biases, including selection, performance, detection, attrition, and reporting, as shown in Figure 5. The included studies were found to exhibit a low-to-moderate risk in bias assessment, indicating their sound methodology. Notably, no studies exhibited a high risk of bias.
Figure 2.
Forest plot of mortality in patients cooled with Arctic Sun (n = 101) compared to standard cooling techniques (n = 86) for targeted temperature management
Figure 3.
Forest plot of good neurologic outcomes in postcardiac arrest patients cooled with Arctic Sun (n = 101) versus standard cooling techniques (n = 86)
Figure 4.
Forest plots of complications of targeted temperature management by means of Arctic Sun compared to standard cooling techniques for therapeutic hypothermia. (a) Infections, (b) shock, (c) bleeding
Figure 5.

Risk of bias evaluation for the included studies
Discussion
The European Society of Intensive Care Medicine and the European Resuscitation Council provide essential recommendations for managing patients after resuscitation.[17] These guidelines propose TTM as an effective strategy for managing unresponsive postcardiac arrest patients who have achieved ROSC, regardless of their initial cardiac rhythm. To achieve this, maintaining a target temperature within the limit of 32°C–36°C for at least 24 h is strongly recommended. It should be noted that prehospital intravenous cold fluids are not recommended for inducing therapeutic hypothermia. Therefore, it is crucial to select appropriate methods to induce therapeutic hypothermia when managing postcardiac arrest patients.
The realm of TTM for postcardiac arrest patients necessitates careful consideration of various cooling techniques to effectively mitigate the adverse effects of hypoxic-ischemic encephalopathy. Among the myriad options, surface cooling systems have emerged as an enticing avenue for initiating treatment due to their convenience and electronic auto-feedback mechanisms. The Arctic Sun device, with adhesive pads and an auto-feedback system based on the patient’s skin and core temperatures, allows for precise control of the therapeutic hypothermia process. TTM for postcardiac arrest patients involves induction, maintenance, and rewarming phases. Precise temperature control is critical during all three phases, particularly during the rewarming phase, where uncontrolled temperature increases can harm patients.[18] Several cooling techniques are available for TTM, each with varying degrees of effectiveness for each cooling stage. Although cheaper and more accessible, cold saline and ice packs are not as effective as surface or intravascular cooling systems in maintaining target temperatures.[19] Surface cooling systems are an effective means of initiating treatment, equipped with electronic auto-feedback mechanisms that allow users to establish target temperatures by adjusting water temperature based on sensors that monitor the patient’s skin and core temperatures. Conventional cooling blankets and ice packs are not as effective as surface or endovascular cooling devices in maintaining temperature and may result in inadvertent cooling lower than the target temperature.[20,21] Gel-coated cold water circulation pads, such as the Arctic Sun device, are more effective than Cincinnati Sub Zero cooling blankets for fever management in neurocritical care settings.[22] However, surface cooling techniques may occasionally lead to skin burns.[23] In the realm of pediatric neurocritical care, Alcamo et al. investigated the feasibility and performance of the Arctic Sun system in critically ill pediatric patients undergoing TTM and concluded that the Arctic Sun gel-adhesive pad system proved both feasible and efficacious, ensuring prompt attainment and maintenance of target temperatures while exhibiting a minimal incidence of major adverse events.[24] In a comparison study between the Arctic Sun noninvasive device and the endovascular CoolGard device in patients resuscitated after cardiac arrest, no significant difference in survival rates and favorable neurologic outcomes was observed.[25] Nonetheless, endovascular cooling systems pose additional risks of catheter insertion-related adverse events, bloodstream infections, and venous thromboembolism. It is therefore important to evaluate the risks and benefits of each cooling technique when selecting the most appropriate method for TTM for patients resuscitated after cardiac arrest.
A thorough understanding of the possible risks and adverse effects related to each approach is critical to making an informed decision. Although the present study did not focus on shivering as a complication of therapeutic hypothermia, shivering poses a significant challenge during induced hypothermia, impacting patient outcomes due to increased metabolic demands.[26] Mayer et al. described shivering in 39% of patients treated with Arctic Sun for fever control in neurocritical care patients,[22] whereas Jarrah et al. reported shivering in 94% of resuscitated cardiac arrest patients treated with therapeutic hypothermia using Arctic Sun.[13] Tømte et al. observed that there was no difference in the occurrence of shivering between patients who underwent surface cooling and those who underwent endovascular cooling.[14] They posited that this lack of discrepancy could potentially be attributed to the administration of deep sedation to all patients, irrespective of the cooling method employed, with the primary goal of mitigating shivering. Management protocols for shivering include various pharmacologic options including buspirone, magnesium sulfate, dexmedetomidine, opioids, propofol, or neuromuscular blockade.[15,27]
In their study, Leclerc et al. examined the heat transfer characteristics of three surface cooling systems for the induction of hypothermia comparing Arctic Sun versus Blanketrol combined with nonadhesive Maxi-Therm Lite surface cooling blankets, and Blanketrol coupled with the tight-fitting nonadhesive Kool Kit.[28] The study findings contribute to the understanding of surface cooling systems and their role in therapeutic hypothermia induction. The results revealed that Kool Kit exhibited higher skin temperature and lower heat loss in the 1st h, but no significant difference was observed after 120 min. Arctic Sun demonstrated a greater core temperature decrease (0.57°C) compared to blankets (0.14°C; P = 0.035), but no significant difference was found between Arctic Sun and Kool Kit (0.24°C; P = 0.1). Although Arctic Sun initially showed an advantage in heat removal, this effect diminished over time, reducing any potential benefit during extended periods of cooling commonly encountered in clinical therapeutic hypothermia.
This systematic review and meta-analysis sought to compare the effectiveness and potential drawbacks of the Arctic Sun noninvasive surface cooling device with adhesive pads and an auto-feedback mechanism, with traditional cooling methods such as intravenous cold saline, ice packs, and surface cooling blankets for TTM for the purpose of managing hypoxic-ischemic brain injury in patients who have suffered cardiac arrest. Our findings shed light on the nuances of therapeutic hypothermia and contribute to the broader understanding of optimal cooling strategies for this vulnerable population. The present study examined clinically relevant endpoints such as mortality rates, neurological outcomes, and any adverse effects of the treatments. The results did not indicate a significant difference in mortality rates or favorable neurological outcomes with the use of the Arctic Sun device compared to conventional cooling techniques in resuscitated cardiac arrest patients. This suggests that the precision in temperature control provided by the Arctic Sun device may not offer additional benefits in reducing secondary brain damage over commonly used conventional methods of therapeutic hypothermia. It is important to take into account the cost of deploying surface cooling systems with automated temperature control for broader emergency use compared to conventional cooling methods.[29,30] Another crucial factor to consider is the frequency of adverse effects with different methods of therapeutic hypothermia. The results of our meta-analysis revealed a tale of equipoise, where the Arctic Sun device failed to demonstrate a significant difference in mortality rates or the achievement of favorable neurological outcomes when compared to its conventional counterparts. These findings suggest that the Arctic Sun’s precision in temperature control, while commendable, does not offer additional benefits in curtailing secondary brain damage beyond what can be achieved with conventional therapeutic hypothermia methods.
Furthermore, our meta-analysis unearthed an intriguing facet of the safety profile associated with different cooling techniques. The Arctic Sun device, while adept at regulating temperatures, carried a heightened risk of infections when contrasted with traditional cooling methods. The study found an increased incidence of infections with the use of the Arctic Sun device versus conventional cooling methods, without a significant difference in the incidence of shock among the two groups. This revelation demands vigilance on the part of health-care providers, urging them to exercise meticulous infection control measures while utilizing surface cooling systems. Patients undergoing TTM are at an increased risk of infections due to the presence of central venous catheters, urinary catheters, endotracheal tubes, and impaired immune function in postcardiac arrest patients. The reasons for the increased rate of infections observed in patients receiving therapeutic hypothermia with the Arctic Sun device compared to conventional cooling methods remain unclear. Potential factors such as skin integrity compromise, inadequate infection control practices, and patient-related factors may contribute to this increased risk. The extended exposure to the cooling device and the associated equipment (e.g., temperature sensors and connectors) could provide more opportunities for contamination and the introduction of pathogens. Inadequate cleaning and disinfection practices or insufficient infection control measures during the use of the device may also contribute to the increased infection risk. Health-care providers must prioritize stringent infection control measures such as appropriate catheter care, aseptic techniques during device insertion, and diligent monitoring for signs of infection to mitigate the risk of complications associated with infections.[31] The most common infections reported by Heard et al. in patients treated with the Arctic Sun device were pneumonia (22%), sepsis (9%), urinary tract infection (6%), and sinusitis (3%).[9] Jarrah et al. reported comparable rates of pneumonia (23%), bacteremia (9%), and urinary tract infection (1%), whereas Sonder et al. recorded respiratory tract infection in 26% and urinary tract infection in 15% of cardiac arrest survivors treated with therapeutic hypothermia using Arctic Sun.[11,13] This highlights the need for careful monitoring and appropriate infection control measures when employing the Arctic Sun device for TTM. The risk–benefit analysis must be delicately balanced, emphasizing the importance of vigilant surveillance and infection prevention strategies to ensure patient safety. However, the Arctic Sun device was linked with a significant decrease in bleeding complications compared to standard cooling methods. This suggests that the Arctic Sun surface cooling device may be particularly beneficial for patients at high risk of bleeding complications. While the exact mechanisms responsible for this observation were not explored in the included studies of this meta-analysis, several factors could contribute to the reduced risk of bleeding. Besides the noninvasive nature of the device, a plausible explanation is the precise temperature control provided by the Arctic Sun device. By avoiding temperature fluctuations and undesirable overcooling, the Arctic Sun device may help mitigate the potential negative effects of hypothermia on the coagulation system, reducing the risk of bleeding.[20,32,33]
The present study has several limitations that should be taken into consideration when interpreting the results. First, the meta-analysis included a limited number of studies with small sample sizes, which may have compromised the statistical power and the ability to detect significant differences between the intervention and control groups. A larger number of studies with larger sample sizes would provide more robust evidence. Second, the lack of blinding of healthcare personnel and outcome assessment in the included studies may have introduced performance and detection bias. Blinding is important to minimize the potential influence of subjective factors on the assessment of outcomes. Future studies should consider incorporating blinding procedures to enhance the validity of the results. Overall, the results of the meta-analysis have a moderate level of certainty of evidence. Moreover, the generalizability of the findings may be limited to the specific population included in the analyzed studies focused on adult cardiac arrest patients undergoing postresuscitation therapeutic hypothermia. The meta-analysis did not evaluate the use of TTM in diverse settings such as for ischemic stroke or pediatric neurocritical care settings or for normothermia maintenance in febrile patients in neurointensive care. It is important to consider the characteristics of the patient population and the clinical context when applying the findings to different settings.
Conclusions
Our meta-analysis suggests that the use of the Arctic Sun surface cooling device does not confer additional benefits in terms of mortality rates or neurological outcomes compared to conventional cooling methods for hypoxic-ischemic brain injury in adult patients following cardiac arrest. This realization compels us to reevaluate the role of precision in temperature management and underscores the importance of holistic therapeutic approaches that extend beyond the realm of temperature control. However, the Arctic Sun device may have advantages in terms of decreased bleeding complications, making it a suitable option for patients at high risk of such complications. Nevertheless, it is important to consider the increased risk of infections associated with the use of the Arctic Sun device, emphasizing the need for vigilant monitoring and infection control measures. Ultimately, the selection of an appropriate cooling technique should be based on a comprehensive evaluation of the risks and benefits associated with each method, considering patient-specific factors and the clinical context.
Data availability statement
All data generated and/or analyzed during this study are included in this published article [and its supplementary information files].
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33° C versus 36° C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 3.Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, et al. Temperature management after cardiac arrest: An advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the American Heart Association emergency cardiovascular care committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation. 2015;132:2448–56. doi: 10.1161/CIR.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 4.Geocadin RG, Carhuapoma JR. Medivance arctic sun temperature management system. Neurocrit Care. 2005;3:63–7. doi: 10.1385/ncc:3:1:063. [DOI] [PubMed] [Google Scholar]
- 5.Jung YS, Kim KS, Suh GJ, Cho JH. Comparison between gel pad cooling device and water blanket during target temperature management in cardiac arrest patients. Acute Crit Care. 2018;33:246–51. doi: 10.4266/acc.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JP, Savovic J, Page MJ, Sterne JA. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) 2019. [[Last accessed on 2021 Jun 01]]. Available from: https: //www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 .
- 7.OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. [[Last accessed on 2021 Jun 01]]. Available from: https: //www.cebm.ox.ac.uk/resourcees/levels-of-evidence/ocebm-levels-of-evidence .
- 8.Hsu CH, Li J, Cinousis MJ, Sheak KR, Gaieski DF, Abella BS, et al. Cerebral performance category at hospital discharge predicts long-term survival of cardiac arrest survivors receiving targeted temperature management* . Crit Care Med. 2014;42:2575–81. doi: 10.1097/CCM.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heard KJ, Peberdy MA, Sayre MR, Sanders A, Geocadin RG, Dixon SR, et al. Arandomized controlled trial comparing the Arctic sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation. 2010;81:9–14. doi: 10.1016/j.resuscitation.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinada T, Hata N, Yokoyama S, Kobayashi N, Tomita K, Shirakabe A, et al. Usefulness of a surface cooling device (Arctic Sun® ) for therapeutic hypothermia following cardiac arrest. J Cardiol. 2014;63:46–52. doi: 10.1016/j.jjcc.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Sonder P, Janssens GN, Beishuizen A, Henry CL, Rittenberger JC, Callaway CW, et al. Efficacy of different cooling technologies for therapeutic temperature management: A prospective intervention study. Resuscitation. 2018;124:14–20. doi: 10.1016/j.resuscitation.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Haugk M, Sterz F, Grassberger M, Uray T, Kliegel A, Janata A, et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation. 2007;75:76–81. doi: 10.1016/j.resuscitation.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Jarrah S, Dziodzio J, Lord C, Fraser GL, Lucas L, Riker RR, et al. Surface cooling after cardiac arrest: Effectiveness, skin safety, and adverse events in routine clinical practice. Neurocrit Care. 2011;14:382–8. doi: 10.1007/s12028-011-9506-y. [DOI] [PubMed] [Google Scholar]
- 14.Tø mte Ø , Dræ gni T, Mangschau A, Jacobsen D, Auestad B, Sunde K. A comparison of intravascular and surface cooling techniques in comatose cardiac arrest survivors. Crit Care Med. 2011;39:443–9. doi: 10.1097/CCM.0b013e318206b80f. [DOI] [PubMed] [Google Scholar]
- 15.Khan S, Meyers CM, Bentley S, Manini AF. Impact of targeted temperature management on ED patients with drug overdose-related cardiac arrest. J Med Toxicol. 2019;15:22–9. doi: 10.1007/s13181-018-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pé rez G, Manrique G, Garcí a J, de la Mata S, Sanz D, Ló pez-Herce J. Use of a servo-controlled cooling gel pad system to regulate body temperature in critically ill children. Pediatr Crit Care Med. 2020;21:e1094–8. doi: 10.1097/PCC.0000000000002563. [DOI] [PubMed] [Google Scholar]
- 17.Nolan JP, Sandroni C, Bö ttiger BW, Cariou A, Cronberg T, Friberg H, et al. European resuscitation council and European society of intensive care medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021;47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaity C, Al-Subaie N, Cecconi M. Cooling techniques for targeted temperature management post-cardiac arrest. Crit Care. 2015;19:103. doi: 10.1186/s13054-015-0804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliegel A, Janata A, Wandaller C, Uray T, Spiel A, Losert H, et al. Cold infusions alone are effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation. 2007;73:46–53. doi: 10.1016/j.resuscitation.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Merchant RM, Abella BS, Peberdy MA, Soar J, Ong ME, Schmidt GA, et al. Therapeutic hypothermia after cardiac arrest: Unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34:S490–4. doi: 10.1097/01.CCM.0000246016.28679.36. [DOI] [PubMed] [Google Scholar]
- 21.Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: A prospective intervention study. R91. Crit Care. 2007;11 doi: 10.1186/cc6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508–15. doi: 10.1097/01.ccm.0000147441.39670.37. [DOI] [PubMed] [Google Scholar]
- 23.Varon J, Acosta P, Wintz R, Mendoza N. Unusual side effect from hydrogel pads during therapeutic hypothermia. Resuscitation. 2008;78:248–9. doi: 10.1016/j.resuscitation.2008.03.223. [DOI] [PubMed] [Google Scholar]
- 24.Alcamo AM, Lavezoli R, Dezfulian C, Simon DW, Aneja RK, Clark RS, et al. Feasibility and performance of a gel-adhesive pad system for pediatric targeted temperature management: An exploratory analysis of 19 pediatric critically ill patients. Ther Hypothermia Temp Manag. 2021;11:19–27. doi: 10.1089/ther.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: A retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010;81:1117–22. doi: 10.1016/j.resuscitation.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 26.May TL, Riker RR, Gagnon DJ, Duarte C, McCrum B, Hoover C, et al. Continuous surface EMG power reflects the metabolic cost of shivering during targeted temperature management after cardiac arrest. Resuscitation. 2018;131:8–13. doi: 10.1016/j.resuscitation.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Cook CJ. Induced hypothermia in neurocritical care: A review. J Neurosci Nurs. 2017;49:5–11. doi: 10.1097/JNN.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 28.Leclerc C, Talebian Nia M, Giesbrecht GG. Heat Transfer Capabilities of Surface Cooling Systems for Inducing Therapeutic Hypothermia. Ther Hypothermia Temp Manag. 2023 doi: 10.1089/ther.2023.0003. doi: 10.1089/ther.2023.0003. Epub ahead of print. PMID: 37276032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javanbakht M, Mashayekhi A, Hemami MR, Branagan-Harris M, Keeble TR, Yaghoubi M. Cost-effectiveness analysis of intravascular targeted temperature management after cardiac arrest in England. Pharmacoecon Open. 2022;6:549–62. doi: 10.1007/s41669-022-00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damluji AA, Al-Damluji MS, Pomenti S, Zhang TJ, Cohen MG, Mitrani RD, et al. Health care costs after cardiac arrest in the United States. Circ Arrhythm Electrophysiol. 2018;11:e005689. doi: 10.1161/CIRCEP.117.005689. [DOI] [PubMed] [Google Scholar]
- 31.Broessner G, Fischer M, Lackner P, Pfausler B, Schmutzhard E. Complications of hypothermia: Infections. Crit Care. 2012;16:A19. [Google Scholar]
- 32.Wang CH, Chen NC, Tsai MS, Yu PH, Wang AY, Chang WT, et al. Therapeutic hypothermia and the risk of hemorrhage: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2152. doi: 10.1097/MD.0000000000002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kander T, Schö tt U. Effect of hypothermia on haemostasis and bleeding risk: A narrative review. J Int Med Res. 2019;47:3559–68. doi: 10.1177/0300060519861469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article [and its supplementary information files].