Summary
Pyroptosis is a type of regulated necrosis executed by gasdermin. Osmotic cell swelling and membrane perforation are the key features of pyroptosis. This protocol presents time-lapse imaging of morphological changes during pyroptosis using a confocal microscope. We describe the step-by-step ectopic expression of gasdermin, cell staining with nuclear and membrane probes, and visualization of pyroptosis by time-lapse imaging. This protocol is applicable to monitoring pyroptosis in various situations.
For complete details on the use and execution of this protocol, please refer to Qin et al. (2023).1
Subject areas: Cell Biology, Cell-based Assays, Immunology, Microscopy
Graphical abstract

Highlights
-
•
Time-lapse imaging illustrates cell osmotic swelling during pyroptosis
-
•
Cell nuclear and membrane staining demonstrate membrane perforation during pyroptosis
-
•
This protocol is applicable to imaging pyroptosis under different situations
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Pyroptosis is a type of regulated necrosis executed by gasdermin. Osmotic cell swelling and membrane perforation are the key features of pyroptosis. This protocol presents time-lapse imaging of morphological changes during pyroptosis using a confocal microscope. We describe the step-by-step ectopic expression of gasdermin, cell staining with nuclear and membrane probes, and visualization of pyroptosis by time-lapse imaging. This protocol is applicable to monitoring pyroptosis in various situations.
Before you begin
Pyroptosis is a type of programmed necrosis executed by gasdermin (GSDM) family members.2 During pyroptosis, gasdermin is cleaved by protease and releases the pore-forming N-terminal (NT) fragment, which perforates the cell membrane and induces osmotic lysis. For the inflammasome-mediated pyroptosis, the canonical and non-canonical inflammasome pathways activate caspase-1/4/5/11, which cleave gasdermin D (GSDMD) to produce the pyroptosis-inducing NT fragment.3,4,5 Different from GSDMD, gasdermin E (GSDME) is cleaved by caspase-3 and granzyme B to release the NT fragment that initiates pyroptosis.6,7,8 In this protocol, we use a GSDME from Pacific abalone Haliotis discus for the induction of pyroptosis,1 and describe step-by-step GSDME ectopic expression, cell staining and time-lapse imaging of pyroptosis by confocal microscopy. Before starting the experiment, the GSDME NT (GSDME-NT) domain, which possesses pyroptosis-inducing activity, is cloned into a mammalian expression vector pmCherry-N1. In this protocol, the immortalized human embryonic kidney (HEK293T) cells are used for the ectopic expression of GSDME-NT. Besides HEK293T cells, we have also used HeLa cells for ectopic expression and time-lapse imaging of pyroptosis. Since time-lapse imaging of pyroptosis usually lasts 2–3 days, the researchers should have a confocal microscope equipped with an environmental control chamber to maintain 37°C and humidified 5% CO2 to support cell growth. We also recommend to prepare the commercially available nuclear dyes (e.g., Sytox Green) and inner membrane fluorescent probes (e.g., Annexin V), which are often used to observe membrane perforation and osmotic swelling during pyroptosis. This protocol is also useful to examine pyroptosis in other situations, such as infection, cancer and inflammatory diseases.
Preparation of cells
Timing: 5–7 days
-
1.
Thaw the frozen HEK293T cells rapidly in a 37°C water bath. After centrifugation at 500 g at 24°C for 5 min, discard the freezing medium and re-suspend the cells with pre-warmed Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum.
-
2.
Culture the cells in a T-25 flask at 37°C in a 5% CO2 incubator. Grow the cells to 80%–90% confluence, and culture the cells at least two passages to reach the exponential growing phase.
Endotoxin-free plasmid DNA purification
Timing: 2 days
-
3.
Grow the Escherichia coli (E. coli) Trelief 5α cells that harbor the recombinant pmCherry-N1-GSDME-NT expression vector in Luria-Bertani broth supplemented with 50 μg/mL of kanamycin and shaken at 220 rpm at 37°C for 12–17 h.
-
4.
Pellet the E. coli cells by centrifugation at 3000 g at 24°C for 15 min.
-
5.
Obtain the endotoxin-free plasmid DNA (endotoxin < 0.1 EU/μg plasmid DNA) by using Endo-Free Plasmid DNA Kit (OMEGA) according to manufacturer’s instruction (http://www.omegabiotek.com.cn/template/productShow.aspx?m=129002&i=100000012774922).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli Trelief 5α | Tsingke Biological Technology | Cat.#TSC01 |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s modified Eagle’s medium | Corning | Cat.#10-013-CVRC |
| Fetal bovine serum | Sigma-Aldrich | Cat.#F8687 |
| Trypsin-EDTA | Sigma-Aldrich | Cat.#SM-2003 |
| PolyJet transfection reagent | SignaGen Laboratories | Cat.#L100688 |
| Annexin V Alexa Fluor 647 | Thermo Fisher Scientific | Cat.#R37175 |
| Sytox Green | Thermo Fisher Scientific | Cat.#S7020 |
| CaCl2 | Sigma-Aldrich | Cat.#C5670 |
| Critical commercial assays | ||
| Endo-Free plasmid DNA kit | OMEGA | Cat.#D6950-02 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | Cat.#CRL-3216 |
| Recombinant DNA | ||
| pmCherry-N1-GSDME-NT | This paper | N/A |
| Software and algorithms | ||
| Zen | Zeiss | http://www.zeiss.com |
| Other | ||
| LSM710 confocal microscope system equipped with environmental control chamber | Zeiss | http://www.zeiss.com |
| Steri cycle CO2 incubator | Thermo Fisher Scientific | https://www.thermofisher.com/ |
| NanoPhotometer N60 | Implen Spectrophotometer | https://www.implen.de/ |
| Eppendorf 5424 | Eppendorf | https://www.eppendorf.com |
| 35-mm glass-bottom dish | Corning | Cat.#801002 |
| T-25 flask | Corning | Cat.#3289 |
Step-by-step method details
Ectopic expression of GSDME-NT
Timing: 1 day
-
1.
Digest the exponentially growing HEK293T cells by trypsin-EDTA to single cells. Plate the cells (approximately 5 ×105 cells) in 35-mm glass-bottomed culture dishes for 12–17 h to reach 60%–70% confluency before transfection.
-
2.
Discard the medium, and add fresh DMEM (supplemented with 10% FBS) to the cells at 30 min before transfection.
-
3.Prepare the transfection complex by using PolyJet transfection reagent.
-
a.Gently dilute the plasmid DNA (1 μg) and PolyJet transfection reagent (3 μL) with 50 μL serum-free DMEM.
-
b.Add the diluted transfection reagent to the diluted plasmid DNA and mix gently.
-
c.Incubate the mixture for 15 min to prepare the transfection complex.
-
a.
-
4.
Add the transfection complex drop-wise to the cells and gently swirl the cell culture dishes.
-
5.
After transfection for 24 h, check the expression level of GSDME-NT using a fluorescence microscope.
Alternatives: Some other commercially available transfection reagents, e.g., lipofectamine 3000 transfection reagent (Thermo Fisher Scientific), can also be used for cell transfection. In addition, HeLa cells can also be used for the ectopic expression of GSDME-NT.
CRITICAL: To prepare the transfection complex for the negative control transfection, add the diluted transfection reagent to serum-free DMEM and incubate the mixture as described in step 3.
CRITICAL: Because HEK293T cells are semi-adhesive, all steps, including medium replacement, transfection, cell staining, and microscopic observation, should be careful to avoid cell detachment or damage.
Staining of cell nucleus and membrane
Timing: 1 h
-
6.
Dilute Sytox Green and Annexin V-Alexa Fluor 647 in the cell culture medium to the final concentration of 1 μM (Sytox Green) or add one drop Annexin V-Alexa Fluor 647 solution per 105 cells according to the manufacturer’s instruction (https://www.thermofisher.com/order/catalog/product/R37175).
-
7.
Incubate the cells with the Sytox Green and Annexin V-Alexa Fluor 647 at 37°C in dark for 15 min.
CRITICAL: Due to the calcium-dependent binding to phosphatidylserine, add sterile CaCl2 stock solution to the cell culture medium to reach a final concentration of 2.5 mM before Annexin V staining.
Time-lapse imaging of pyroptosis
Timing: 3–4 days
-
8.
Start the LSM710 confocal microscope, and pre-warm the laser source.
-
9.
Equip the microscope with an environmental control chamber. Warm up the environment chamber to 37°C, and maintain a supply of humidified 5% CO2.
-
10.
Place the cell culture dish into the environmental control chamber.
-
11.
Choose the desired objective for cell imaging. Typically, 40 × (NA1.2) magnification objective is preferred.
-
12.
Locate a view field of interest and then go to the software operation panel. Choose the fluorescence channels and pseudo-colors that match the dyes used in the experiment. To view the brightfield image, select the channel corresponding to brightfield.
-
13.
Adjust the z-axis to get a clear view of interest. Set the excitation laser power, and optimize the gain of fluorescence to avoid low fluorescent intensity or overexposure. The “Pinhole” for each fluorescent channel is set at 1 Airy Unit (AU). Adjust the gain and offset of each fluorescent channel to as required before imaging.
-
14.
Take 512 × 512 time-lapse images at 3–5 min intervals for a total of 7 h or longer.
-
15.
Save the time series images, and represent the images as “Contents of Image window-series” or “Full resolution image window-series”. Adjust frames per second and export the time-lapse imaging video. The expected outcome could be the Method video S1.
Alternatives: The confocal microscope from Leica and Nikon can be used as an alternative to that of Zeiss.
CRITICAL: Warm up the environmental control chamber and supply humidified 5% CO2 at least 1 h before imaging.
CRITICAL: The concentrations of the fluorescent dyes/probes and the gain and offset of each fluorescent channel should be adjusted by using positive and negative control before time-lapse imaging.
CRITICAL: Repeat time-lapse imaging (steps 11–15) to generate a high-quality video.
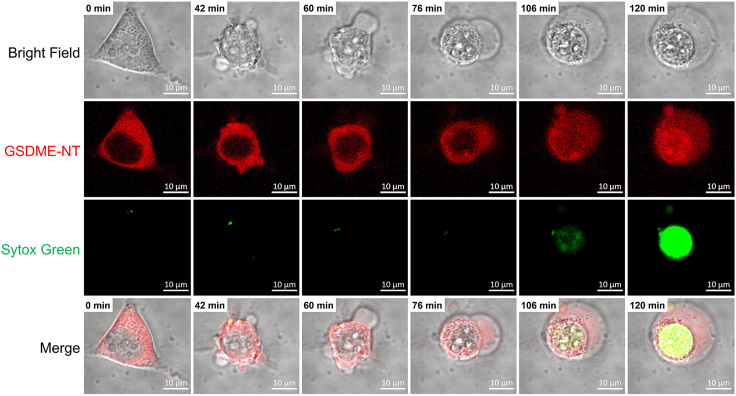
GSDME-NT and Sytox Green are indicated by red and green colors, respectively. Scale bar: 10 μm.
Expected outcomes
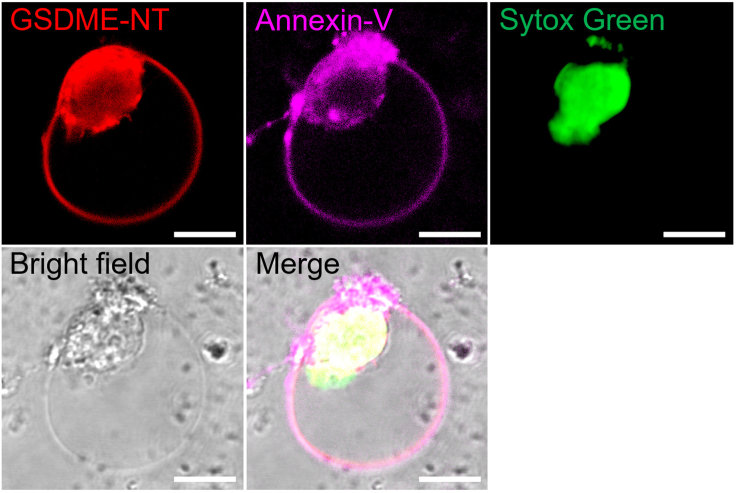
Pyroptosis is an inflammatory type of regulated necrotic cell death.9 GSDM executes pyroptosis via its NT pore-forming domain.3,4,5 Cell swelling and perforation are the hall-markers of pyroptosis induced by GSDM-NT.10 In this protocol, we transfect HEK293T cells with the vector expressing abalone GSDME-NT, which possesses robust pyroptosis-inducing activity.1 Compared with the negative control, abalone GSDME-NT is expressed in cells and could translocate to the cytoplasmic membrane (Figures 1 and S1), and induces rapid cell membrane perforation and osmotic swelling (Figure 2, Method video S1), characterized by Sytox Green uptake and Annexin V staining (Figures 1 and 2). This protocol can be used to determine the pyroptosis-inducing activity of GSDM fragments or GSDM homologs, and can also be adapted for imaging pyroptosis under pathological conditions.
Figure 1.
Morphological characteristics of a pyroptotic cell expressing abalone GSDME-NT
HEK293T was transfected with the vector expressing mCherry tagged abalone GSDME-NT for 24 h. The cells were then stained with Sytox Green and Alexa Fluor 647-Annexin V. Images were captured with a confocal microscope. Scale bar: 10 μm.
Figure 2.
Time-lapse imaging of a cell expressing abalone GSDME-NT
mCherry tagged GSDME-NT was overexpressed in HEK293T cells. After transfection for 24 h, the cells were incubated with Sytox Green, and the time-lapse images of pyroptosis were captured with a confocal microscope. Scale bar: 10 μm.
Limitations
This step-by-step protocol for time-lapse imaging of pyroptosis is straightforward to visualize rapid membrane swelling and perforation, which are typical morphological features of pyroptotic cell death. Although this protocol is suitable for imaging pyroptosis under different conditions, several issues need to be considered.
First, the time-lapse imaging is time consuming and usually takes 3–4 days to acquire high-qualified images. For the canonical inflammasome activated pyroptosis, detecting the inflammasome (e.g., NLRP3) and ASC speck assembly is a faster way to study the early stage of pyroptosis.11 Second, an environmental control chamber with accurate control of heat and CO2 is required to support the long-term survival of cells, and the chamber should be germ-free during imaging. Third, the concentration of the fluorescent dye/probe and the gain and offset of each fluorescent channel should be adjusted by using positive and negative controls before imaging. Fourth, the release of lactate dehydrogenase and generation of pore-forming GSDM-NT are other key markers of pyroptosis, and can serve as complementary evidences to the occurrence of pyroptosis.
Troubleshooting
Problem 1
The cultured cells are contaminated with microbes.
Potential solution
-
•
Always wear clean gloves and lab coat, and use sterile labware during the experiment (all steps mentioned in this protocol except for image/video processing).
-
•
Clean the environmental control chamber of the confocal microscope with 70% ethanol or other disinfectants before imaging (step 9).
-
•
Minimize the exposure of cells placed in non-sterile conditions (steps 6, 7 and 10).
-
•
The fluorescent probes or reagents should be filtered through 0.22 μm sterile filters to remove bacteria and fungi (steps 6 and 7).
-
•
Add antibiotics, such as penicillin and streptomycin, to the cell culture to prevent bacterial contamination. Anti-mycoplasma reagents, for example plasmocin (Invivogen), can also be used to prevent mycoplasma contamination (step 2).
Problem 2
The dead cells fluctuate and cause focus drift during time-lapse imaging.
Potential solution
Adjust the z-axis to re-focus on the pyroptotic cells during imaging. Autofocus devices, such as Definite Focus (Carl Zeiss), are also recommended to maintain stable focus during imaging (step 14).
Problem 3
The fluorescence is quenched during imaging.
Potential solution
Use stable fluorescent protein tags, such as enhanced green fluorescent protein (EGFP) and mCherry, to the target protein during time-lapse imaging. For cell nucleus or membrane staining, photostable reagents or conjugates should be used (steps 6, 7).
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Li Sun (lsun@qdio.ac.cn).
Materials availability
Plasmids generated in this study will be made available on request to the lead contact. This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze any datasets or code.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (42276115), the Science & Technology Innovation Project of Laoshan Laboratory (LSKJ202203000), the Key Deployment Project of Centre for Ocean Mega-Research of Science (no. COMS2020Q03), the Taishan Scholar Program of Shandong Province (2018 and 2021), and the Youth Innovation Promotion Association CAS (2021204).
Author contributions
S.J. and L.S. conceived the idea, designed the study, and obtained the funding. S.J. and K.Q. conducted the experiments and analyzed the data. S.J. wrote the first draft of the manuscript. L.S. revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102708.
Contributor Information
Shuai Jiang, Email: sjiang@qdio.ac.cn.
Li Sun, Email: lsun@qdio.ac.cn.
Supplemental information
References
- 1.Qin K., Jiang S., Xu H., Yuan Z., Sun L. Pyroptotic gasdermin exists in Mollusca and is vital to eliminating bacterial infection. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112414. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs S.B., Miao E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 4.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 5.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.H., Zhong C.Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., Wang K., Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Zhang Y., Xia S., Kong Q., Li S., Liu X., Junqueira C., Meza-Sosa K.F., Mok T.M.Y., Ansara J., et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8 doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 11.Dong X., Chen L.F. Protocol for measuring NLRC4 inflammasome activation and pyroptosis in murine bone-marrow-derived macrophages. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GSDME-NT and Sytox Green are indicated by red and green colors, respectively. Scale bar: 10 μm.
Data Availability Statement
This study did not generate/analyze any datasets or code.