Abstract
Introduction
As the only market-authorized allergen immunotherapy (AIT) for peanut allergy is accompanied by a high risk of side effects and mainly induces robust desensitization without sustained efficacy, novel treatment options are required. Peanut-specific plant-derived eBioparticles (eBPs) surface expressing Ara h 2 at high density have been shown to be very hypoallergenic. Here, we assessed the dendritic cell (DC)-activating and T cell polarization capacity of these peanut-specific eBPs.
Methods
Route and kinetics of eBP uptake were studied by (imaging) flow cytometry using monocyte-derived DCs incubated with fluorescently-labelled Ara h 2 eBPs or natural Ara h 2 (nAra h 2) in the presence or absence of inhibitors that block pathways involved in macropinocytosis, phagocytosis, and/or receptor-mediated uptake. DC activation was monitored by flow cytometry (maturation marker expression) and ELISA (cytokine production). T cell polarization was assessed by co-culturing DCs exposed to Ara h 2 eBPs or nAra h 2 with naïve CD4+ T cells, followed by flow cytometry assessment of intracellular IFNγ+ (Th1) and IL-13+ (Th2), and CD25+CD127-Foxp3+ regulatory T cells (Tregs). The suppressive activity of Tregs was tested using a suppressor assay.
Results
Ara h 2 eBPs were taken up by DCs through actin-dependent pathways. They activated DCs demonstrated by an induced expression of CD83 and CD86, and production of TNFα, IL-6, and IL-10. eBP-treated DCs polarized naïve CD4+ T cells towards Th1 cells, while reducing Th2 cell development. Furthermore, eBP-treated DCs induced reduced the frequency of Foxp3+ Tregs but did not significantly affect T cell IL-10 production or T cells with suppressive capacity. In contrast, DC activation and Th1 cell polarization were not observed for nAra h 2.
Conclusion
Ara h 2 eBPs activate DCs that subsequently promote Th1 cell polarization and reduce Th2 cell polarization. These characteristics mark Ara h 2 eBPs as a promising novel candidate for peanut AIT.
Keywords: Peanut allergy, Allergen immunotherapy, Dendritic cell, T cell, Microparticle
Introduction
Allergen immunotherapy (AIT) is still the only treatment for IgE-mediated hypersensitivity that modulates allergen-specific immune responses to a tolerogenic and desensitized state. Successful desensitization is accompanied by a reduction in allergen-specific memory Th2 cells, induction of regulatory T and B cells, and production of allergen-specific IgG4.1, 2, 3, 4, 5, 6 Recently, the first AIT for peanut allergy, received market authorization to treat patients from 4 to 17 years of age using oral administration.7 Interestingly, adult patients showed no significant improvement in a phase III trial (PALISADE study), thereby limiting the group of patients that can be helped with this approach.8 Furthermore, for oral immunotherapy (OIT) there is no convincing evidence of sustained efficacy, treatment can take up to 3 to 5 years, and there is a significant risk of developing severe side effects.9, 10, 11, 12 Therefore, novel treatment strategies for peanut allergy are needed.
Besides OIT, alternative routes of administration are being studied for peanut allergy AIT, eg, epicutaneous immunotherapy (EPIT) in the form of a skin patch for non-invasive and continuous delivery of the allergens, and sublingual immunotherapy (SLIT) with drops or tablets.13, 14, 15 Additionally, other novel approaches are being explored. For example, toll-like receptor 4 (TLR-4) agonist monophosphoryl lipid A (MPLA), received market authorization to treat grass and tree pollen allergy. For peanut allergy, another TLR-4 agonist, glucopyranosyl lipid A, is currently being tested as an adjuvant therapy to peanut SLIT (phase I, NCT03463135). Vaccine approaches for peanut allergy, using micro- or nanoparticles, include a DNA vaccine targeting peanut allergens to lysosomes to enhance immunogenicity (phase I, NCT02851277; phase I, NCT03755713), PLGA particles loaded with purified peanut extract (phase II, NCT05250856), or Cucumber Mosaic Virus-derived (CuMVtt) virus-like particles (VLPs) coupled to either Ara h 1 or Ara h 2.16
Recently, a new micro-particulate platform has been introduced, namely, enveloped plant-based Bioparticles (eBPs). These eBPs express approximately 3000 copies of recombinant allergen on the surface of the eBPs in a trimeric conformation.17 Previously, it has been demonstrated that peanut Ara h 2 and cat Fel d 1 eBPs are very hypoallergenic in functional assays with basophils and mast cells.18,19 Furthermore, house dust mite Der p 2 eBPs induced stronger IgG responses in mice, with increased Th1-associated IgG2a, compared to alum-absorbed Der p 2.17 Moreover, Fel d 1 eBPs, but not soluble Fel d 1, upregulated the expression of maturation markers (CD80, CD83, CD86) and induced cytokine production (IL-6, IL-10, IL-12p70) in human monocyte-derived dendritic cells (DCs).19
In this study, we investigated the DC and T cell modulating capacity of Ara h 2 eBPs. Ara h 2 was selected as the antigen, as it is 1 of the major peanut allergens and has the highest potential to cross-link IgE and subsequently activate allergy effector cells.20, 21, 22, 23, 24, 25 Here, we studied uptake of Ara h 2 eBPs by moDCs and their capacity to activate these antigen-presenting cells. Furthermore we showed that Ara h 2 eBPs modulate T cell responses that favour Th1 cell development and reduce Th2 cell polarization, a novel finding, as Th1 polarization mediated by the eBPs was previously only suggested based on IgG2a responses in mice and IL-12p70 induction in human moDCs.17,19 Together with the exceptional hypo-allergenicity of Ara h 2 eBPs, as determined by IgE-induced effector responses, these data mark Ara h 2 eBPs as a promising therapeutic candidate for peanut allergy and provide a solid foundation for clinical studies.18
Methods
Ara h 2-eBioparticle production and quantification
Ara h 2 eBPs were generated as described before.17,18 In short, Nicotiana benthamiana was transfected with Agrobacterium tumefaciens carrying Ara h 2 cDNA linked to oligomerization and membrane sequences (fusion protein sequence in Supplementary Fig. 1). Quantification of Ara h 2 was performed by immune-slot blot using an in-house polyclonal rabbit anti-nAra h 2 serum and by IgE dot-blot using serum from an Ara h 2-sensitized patient. Dilutions of Ara h 2 eBPs were compared by densitometric scanning to a standard curve of titrated natural purified Ara h 2 (nAra h 2; Indoor Biotechnologies, Cardiff, Wales, UK) that had been quantified by a BCA protein assay (Pierce, Thermo Fisher Scientific, Waltham, MA, USA). The size of the eBPs was determined by dynamic light scattering (DLS) using a NanoZS Zetasizer (Malvern Ltd., Malvern, UK), measuring the Z-average diameter (Zave) and polydispersity index (PdI). The surface charge of the eBPs was measured on the same instrument using laser Doppler electrophoresis. Endotoxin levels were measured using a LAL quantification kit (Thermo Scientific, IL, USA) according to the manufacturer's instructions. Bacterial contamination was determined by plating 50 μL of eBPs on yeast extract beef (YEB) medium, followed by incubation at 28 °C for 48 h, after which the colony-forming unit (CFU) was determined.
Fluorescent labelling of Ara h 2 eBioparticle and nAra h 2
For labeling, the pH of either the Ara h 2 eBP or nAra h 2 (Indoor) was adjusted to 9 with a bicarbonate buffer (1 M NaHCO3, pH = 9). Fluorescein isothiocyanate isomer I (FITC; Sigma-Aldrich, St Louis, MO, USA) in DMSO (10 mg/mL) was added to the allergens in a molar ratio 1:25 (Ara h 2:FITC). After overnight incubation at 4°C, unbound fluorophore was removed from nAra h 2 with a PD-10 desalting column (Cytiva, Marlborough, MA, USA) following the manufacturer's instructions. For the Ara h 2 eBPs, unbound FITC was removed by repeated centrifugation (8 h, 3600g, 4 °C).
Monocyte-derived dendritic cell generation
Monocyte-derived dendritic cells (moDCs) were differentiated from peripheral blood monocytes obtained from fresh blood using density gradient centrifugation on Lymphoprep (d = 1.077 ± 0.001 g/mL; Serum Bernburg AG, Bernburg, Germany), as described elsewhere.26 In short, monocytes were isolated from PBMCs using density gradient centrifugation on Percoll (d = 1.130 g/mL; GE Healthcare, Chicago, IL, USA). Immature moDCs were generated by culturing monocytes for 6 days in Iscove's Modified Dulbecco's Medium (IMDM; BioWhittaker, Lonza, Basel, Switzerland) supplemented with gentamicin (86 μg/L; Duchefa, Haarlem, The Netherlands), GM-CSF (500 U/mL; Schering-Plough, NY, USA), IL-4 (10 U/mL; Miltenyi Biotec, Gladbach, Germany), and 5% fetal bovine serum (FBS; Sigma-Aldrich).
Ara h 2-eBioparticle internalization
Immature moDCs were harvested after 15–30 min incubation on ice. To evaluate uptake kinetics, 50.000 moDCs were incubated with 10 μg/mL fluorescently labelled nAra h 2 or Ara h 2 eBPs for 15, 30, 60, 120, 240, or 360 min in IMDM 5% FBS, either at 4°C or 37°C. To evaluate the mode of uptake, 50 000 moDCs were pre-incubated for 1 h at 37 °C with inhibitors (all from Sigma-Aldrich) for macropinocytosis (5, 25, or 50 μM imipramine hydrochloride), actin-polymerization (0.1, 1.0 or 10 μM cytochalasin D), clathrin-mediated endocytosis (2.5, 5.0.10 μM chlorpromazine hydrochloride), and calcium dependent uptake (0.5, 1.0, or 2.5 mM EDTA). Followed by 1 h incubation at 37 °C using 10 μg/mL fluorescently-labelled Ara h 2 eBPs. Natural Ara h 2 and Ara h 2 eBP uptake was measured with the FACSCanto cell analyser (BD Biosciences, Franklin Lake, NJ, USA).
Internalization was determined by acquiring the sample on the Amnis ImageStreamX MK II (Luminex Corporation, Austin, TX, USA). Here, 300 000–500 000 moDCs were incubated with fluorescently-labelled Ara h 2 eBPs for 15, 30, 60, 120, and 240 min in IMDM 5% FBS, either at 4°C or 37C. For each sample 20 000 cells were recorded at a flow rate between 50 and 100 cells/second at 60x magnification. Data were analyzed using the software package IDEAS (Amnis). Cells were gated as follows: single cells > in focus cells > non-saturated cells (Supplementary Fig. 2). Internalization was calculated using the bright field images of the cells to apply a morphological mask representing the inside of the cell. The IDEAS software calculated the internalization score based on the log-scale ratio between the mean fluorescent intensity (MFI) of the FITC signal falling inside the morphological mask, divided by the MFI of the FITC signal in the whole cell. The following formula was used:
i = input mask, β = area of the segmentation mask outside the input mask i, Mi = mean intensity of upper quartile pixels in i, Mβ = mean intensity of upper quartile pixels in β, Pi = peak intensity of upper quartile pixels in i, Pβ = peak intensity of upper quartile pixels in β.
moDC cytokine production with and without TLR-4 blocking
There were 30 000–50 000 immature moDCs stimulated with 1 of the following components alone or in combination: 1, 3, or 10 μg/mL Ara h 2 eBPs, 1, 3, or 10 μg/mL nAra h 2, and 10 ng/mL E.coli LPS (strain 0111-B4; Sigma-Aldrich). After 24 h, supernatants were collected, and IL-6, Il-10, and TNFα were measured by ELISA. To assess TLR-4 involvement, moDCs were pre-incubated with an TLR-4 blocking antibody (clone 7E3, mouse IgG1, Hycult Biotech, Noord-Brabant, The Netherlands), an isotype antibody (MOPC-21, mouse IgG1, Hycult), or with medium for 30 min in IMDM 5% FBS at 37°C.
moDC maturation and T cell co-cultures
moDCs were matured by culturing them for 48 h in the presence of medium, 10 μg/mL Ara h 2 eBPs, 10 μg/mL nAra h 2, in the presence or absence of 10 ng/mL E.coli LPS. Mature controls were stimulated with maturation factors (MF; 25 ng/mL TNFα (Miltenyi) and 10 ng/mL Il-1β (Miltenyi)) and 100 ng/mL E.coli LPS. DCs were stained using anti-CD83-APC (clone HB1e, mouse IgG1, BD Biosciences, NJ, USA), anti-CD86-PE (clone 2331, mouse IgG1, BD Biosciences), and anti-HLA-DR-PerCP (clone L243, mouse IgG2a, BD Biosciences). Maturation was assessed using flow cytometry performed with the FACS Canto II (BD Biosciences).
To assess T cell polarization and induction of regulatory T cells, 5000 matured moDCs were co-cultured with 20 000 allogeneic naïve CD4 T cells (isolated from buffy coats using a CD4 T cell isolation kit (Miltenyi), LS Columns, and a MACS Separator) in IMDM 10% FBS and 10 pg/mL Staphylococcal enterotoxin B (SEB; Toxin Technology Inc., FL, USA). T cells were kept in IMDM 10% FBS supplemented with 10 U/mL human recombinant IL-2 (Novartis AG, Basel, Switzerland) till they were resting. To determine IL-10 production, 100.000 T cells were restimulated with anti-CD3 and anti-CD28 for 48 h in IMDM 10% FCS. IL-10 was measured in the supernatant by ELISA. To determine regulatory T cell induction, T cells were stained with anti-CD25-FITC (clone 2A3, mouse IgG1, BD Biosciences), anti-CD127-PE (clone hIL-7R-M2, mouse IgG1, BD Biosciences), and anti-Foxp3-AF647 (clone 259D, mouse IgG1, Biolegend, CA, USA) using a Transcription Factor Buffer Set (BD Biosciences) for fixation and permeabilization. To determine T cell polarization, T cells were restimulated for 5 h with 10 ng/mL phorbol-12-myristate 13-acetate (PMA; Sigma-Aldrich), 1 μg/mL ionomycin (Sigma-Aldrich), and 10 μg/mL brefeldin A (Sigma-Aldrich) in IMDM 10% FBS. T cells were fixed with 3.7% formaldehyde (Sigma-Aldrich) in PBS, permeabilized with saponin (Sigma-Aldrich), and stained with anti-IFNγ-FITC (clone 25723.11, mouse IgG2b, BD Biosciences) and anti-IL-13-PE (clone JES10-5A2, rat IgG1, BD Biosciences). All flow cytometry samples were acquired on the FACS Canto II (BD Biosciences).
Suppressor assay
To assess the suppressive activity of the T cells, 30 000 matured moDCs (as described before, additional control: moDCs matured with MF, 100 ng/mL LPS and 2.5 μM 1α,25-dihydroxyvitamin D3 (VitD3; Sigma-Aldrich)) were co-cultured with 300 000 allogeneic naïve CD4 T cells in IMDM 10% FBS. After 5 days, T cells were harvested and irradiated with 30 Gy (CellRad Benchtop X-Ray Irradiation, CT, USA). There were 25 000 allogeneic CFSE-labelled memory CD4 T cells (isolated from buffy coat) co-cultured with 50 000 irradiated T cells and 1500 mature moDCS (matured with MF + 100 ng/mL LPS). After 5 days, T cell proliferation was determined with flow cytometry on the FACS Canto II (BD Biosciences).
HEK293 cell culture and stimulation
Human Embryonic Kidney (HEK) 293 cells expressing either CD14 or CD14 and TLR-4 were kindly donated by Prof. Dr. H.H. Smits, Department of Parasitology, Leiden University Center for Infectious Diseases, Leiden University Medical Center, Leiden, the Netherlands. HEK293 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco) 10% FBS, supplemented with 10 μg/mL ciprofloxacin (Thermo Scientific) and 5 μg/mL puromycin (InvivoGen, CA, USA). To assess TLR-4 signaling 50 000 HEK293 cells were stimulated with E.coli LPS, or Ara h 2 eBPs, in the presence of 12.5% myeloid differentiation factor 2. Supernatants were collected after 24 h and IL-8 production was determined by ELISA.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.3.1 (GraphPad Software, Inc., San Diego, CA, USA). The shown p-values were derived from the mixed effect analysis (Fig. 1A and B; Fig. 3A, B, C, D; Fig. 4C and D), the Friedman test (Fig. 1F; Fig. 2A; Fig. 3E and F; Supplementary Fig. 3A and B), and the two-way ANOVA (Fig. 2B; Fig. 4B).
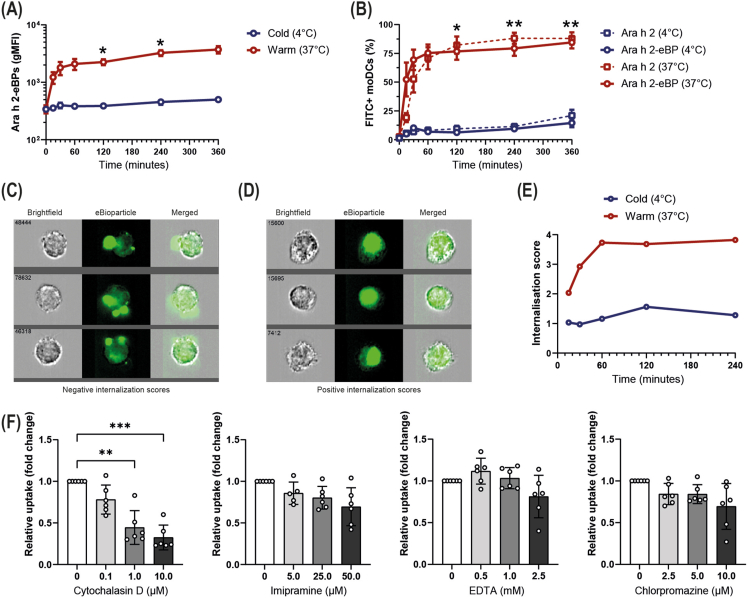
Fig. 1.
Uptake of Ara h 2 eBioparticles by dendritic cells. FITC-labelled eBPs internalization by DCs was assessed using either imaging flow cytometry by determining the internalization score or traditional flow cytometry, determining the geometric mean fluorescent intensity (gMFI) or the % of cells that are FITC-positive. (A) The gMFI of DCs after eBPs exposure over time, either at 4°C or 37C. (n = 3–4, mean ± SEM, mixed effect analysis post-hoc Bonferroni test). (B) The percentage of DCs that have taken up either the Ara h 2 eBP or nAra h 2 over time at 4°C or 37°C. (n = 3–4, mean ± SEM, mixed effect analysis post-hoc Bonferroni test). (C) Example images of DCs having a negative internalization score. (D) Example images of DCs having a positive internalization score. (E) The internalization score of the eBPs over time, either at 4C or 37C. (F) The relative uptake of the eBPs by DCs in the presence of cytochalasin D (inhibiting actin-polymerization), imipramine (inhibiting macropinocytosis), EDTA (capturing extracellular calcium), or chlorpromazine (inhibiting clathrin-mediated endocytosis). (n = 6, mean ± SD, Friedman test). ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001
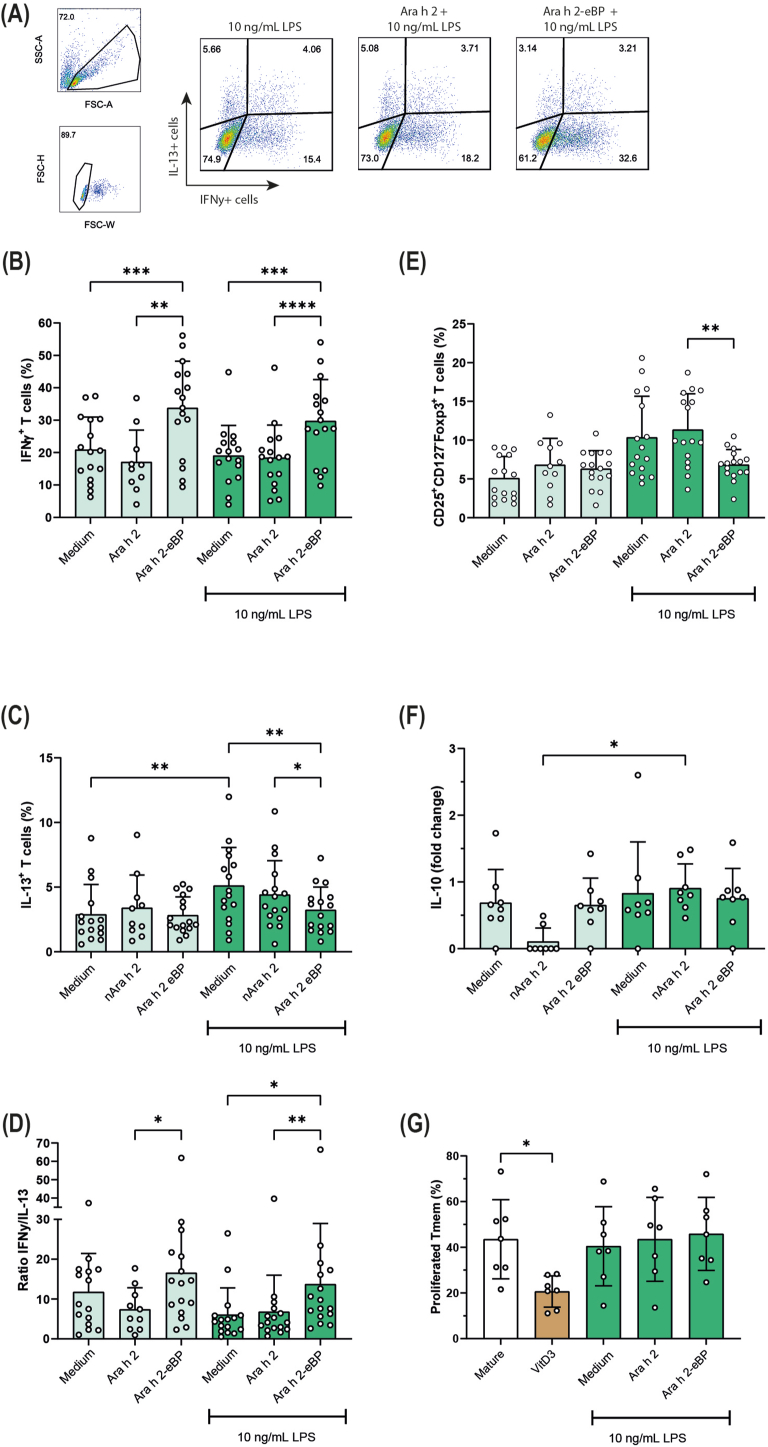
Fig. 3.
Ara h 2 eBioparticles induce Th1 polarization.
DCs were stimulated with medium, nAra h 2, or the Ara h 2 eBP either in the absence or presence 10 ng/mL E.coli LPS, and co-cultured with CD4+ naïve T cells. (A) Flow cytometry gating example of T cell polarization, gating for IL-13+ T cells or IFNγ+ T cells, representing Th2 and Th1 cells, respectively. T cell polarization is depicted as the percentage of IFNγ+ T cells (B), the percentage of IL-13+ T cells (C), and the ratio of IFNγ+/IL-13+ T cells (D) (n = 10–16, mean ± SD, mixed-effect analysis post-hoc Tukey test). Furthermore, the induction of CD25+CD127-Foxp3+ regulatory T cells (n = 11–16, mean ± SD, mixed-effect analysis post-hoc Tukey test) (E), IL-10 production of T cells (n = 8, mean ± SD, Friedman test) (F), and the percentage of proliferation of memory T cells after stimulation with mature DCs and T cells obtained from the co-cultures (suppressor assay; n = 7, mean ± SD, Friedman test) (G) is shown. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, ∗∗∗∗P ≤ 0.0001
Fig. 4.
Dendritic cell activation by Ara h 2 eBioparticles is partly mediated by TLR-4 signaling. (A) Overview of endotoxin content (EU/μg) and bacterial contamination (CFU) of 3 different batches of Ara h 2 eBPs. (B) Il-8 production in HEK-293 cells transfected with CD14 alone or CD14 and TLR-4 together. HEK-293 cells were incubated with E. coli LPS or the Ara h 2 eBP (n = 6, mean ± SD, two-way ANOVA). (C–D) IL-6 and TNFα production by DCs after stimulation with E. coli LPS or Ara h 2 eBPs in the absence or presence of either a blocking TLR-4 antibody or the isotype control (n = 5–6, mean ± SD, mixed-effect analysis post-hoc Tukey test). ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, ∗∗∗∗P ≤ 0.0001
Fig. 2.
Activation of dendritic cells by Ara h 2 eBioparticles. (A) Maturation marker expression expressed in geometric mean fluorescence (gMFI) of DCs incubated with nAra h 2 or the Ara h 2 eBP in the absence of E. coli LPS (n = 8–9, mean ± SD, Friedman test). The DCs in the mature condition were matured with 25 ng/mL TNFα, 10 ng/mL IL-1β, and 100 ng/mL E. coli LPS. (B) Cytokine production by DCs which were incubated with nAra h 2 or the Ara h 2 eBP in the absence of E. coli LPS (n = 4–6, mean ± SD, two-way ANOVA post-hoc Bonferroni test). As a positive control for cytokine production by DCs, 10 ng/mL E.coli LPS was used. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, ∗∗∗∗P ≤ 0.0001
Results
Ara h 2 eBioparticles are taken up by dendritic cells through actin-dependent pathways
To efficiently induce Ara h 2-specific T cell responses, Ara h 2 eBPs need to be taken up by moDCs, followed by Ara h 2 processing and loading on MHC molecules. Previously, it has been shown that uptake efficiency of nano- and microparticulate vehicles depends on their charge and size.27 DLS analysis showed that the Ara h 2 eBPs are slightly negative (zeta potential of −1.34 mV) and have a diameter of ∼160 nm (polydispersity index of 0.21). Flow cytometry analysis demonstrated that Ara h 2 eBPs were taken up by moDCs at 37°C but not at 4°C, indicating that the eBPs are taken up via energy-depending pathways (Fig. 1A). Furthermore, the uptake kinetics of the BP was comparable to natural Ara h 2 (nAra h 2; Fig. 1B). To check whether this increase in fluorescent signal was caused by internalization of the eBPs and not cell membrane adherence, Ara h 2 eBP internalization was assessed with imaging flow cytometry, where we calculated the internalization score. This score is negative upon cell membrane adherence and positive upon internalization of the fluorescent probe (Fig. 1 C, D). The internalization score increased over time (up to 60 min) when moDCs were incubated with Ara h 2 eBPs at 37C, while the internalization score remained stable and around 1 over time at 4°C (Fig. 1E), indicating that the eBPs are efficiently internalized at 37°C. To gain further insights into the processes involved in BP uptake, we studied the route of uptake. MoDCs were pre-incubated with inhibitors targeting molecules required to facilitate macropinocytosis, phagocytosis and receptor-mediated uptake. Ara h 2 eBP uptake was significantly inhibited by cytochalasin D, preventing actin polymerization, required for both macropinocytosis and phagocytosis (Fig. 1F). Although a trend towards a decrease in uptake was observed at the highest dose of imipramine, EDTA, and chlorpromazine, BP uptake was not significantly reduced using these agents that inhibit macropinocytosis, clathrin-mediated endocytosis, or calcium-dependent uptake, respectively (Fig. 1F). Altogether, these data demonstrated that Ara h 2 eBPs are efficiently internalized by moDCs via actin-dependent pathways that mediate phagocytosis.
Ara h 2 eBioparticles induce dendritic cell maturation and cytokine production
Following uptake of eBPs, maturation of DCs is required to allow activation and polarization of naïve T cells. Exposure of moDCs to Ara h 2 eBPs increased expression levels of CD83 and CD86 compared to immature moDCs, reaching similar expression levels as mature moDCs, which were stimulated with maturation factors and 100 ng/mL E.coli LPS (Fig. 2A). Using nAra h 2, no induction of these markers was observed. (Fig. 2A). Analysis of HLA-DR expression demonstrated that immature moDCs of some donors already expressed high levels of HLA-DR comparable to mature moDCs; therefore, no statistically significant increase in HLA-DR expression was observed using the Ara h 2 eBPs (Fig. 2A). Next, we determined whether the Ara h 2 eBPs induced cytokine production. Ara h 2 eBPs-exposed moDCs secreted TNFa, IL-6, and IL-10 in a dose-dependent manner, while nAra h 2 did not stimulate cytokine production (Fig. 2B). Thus, Ara h 2 eBPs induced moDC maturation and cytokine production, while nAra h 2 did not.
Ara h 2 eBioparticles polarize T cell responses to Th1
The primary function of DCs is initiation and regulation of adaptive immune responses. Therefore, we assessed T cell polarization by moDCs that were cultured with Ara h 2 eBPs or nAra h 2. T cell polarization was assessed by intracellular measurement of IFNγ expression, identifying Th1 cells, and IL-13 expression, identifying Th2 cells (Fig. 3A, B, C). T cells stimulated with moDCs exposed to the Ara h 2 eBP showed an increase in the percentage of IFNγ+ T cells and no change in the percentage of IL-13+ T cells compared to moDCs exposed to medium or nAra h 2 (Fig. 3B and C). Even when the moDCs were matured in the presence of 10 ng/mL of LPS, resulting in similar expression levels of maturation markers CD83 and CD86 when exposed to medium, nAra h 2 or Ara h 2 eBPs (Supplementary Fig. 3), the Ara h 2 eBP still increased the number of IFNγ+ T cells (Fig. 3B). In the presence of LPS, the Ara h 2 eBP also significantly reduced the IL-13+ T cells, which was not observed in conditions without LPS (Fig. 3C). This difference may be caused by an increase in the percentage of IL-13+ T cells after stimulation with LPS alone, allowing for IL-13+ T cell suppression with the eBP (Fig. 3C). The net result is an increased ratio of IFNγ+ over IL-13+ T cells, observed either without LPS or in the presence of 10 ng/mL LPS (Fig. 3 D). Additionally, we assessed the number of Foxp3+ Tregs, as they also play an essential role during the process of desensitization. Interestingly, LPS and Ara h 2 eBP-treated moDCs reduced the percentage of CD25+CD127-Foxp3+ T cells compared to the medium control and nAra h 2, which was not observed for the moDCs treated with eBP in the absence of LPS (Fig. 3E). Furthermore, no effects of the eBPs were observed on T cell IL-10 secretion and the ability of these T cells to suppress memory T cell proliferation (Fig. 3 F, G). While for the control vitamin D3, an immunosuppressive compound known for its potential to induce tolerogenic moDCs and subsequently induce T cell that suppress memory T cell proliferation, significant suppression of memory T cells was observed compared to the mature condition, which for some donors was accompanied by induction of IL-10 secretion (Fig. 3 F, G).
moDC activation by Ara h 2-eBioparticles is partly mediated by TLR-4 signaling
As Ara h 2 eBPs activate moDCs, we aimed to determine the mechanism of activation. Ara h 2 eBPs were produced in N. benthamiana plants by transfection of a vector using A. tumefaciens. The final eBP product contained 2–3 EU/μg endotoxin and was not contaminated with agrobacteria, determined by a LAL assay and culturing the eBPs on YEB medium, respectively (Fig. 4A). Next, we tested whether the Ara h 2 eBPs could signal through TLR-4 by incubating HEK293-CD14 and HEK293-CD14-TLR-4 cells with MD-2 and eBPs, after which IL-8 production was measured. In this cell model, the presence of CD14, TLR-4 and MD-2 are all required to become sensitive to LPS,28 as observed after incubation with LPS (Fig. 4B). Incubation with the highest dose of Ara h 2 eBPs (10 μg/mL) induced IL-8 production, while lower doses (1 and 0.1 μg/mL) did not (Fig. 4B). An effect that was only observed in HEK293-CD14-TLR4 cells and not in HEK293-CD14 cells, indicating that the eBP signals via TLR-4. To assess whether Ara h 2 eBP-induced DC cytokine production was mediated by TLR-4 signaling, moDCs were incubated with a blocking TLR-4 antibody or an isotype control. Although CD14 expression is low on immature moDCs, they still respond to LPS, shown by production of IL-6 and TNFα (Fig. 4C–D). In the presence of anti-TLR-4, there was a reduced production of IL-6 and TNFα after stimulation with either E. coli LPS or the Ara h 2 eBPs compared to the medium control or the isotype control (Fig. 4C–D). These data demonstrated that the Ara h 2 eBPs signal via TLR-4, inducing cytokine production by moDCs.
Discussion
DCs are important sentinels of the body that orchestrate immune responses. Therefore, they form an essential target in AIT, where remodeling of the allergen-specific immune response is required. In this study, we extensively examined the effect of Ara h 2 eBPs, a novel AIT therapeutic, on moDC function and subsequent T cell polarization. We demonstrated that Ara h 2 eBPs are taken up by phagocytosis and fully activate moDCs. The latter is partly mediated by TLR-4 signaling. Additionally, we showed that eBP-exposed moDCs induce Th1 cell polarization, a novel finding as this was so far only suggested in mice and human moDC experiments using Der p 2 and Fel d 1 eBP.17,19 Yet, we did not observe an induction of Foxp3+ or IL-10-producing Tregs.
A crucial step in antigen presentation by DCs is the process of antigen internalization. Previously, it has been shown that negatively charged and neutral, small-sized particles (diameter <500 nm) are efficiently taken up by DCs, especially compared to positively charged particles, which predominantly adhere to the cell membrane.27,29 Indeed, slightly negative Ara h 2 eBPs with a diameter of approximately 160 nm were efficiently taken up by DCs, a process that was predominantly mediated by actin polymerization. This aligns with our previously published data showing that internalization of anionic and neutral liposomes also depends on actin polymerization.29 In contrast, nAra h 2 uptake is dependent on multiple pathways, including macropinocytosis, receptor-mediated uptake, and possibly phagocytosis (Castenmiller et al, manuscript in preparation).
For proper stimulation of naïve T cells, activation of DCs leading to the expression of costimulatory molecules and cytokine production is needed. Ara h 2 eBPs indeed induced full activation of human DCs as determined by upregulated expression of CD86 and CD83, as well as induced cytokine production (IL-6, TNFα, IL-10), whereas nAra h 2 did not. Similarly, it has been demonstrated before that Fel d 1 eBPs, but not natural Fel d 1, fully activated DCs.19 Together, these results indicate that the route of uptake of eBPs by DCs and activation of DCs by eBPs is rather mediated by the formulation of the eBPs than by the allergen expressed on eBPs. This principle of particulate-mediated DC activation has also been described for other particulate delivery systems, for example PLGA and poly(γ-glutamic acid) NPs.30,31
A key cellular immunological change during the onset of AIT-induced desensitization is reducing the number of allergen-specific Th2 cells, accompanied by a shift towards Th1 cells or induction of Tregs.2,3,6,32,33 Furthermore, Th1 polarization has been shown to be a crucial factor in inducing and maintaining AIT effectiveness.34, 35, 36, 37, 38, 39 Therefore, multiple AIT adjuvants have been tested, for example TLR ligands, which are extensively reviewed by Kirtland et al.37 One of these adjuvants, MPLA, has been studied for multiple pollen allergies and is already used in a market-authorized SCIT for tree and grass pollen.40, 41, 42, 43, 44, 45, 46 Interestingly, the immune modulating mechanism of MPLA is comparable to the eBPs. MPLA induced CD80 and CD86 expression on monocytes via TLR-4 and TLR-2 signaling, resulting in IL-10 and IL-12 production.47 Additionally, stimulating grass pollen allergic patients’ PBMCs with MPLA and grass pollen extract suppressed allergen-induced Th2 cell responses and favored Th1 cell responses.48 Another TLR-activating AIT approach, designed for peanut allergy, are poly(anhydride) NPs. These NPs have been shown to activate TLR-2 and TLR-4 signaling and induce Th1 cell responses.49 Intradermal injection of peanut extract-incorporated poly(anhydride) NPs in mice resulted in strong Th1 cell responses, using spray-dried NPs, and mixed Th1/Th2 cell responses using freeze-dried NPs.50 The authors speculate that the higher stability of spray-dried NPs compared to the freeze-dried NPs results in higher antigen allergen doses when interacting with APCs, possibly facilitating the stronger Th1 cell response. In this study, we demonstrated that activation of DCs by Ara h 2 eBPs is mediated by TLR-4 signaling, similar to MPLA and poly(anhydride) NP-based strategies. Whether low agrobacterium-derived levels of endotoxin or other plant-derived constituents present in the eBPs cause TLR-4 activation remains to be determined. Furthermore, Ara h 2 eBPs reduced Th2 cell polarization and induced Th1 cell polarization, which has also been suggested for Der p 2 eBPs in mice based on IgG2a induction.17 Future stability tests and mouse studies should confirm whether similar immunological effects are observed for the Ara h 2 eBPs in vivo. Altogether, these findings support the potential of Ara h 2 eBPs to serve as an effective AIT for peanut allergy. Therefore, plant-derived eBPs can be added to the list of promising Th1 cell polarizing formulations.
Other particulate approaches that have been studied for peanut allergy include the CuMVtt VLPs, a DNA vaccine targeting peanut allergens to lysosomes to enhance immunogenicity (Ara-LAMP-Vax), and PLGA NPs loaded with peanut extract. It has been reported that vaccination of mice with Ara h 2 CuMVtt VLPs induced strong IgG responses sufficient to reduce allergic symptoms initiated by peanut extract, which contains all peanut allergens.16 The authors propose that high IgG titers against a single allergen could inhibit FcεRI signaling induced by multiple allergens in mast cells and basophils via intracellular signaling of the inhibitory FcγRIIb. This would suggest that eBPs expressing only Ara h 2 will also be sufficient to dampen activation of allergic effector cells by whole peanut, provided that strong Ara h 2-specific IgG responses are induced by eBPs. The Ara-LAMP-Vax platform utilizes an entirely different strategy, by introducing a DNA construct of Ara h 1, 2, and 3 linked to the lysosomal protein sequence LAMP-1, which shuttles the proteins directly to the lysosomal compartment. This approach promotes antigen presentation circumventing allergen exposure and was promising in a murine model.51 The FDA-approved and biodegradable PLGA NP platform has been widely studied as an AIT for multiple allergic disorders, where it shifts, comparable to the eBPs, Th2 cell responses to Th1 cell responses.52, 53, 54, 55, 56, 57, 58 Both PLGA NPs loaded with peanut extract and Ara-LAMP-Vax, are now tested in clinical trials (NCT05250856, NCT02851277, NCT03755713). Additionally, the Ara h 2 CuMVtt VLPs will soon enter clinical trials (personal communication with Martin Bachmann).
The induction of regulatory T cells has been demonstrated to contribute to tolerance induction during AIT. Interestingly, multiple clinical studies demonstrated that no change in peanut-specific Treg frequencies were detected in patients who responded to AIT.32,59,60 Instead, desensitization correlated to selective T cell exhaustion and deletion of pathogenic Th2 cells. These findings, together with the above mentioned promising results using Th1-polarizing strategies, indicate the Ara h 2 eBPs, despite the absence of Treg induction, still hold potential as a promising AIT therapeutic. Furthermore, in this study the Treg population was identified as CD25+CD127-Foxp3+, which are markers that exist on thymic and peripheral Tregs. However, additional Treg markers (such as Helios, CTLA-4, ICOS, GITR, PD-1, CD39, CD73, GARP, TIGIT, and GITR) and Treg subsets (such as CD49b+LAG-3+ T regulatory type 1 (Tr1) cells) were not included in the analysis.61 Additional phenotyping may explain the discrepancy between eBP-mediated reduction of CD25+CD127-Foxp3+ T cells while IL-10 production and the suppressive capacity of the T cells remains unchanged. For example, Tr1 cells are known for its high IL-10 production,62 and Helios+Foxp3+ Tregs have been shown to have a stronger suppressive capacity than Helios−Foxp3+ Tregs in mice.63 Extensive flow cytometry panels or unbiased single cell approaches may help to characterize the complete eBP-induced T cell profile. Therefore, it remains to be determined whether eBPs have the capacity to induce Tregs in an antigen-specific fashion in an in vitro or in vivo setting. Moreover, the route of administration determines which subpopulation of DCs interacts with the eBPs, as each tissue contains its own tissue-specialized APCs. Some of these local subpopulations, e.g., CD103+ intestinal DCs and CD14+ dermal DCs are prone to induce regulatory T cell responses via production of IL-10, TGF-β, or retinoic acid.64, 65, 66, 67, 68, 69 In this study, in vitro-generated moDCs were used as a model for DCs. However, these DCs do not fully mimic DC responses of all different subsets present in their niche environment and should be taken into consideration when translating the findings of this study to human in vivo applications.70
Altogether, we have shown that Ara h 2 eBPs activate moDCs, promote Th1 cell polarization of naïve T cells, and could reduce Th2 cell polarization. These effects are rather caused by the formulation of the eBPs than by the allergen expressed on the eBPs, which allows translation of the plant-derived eBP platform to other food and respiratory allergic diseases. These characteristics, together with the previously shown hypo-allergenicity, potentially reduce side effects during AIT, but could potentially also induce stronger and quicker immune responses.18
Abbreviations
AIT, allergen immunotherapy; CFU, colony-forming unit; CuMVtt, Cucumber Mosaic Virus-derived; DCs, dendritic cells; DLS, dynamic light scattering; eBPs, plant-based Bioparticles; EPIT, epicutaneous immunotherapy; FBS, fetal bovine serum; HEK, Human Embryonic Kidney; IMDM, Iscove's Modified Dulbecco's Medium; MFI, mean fluorescent intensity; MPLA, monophosphoryl lipid A; moDCs, monocyte-derived dendritic cells; OIT, oral immunotherapy; SLIT, sublingual immunotherapy; TLR, toll-like receptor; VLP, virus-like particles; YEB, yeast extract beef.
Data availability
All data available are included in this paper and in the supplemental material.
Author contributions
C. Castenmiller, E.C. de Jong, and R. van Ree conceptualized the study and wrote, revised, and edited the manuscript. C. Castenmiller, P.Z. kroon and N.A. Nagy designed experiments and acquired, interpreted, and analyzed the data. L. Auger, R. Desgagnés, C. Martel, L. Mirande, B. Morel, J. Roberge, V. Stordeur, G. Tropper, L.P. Vézina, and V. Gomord contributed to the concept design, development, or manufacturing of the plant-derived bioparticle platform. R. van Ree and E.C. de Jong supervised experiments. E.C. de Jong supervised the study. All authors critically reviewed the manuscript.
Ethics statement
Following approval of the Medical Research Ethics Committee of the Amsterdam UMC, The Netherlands (NL71330.018.19; NL73819.018.21) donors were recruited to for blood donation.
Consent for publication
All authors have agreed with this publication in the World Allergy Organization Journal.
Declaration of competing interest
C. Castenmiller received contract research funding and study material from Angany Inc. L.P. Vézina, G. Tropper and V. Gomord are a cofounder, board member, and respectively CSO, EVP and CIO of Angany Inc. R. Desgagnés, C. Martel, L. Auger, J. Roberge, B. Morel, V. Stordeur, and L. Mirande are employees of Angany Inc. R. van Ree received contract research funding and research material form Angany Inc. and besides received consulting fees and speaker's fees from Angany Inc. HAL Allergy BV, Citeq BV, ThermoFisher Scientific, Reacta Healthcare Ltd., Mission MightyMe, The Protein Brewery and AB Enzymes, and has stock options from Angany.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100839.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wambre E., Bajzik V., DeLong J.H., et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401) doi: 10.1126/scitranslmed.aam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luce S., Chinthrajah S., Lyu S.C., Nadeau K.C., Mascarell L. Th2A and Th17 cell frequencies and regulatory markers as follow-up biomarker candidates for successful multifood oral immunotherapy. Allergy Eur J Allergy Clin Immunol. 2020;75(6):1513–1516. doi: 10.1111/all.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monian B., Tu A.A., Ruiter B., et al. Peanut oral immunotherapy differentially suppresses clonally distinct subsets of T helper cells. J Clin Invest. 2022;132(2) doi: 10.1172/JCI150634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos A.F., James L.K., Bahnson H.T., et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135(5):1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickery B.P., Lin J., Kulis M., et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131(1) doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barshow S.M., Kulis M.D., Burks A.W., Kim E.H. Mechanisms of oral immunotherapy. Clin Exp Allergy. 2021;51(4):527–535. doi: 10.1111/cea.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrawala M., Shih J., Lee G., Vickery B. Peanut oral immunotherapy: a current perspective. Curr Allergy Asthma Rep. 2020;20(5) doi: 10.1007/s11882-020-00908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickery B.P., Vereda A., Casale T.B., et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379(21) doi: 10.1056/NEJMoa1812856. [DOI] [PubMed] [Google Scholar]
- 9.Chinthrajah R.S., Purington N., Andorf S., et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394(10207):1437–1449. doi: 10.1016/S0140-6736(19)31793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones S.M., Kim E.H., Nadeau K.C., et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399(10322):359–371. doi: 10.1016/S0140-6736(21)02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu D.K., Wood R.A., French S., et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–2232. doi: 10.1016/S0140-6736(19)30420-9. [DOI] [PubMed] [Google Scholar]
- 12.Yu W., Freeland D.M.H., Nadeau K.C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16(12):751–765. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alghamdi R., Alshaier R., Alotaibi A., et al. Immunotherapy effectiveness in treating peanut hypersensitivity: a systemic review. Cureus. 2022 doi: 10.7759/cureus.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schworer S.A., Kim E.H. Sublingual immunotherapy for food allergy and its future directions. Immunotherapy. 2020;12(12):921–931. doi: 10.2217/imt-2020-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E.H., Burks A.W. Food allergy immunotherapy: oral immunotherapy and epicutaneous immunotherapy. Allergy Eur J Allergy Clin Immunol. 2020;75(6):1337–1346. doi: 10.1111/all.14220. [DOI] [PubMed] [Google Scholar]
- 16.Storni F., Zeltins A., Balke I., et al. Vaccine against peanut allergy based on engineered virus-like particles displaying single major peanut allergens. J Allergy Clin Immunol. 2020;145(4):1240–1253.e3. doi: 10.1016/j.jaci.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Gomord V., Stordeur V., Fitchette A.C., et al. Design, production and immunomodulatory potency of a novel allergen bioparticle. PLoS One. 2020;15(12 December) doi: 10.1371/journal.pone.0242867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castenmiller C., Stigler M., Kirpas M.E., et al. Plant-based enveloped Ara h 2 bioparticles display exceptional hypo-allergenicity. Clin Exp Allergy. 2023 doi: 10.1111/cea.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busold S., Aglas L., Menage C., et al. Fel d 1 surface expression on plant-made eBioparticles combines potent immune activation and hypoallergenicity. Allergy Eur J Allergy Clin Immunol. 2022 doi: 10.1111/all.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmings O., Du Toit G., Radulovic S., Lack G., Santos A.F. Ara h 2 is the dominant peanut allergen despite similarities with Ara h 6. J Allergy Clin Immunol. 2020;146(3):621–630.e5. doi: 10.1016/j.jaci.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazebrouck S., Guillon B., Paty E., Dreskin S.C., Adel-Patient K., Bernard H. Variable IgE cross-reactivity between peanut 2S-albumins: the case for measuring IgE to both Ara h 2 and Ara h 6. Clin Exp Allergy. 2019;49(8):1107–1115. doi: 10.1111/cea.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang Y., Dreskin S.C. Redefining the major peanut allergens. Immunol Res. 2013;55(1-3):125–134. doi: 10.1007/s12026-012-8355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppelman S.J., Wensing M., Ertmann M., Knulst A.C., Knol E.F. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34(4):583–590. doi: 10.1111/J.1365-2222.2004.1923.X. [DOI] [PubMed] [Google Scholar]
- 24.Palmer G.W., Dibbern D.A., Burks A.W., et al. Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin Immunol. 2005;115(3):302–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Porterfield H.S., Murray K.S., Schlichting D.G., et al. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39(7):1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jong EC de, Vieira P.L., Kalinski P., et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168(4):1704–1709. doi: 10.4049/JIMMUNOL.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 27.Foged C., Brodin B., Frokjaer S., Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Medvedev A.E., Vogel S.N. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res. 2003;9(1):60–64. doi: 10.1179/096805103125001360. [DOI] [PubMed] [Google Scholar]
- 29.Nagy N.A., Castenmiller C., Vigario F.L., et al. Uptake kinetics of liposomal formulations of differing charge influences development of in vivo dendritic cell immunotherapy. J Pharmaceut Sci. 2022;111(4):1081–1091. doi: 10.1016/j.xphs.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Waeckerle-Men Y., Allmen EU Von, Gander B., et al. Encapsulation of proteins and peptides into biodegradable poly(D,L-lactide-co-glycolide) microspheres prolongs and enhances antigen presentation by human dendritic cells. Vaccine. 2006;24(11):1847–1857. doi: 10.1016/j.vaccine.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Broos S., Lundberg K., Akagi T., et al. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine. 2010;28(31):5075–5085. doi: 10.1016/j.vaccine.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Berin M.C., Agashe C., Burks A.W., et al. Allergen-specific T cells and clinical features of food allergy: lessons from CoFAR immunotherapy cohorts. J Allergy Clin Immunol. 2022;149(4):1373–1382.e12. doi: 10.1016/j.jaci.2021.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turcanu V., Maleki S.J., Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111(7):1065–1072. doi: 10.1172/jci16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durham S.R., Ying S., Varney V.A., et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for Interferon-γ. J Allergy Clin Immunol. 1996;97(6):1356–1365. doi: 10.1016/S0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 35.Möbs C., Ipsen H., Mayer L., et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol. 2012;130(5) doi: 10.1016/j.jaci.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 36.Wachholz P.A., Nouri-Aria K.T., Wilson D.R., et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1: Th2 cytokine ratios. Immunology. 2002;105(1):56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirtland M.E., Tsitoura D.C., Durham S.R., Shamji M.H. Toll-like receptor agonists as adjuvants for allergen immunotherapy. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Möbs C., Slotosch C., Löffler H., Jakob T., Hertl M., Pfützner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184(4):2194–2203. doi: 10.4049/jimmunol.0901379. [DOI] [PubMed] [Google Scholar]
- 39.Cosmi L., Santarlasci V., Angeli R., et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-γ- and interleukin-10-production. Clin Exp Allergy. 2006;36(3):261–272. doi: 10.1111/j.1365-2222.2006.02429.x. [DOI] [PubMed] [Google Scholar]
- 40.Pfaar O., Barth C., Jaschke C., Hörmann K., Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol. 2011;154(4):336–344. doi: 10.1159/000321826. [DOI] [PubMed] [Google Scholar]
- 41.Pfaar O., Lang S., Pieper-Fürst U., et al. Ultra-short-course booster is effective in recurrent grass pollen-induced allergic rhinoconjunctivitis. Allergy Eur J Allergy Clin Immunol. 2018;73(1):187–195. doi: 10.1111/all.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel P., Holdich T., Fischer Von Weikersthal-Drachenberg K.J., Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133(1) doi: 10.1016/j.jaci.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 43.Rosewich M., Girod K., Zielen S., Schubert R., Schulze J. Induction of bronchial tolerance after 1 cycle of monophosphoryl-A-adjuvanted specific immunotherapy in children with grass pollen allergies. Allergy, Asthma Immunol Res. 2016;8(3):257–263. doi: 10.4168/aair.2016.8.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosewich M., Schulze J., Eickmeier O., et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010;160(3):403–410. doi: 10.1111/j.1365-2249.2010.04106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mothes N., Heinzkill M., Drachenberg K.J., et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33(9):1198–1208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 46.Drachenberg K.J., Wheeler A.W., Stuebner P., Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy Eur J Allergy Clin Immunol. 2001;56(6):498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 47.Martin M., Michalek S.M., Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71(5):2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puggioni F., Durham S.R., Francis J.N. Monophosphoryl lipid A (MPL®)∗ promotes allergen-induced immune deviation in favour of Th1 responses. Allergy Eur J Allergy Clin Immunol. 2005;60(5):678–684. doi: 10.1111/j.1398-9995.2005.00762.x. [DOI] [PubMed] [Google Scholar]
- 49.Tamayo I., Irache J.M., Mansilla C., Ochoa-Repáraz J., Lasarte J.J., Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17(9):1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebouças J.D.S., Irache J.M., Camacho A.I., et al. Development of poly(anhydride) nanoparticles loaded with peanut proteins: the influence of preparation method on the immunogenic properties. Eur J Pharm Biopharm. 2012;82(2):241–249. doi: 10.1016/j.ejpb.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Li X.-M., Song Y., Su Y., Heiland T., Sampson H.A. Immunization with ARA h1,2,3-lamp-vax peanut vaccine blocked IgE mediated-anaphylaxis in a peanut allergic murine model. J Allergy Clin Immunol. 2015;135(2):AB167. doi: 10.1016/j.jaci.2014.12.1482. [DOI] [Google Scholar]
- 52.Schöll I., Weissenböck A., Förster-Waldl E., et al. Allergen-loaded biodegradable poly(D,L-lactic-co-glycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy. 2004;34(2):315–321. doi: 10.1111/j.1365-2222.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 53.Xiao X., Zeng X., Zhang X., et al. Effects of Caryota mitis profilin-loaded PLGA nanoparticles in a murine model of allergic asthma. Int J Nanomed. 2013;8:4553–4562. doi: 10.2147/IJN.S51633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajavi J., Hashemi M., Sankian M. Evaluation of size and dose effects of rChe a 3 allergen loaded PLGA nanoparticles on modulation of Th2 immune responses by sublingual immunotherapy in mouse model of rhinitis allergic. Int J Pharm. 2019;563:282–292. doi: 10.1016/j.ijpharm.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 55.Marazuela E.G., Prado N., Moro E., et al. Intranasal vaccination with poly(lactide-co-glycolide) microparticles containing a peptide T of Ole e 1 prevents mice against sensitization. Clin Exp Allergy. 2008;38(3):520–528. doi: 10.1111/j.1365-2222.2007.02922.x. [DOI] [PubMed] [Google Scholar]
- 56.Kostadinova A.I., Middelburg J., Ciulla M., et al. PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow's milk allergy prevention. Eur J Pharmacol. 2018;818:211–220. doi: 10.1016/j.ejphar.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 57.Joshi V.B., Adamcakova-Dodd A., Jing X., et al. Development of a poly (lactic-co-glycolic acid) particle vaccine to protect against house dust mite induced allergy. AAPS J. 2014;16(5):975–985. doi: 10.1208/s12248-014-9624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gómez J.M.M., Fischer S., Csaba N., et al. A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm Res (N Y) 2007;24(10):1927–1935. doi: 10.1007/s11095-007-9318-0. [DOI] [PubMed] [Google Scholar]
- 59.Bajzik V., DeBerg H.A., Garabatos N., et al. Oral desensitization therapy for peanut allergy induces dynamic changes in peanut-specific immune responses. Allergy Eur J Allergy Clin Immunol. 2022;77(8):2534–2548. doi: 10.1111/all.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim E.H., Bird J.A., Kulis M., et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–646.e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boonpiyathad T., Sözener Z.C., Akdis M., Akdis C.A. The role of treg cell subsets in allergic disease. Asian Pac J Allergy Immunol. 2020;38(3):139–149. doi: 10.12932/AP-030220-0754. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda M., Terada T., Kitatani K., Kawata R., Nabe T. Roles of type 1 regulatory T (Tr1) cells in allergen-specific immunotherapy. Front Allergy. 2022;3 doi: 10.3389/falgy.2022.981126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornton A.M., Lu J., Korty P.E., et al. Helios + and Helios − Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol. 2019;49(3):398–412. doi: 10.1002/eji.201847935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaensson E., Uronen-Hansson H., Pabst O., et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205(9):2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tordesillas L., Berin M.C. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. 2018;55(2):107–117. doi: 10.1007/s12016-018-8680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamazaki S., Morita A. Dendritic cells in the periphery control antigen-specific natural and induced regulatory T cells. Front Immunol. 2013;4(JUN) doi: 10.3389/fimmu.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matteoli G., Mazzini E., Iliev I.D., et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59(5):595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 68.Chu C.C., Ali N., Karagiannis P., et al. Resident CD141 (BDCA3) + dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. 2012;209(5):935–945. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klechevsky E., Morita R., Liu M., et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.León B., López-Bravo M., Ardavín C. Monocyte-derived dendritic cells. Semin Immunol. 2005;17(4):313–318. doi: 10.1016/j.smim.2005.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data available are included in this paper and in the supplemental material.