Key Points
-
•
With longer follow-up, zanubrutinib showed durable disease control (2-year DOR rate of 72.9%) in patients with R/R MZL.
-
•
Atrial fibrillation/flutter and hypertension were uncommon, constituting an improvement over earlier-generation BTK inhibitors.
Visual Abstract
Abstract
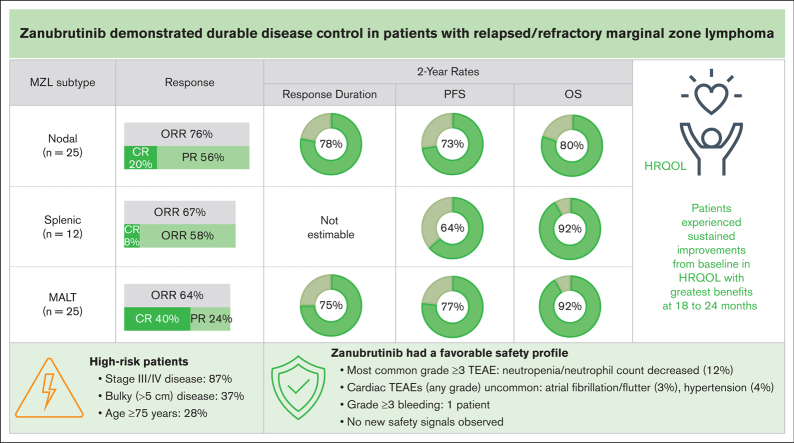
The primary analysis of MAGNOLIA, an open-label, single-arm, multicenter, phase 2 study, demonstrated that the next-generation Bruton tyrosine kinase (BTK) inhibitor zanubrutinib provided a high overall response rate (ORR) in patients with relapsed/refractory marginal zone lymphoma (R/R MZL), with a favorable safety/tolerability profile. Presented here, is the final analysis of MAGNOLIA, performed to characterize the durability of response and longer-term safety and tolerability. Zanubrutinib (160 mg twice daily) was evaluated in 68 patients with R/R MZL who had received at least 1 anti-CD20–directed regimen. The primary end point was independent review committee (IRC)-assessed ORR. Secondary end points included investigator-assessed ORR, duration of response (DOR), progression-free survival (PFS), overall survival (OS), health-related quality of life, safety, and tolerability. With a median follow-up of 27.4 months, the IRC-assessed ORR was 68.2% (95% confidence interval [CI], 55.6-79.1), with a 24-month DOR event-free rate of 72.9% (95% CI, 54.4-84.9). PFS and OS at 24 months were 70.9% (95% CI, 57.2-81.0) and 85.9% (95% CI, 74.7-92.4), respectively. The zanubrutinib safety profile was consistent with the primary analysis, with no new safety signals observed. Atrial fibrillation/flutter (n = 2 [2.9%]) and hypertension (n = 3 [4.4%]) were uncommon. Neutropenia (n = 8 [11.8%]) was the most common grade ≥3 adverse event. In this final analysis of MAGNOLIA, zanubrutinib demonstrated sustained clinical responses beyond 2 years, with 73% of responders alive and progression free. Zanubrutinib continued to demonstrate a favorable safety/tolerability profile with the additional time on treatment. This trial was registered at www.clinicaltrials.gov as #NCT03846427.
Introduction
Marginal zone lymphoma (MZL) is an indolent non-Hodgkin lymphoma, which accounts for ∼7% of mature B-cell non-Hodgkin lymphoma and has 3 main subtypes: (1) extranodal MZL of mucosa-associated lymphoid tissue, which is the most common, occurring in ∼60% of cases; (2) nodal MZL, occurring in ∼30% of cases; and (3) splenic MZL, occurring in ∼10% of cases.1,2 Conventional treatments for relapsed/refractory (R/R) MZL include immunotherapy (eg, rituximab), chemoimmunotherapy (eg, rituximab/cyclophosphamide/vincristine/prednisolone [RCVP], rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone [RCHOP]), bendamustine plus rituximab, and lenalidomide plus rituximab.3 In recent years, advances in the understanding of MZL have revealed the importance of the B-cell receptor signaling pathway and the involvement of Bruton tyrosine kinase (BTK) in the B-cell receptor’s downstream transduction pathway.4 This led to the development and approval of the first BTK inhibitor, ibrutinib, which changed the treatment landscape for indolent B-cell malignancies.3,5 However, ibrutinib is associated with significant toxicity including cardiac toxicity and hemorrhage6, 7, 8, 9; with 17% of patients in a phase 2 study of ibrutinib for the treatment of R/R MZL discontinuing treatment because of adverse events (AEs).7
Zanubrutinib is an irreversible, next-generation, covalent BTK inhibitor, developed to ensure greater BTK specificity, thereby minimizing off-target inhibition of epidermal growth factor receptor and TEC family kinases and the associated toxicities.10 In the primary analysis of this phase 2 MAGNOLIA study in 66 patients with R/R MZL who were evaluable, and a median follow-up of 15.7 months, zanubrutinib demonstrated efficacy (independent review committee [IRC]-assessed overall response rate [ORR], 68.2%; complete response [CR], 25.8%; and median duration of response [DOR] and median progression-free survival [PFS] were not reached) and a favorable safety/tolerability profile.11 Based on these early data, zanubrutinib was approved in the United States (accelerated approval), Canada, and the European Union for the treatment of patients with R/R MZL who have received at least 1 anti-CD20–directed regimen.11, 12, 13, 14
Here, we present the final analysis of the MAGNOLIA study with a median follow-up of an additional 12 months to characterize the durability of response and longer-term safety and tolerability of zanubrutinib in patients with R/R MZL.
Methods
Study design
The study methods, including study design and treatment, full inclusion/exclusion criteria, end points, and statistical analyses have been published previously.11 Briefly, MAGNOLIA (clinicaltrials.gov; #NCT03846427) is an open-label, single-arm, multicenter, phase 2 study evaluating the efficacy and safety of zanubrutinib for the treatment of patients with R/R MZL who had received at least 1 anti-CD20–directed regimen.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for good clinical practice, and all applicable local regulatory requirements. All patients provided written informed consent. All authors had access to the data.
Patients
Eligible patients were aged ≥18 years with R/R MZL, had previously received at least 1 anti-CD20–directed regimen, had measurable disease (≥1 nodal lesion of >1.5 cm in the longest diameter and/or ≥1 extranodal lesion of >1.0 cm) by computed tomography (CT) or magnetic resonance imaging, were in need of systemic therapy for MZL in the opinion of the study investigator, had an Eastern Cooperative Oncology Group performance status score of 0 to 2, and had adequate bone marrow (BM) and organ function. Patients were excluded if they had previously received a BTK inhibitor, were receiving a strong CYP3A inhibitor/inducer, had central nervous system MZL involvement, had a known transformation to aggressive lymphoma, had clinically significant cardiovascular disease, or had active infection. Patients receiving antiplatelet therapy and/or anticoagulants, including warfarin, were eligible.
Patients with Waldenström macroglobulinemia were not eligible. To assess for Waldenström macroglobulinemia, serum protein electrophoresis was required at screening. Patients with a monoclonal spike (M spike or paraprotein) were required to have an MYD88 mutational analysis. In addition, histologic review by an independent central laboratory was performed for all enrolled patients to confirm the diagnosis of MZL.
Procedures and end points
All patients received oral zanubrutinib (160 mg twice daily), until disease progression, unacceptable toxicity, or patient withdrawal. Disease response was assessed in accordance with the Lugano classification for non-Hodgkin lymphoma.15 Positron emission tomography (PET)-based criteria for patients with fluorodeoxyglucose (FDG)-avid disease were used, whereas CT-based criteria were used for non–FDG-avid disease. Scans were performed at screening, weeks 12, 24, 36, and 48, and every 24 weeks thereafter. At screening, BM assessment was required whereas endoscopy was optional for patients with gastrointestinal (GI) involvement. Repeat BM and/or endoscopy were required for confirmation of CR in patients with BM and/or GI involvement at baseline.
The primary efficacy end point was IRC-assessed ORR, defined as the proportion of patients who achieved a best overall response of partial response (PR) or CR. Secondary efficacy end points included investigator-assessed ORR, DOR (time [months] from first PR or CR to disease progression or death), PFS (time [months] from first dose of zanubrutinib to disease progression or death), and overall survival (OS; time [months] from first dose of zanubrutinib to death).
Health-related quality of life was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30) and the EuroQol 5-dimension 5-level questionnaire (EQ-5D-5L).16,17 These patient-reported questionnaires were completed before the first dose of zanubrutinib, at the end of treatment cycle 3, every 12 weeks for the next 12 months, then every 24 weeks thereafter. Both the EORTC QLQ-C30 and the EQ-5D-5L global scales range from 0 to 100, with 0 representing the worst possible health status/quality of life, and 100 representing the best.
Safety/tolerability was assessed from the first treatment day until 30 days after the last dose of zanubrutinib, and included the monitoring of AEs, clinical laboratory parameters, and vital signs. AE severity was graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03), and AEs were coded using Medical Dictionary for Regulatory Activities terminology (version 24.0). AEs of special interest were defined based on the known and theoretical toxicities associated with the BTK inhibitor class; these included bleeding (minor and major hemorrhage), hypertension, atrial fibrillation and flutter, second primary malignancies (including skin cancers), tumor lysis syndrome, infections (including opportunistic infections), and cytopenia (eg, neutropenia, thrombocytopenia, and anemia).
In a biomarker substudy by the Australasian Leukaemia & Lymphoma Group, whole-exome sequencing was performed, focusing on 48 genes currently known to be mutated in MZL.18
Statistical analysis
The study planned to enroll ∼65 patients to provide 82% power to detect a significant difference with a predicted ORR of 48% for zanubrutinib (predicted ORR based on previously published data for ibrutinib6) against a null hypothesis ORR of 30%.
Efficacy analyses were performed using the efficacy analysis set, defined as all patients enrolled in the study who received at least 1 dose of zanubrutinib and had a centrally confirmed diagnosis of MZL. For the primary efficacy end point (IRC-assessed ORR per Lugano classification), superiority against the null hypothesis was evaluated using a binomial exact test with a 1-sided significance level of .025. For PFS, DOR, and OS, Kaplan-Meier analyses were used to determine medians, event-free rates, and 95% confidence intervals (CIs), with reverse Kaplan-Meier analyses used to determine follow-up times. A sensitivity analysis was performed (efficacy analysis set) to evaluate disease response via the CT-based Lugano criteria, regardless of PET status at baseline. Health-related quality of life was analyzed using data from all patients in the efficacy analysis set who had a baseline and at least 1 postbaseline measurement.
Safety analyses were performed using the safety analysis set, defined as all patients enrolled in the study who received at least 1 dose of zanubrutinib. Safety data were summarized using standard descriptive statistics (absolute values and percentages), as were quality of life measures.
Predefined subgroup analyses were performed on the primary efficacy end point (using the same methodology) in subgroups of patients defined by baseline characteristics including age, disease subtype and stage, Eastern Cooperative Oncology Group performance status, prior therapies, R/R status, and disease bulk.
Results
Patients and baseline characteristics
The MAGNOLIA study enrolled and treated 68 patients at 31 sites across 9 countries (Australia, China, Czech Republic, France, Italy, New Zealand, South Korea, the United Kingdom, and the United States) between February 2019 and January 2020. The data cut-off date for this final analysis was 4 May 2022. All 68 patients were included in the safety analysis set, whereas 66 (97.1%) patients had a centrally confirmed diagnosis of MZL and were therefore included in the efficacy analysis set. At study completion, 34 (50.0%) patients remained on treatment: 31 (50.0%) patients rolled over into a long-term extension study (ClinicalTrials.gov; #NCT04170283), 3 (4.4%) patients were being treated with zanubrutinib but did not enroll in the long-term extension study; and 34 (5.650.0%) were off treatment. Twenty-four (35.3%) patients discontinued zanubrutinib because of investigator-assessed disease progression, and 5 (7.4%) discontinued because of AEs (supplemental Figure 1).
Most patient baseline demographics and characteristics have been published previously.11 Briefly, the median age was 70 years (interquartile range [IQR], 59.5-77.0) and 19 (27.9%) patients were aged ≥75 years (supplemental Table 1). The same proportion of patients had extranodal and nodal MZL subtypes (n = 26 [38.2% for each]; 73.1% [19 of 26] patients with extranodal MZL had nongastric/noncutaneous disease), 12 (17.6%) patients had splenic MZL, and 4 (5.9%) presented with both nodal and extranodal lesions, meaning investigators were unable to classify the primary subtype. The majority of patients had FDG-avid disease (n = 61 [89.7%]), stage III/IV disease (n = 59 [86.8%]), or extranodal site involvement (n = 53 [77.9%]). The median number of prior therapies was 2 (IQR, 1.0-3.0), and 22 (32.4%) patients had disease refractory to their last therapy; most patients (n = 61 [89.7%]) received chemoimmunotherapy whereas 7 (10.3%) patients received rituximab as their only prior treatment. The median duration of study follow-up was 28.0 months (IQR, 24.9-30.5), the median duration of treatment was 24.2 months (IQR, 7.2-29.3), and the median number of treatment cycles was 26.3 (IQR, 7.8-31.9).
Efficacy
The IRC-assessed ORR was 68.2% (95% CI, 55.6-79.1; P < .001 vs the null hypothesis), with a CR rate of 25.8% (n = 17) and PR rate of 42.4% (n = 28), consistent with the primary analysis.11 Efficacy was observed across all MZL subtypes, with an ORR of 76.0% in patients with nodal MZL, 66.7% in those with splenic MZL, and 64.0% in those with extranodal mucosa-associated lymphoid tissue (Table 1). Median time to overall response was 2.8 months, as shown in Table 1. Median time to CR was 2.9 months (IQR, 2.8-5.5) in the total population, and 3.0 months (IQR, 2.7-3.8) in patients with nodal MZL, 6.3 months (IQR, 6.3-6.3) in the patient with splenic MZL, and 2.9 months (IQR, 2.8-5.3) in those with extranodal mucosa-associated lymphoid tissue. A sensitivity analysis of IRC-assessed ORR based on CT assessments showed an ORR of 66.7% (95% CI, 54.0-77.8) and a CR rate of 24.2% (Table 2). Investigator-assessed ORR was slightly higher than IRC-assessed ORR (Table 2).
Table 1.
Summary of IRC-assessed disease responses by MZL subtypes (efficacy analysis set)
| Extranodal (MALT) (n = 25) | Nodal (n = 25) | Splenic (n = 12) | Unknown∗ (n = 4) | Total† (N = 66) | |
|---|---|---|---|---|---|
| ORR, % (95% CI)‡ | 64.0 (42.5-82.0) | 76.0 (54.9-90.6) | 66.7 (34.9-90.1) | 50.0 (6.8-93.2) | 68.2 (55.6-79.1) |
| Best overall response, n (%) | |||||
| CR | 10 (40.0) | 5 (20.0) | 1 (8.3) | 1 (25.0) | 17 (25.8) |
| PR | 6 (24.0) | 14 (56.0) | 7 (58.3) | 1 (25.0) | 28 (42.4) |
| Stable disease | 4 (16.0) | 5 (20.0) | 3 (25.0) | 1 (25.0) | 13 (19.7) |
| Progressive disease | 3 (12.0) | 1 (4.0) | 1 (8.3) | 1 (25.0) | 6 (9.1) |
| Nonprogressive disease§ | 1 (4.0) | 0 | 0 | 0 | 1 (1.5) |
| Discontinued study before first assessment | 1 (4.0) | 0 | 0 | 0 | 1 (1.5) |
| Median time to response, mo (IQR) | 2.8 (2.7-2.9) | 2.8 (2.7-3.8) | 3.6 (2.7-6.0) | 2.7 (2.6-2.8) | 2.8 (2.7-3.7) |
MALT, mucosa-associated lymphoid tissue.
These patients presented with both nodal and extranodal lesions; therefore, the study sites were unable to classify the MZL subtype.
Two patients were excluded from the efficacy analysis set because central review determined their diagnosis as diffuse large B-cell lymphoma.
95% CIs were calculated using 2-sided Clopper-Pearson methodology.
One patient was classified as having “nonprogressive disease” because of a missed PET scan at cycle 3 (CT scan showed stable disease).
Table 2.
Summary of disease responses (efficacy analysis set)
| IRC-assessed by PET and/or CT (n = 66)∗ | IRC-assessed by CT only (n = 66)∗ | Investigator-assessed by PET and/or CT (n = 66)∗ | |
|---|---|---|---|
| ORR, % (95% CI)† | 68.2 (55.6-79.1) | 66.7 (54.0-77.8) | 75.8 (63.6-85.5) |
| Best overall response, n (%) | |||
| CR | 17 (25.8) | 16 (24.2) | 19 (28.8) |
| PR | 28 (42.4) | 28 (42.4) | 31 (47.0) |
| Stable disease | 13 (19.7) | 16 (24.2) | 10 (15.2) |
| Progressive disease | 6 (9.1) | 5 (7.8) | 5 (7.8) |
| Nonprogressive disease‡ | 1 (1.5) | 0 | 0 |
| Discontinued study before first assessment, n (%) | 1 (1.5) | 1 (1.5) | 1 (1.5) |
| Median time to response, mo (range) | 2.8 (1.7-11.1) | 3.0 (1.8-22.2) | 2.8 (1.7-16.6) |
Two patients were excluded from the efficacy analysis set because central review determined their diagnosis as diffuse large B-cell lymphoma.
95% CIs were calculated using 2-sided Clopper-Pearson methodology.
One patient with FDG-avid disease who missed the PET scan at cycle 3 and was assessed as nonprogressive disease (CT scan showed stable disease).
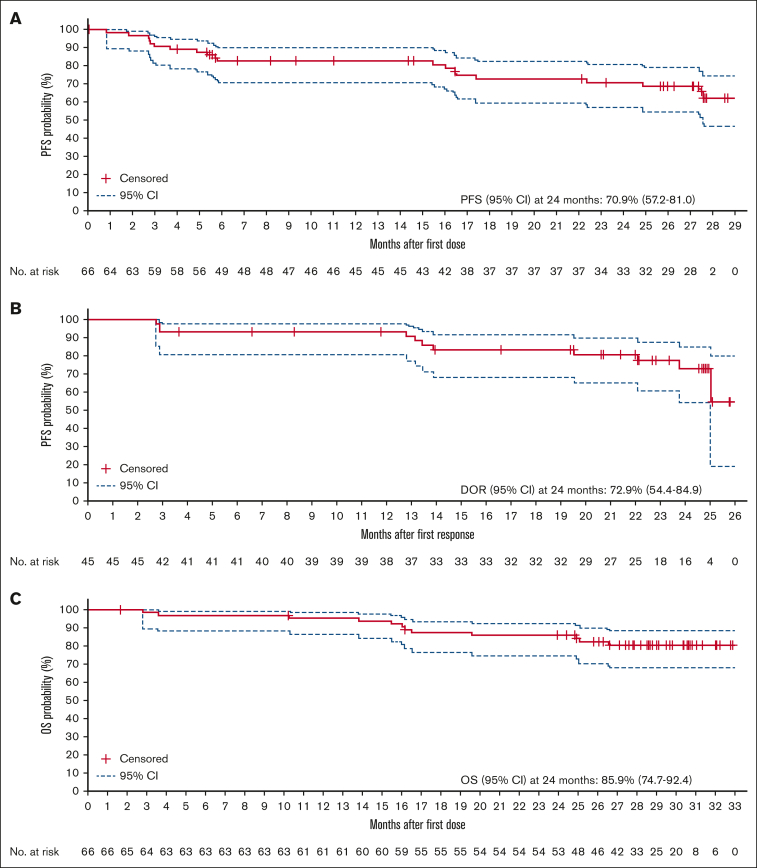
Median PFS and DOR were not reached at a median follow-up of 27.4 months (IQR, 16.5-not reached) and 23.4 months (IQR, 23.8 to not reached), respectively. At 24 months, PFS and DOR rates were 70.9% (95% CI, 57.2-81.0) and 72.9% (95% CI, 54.4-84.9), respectively (Figure 1A-B). PFS rates at 24 months were higher in those with nodal MZL and extranodal mucosa-associated lymphoid tissue than in those with splenic MZL. Of patients with a response, 78.0% of those with nodal MZL and 74.6% of those with extranodal mucosa-associated lymphoid tissue maintained the response at 24 months. Response duration was not reached in the splenic MZL subgroup (supplemental Figure 2). A larger proportion of patients who attained a CR were progression free and alive at 24 months (76.5% [13 of 17] of patients; PFS rate, 87.4%) compared with patients who did not attain a CR (40.8% [20 of 49] of patients; PFS rate, 64.7%). The estimated 24-month OS rate was 85.9% (95% CI, 74.7-92.4) (Figure 1C). The highest 24-month survival rates were observed in patients with extranodal mucosa-associated lymphoid tissue (91.7%) or splenic MZL (91.7%), and the lowest rate was seen in patients with nodal MZL (80.0%; supplemental Figure 2).
Figure 1.
Kaplan-Meier analyses. (A) PFS, (B) DOR, and (C) OS (efficacy analysis set). NR, not reached.
Seventeen patients (25.8%) started a new anticancer therapy for MZL. At 24 months, 74.5% (95% CI, 61.7-83.6) of patients had not started a new anticancer treatment for MZL. The median time to next line of therapy was not reached (IQR, 20.7-not reached).
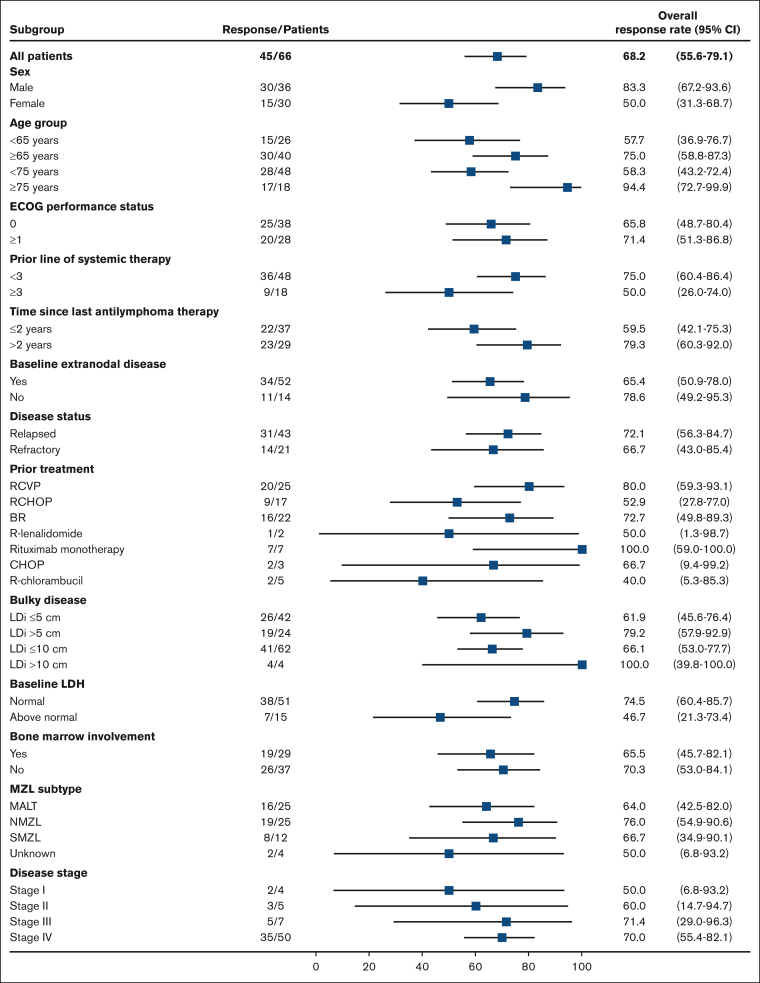
IRC-assessed ORRs for prespecified subgroups are presented in Figure 2. Response rates higher than those seen in the overall study population were observed in several patient subgroups, including those who traditionally respond poorly to therapy.19 Specifically, higher response rates were observed in the following subgroups: patients aged ≥75 years (94.4%), male patients (83.3%), patients with >2 years since last antilymphoma therapy (79.3%), patients with relapsed disease (72.1%), patients with at least 1 target lesion of >5 cm (79.2%), patients with nodal MZL subtype (76.0%), patients with stage IV disease (70.0%), patients who received prior treatment with rituximab monotherapy (100.0%), and patients who received prior treatment with RCVP (80.0%).
Figure 2.
IRC-assessed ORR in prespecified subgroups (efficacy analysis set). Figure is adapted and updated from Opat S et al.11(Fig2) BR, bendamustine + rituximab; CHOP, cyclophosphamide + doxorubicin + vincristine + prednisone; ECOG, Eastern Cooperative Oncology Group; LDi, longest diameter; LDH, lactate dehydrogenase; NMZL, nodal marginal zone lymphoma; R, rituximab; SMZL, splenic marginal zone lymphoma.
Biomarker substudy
Whole-exome sequencing was performed at baseline in 17 patients treated with zanubrutinib. Patients with mutations in genes associated with the NF-κB pathway (MYD88 or TNFAIP3 mutations [n = 8]) had longer PFS compared with those with a wildtype phenotype (n = 9): median PFS was not reached vs 11.1 months, respectively; P = .008 (hazard ratio 0.09; 95% CI, 0.01-0.52). Two patients with disease progression on therapy also had acquired BTK and PLCy2 mutations.18
Health-related quality of life
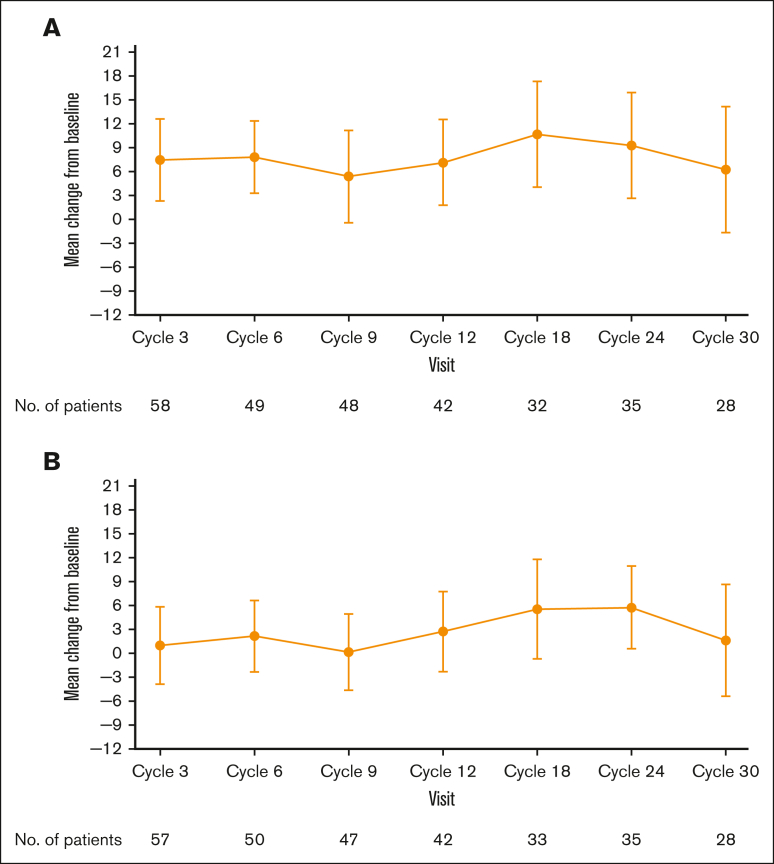
Global EORTC QLQ-C30 and EQ-5D-5L scores indicated an improvement from baseline in patient health status/quality of life, which was observed at cycle 3 of treatment and was broadly maintained throughout the study (Figure 3A-B). The greatest improvements in both patient-reported questionnaires occurred during cycles 18 to 24 (18-24 months), during which the mean (standard deviation) EORTC QLQ-C30 scores were 10.7 (18.5) and 9.3 (19.3) points above baseline at cycles 18 and 24, respectively, and the mean (standard deviation) EQ-5D-5L visual analog score was 5.6 (17.7) points above baseline at cycle 18.
Figure 3.
Mean change from baseline over time. (A) EORTC QLQ-C30 and (B) EQ-5D-DL global health status/quality of life scores (efficacy analysis set).
Safety/tolerability
All patients (n = 68) reported at least 1 treatment-emergent AE of any grade, irrespective of relationship to treatment (Table 3). The most common AEs occurring in ≥10% of patients were contusion (n = 16 [23.5%]), diarrhea (n = 15 [22.1%]), and constipation (n = 12 [17.6%]). Grade ≥3 AEs occurred in 33 (48.5%) patients, with neutropenia/neutrophil count decrease (n = 8 [11.8%]) and COVID-19 pneumonia (n = 4 [5.9%]) occurring in ≥5% of patients (Table 3).
Table 3.
Summary of AEs and most common AEs (safety analysis set)
| MAGNOLIA study (N = 68) | |
|---|---|
| Any treatment-emergent AE | 68 (100.0) |
| Grade ≥3 AE | 33 (48.5) |
| Serious AE | 30 (44.1) |
| AE leading to dose reduction | 0 |
| AE leading to dose interruption | 25 (36.8) |
| AE leading to treatment discontinuation | 5 (7.4) |
| AE leading to death | 5 (7.4) |
| Any grade AE occurring in ≥10% of patients | |
| Contusion | 16 (23.5) |
| Diarrhea | 15 (22.1) |
| Constipation | 12 (17.6) |
| Arthralgia | 10 (14.7) |
| Pyrexia | 10 (14.7) |
| Upper respiratory tract infection | 9 (13.2) |
| Back pain | 8 (11.8) |
| Nausea | 7 (10.3) |
| Cough | 7 (10.3) |
| Grade ≥3 AEs occurring in at least 2 patients | |
| Neutropenia | 6 (8.8) |
| COVID-19 pneumonia | 4 (5.9) |
| Diarrhea | 3 (4.4) |
| Pneumonia | 3 (4.4) |
| Syncope | 3 (4.4) |
| Anemia | 2 (2.9) |
| Hypertension | 2 (2.9) |
| Neutrophil count decreased | 2 (2.9) |
| Pyrexia | 2 (2.9) |
| Thrombocytopenia | 2 (2.9) |
Most common AEs are any grade AEs occurring in >10% of patients and grade ≥3 AEs occurring in at least 2 patients.
Data are n (%).
Grade ≥3 AEs of special interest were infrequent (Table 4). The only specific infection reported in >5% of patients was COVID-19. A grade 3 GI bleed occurred in 1 patient who was receiving concomitant enoxaparin and rivaroxaban for a bilateral pulmonary embolism. The patient recommenced zanubrutinib once the hemorrhage had subsided and experienced no recurrent bleeding. New-onset grade 3 hypertension was reported in 2 (2.9%) patients; atrial fibrillation (grade 3), atrial flutter (grade 2), and ventricular extrasystole (grade 2) occurred in 1 (1.5%) patient each. The atrial fibrillation occurred 21 days after the last dose of zanubrutinib and after disease progression, in a patient with a preexisting history of atrial fibrillation. Grade ≥3 anemia was reported in 2 (2.9%), neutropenia in 8 (11.8%), and thrombocytopenia in 3 (4.4%) patients. The median time to onset of anemia, neutropenia, and thrombocytopenia (all grades) was 102.5 days (IQR, 64.0-108.5), 86.0 days (IQR, 45.0-339.0), and 84.0 days (IQR, 28.0-343.0), respectively. Three (4.4%) patients had dose interruptions for neutropenia whereas 4 (5.9%) received growth factor support. No platelet transfusions were required for thrombocytopenia. No patient discontinued zanubrutinib because of cytopenia, cardiac arrhythmia, or bleeding events. There were no reports of tumor lysis syndrome or febrile neutropenia.
Table 4.
Summary of AEs of special interest (safety analysis set)
| AE of special interest | MAGNOLIA study (N = 68) |
|
|---|---|---|
| Any Grade AE | Grade ≥3 AE | |
| Any AE of special interest | 54 (79.4) | 23 (33.8) |
| Infections | 38 (55.9) | 15 (22.1) |
| Opportunistic infections | 3 (4.4) | 2 (2.9) |
| Bleeding | 28 (41.2) | 1 (1.5) |
| Major hemorrhage∗ | 1 (1.5) | 1 (1.5) |
| Second primary malignancies | 5 (7.4) | 3 (4.4) |
| Skin cancers | 2 (2.9) | 0 |
| Neutropenia† | 11 (16.2) | 8 (11.8) |
| Thrombocytopenia‡ | 11 (16.2) | 3 (4.4) |
| Anemia | 4 (5.9) | 2 (2.9) |
| Hypertension | 3 (4.4) | 2 (2.9) |
| Atrial fibrillation/flutter | 2 (2.9) | 1 (1.5) |
| Ventricular arrhythmia | 1 (1.5) | 0 |
Data are number (%).
Defined as any serious or grade ≥3 bleed at any site, or central nervous system bleed of any grade.
“Neutropenia” included the terms “neutropenia” and “neutrophil count decreased.”
“Thrombocytopenia” included the terms “thrombocytopenia” and “platelet count decreased.”
Of the 5 (7.4%) patients who developed second primary malignancies, 2 (2.9%) developed skin cancer (both had a prior history of skin cancer), 1 (1.5%) had a recurrence of bladder/prostate cancer, 1 (1.5%) with a preexisting thyroid mass was subsequently diagnosed with papillary thyroid cancer, and 1 (1.5%) who previously received alkylating agents developed acute myeloid leukemia.
Serious AEs were reported in 30 (44.1%) patients; those occurring in >1 patient included COVID-19 pneumonia (n = 4 [5.9%]), pneumonia (n = 3 [4.4%]), pyrexia (n = 3 [4.4%]), syncope (n = 2 [2.9%]), and fall (n = 2 [2.9%]). Twenty-five (36.8%) patients had treatment interruption because of AEs. The median duration of treatment interruption was 22.0 days (IQR, 6-53) and the most common AEs leading to treatment interruption were COVID-19 pneumonia (n = 4 [5.9%]) and neutropenia (n = 3 [4.4%]).
Five (7.4%) patients discontinued treatment because of AEs (all fatal), including 2 (2.9%) cases of COVID-19 pneumonia, myocardial infarction in a patient with a history of coronary artery disease, acute myeloid leukemia in a patient with prior exposure to an alkylating agent, and septic encephalopathy after radical cystectomy in a patient diagnosed with recurrent bladder/prostate cancer.
Discussion
Advanced-stage MZL is a generally incurable disease characterized by a continuing pattern of relapse and remission.20,21 Although immunotherapy (such as rituximab) and chemoimmunotherapy are standard treatments for R/R MZL,3 treatment outcomes are generally poor because of inadequate response and/or toxicities.22,23 Targeted oral therapies such as ibrutinib and lenalidomide have provided treatment alternatives; however, these agents have a limited duration of benefit, in part because of suboptimal tolerability.6,7,22 This highlights the need for regimens with fewer toxicities, better tolerability, and durable disease control.
The primary analysis of the MAGNOLIA study, with a median follow-up of 15.7 months, showed that the primary end point was met with an IRC-assessed ORR of 68.2%, which was substantially higher than that of the null hypothesis. These results led to the global approval of zanubrutinib for the treatment of R/R MZL.12, 13, 14 With a median follow-up of 28.0 months, this final MAGNOLIA analysis confirms that zanubrutinib is an efficacious, generally well-tolerated treatment in patients with R/R MZL, thereby supporting its use as a chemotherapy-free therapeutic option. The IRC-assessed disease response remained high regardless of imaging modality, with an ORR of 68.2%, a CR of 25.8% by PET and/or CT, and an ORR of 66.7%, with a CR of 24.2% by CT only. Of note, 13 (19.7%) patients with stable disease remained on treatment after >18 cycles of zanubrutinib. In addition, 42.9% (12 of 28) of patients with a best response of PR had radiographic evidence of CR; however, they were missing repeat BM examination or endoscopy required for CR confirmation as per the protocol. This high ORR was generally consistent across MZL subtypes and prespecified subgroups, including patients who were characterized as difficult to treat such as those with advanced age and advanced-stage MZL.19 These updated results provide additional evidence of the durability of disease control provided by zanubrutinib, with 72.9% of responders maintaining remission, 70.9% remaining progression-free and alive, and an OS rate of 85.9% at 24 months. In addition, a numerically higher PFS rate at 24 months was observed in patients who attained CR vs those who did not attain a CR (87.4% vs 64.7%). This finding suggests that achieving a CR may be indicative of longer remission and survival in MZL. Further research with larger sample sizes may be warranted to investigate this observation, and if confirmed, achieving a high CR rate could be considered an efficacy end point in future trials of R/R MZL.
In the correlative biomarker substudy, mutations in genes associated with the NF-κB pathway (MYD88 and TNFAIP3 mutations) appeared to lead to an improved PFS, whereas acquired BTK and PLCy2 mutations appeared to herald disease progression.18
Similar to the efficacy data, the safety profile of zanubrutinib in this final analysis remained consistent with that observed during the primary analysis, as well as in pooled safety analyses of zanubrutinib.8,11 No patients required dose reduction, and treatment discontinuations because of AEs were infrequent (7.4%). Although the study enrolled predominantly patients who were older with preexisting comorbidities, the incidence of cardiac events, including hypertension and arrhythmias, was low, with none leading to treatment withdrawal. Notably, the incidence of cardiac events did not increase with longer treatment. Bleeding events were uncommon, with only 1 grade 3 GI bleed reported in a patient receiving concomitant anticoagulant therapy. Anemia, neutropenia, and thrombocytopenia were also infrequent and manageable with supportive care; only 3 patients had treatment interruptions because of neutropenia and none of the cytopenia events led to treatment withdrawal.
The favorable toxicity profile observed in this study is consistent with zanubrutinib’s greater selectivity for BTK compared with earlier-generation BTK inhibitors.24 By comparison, a phase 2 ibrutinib study reported grade ≥3 infections in 22%, atrial fibrillation in 8%, grade ≥3 bleeding events in 3%, and serious AEs in 46% of patients; 17% of patients discontinued treatment because of AEs.7 These findings are consistent with the results of a pooled analysis of 2 head-to-head studies of zanubrutinib and ibrutinib for the treatment of B-cell malignancies, which demonstrated a fourfold lower incidence of atrial fibrillation in patients receiving zanubrutinib vs those receiving ibrutinib.8 In addition, the response rates for zanubrutinib in this study also compare favorably with ibrutinib (ORR 68% vs 48%, respectively; CR rate of 26% vs 3%, by independent review).6
Certain limitations should be considered when interpreting the MAGNOLIA study data. The single-arm design of the study, as well as the small number of patients in some subgroups, may limit efficacy and safety comparisons with similar research. Nevertheless, the consistently high response rates seen across MZL subtypes and prespecified subgroups support the primary findings.
Conclusions
The final analysis of the MAGNOLIA study shows that, in patients with R/R MZL, zanubrutinib provides durable disease control with a favorable safety profile. Compared with the primary findings, there were no additional late-onset toxicities and no new safety signals observed after longer treatment duration and follow-up. The recent approval of zanubrutinib offers a new treatment option for patients with R/R MZL.
Conflict-of-interest disclosure: S.O. has acted as a consultant/adviser for AbbVie, BeiGene, Janssen, Gilead, Roche, Mundipharma, Merck, and Bristol Myers Squibb; has received research funding from AbbVie, BeiGene, Janssen, Gilead, Roche, and Epizyme; and has received honoraria from AbbVie, BeiGene, Janssen, Gilead, Roche, Merck, and Bristol Myers Squibb. A.T. reports consulting fees from AbbVie, AstraZeneca, and BeiGene; payment of honoraria for lectures, presentations, speaker’s bureaus, and manuscript writing of educational events from AbbVie, AstraZeneca, and BeiGene; and support for attending meetings and/or travel from Janssen, AstraZeneca, and AbbVie. B.H. reports receiving consulting fees from BeiGene, ADC Therapeutics, Janssen, and Incyte. K.M.L. reports payment for expert testimony at the National Institute for Health and Care Excellence Office for Market Access (Zanubrutinib in MZL) and as a member of BeiGene consultancy advisory boards. P.M. reports consulting fees from AbbVie, AstraZeneca, BeiGene, Celgene/ Bristol Myers Squibb, Epizyme, Gilead/Kite, Incyte, Janssen, Roche, and Takeda; payment of honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Gilead/Kite, Incyte, and Janssen; support for attending meetings and/or travel from Takeda and Janssen; and participation in noncommercial trials. P.L.Z. reports consulting fees from Takeda, Roche, Janssen, Merck Sharpe & Dohme, Novartis, Gilead, Kyowa Kirin, Incyte, and BeiGene; and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Takeda, Sanofi, BeiGene, AstraZeneca, Gilead, Novartis, Bristol Myers Squibb, Roche, Kyowa Kirin, Incyte, Janssen, and Merck Sharpe & Dohme. C.T. reports payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Kite, Novartis, Roche, Incyte, AbbVie, Gilead, Roche, and Janssen; and support for attending meetings and/or travel from Kite, Novartis, Roche, Incyte, AbbVie, Gilead, Roche, and Janssen. K.A. reports support for attending meetings and/or travel from Gilead, Novartis, and Bristol Myers Squibb. P.W. reports consulting or advisory roles for BeiGene and Acerta. E.A.H. reports research funding to her institution from Roche, Bristol Myers Squibb, Merck KGaA, AstraZeneca, and Merck; and advisory board and speaker fees (institutional or personal) from Roche, Merck Sharpe & Dohme, AstraZeneca, Gilead, Antigene, Novartis, Regeneron, Janssen, and Specialised Therapeutics, outside the submitted work. Z.L., J.X., C.T., R.D., and M. Co are employees of, and own stock in, BeiGene Co, Ltd. J.T. reports research funding to her institution from BeiGene. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers.
This study was supported by research funding from BeiGene (Beijing) Co, Ltd. Medical writing support was funded by BeiGene and provided by Stuart Wakelin and Holly Strausbaugh on behalf of Twist Medical.
The study sponsor confirmed the accuracy of the data and compiled the data for analysis.
Authorship
Contribution: All authors made substantial contributions to the conception of the study, were involved in the analysis and interpretation of the data, drafted or substantively revised the manuscript, and approved the manuscript for submission; S.O., A.T., B.H., K.M.L., P.M., S.L., M. Coleman, P.L.Z., J.J., M.S., M.S.-T., P.B., X.K., C.T., K.A., F.B., P.W., E.A.H., S.-J.H., K.Z., and J.T. (study investigators) were involved in data collection; Z.L., J.X., C.T., R.D., and M. Co (BeiGene employees) were involved in the study design and further contributed to data interpretation and analysis; all authors had access to the data and vouch for its accuracy and completeness, and for adherence to the protocol; and J.T had the final responsibility of submitting the manuscript for publication, and is responsible for the integrity of the work as a whole; J.X. performed the statistical analysis and all authors were responsible for interpreting the data.
Footnotes
On request, and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved, or (2) in programs that have been terminated. Data requests may be submitted to datadisclosure@beigene.com.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2.Cerhan JR, Habermann TM. Epidemiology of marginal zone lymphoma. Ann Lymphoma. 2021;5:1. doi: 10.21037/aol-20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: B-cell lymhomas, version 5. 2022. https://www.nccn.org/guidelines/category_1
- 4.Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaCasce AS, Noy A. Updates to the management of patients with relapsed or refractory indolent follicular and marginal zone lymphomas. J Natl Compr Canc Netw. 2022;20(5.5):578–580. [Google Scholar]
- 6.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–2232. doi: 10.1182/blood-2016-10-747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noy A, de Vos S, Coleman M, et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long-term follow-up and biomarker analysis. Blood Adv. 2020;4(22):5773–5784. doi: 10.1182/bloodadvances.2020003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam CS, Wallis N, Zhang M, et al. Rate of atrial fibrillation in patients with B-cell malignancies who undergo treatment with zanubrutinib. https://epostersonline.com/llm2022/poster/p-040?view=true Poster presented at: Lymphoma, Leukemia, and Myeloma (LLM) Congress; 18 to 22 October 2022; New York, NY.
- 9.European Medicines Agency Imbruvica (ibrutinib) SMPC. https://www.ema.europa.eu/en/medicines/human/EPAR/imbruvica
- 10.Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem. 2019;62(17):7923–7940. doi: 10.1021/acs.jmedchem.9b00687. [DOI] [PubMed] [Google Scholar]
- 11.Opat S, Tedeschi A, Linton K, et al. The MAGNOLIA Trial: zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res. 2021;27(23):6323–6332. doi: 10.1158/1078-0432.CCR-21-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BRUKINSA (zanubrutinib). Prescribing information. BeiGene Ltd. 2023 www.brukinsa.com/prescribing-information.pdf [Google Scholar]
- 13.BeiGene Ltd Press release: BeiGene announces Health Canada approval for BRUKINSA® (zanubrutinib) in relapsed or refractory marginal zone lymphoma. https://ir.beigene.com/news/beigene-announces-health-canada-approval-for-brukinsa-zanubrutinib-in-relapsed-or-refractory-marginal-zone-lymphoma/e02c3862-c6f8-4588-bf56-6411bc77afbc/
- 14.BeiGene Ltd Press release: BeiGene receives European Commission approval for BRUKINSA® (zanubrutinib) for the treatment of adults with marginal zone lymphoma. https://ir.beigene.com/news/beigene-receives-european-commission-approval-for-brukinsa-zanubrutinib-for-the-treatment-of-adults-with-marginal/66b2b907-4d3c-466f-836d-43e225f2a7b2/
- 15.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. 3rd ed. European Organisation for Research and Treatment of Cancer; 2001. EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatarczuch M, Waltham M, Shortt J, et al. Molecular associations of response to the new generation BTK inhibitor zanubrutinib in marginal zone lymphoma. Blood Adv. 2023;7(14):3531–3539. doi: 10.1182/bloodadvances.2022009412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thieblemont C, Cascione L, Conconi A, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409–1417. doi: 10.1182/blood-2017-03-771915. [DOI] [PubMed] [Google Scholar]
- 20.Cheah CY, Zucca E, Rossi D, Habermann TM. Marginal zone lymphoma: present status and future perspectives. Haematologica. 2022;107(1):35–43. doi: 10.3324/haematol.2021.278755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denlinger NM, Epperla N, William BM. Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res. 2018;10:615–624. doi: 10.2147/CMAR.S133291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37(14):1188–1199. doi: 10.1200/JCO.19.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andorsky DJ, Coleman M, Yacoub A, et al. MAGNIFY: phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2020;38(15 suppl):8046. https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.8046 Abstract 8046. [Google Scholar]
- 24.Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi: 10.1182/blood.2019001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.