Summary
The transplantation model provides the opportunity to assess the relevance of a molecule of interest for tumor cell extravasation by using a respective genetically modified donor animal. Here, we present a protocol for orthotopic single-lung transplantation in mice as a tool for lung metastasis studies. We describe steps for animal preparation, lung transplantation, and tumor cell injection. We then detail procedures for the direct comparison of tumor cell spreading between the genetically modified left lung and the naive right lung parenchyma.
For complete details on the use and execution of this protocol, please refer to Giannou et al. (2023).1
Subject areas: Cancer, Immunology, Model Organisms
Graphical abstract

Highlights
-
•
Model to study extravasation of colorectal cancer cells to the lung
-
•
Steps for murine lung preparation and lung implantation
-
•
Tail vein injection of MC38-GFP cells followed by quantification of cells in the lungs
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The transplantation model provides the opportunity to assess the relevance of a molecule of interest for tumor cell extravasation by using a respective genetically modified donor animal. Here, we present a protocol for orthotopic single-lung transplantation in mice as tool for lung metastasis studies. We describe steps for animal preparation, lung transplantation, and tumor cell injection. We then detail procedures for the direct comparison of tumor cell spread between the genetically modified left lung and the naive right lung parenchyma.
Before you begin
A key feature of the malignant phenotype is the ability of cancer cells to spread from the primary tumor site and colonize different organ targets, a process resulting in metastasis formation. A crucial step of this process is cancer cell intravasation from the primary tumor and circulation in the bloodstream.2 When reaching the lung, cancer cells are able to extravasate and seed the lung parenchyma, where they will struggle to adapt and progress into metastasis.3
To investigate metastatic formation, mouse models of lung metastasis have been used for decades. A valuable experimental model to study the process of transendothelial migration of metastatic cells is the model of single lung transplantation in the mouse.4 Using this model, we can assess the extravasation of cancer cells upon lung metastasis formation in a mouse that has in parallel a wild type and a knockout lung for a specific endothelial gene. This gives us the opportunity to investigate the role of a specific gene in the extravasation step of lung metastasis.
Institutional permissions
All procedures performed on mice were approved by the veterinary review committee at the Institutional Review Board “Behörde für Justiz und Verbraucherschutz (Veterinärwesen/Lebensmittelsicherheit)” (Hamburg and Freiburg, Germany), and are in compliance with the ethical principles of the 3R’s for humane animal research and under the conduction of the EU Directive 2010/63/EU. Likewise, the readers should acquire permission from the relevant institutions before carrying out any procedures in this protocol.
Cell preparation
Timing: 1 week
-
1.
Bring 1 mL of cryopreserved MC38 cells (1 million cells/mL in 10% DMSO/90% FBS) to 37°C using a water bath.
-
2.
Transfer the solution of cells into 9 mL of cell culture medium and spin down at 300 × g for 5 min in order to wash off the DMSO.
-
3.
Seed cells onto a 100 mm petri dish by resuspending the MC38 cells with 10 mL of cell culture medium.
-
4.
Incubate cells for 24 h in an incubator (5% CO2) at 37°C.
-
5.
Replace the medium with 10 mL fresh cell culture medium and incubate cells for another 24 h in an incubator (5% CO2) at 37°C.
-
6.
Check cells daily under a microscope and proceed only when a confluence of 70% is reached.
-
7.Split cells.
-
a.Remove and discard all medium within the petri dish. Since the cancer cells are adherent, they should stick to the bottom of the plate.
-
b.Rinse the plate with 10 mL PBS and discard what is washed off.
-
c.Add 1 mL of Trypsin to the cells and incubate the cells for 3 min in an incubator (5% CO2) at 37°C.
-
d.Add 19 mL of fresh cell culture medium (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks) and resuspend the cells.
-
e.Centrifuge the cells, discard the supernatant and resuspend the pellet in 20 mL medium.
-
f.Divide the 20 mL medium with the cells onto two fresh culture plates.
-
g.Incubate cells for a further 24 h in an incubator (5% CO2) at 37°C.
-
a.
-
8.
Cells should be split at least 2–3 times before use in in vivo experiments.
-
9.
Split cells 1:2 24 h before conducting the rodent surgery.
-
10.
Before harvesting, check that a confluence of 50%–70% is reached.
CRITICAL: Cells have to be confluent at around 50%–70% to ensure that they are in their exponential growth phase. Otherwise, mice may not develop any metastasis.
-
11.Harvest cells.
-
a.Discard the medium completely.
-
b.Rinse the plate with 10 mL PBS and discard what is washed off.
-
c.Add 1 mL of Trypsin to the cells and incubate the cells for 3 min in an incubator (5% CO2) at 37°C.
-
d.Add 9 mL of fresh cell culture medium and resuspend the cells.
 CRITICAL: Full culture medium has to be added at this point, since the FBS included stops the enzymatic activity of Trypsin.
CRITICAL: Full culture medium has to be added at this point, since the FBS included stops the enzymatic activity of Trypsin. -
e.Transfer cells with cell culture medium (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks) to a 50 mL Falcon and add 40 mL of PBS.
-
f.Pellet by centrifugation at 300 × g for 5 min.
-
g.Discard the supernatant.
-
h.Resuspend the pellet in 1 mL PBS.
-
i.Take a 10 μL aliquot of the cells and dilute it with 80 μL PBS and 10 μL trypan blue.
-
j.Count cells.Note: A general yield of 5–10 million cells per plate should be expected.
 CRITICAL: Ensure a viability of >90% of counted cancer cells.
CRITICAL: Ensure a viability of >90% of counted cancer cells. -
k.Dilute cells with PBS to a concentration of 5 million cells per mL for intravenous injections.Note: Alternative cancer cell lines might require different cell concentrations for appropriate metastatic yield.
-
l.Aliquot cells into 1 mL aliquots. Transport and store the cells on ice for the entirety of the experiment.
 CRITICAL: Do not store cancer cells on ice for more than 2 h. If more than 40–50 mice are desired to be injected, harvest cells multiple times.
CRITICAL: Do not store cancer cells on ice for more than 2 h. If more than 40–50 mice are desired to be injected, harvest cells multiple times.
-
a.
Prepare the microsurgical workspace
Timing: 30 min
-
12.Provide sufficient anesthetics and prepare the ventilator.
-
a.Make sure that the isoflurane vaporizer(s) are sufficiently filled.
-
b.Set up the ventilator and check its functionality with a 20 G i.v. catheter and a small artificial lung. Pre-set the frequency to 130 breaths/min and aim for a tidal volume of 0.5–1.0 mL.
-
a.
-
13.Prepare the preservation fluid for perfusion.
-
a.Remove the stamp from a 10 mL syringe and place it on the laboratory stand.
-
b.Attach a 21G butterfly cannula to the syringe.
-
c.Fill the syringe with Perfadex Plus (temperature 4°C and maximum storage 1 day) and hang the butterfly cannula facing upwards to avoid further leakage once the air is fully evacuated from it.
-
a.
-
14.Prepare plastic cuffs from 20 G, 22 G and 26 G intravenous catheters for anastomosis of the bronchus, vein, and artery, respectively.
-
a.Roughen the outside surface of the catheters with fine-grained sandpaper.
-
b.Cut off the conical end of the catheters with a scalpel.
-
c.Incise the catheters longitudinally in two opposite regions and then perform a vertical incision for half of the catheter circumference. This way, a small handle is generated (Figure 1).
-
d.Finish the preparation of the cuff with a circumferential vertical cut.
-
a.
-
15.
Have ice readily available at your operating table.
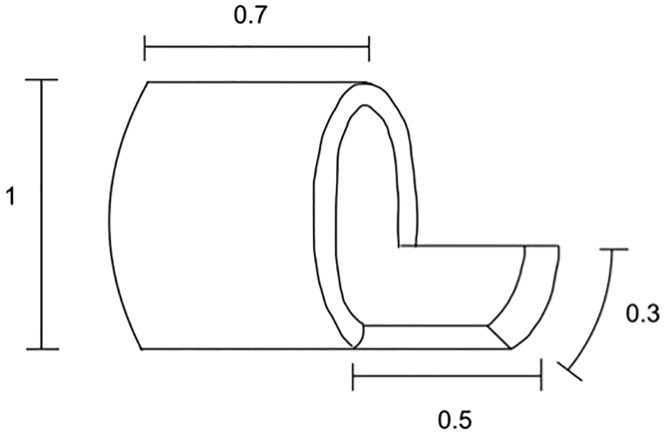
Note: Small handles on the cuffs are necessary in order to hold them in place with the adjustable helping hand tool. The size of the handles can hinder the introduction of the donor structures through the cuff. If the reader experiences this difficulty, narrower cuff handles of only a quarter circumference can be a good solution. Figure 1 depicts the sizing of the cuff components that works well in our hands.
Figure 1.
Dimensions of plastic cuffs to be prepared from 20 G, 22 G and 26 G intravenous catheters
The size of the respective parts of the cuff are normalized to its diameter.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Cell lines | ||
| MC38 GFP-labeled cells | Giannou et al.1 | N/A |
| MC38 shC | Giannou et al.1 | N/A |
| Experimental models: Organisms/strains | ||
| C57BL/6J wild-type mice, adult, 24–30 g BW, used as recipient animal | The Jackson Laboratory | N/A |
| Knockout mouse for the respective structure of interest, e.g., Il22ra1−/− mice on a B6 background, adult, 24–30 g BW, used as donor animal | Till Strowig Lab – Helmholtz Center for Infection Research, Braunschweig, Germany | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| PBS | Dirk Hindorf Anprotec | AC-BS-0002 |
| FBS | Capricorn Scientific | CS-HI-1A |
| EDTA | Carl Roth GmbH | 8043.2 |
| DMEM (GlutaMAX) | Gibco | 31966047 |
| Penicillin/Streptomycin | Gibco | 15070-063 |
| Metamizole | Ratiopharm GmbH | 9051799 |
| HBSS (with Ca2+ and Mg2+) | Life Technologies GmbH | 14065-049 |
| Collagenase | Sigma | C2139-5G |
| DNase I | AppliChem über Th. Geyer | A3778-50MG |
| 0.05% Trypsin-EDTA | Gibco | 25300-054 |
| Other | ||
| Leica M620 TTS tabletop surgical microscope | Leica Microsystems | |
| Induction chamber for small animals | UNO Roestvaststaal BV | 180000232 |
| Ventilator for small animal use, e.g., UMV-03 UNO microventilator | UNO Roestvaststaal BV | 180000023 |
| Isoflurane vaporizer | UNO Roestvaststaal BV | 180000008 |
| Flowmeter CM2 | UNO Roestvaststaal BV | 180000002 |
| Artificial lung | UNO Roestvaststaal BV | 180000009 |
| Heating plate | UNO Roestvaststaal BV | 180000028 |
| Control unit 01 for heating plate | UNO Roestvaststaal BV | 180000123 |
| Laboratory stand with clamp, e.g., Wisamic lab stand | Amazon | 18-01-22-0001-EU |
| Small surgical scissors, e.g., fine scissors, sharp | Fine Science Tools | 14060-09 |
| Small blunt surgical scissors, e.g., Strabismus scissors | Fine Science Tools | 14074-09 |
| Small surgical forceps, e.g., Mueller micro forceps, straight, 110 mm | Braun Aesculap | FM001R |
| Curved surgical forceps, e.g., Student fine forceps, curved | Fine Science Tools | 91117-10 |
| Fine angled forceps, e.g., Dumont #5/45 forceps | Fine Science Tools | 11251-35 |
| Fine spring scissors, straight, e.g., micro scissors, straight, sharp/sharp, 105 mm | Braun Aesculap | OC496R |
| Fine spring scissors, curved, e.g., micro scissors, curved, sharp/sharp, 105 mm | Braun Aesculap | OC297R |
| Micro needle holder, e.g., Mueller micro needle holder, curved, 180 mm | Braun Aesculap | FM061R |
| Adjustable helping hand tool | Toolcraft | ZD-10F |
| Retractor, e.g., Alm retractor (self-retaining), 70 mm, 4 × 4 prongs, semi-sharp | Braun Aesculap | BV010R |
| Vascular clips, e.g., Biemer micro vascular clip, oblique serrated, 5 mm opening width, 9 mm | Braun Aesculap | FD562R |
| Small electrocautery, e.g., cautery high temp 2″ extended shaft loop tip | Bovie Medical | AA05 |
| LOOK 6-0 silk suture spool, black braid, 100 yards | Corza Medical | SP114 |
| Dafilon 10-0/30 cm/DLM6 | B. Braun | G1118749 |
| Prolene 5-0/90 cm/2xRB1 | B. Braun | 8556H |
| Vasofix Safety, 20 G, PUR | B. Braun | 4269110S-01 |
| Vasofix Safety, 22 G, PUR | B. Braun | 4269098S-01 |
| Versatus-W, winged i.v. catheter, 26 G | Terumo | SR + DU2619WX |
| Sandpaper, 240 grit | BAHAG AG | N/A |
| Injekt Solo, 10 mL syringe | B. Braun | 4606108V |
| Injekt F, 1 mL syringe | B. Braun | 9166017V |
| Berpu cannula 25 G, 30 mm | B. Braun | BP2530430 |
| Venofix Safety 21 G, 30 cm length | B. Braun | 4056504-01 |
| Tape, e.g., Durapore surgical tape | 3M | 1538-1 |
| Gauze compresses, 5 × 5 cm | Fuhrmann GmbH | 31501 |
| Petri dishes, e.g., Sterilin Petri dishes | Thermo Fisher Scientific | 501V |
| Cotton swabs, wood, 15 cm | NOBA Verbandmittel | 10859 |
| Ointment, e.g., Bepanthen Augen- und Nasensalbe | Bayer Vital GmbH | N/A |
| Isofluran-Piramal (isoflurane) 250 mL | Piramal Critical Care BV | N/A |
| Temgesic Ampullen (buprenorphine hydrochloride, 0.3 mg/mL) | Indivior Europe Ltd. | N/A |
| Thiopental Inresa 0.5 g (sodium thiopental) | Inresa Arzneimittel GmbH | N/A |
| Heparin-Natrium-25000-ratiopharm (sodium heparin, 25,000 IU/5 mL) | Ratiopharm | N/A |
| Preservation fluid (Perfadex Plus) | XVIVO Perfusion | N/A |
| Neubauer hemocytometer (cell counting) | Neubauer | 68052-14, 68052-15 |
Note: For orthotopic lung transplantation, we recommend using adult mice of 24–30 g of body weight. The protocol can be adapted according to your needs regarding specific mouse and tumor cell strains.
Materials and equipment
Analgetic drinking water
| Reagent | Final concentration | Amount |
|---|---|---|
| Autoclaved drinking water | 95% | 200 mL |
| Glucose | 5% | 10 g |
| Metamizole | <1% | 240 mg |
| Buprenorphine | 0.1 mg/kg | depending on mouse weight |
The analgetic drinking water should be prepared fresh and replaced daily.
Cell culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM medium | 90% | 450 mL |
| FBS | 10% | 50 mL |
| Penicillin/Streptomycin (5000 unit/mL Pen and 5000 μg/mL Strep) | 1% | 5 mL |
The cell culture medium has to be prepared and stored under sterile conditions.
Lung digesting medium
| Reagent | Final concentration | Amount |
|---|---|---|
| HBSS (with Ca2+ and Mg2+) | 99% | 100 mL |
| Collagenase | 1 mg/mL | 0.5 mL |
| DNase I | 10 U/mL | 0.2 mL |
The lung digesting medium has to be prepared fresh every time.
MACS buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| PBS | 98.5% | 492.5 mL |
| FBS | 1% | 5 mL |
| EDTA (0.5 M, PH 8.0) | 0.5% | 2.5 mL |
Step-by-step method details
Explant the heart-lung block from the donor animal
Timing: 30 min
Note: This section describes the removal of the heart-lung block from the donor animal that is sacrificed during this step. Careful dissection and thorough perfusion of the donor lung are indispensable to obtain a functional donor graft.
-
1.Anesthetize the donor mouse.
-
a.Weigh the donor animal and administer Buprenorphine (0.1 mg/kg) subcutaneously.
-
b.Place the mouse in an induction chamber and administer isoflurane (5 L O2/min, 5%) until the breathing rate is visibly reduced to 30–40 breaths/min and the animal is still.
-
c.Quickly remove the animal from the induction chamber and perform orotracheal intubation with a 20 G catheter. The inhalative anesthesia leaves approximately 20 s to complete this step.
-
d.Connect the intubated animal to the ventilator, and ventilate the animal at a frequency of 130/min with a tidal volume of 0.5 mL (for animals of 25–27 g) or 1.0 mL (for animals of 28–30 g), continuously administering 2%–3% isoflurane.
-
a.
Note: Orotracheal intubation in mice can be performed in different ways. Readers should be aware that the preparation of the animal by inhalation anesthesia only significantly shortens the available time to perform the intubation. In our hands, the anesthesia leaves enough time for using an intubation aid, suspending the animal from its incisors and placing the catheter intratracheally via a guidewire. Other methods of intubation may take more time and the addition of an intraperitoneal anesthetic should be discussed.
-
2.
Place the mouse in a supine position, fix it to the operating plate with tape, and administer Thiopental (50 mg/kg) intraperitoneally to deepen the anesthesia.
-
3.
Broadly shave the thorax and abdomen.
-
4.
Perform a skin incision from the caudal abdomen up to the lower jaw using small surgical scissors.
-
5.
Carefully separate the salivary glands and dissect the trachea in between them. During this step, the correct placement of the endotracheal tube is visible through the tracheal wall.
-
6.
Loosely place a long ligature (5-0 silk, 20 cm length) around the trachea using rounded forceps.
-
7.
Perform a median laparotomy and gently push the intestine aside to display the inferior vena cava.
-
8.
Gently push down the liver and gall bladder and perform a median sternotomy.
-
9.
Incise the diaphragm on both sides, push both sides of the ribcage aside and fix them to the operating plate using small pins.
Note: Be careful not to cause rib fractures during this step as they may injure the lung parenchyma.
-
10.
Remove the thymic gland to expose the roots of the aorta and pulmonary artery.
CRITICAL: Take care not to damage any underlying blood vessels (innominate and subclavian arteries, superior venae cava) during this step. To avoid bleeding, do not apply too much tension on the thymus while removing it. It is not necessary to remove the thymus in its entirety during this step, as long as the aorta and pulmonary artery are clearly identified. After this step, the animal is now ready for perfusion. The relevant structures are depicted in Figure 2.
Figure 2.
Donor animal prepared for perfusion
The pulmonary artery, the aorta, and the inferior vena cava are clearly visible.
-
11.
Inject Heparin (100 U/kg) in the inferior vena cava.
-
12.Perfuse the animal with 5–10 mL Perfadex Plus. Be aware that the following sub-steps (12 a-c) have to be performed in quick succession as the animal starts bleeding out.
-
a.Quickly incise the inferior vena cava and the left auricular appendix.
-
b.Insert a 21 G butterfly cannula at the root of the pulmonary artery.
-
c.Perfuse the animal with 5–10 mL Perfadex Plus until the lungs are flushed bloodless and turn white. It is important to keep the lungs sufficiently ventilated during this step to achieve good perfusion. By the end of the procedure, a remaining slight redness along the periphery of the lung is normal (troubleshooting 1).
-
a.
-
13.Ligate the trachea and take the animal off the ventilator.
-
a.Change the ventilator settings to a frequency of 100 breaths/min or lower and turn off the isoflurane addition.
-
b.Gently pull back the intubation cannula and ligate the trachea when the lung is at approximately 2/3 of its maximum expansion.
-
c.Remove the intubation cannula and turn off the ventilator.
-
a.
-
14.
Cut the trachea above the ligature, and gently pull on the ligature while removing the heart-lung block from the donor animal by careful dissection.
Note: We recommend working close along the spine as a guiding structure for this step, to avoid damage to the graft.
-
15.
Place the heart-lung block on wet gauze on ice.
Prepare the lung graft (Methods Video S1)
Timing: 60 min
Note: After the excision of the heart-lung block, the pulmonary graft is prepared. This step requires the microsurgical dissection of the pulmonary artery, pulmonary vein, and main bronchus. The three structures are then fixed on plastic cuffs to prepare the graft for implantation.
-
16.Place the heart-lung block on wet gauze in a petri dish filled with ice under the operating microscope.
-
a.To stabilize the tissue, place it on top of the wooden stem of a cotton swab.
-
b.To provide enough tension on the tissue for subsequent dissection, attach the trachea to the operating table by the ligature ends. This setup is depicted in Figure 3.
-
a.
-
17.
Gently cover the left lung with wet gauze to avoid injury to the parenchyma during the following operative steps.
-
18.
Identify the pulmonary artery at its root and remove the surrounding fatty tissue using fine microsurgical forceps and spring scissors.
-
19.
Cut the artery close to the bifurcation and separate it from the left main bronchus, all the way down to the left lung hilum.
-
20.
Identify the bronchial bifurcation, cut the left main bronchus close to the bifurcation and separate it from the underlying esophagus down to the hilum (troubleshooting 2).
-
21.
Identify the left pulmonary vein, remove the surrounding fatty tissue, and cut off the vein at the influx to the left atrium. The left lung is now successfully separated from the rest of the heart-lung block (troubleshooting 3).
Note: While the pulmonary artery is quite elastic, and therefore resistant to tear strength, caution should be taken during the dissection of the bronchus and pulmonary vein. Regarding the bronchus, a tear in the membranous part is to be avoided. The pulmonary vein easily tears when not handled with care. We advise cutting the vein deep inside the left atrium, as the atrial tissue does not tear as easily as the venous wall. Generally, in order to avoid damage to the vessels and the bronchus, we advise to either grab structures as a whole or to manipulate the surrounding connective tissue.
-
22.
Place the left lung on wet gauze on ice.
-
23.
Place the 22 G cuff in the adjustable helping hand tool by suspending it from a needle holder. The cuff should be placed above the hilum of the isolated left lung in a way that allows for a straight top view onto the cuff entrance, as depicted in Figure 4A.
-
24.
Grab the pulmonary vein and gently pull it through the cuff from below with angled forceps.
Note: As the thin venous walls are prone to tearing, we recommend handling the donor vein by grabbing it primarily at the attached atrial tissue. However, make sure that there is not too much excess tissue at the distal end of the vein, as this may hinder the passage through the narrow cuff.
-
25.
Identify the venous lumen and pull the venous walls over the whole circumference of the cuff.
-
26.
Fasten the vein on the cuff with a 10-0 ligature. The desired result is depicted in Figure 4B.
-
27.
Repeat steps 21–24 with the 26 G cuff for the pulmonary artery and the 20 G cuff for the bronchus, respectively. The recommended orientation of the cuffs on the donor organ is depicted in Figure 4C.
-
28.
Remove excess tissue around the cuffs at the bronchus and the vein.
Note: Removal of the excess atrial tissue around the vein is critical in order to obtain a functional venous anastomosis, as it may cause obstruction of venous blood flow. Thus, make sure that the ligature is placed strictly around the thin venous wall and remove all excess atrial tissue after attaching the cuff.
Figure 3.
Placement of the heart-lung block for back-table preparation
The position of the heart-lung block on a wooden stick as an abutment and stretching out the trachea provides the tissue with the necessary tension for further dissection (A). During preparation, the pulmonary ligament, pulmonary artery and the aortic arch should be visualized (B).
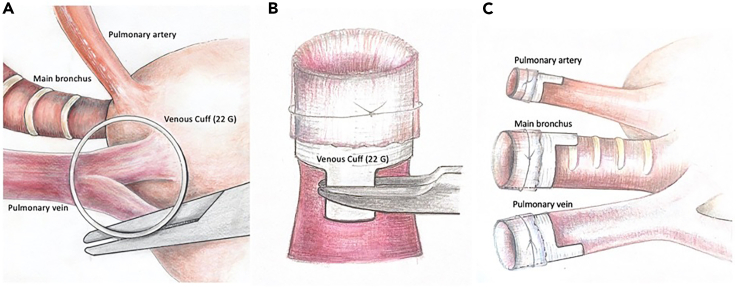
Figure 4.
Position of the 22 G cuff suspended from a micro needle holder above the pulmonary vein
The straight top view through the cuff allows for a tension-free introduction of the vein through the cuff (A). The vein is then pulled through the cuff, slipped over it and fixed with a 10-0 ligature (B). In order to avoid an impairment of the venous flow after implantation, a caudal position of the venous cuff handle is recommended (C).
Implantation of the lung graft to the recipient animal (Methods Video S2)
Timing: 60 min
Note: The following section describes the procedure of implanting the prepared graft to the recipient animal. This is the most challenging aspect of the lung transplant procedure. It requires the dissection of the recipient pulmonary artery, main bronchus, and pulmonary vein, a transient closure of the vasculature, and introduction of the donor cuffs into the respective recipient structures. During this procedure, the continuous visual control of the heartbeat and respective adjustments of the inhalation anesthetic is mandatory. Inhalation anesthesia should be adjusted to achieve a continuous heart rate of approx. 260 beats/min.
-
29.
Anesthetize the recipient mouse in the same fashion as described in step 1.
-
30.
Place the mouse in a half-supine position turned toward its right side on a heated plate and attach it with tape.
-
31.
Apply ointment to the eyes of the animal to keep them moist during the surgery.
-
32.
Shave the left side of the thorax.
-
33.
Incise the skin right below the inferior angle of the scapula to perform a left-sided thoracotomy.
-
34.
Dissect the muscle tissue until the intercostal spaces are visible.
Note: During this step, small blood vessels between the muscles are visible. To avoid bleeding, use electrocautery on them.
-
35.
Open the thoracic cavity through the fourth intercostal space right below the scapula, all the way from the sternum to the spine using blunt-tipped scissors, and insert a retractor.
Note: This position offers the best access to the hilar structures of the recipient animal. Be careful not to cause rib fractures during this step, as the sharp edges will damage the lung.
-
36.
Extract the left recipient lung from the thoracic cavity by gently pushing it outwards using wet cotton swabs. Slight twisting movements are required to release the pulmonary ligament.
-
37.
Hold the extracted lung outside the thorax with a vascular clip to obtain the necessary tension for dissection of the recipient hilum. Figure 5 demonstrates the positioning of the animal and the way the lung can be extracted out of the thoracic cavity (troubleshooting 4).
-
38.
Place the animal under the operating microscope.
-
39.
Dissect any remaining parts of the pulmonary ligament by pulling it apart using fine microsurgical forceps.
-
40.
Fixate the lung outside the thoracic cavity so that a horizontal orientation of the hilar structures is achieved. Excessive tilting up- or downwards will impede the successful introduction of the donor cuffs.
-
41.Dissect the recipient hilar structures until a complete separation of pulmonary artery, left main bronchus, and left pulmonary vein is achieved, as depicted in Figure 6.
-
a.To dissect the pulmonary artery, grab the connective tissue around the proximal part of the artery close to the heart and carefully separate the artery from the main bronchus.Note: The murine pulmonary artery is very resistant to tear stress. Once any separation of artery and bronchus is achieved, angled microsurgical forceps can be placed underneath the artery and the structure can be freed easily from the surrounding connective tissue with spreading movements. At the peripheral part of the artery close to the parenchyma, an additional layer of stronger connective tissue can be found that should be pulled apart to achieve a complete separation of the artery.
-
b.Next, the bronchus is separated from the vein by slowly pulling apart the connective tissue between the two structures.Note: For separating the bronchus from the vein, we recommend starting close to the heart and working your way all the way up to the recipient hilum. Make sure not to damage the membranous part of the bronchus that is bulging outwards during ventilation.Note: It is important to dissect the hilar structures for their entire length and circumference. Leftover connective tissue between the structures significantly limits the available space left for placement of the cuffs and ligatures during implantation.
-
a.
-
42.
Place 10-0 ligatures or vessel clips around the vascular structures and tie them down close to the heart.
Note: Transient central closure of the vascular structures can alternatively be performed using vessel clips. However, we have good experience with ligatures, as they leave more space for the anastomosis procedure. Alternatively, slipknots can be used to facilitate reopening of the vascular structures.
-
43.
Loosely place three 10-0 ligatures around the artery, vein and bronchus to prepare for fastening of the donor cuffs (troubleshooting 5).
-
44.
Place the prepared donor graft wrapped in a thin wet piece of gauze on top of the recipient lung in the correct orientation.
-
45.Implant the graft by introducing the vascular donor structures into the respective recipient vessels (troubleshooting 6).Note: The sequence of implantation can be changed according to the individual surgeon’s preference. In this protocol, we describe our preferred sequence. We recommend starting by introducing the vein, as this is usually the shortest donor structure. Thus, greater flexibility of the donor graft can facilitate its introduction. Next, we prefer to introduce the artery and finish the procedure with the bronchial anastomosis. However, others may prefer the introduction of the bronchus before the arterial anastomosis, as the attached bronchial cuff may provide further stability for the introduction of the flexible artery.
-
a.Make a small incision in the recipient vein close to the donor lung parenchyma. The incision should span a quarter of the vessel’s circumference.Note: The size and site of the incision of the recipient structures is critical for successful introduction of the donor cuffs – especially regarding the vein. If the incision is too small, trying to insert the cuff will cause longitudinal tearing of the recipient structure. On the other hand, large incisions make the introduction of the cuff virtually impossible, and may cause further vertical tearing.
-
b.Shortly flush the recipient vein with Perfadex Plus to clearly identify its lumen.
-
c.Insert the venous donor cuff into the recipient vein and push the cuff forward as much as possible to achieve a central anastomosis of the vein.Note: Tears in the recipient structures during introduction of the cuffs can be avoided by applying the following strategy: Clearly visualize the lumen of the recipient structure before attempting to introduce the cuff. Lodge the upper rim of the cuff underneath the top edge of the recipient structure by slightly angling the cuff downwards (Figure 7). Small sideways movements will help to place the cuff in between the side walls of the recipient structure. Before pushing the cuff into the recipient structure, make sure the cuff is orientated strictly parallel to the backside of the recipient structure otherwise the backside of the structure will inevitably tear when trying to introduce the cuff. As the arterial wall is quite resistant to tear stress, this is especially relevant for the vein and bronchus.
-
d.Fixate the cuff inside the recipient vein using the prepared 10-0 ligature (troubleshooting 6).
-
e.Repeat the process (steps 40a-d) for the arterial anastomosis.Note: As the diameter of the artery is smaller than that of the vein, take care not to cut into it too deeply. We recommend flushing the structure in order to clearly identify its lumen before making any attempt to introduce the donor cuff. Following this advice can reduce the likelihood of introducing the cuff into a false lumen created by surrounding connective tissue.
-
a.
-
46.Create the bronchial anastomosis.
-
a.Make a small incision in the recipient left main bronchus directly in between two cartilage braces close to the parenchyma.
-
b.Quickly introduce the donor bronchial cuff into the structure and gently push forward.Note: Be aware that the animal is not sufficiently ventilated once the main bronchus is incised. Therefore, it is critical to quickly introduce the donor cuff to achieve a closure of the airway and sufficient ventilation.
-
c.Fasten the cuff with the prepared ligature. The result is shown in Figure 8.
-
a.
-
47.Initiate reperfusion of the graft by opening the central ligatures.
-
a.Open the central ligature on the vein and check the graft for venous backflow. Rapid venous backflow is a reliable indicator for a functional venous anastomosis.
-
b.Open the central ligature on the artery and observe the immediate arterial perfusion of the graft.
-
a.
-
48.
Carefully observe the color of the graft changing from white to light pink.
Note: The color change of the graft is a reliable predictor of the outcome. A change to a slight pink color shows an optimal match between the arterial blood supply and venous drainage. Insufficient venous drainage due to mechanical constriction causes a red discoloration of the graft. Slight manipulation with a cotton swab or shortening of the venous cuff handle can ameliorate venous blood flow. It is important to check for discoloration of the graft with every change of its position.
-
49.
Place the graft inside the thoracic cavity in the correct anatomic position using moist cotton swabs.
-
50.
Remove the recipient’s lung by cutting off its vessels and bronchus close to its hilum.
-
51.
Remove the retractor and loosely place a continuous pericostal 5-0 Prolene suture across the full length of the thoracotomy.
-
52.
Re-inflate the pulmonary graft by applying positive end-expiratory pressure by shortly closing the expiratory loop of the ventilator.
-
53.
Tighten and close the pericostal suture immediately after the re-inflation of the pulmonary graft to prevent residual pneumothorax.
-
54.
Close the skin incision with metal clips.
-
55.
Turn off the isoflurane addition and slowly let the animal wake up. Remove the tracheal tube once spontaneous breathing resumes.
-
56.
Inject the animal with Buprenorphine (0.1 mg/kg) subcutaneously.
Figure 5.
Position of the recipient animal for implantation
The lung is extracted from the thoracic cavity by a vessel clip attached to the retractor. This way, the pulmonary vessels and bronchus are stretched out for further dissection.
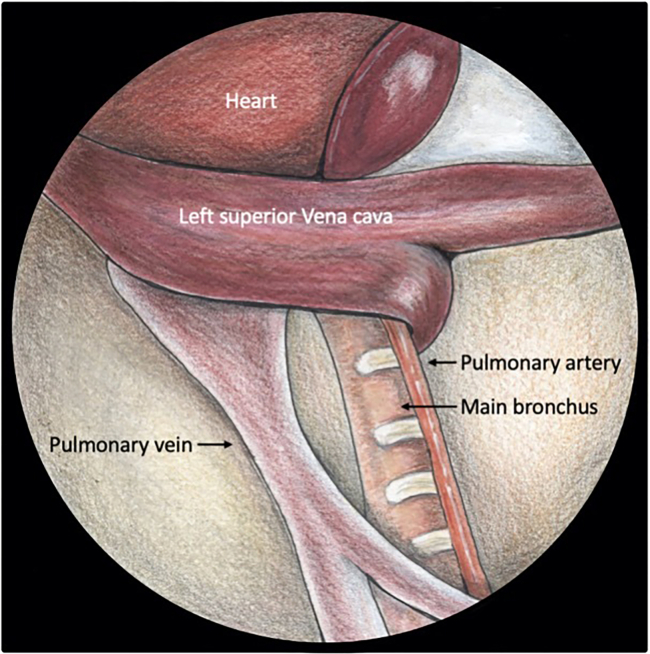
Figure 6.
View of the hilar structures before implantation
Note the complete separation between the vein and bronchus. The superior vena cava crossing the central structures can be gently pushed further towards the heart using cotton swabs.
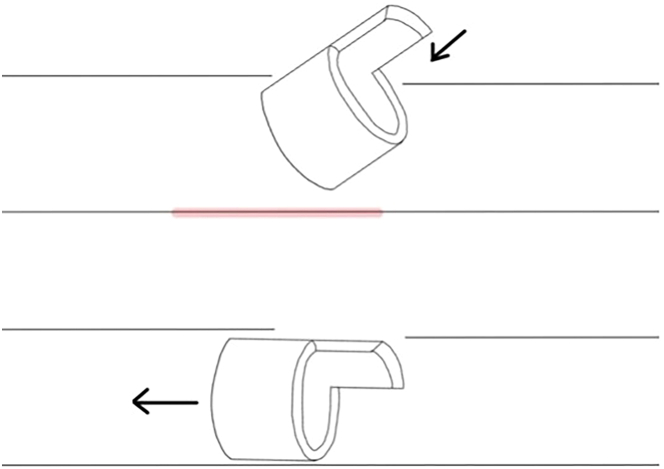
Figure 7.
Movement for the implantation of the donor cuffs
First, angle the cuff downward to lodge it underneath the upper rim of the recipient structure. Then, place the cuff strictly parallel to the recipient structure before pushing it forward in order to avoid injury to the back wall of the respective structure (marked in red).
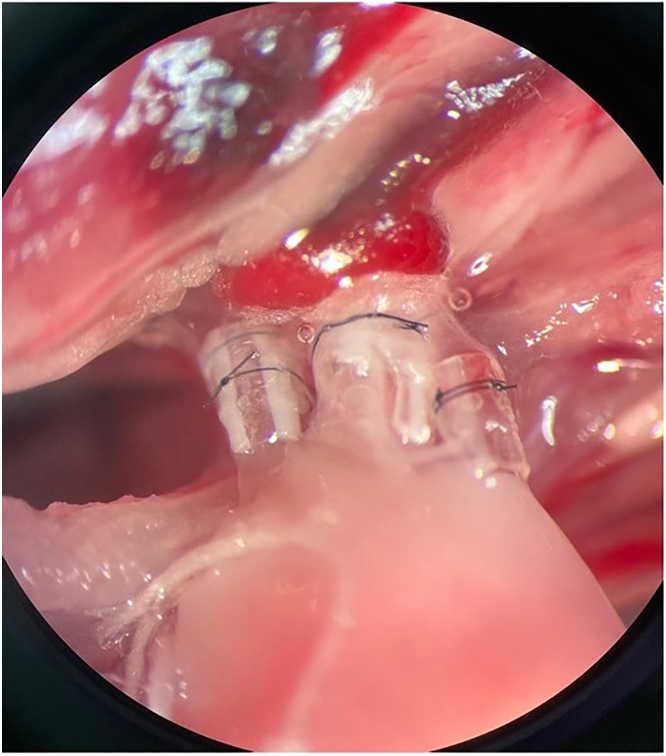
Figure 8.
View of the hilum after implantation of the donor structures
Postoperative care
-
57.
We recommend single housing of the recipient animals during the first postoperative days.
-
58.
Observe the recipient animal regularly over the first 2–4 postoperative days as obstruction of the vascular anastomosis may impair the animal’s well-being during this time.
-
59.
Pain medication should be provided in accordance with your respective institutional permission. In our Institution, 30 min before the start of surgery, the mice are administered Buprenorphine at a concentration of 0.1 mg per kg body weight and Metamizole at a concentration of 200 mg per kg body weight subcutaneously. In the recipient mice for analgesic treatment, Buprenorphine at a concentration of 0.1 mg per kg body weight is administered subcutaneously to the mice every 8 h for 3 days from the end of surgery. For further analgesic treatment, the mice also receive Metamizole via drinking water (see “materials and equipment”; temperature 22°C–23°C and maximum storage 24 h) from one day before surgery, until two days after surgery. Metamizole is administered at a dose of 1200 mg / kg body weight (200 mL water, 5% glucose and 40 drops, equivalent to 1000 mg). Mouse cages are kept on a hot plate for the first 6 postoperative hours. The mice are observed for pain and complications every hour for the first 6 postoperative hours, then every 8 h until the third postoperative day. Thereafter, the mice are examined daily until the time of euthanization.
Extravasation assay
Timing: 1–2 h per mouse
Note: This part of the protocol describes the extravasation assay using intravenous tail vein injection of MC38-GFP cells following the lung transplantation.
-
60.Prepare cancer cells.
-
a.Resuspend aliquoted cancer cells (5 million cells per mL) by aspiring them thrice with a 21-gauge needle and a 1 mL syringe.
-
b.Carefully aspire 100 μL of the resuspended cancer cells with a 27-gauge needle and a 1 mL syringe.
-
a.
CRITICAL: Avoid air bubbles.
-
61.
14 days after lung transplantation, we perform the extravasation assay. Anesthetize one mouse by using an appropriate induction chamber with an oxygen flow of 500 mL/min with 5% nebulized isoflurane until the inter-toe reflex is lost.
-
62.
Maintain anesthesia by using the same oxygen flow with 2%–3% isoflurane provided via a mask.
-
63.
Dilate the tail vein by applying infrared light for approximately 20 s (Figure 9).
CRITICAL: Avoid thermically damaging the tail by holding your finger next to the tail to evaluate if the heat is becoming too strong.
Note: Alternatively, the tail can be positioned in a water bath heated to 38° for 20 s.
-
64.
Position the mouse on its right or left side.
-
65.
Hold the tail with your non-dominant hand and stretch it into a straight line. The tail vein should be visible as a purple, straight line facing up (Figure 9C).
-
66.
Insert the tip of your needle in a 10° angle into the tail vein. The needle should slide in the vein very easily once you are inside the lumen of the vein (Figure 9D).
-
67.
Slowly inject 100 μL of your cell suspension.
CRITICAL: The injection has to feel very smooth, and a short whitening of the tail vein has to be observed. If a resistance can be felt while injecting and if a whitening of one area around the injection side can be observed, intravenous injection was not successful.
-
68.
After injection, wait 30 s with the needle still inside the vein.
-
69.
Quickly remove the needle and immediately press a sterile gauze on the vein for 20 s.
-
70.
Remove the gauze and check that no further bleeding from the vein occurs.
-
71.
Place the mouse back into its cage and monitor it until it is fully awake.
-
72.
24 h later, euthanize mice according to your local guidelines.
-
73.
Disinfect mice using 70% ethanol.
-
74.
Open the thoracic cavity and the cervical part of the mice until the chin.
-
75.
Cut the left ventricle of the heart.
-
76.
Insert a 21-gauge needle into the right ventricle of the heart and flush the mouse with 5–10 mL PBS.
Note: During this step, the lungs will turn from pink to white.
-
77.
Dissect each lung by carefully removing the aorta, esophagus, vena cava, diaphragm, and the heart.
-
78.
Store each lung in RPMI 1640 medium containing 1% FBS on ice.
-
79.
Cut each lung into small pieces and digest the pieces using HBSS (with Ca2+ and Mg2+) containing Collagenase (1 mg/mL) and DNase I (10 U/mL) on a shaker at 37°C for 25 min.
-
80.
Smash each lung into a single cell suspension using a 100 μm cell strainer. Wash the cells using 1% FBS in PBS and fill up to 50 mL.
-
81.
Centrifuge the cells at 400 × g for 8 min and discard the supernatant.
-
82.
Add 1 mL PBS to resuspend the cells and take 100 μL for flow cytometry.
Note: Always prepare a sample from a mouse injected with the same cell line but without fluorescence as a gating control.
-
83.
Add 1 mL MACS buffer (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks) to wash the cells and centrifuge again at 400 × g for 8 min.
-
84.
Resuspend the cells with counting beads diluted in MACS buffer (1:10) and acquire the data using flow cytometry (Figure 10).
Note: Alternatively, another approach to count cell numbers in each sample could be used.
-
85.
Calculate the number of extravasated cells and analyze.
Figure 9.
Intravenous injection of cancer cells
(A) Schematic view of intravenous injection of cancer cells.
(B) Application of infrared light to dilatate the tail vein.
(C) A purple straight line was seen while the mouse tail was stretched.
(D) A smooth insert of needle tip into the tail vein.
Figure 10.
FACS plot of MC38shC and MC38 GFP-labeled cells
Expected outcomes
After the extubation, the recipient animal should quickly recover from the surgery and show a normal breathing pattern. Within the first 2 h, it should move freely around its cage and explore its surroundings. Apathy of the animal on the first postoperative days or abnormal breathing patterns are signs of venous thrombosis or airway damage (see below).
When using this method to induce lung metastasis, nearly 100% of mice are expected to develop lung metastasis in 21–28 days. Using the lung extravasation assay, 100% of mice will have extravasated cancer cells in the lung parenchyma.
Limitations
Despite all the advantages of the intravenous metastasis model outlined above, some limitations of the model must be discussed. The nature of forced metastasis is that cells are forced into the circulation of the host. Thus, the first steps of metastasis, especially intravasation, are bypassed. Therefore, the intravenous model can only be used to study the later phases of metastatic seeding. The success of the lung transplantation critically depends on the functionality of all three anastomoses and the integrity of the donor lung parenchyma. In the following section, we describe the limitations of the lung transplant protocol that can be observed with regard to dysfunction of the vascular and airway anastomoses.
Bronchial anastomosis
In case of an unnoticed anastomotic insufficiency at the bronchus, the animal will develop pneumothorax once the thoracic cavity is closed. As air leakage from the bronchial anastomosis is generally large in volume, the animal will rapidly develop respiratory distress and die from tension pneumothorax. Therefore, we recommend to check the bronchial anastomosis prior to closing the animal’s chest. Small injuries to the recipient bronchus during dissection should be addressed in a timely fashion as they may impair the ventilation of the animal during the surgery. Introduction and placement of the donor cuff proximal to the bronchial injury can provide an easy intraoperative solution.
Parenchymal damage
Damage to the donor graft parenchyma will likewise result in the development of pneumothorax, causing respiratory distress and death of the recipient animal. In order to avoid any damage to the graft, caution should be taken when explanting the heart-lung-block from the donor – especially when cutting the left-sided diaphragm. During the following steps, we recommend covering the donor graft with a moistened gauze swab to avoid accidental damage to the parenchyma with the sharp tips of the microsurgical forceps.
Arterial anastomosis
Due to its length and flexibility, the donor artery is prone to torsion when moving the donor graft around. Implantation of a twisted donor artery results in the absence of arterial perfusion of the graft. Regarding the postoperative outcome, insufficient arterial perfusion does not immediately impair the well-being of the recipient animal. However, it largely affects the results of the experimental study. We recommend to check the donor artery for signs of torsion directly prior to its implantation.
Venous anastomosis
Venous occlusion due to thrombosis or mechanical obstruction causes congestion of the pulmonary graft. The recipient animal will suffer from respiratory distress and circulatory failure. Recipient animals with venous occlusion show a labored breathing pattern and their feet are cold to the touch. Generally, venous occlusion requires euthanasia of the recipient animal. During graft preparation, make sure to remove all excess atrial tissue from the vein after attaching the cuff. We recommend carefully checking the donor vein for torsion before introduction to the recipient vein. Furthermore, flushing the recipient vein can reduce the likelihood of venous thrombosis. The venous cuff should be placed as centrally as possible to avoid kinking. After fixation of the anastomosis, carefully check for leftover fatty tissue around the introduced venous cuff that may obstruct venous blood flow.
Troubleshooting
Problem 1
Perfusion of the donor graft was not sufficient and the lung graft is still significantly red in color (related to Step 12 of the lung transplant procedure).
Potential solution
Proceed with the protocol and prepare a graft from the heart-lung block. After attaching the cuff to the donor vein, gently apply a retrograde perfusion with Perfadex Plus using a 1 mL syringe equipped with a 26 G venous catheter. Make sure to avoid air bubbles. Retrograde perfusion can salvage the graft, achieving a sufficiently perfused lung.
Problem 2
Preparation of the bronchus results in bronchial tears or a short bronchial structure (related to Step 20 of the lung transplant procedure).
Potential solution
-
•
Cut the bronchus closer to the tracheal bifurcation. In order to identify it, carefully dissect the airways from the overlying fatty tissue.
-
•
Make sure that there is appropriate tension on the donor heart-lung block. You can add more tension to the structures by pulling on the ligature around the trachea.
-
•
The bronchial back wall may easily tear. Therefore, carefully dissect it from the esophagus behind it and always remember to work closely to the esophagus.
Problem 3
Preparation of the pulmonary vein results in a short donor vein (related to Step 21 of the lung transplant procedure).
Potential solution
-
•
In order to facilitate the introduction to the recipient structures, enough flexible movement between the bronchus and vein is absolutely necessary.
-
•
When cutting the vein from the heart-lung block, cut deeply inside the atrium to achieve the longest possible donor vein.
-
•
After separating the left lung from the heart-lung block, gently pull the bronchus and vein apart. A fine layer of connective tissue is spread out between the two structures and can be pulled apart without damaging the vein or bronchus.
Problem 4
Exposure of the recipient structures is not optimal (related to Step 37 of the lung transplant procedure).
Potential solution
-
•
Introduction of the donor cuffs can best be achieved when the hilar structures are placed in a horizontal orientation.
-
•
Make sure that the animal is positioned in a half-supine position rather than a true lateral decubitus position. Positioning of the lower body in a supine position and turning the upper body sideways usually achieve better exposure.
-
•
Make sure that the thoracotomy is performed all the way from the sternum to the spine and that the retractor is sufficiently opened to allow for optimal exposure.
Problem 5
Implantation is considerably difficult due to a confusing number of ligatures in the thoracic cavity and tangles between them (related to Step 43 of the lung transplant procedure).
Potential solution
-
•
Central closure of the recipient vessels can be done using vessel clips instead of ligatures. However, this may result in a loss of length of the recipient structures for implantation.
-
•
Before placing the loose ligatures for implantation, be aware of your implantation sequence and prepare the ligatures in reverse order. If you plan on introducing the vein first, place the ligature around the vein last. This way, the ligatures do not entangle easily and can be easily applied in the correct order.
Problem 6
Placing the ligature around the venous/arterial/bronchial anastomosis is not possible (related to Step 45 of the lung transplant procedure).
Potential solution
-
•
Problems in placing the ligatures around the introduced cuffs can be avoided by careful dissection of the recipient structures for their full length.
-
•
Failure to place the ligature firmly around the introduced cuff is often caused by excess connective tissue between or behind the recipient structures.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anastasios Giannou, a.giannou@uke.de.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate datasets or code.
Acknowledgments
The authors thank Tom Blankenburg, Sandra Wende, and Cathleen Haueis for their excellent technical assistance. Furthermore, the authors thank Anke Buck-Ohm for her artistic support in creating the Figures 2, 3, 4, 5, and 6. This work was supported in part by the Deutsche Forschungsgemeinschaft (grants SFB841 and SFB1328 to S.H.), the Deutsche Krebshilfe (no. 70114853 to A.D.G.), Else Kröner Memorial Stipendium (to A.D.G.), Erich und Gertrud Roggenbuck-Stiftung (to A.D.G.), Hamburger Krebsgesellschaft Stiftung (to A.D.G.), and the Jung Foundation for Science and Research (to A.D.G.). S.H. has an endowed Heisenberg Professorship awarded by the Deutsche Forschungsgemeinschaft.
Author contributions
A.D.G. and B.O. performed all rodent surgeries and prepared the figures. A.D.G. and B.O. wrote the manuscript. D.E.Z., J.L., T.Z., and P.S. created the videos and edited the manuscript. J.O., R.G., P.B., T.H., J.R.I., and Y.Y. edited the manuscript and provided intellectual input. S.H. and W.J. provided scientific supervision, revised, and finalized the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102701.
Contributor Information
Anastasios D. Giannou, Email: a.giannou@uke.de.
Wolfgang Jungraithmayr, Email: wolfgang.jungraithmayr@uniklinik-freiburg.de.
Supplemental information
References
- 1.Giannou A.D., Kempski J., Shiri A.M., Lücke J., Zhang T., Zhao L., Zazara D.E., Cortesi F., Riecken K., Amezcua Vesely M.C., et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity. 2023;56:125–142.e12. doi: 10.1016/j.immuni.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Garner H., de Visser K.E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat. Rev. Immunol. 2020;20:483–497. doi: 10.1038/s41577-019-0271-z. [DOI] [PubMed] [Google Scholar]
- 4.Jungraithmayr W.M., Korom S., Hillinger S., Weder W. A mouse model of orthotopic, single-lung transplantation. J. Thorac. Cardiovasc. Surg. 2009;137:486–491. doi: 10.1016/J.JTCVS.2008.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or code.