Abstract
Bacterial enrichment cultures developed with Baltimore Harbor (BH) sediments were found to reductively dechlorinate 2,3,5,6-tetrachlorobiphenyl (2,3,5,6-CB) when incubated in a minimal estuarine medium containing short-chain fatty acids under anaerobic conditions with and without the addition of sediment. Primary enrichment cultures formed both meta and ortho dechlorination products from 2,3,5,6-CB. The lag time preceding dechlorination decreased from 30 to less than 20 days as the cultures were sequentially transferred into estuarine medium containing dried, sterile BH sediment. In addition, only ortho dechlorination was observed following transfer of the cultures. Sequential transfer into medium without added sediment also resulted in the development of a strict ortho-dechlorinating culture following a lag of more than 100 days. Upon further transfer into the minimal medium without sediment, the lag time decreased to less than 50 days. At this stage all cultures, regardless of the presence of sediment, would produce 2,3,5-CB and 3,5-CB from 2,3,5,6-CB. The strict ortho-dechlorinating activity in the sediment-free cultures has remained stable for more than 1 year through several transfers. These results reveal that the classical microbial enrichment technique using a minimal medium with a single polychlorinated biphenyl (PCB) congener selected for ortho dechlorination of 2,3,5,6-CB. Furthermore, this is the first report of sustained anaerobic PCB dechlorination in the complete absence of soil or sediment.
Anaerobic dechlorination of polychlorinated biphenyls (PCBs) has been demonstrated in situ and with laboratory microcosms containing sediment (reviewed in reference 1a). However, sustained PCB dechlorination has never been shown to occur in the absence of soil or sediments. Morris et al. (6) demonstrated a sediment requirement for the stimulation of PCB dechlorination within freshwater sediment slurries. Wu and Wiegel have recently described PCB-dechlorinating enrichments which required soil for the successful transfer of PCB-dechlorinating activity (9). In addition, no anaerobic microorganisms that dechlorinate PCBs have been isolated or characterized, and this may be due in part to the soil or sediment requirement. The inability to isolate dechlorinating organisms or maintain dechlorination without sediment has limited biogeochemical and physiological investigations into the mechanisms of PCB dechlorination.
Dechlorination (ortho, meta, and para) of single PCB congeners has been observed following anaerobic incubation of Baltimore Harbor (BH) sediment under estuarine or marine conditions (2). While sediments from several sites within BH are contaminated with PCBs (1, 5), background contamination of sediment is not necessarily a prerequisite for the development of PCB dechlorination in laboratory microcosms. Wu et al. (8) recently demonstrated meta and ortho dechlorination of Aroclor 1260 when it was added to the same BH sediments. These results showed that more than one dechlorinating activity could be developed with these sediments. It has been proposed that discrete microbial populations are responsible for specific PCB dechlorinations (1a). Consistent with this idea, the ortho dechlorination observed with BH sediments may be catalyzed by discrete microbial populations. In addition, these organisms may be able to couple PCB dechlorination with growth. Therefore we have attempted to select for ortho PCB-dechlorinating organisms by enrichment under minimal conditions with high levels of 2,3,5,6-tetrachlorobiphenyl. We also speculated that given the proper conditions, a PCB-dechlorinating population could be maintained in an actively dechlorinating state in the absence of sediment. Here we report that a distinct PCB-dechlorinating activity, namely, ortho dechlorination, was selected for through sequential transfer initiated with sediments from BH and sustained in the absence of soil or sediment. This is the first report of sustained anaerobic PCB-dechlorinating activity in the total absence of sediment.
MATERIALS AND METHODS
Sediment samples.
Sediment samples were collected with a petite Ponar grab sampler from a subsurface depth of 9.1 m in the northwest branch of BH (39°16.8′N, 76°36.1′W). An oily slick and gas bubbles formed at the surface immediately after the sampler disturbed the sediments. Sediments had a black coloration, a gelatinous texture, and a strong petroleum odor. The combined contents of the sampler were transferred to 0.95-liter canning jars (Ball Corporation, El Paso, Tex.). The jars were filled to the top and immediately sealed with dome tops and ring seals to exclude air. The samples were stored at ambient temperature in the dark prior to use.
Culture conditions.
All sterile media in these experiments included an estuarine salts medium without sulfate (E-Cl) and were prepared anaerobically in an atmosphere that contained N2-CO2 (4:1) as previously described by Berkaw et al. (2). Briefly, the medium contained the following constituents, in grams per liter of demineralized water: Na2CO3, 3.0; Na2HPO4, 0.6; NH4Cl, 0.5; cysteine-HCl · H2O, 0.25; Na2S · 9H2O, 0.25; MgCl2 · 6H2O, 0.1; CaCl2 · 6H2O, 0.1; and resazurin, 0.001. In addition, vitamin and trace element solutions (1% [vol/vol] each) were added (7). The final pH of the medium was 6.8. Media were dispensed into anaerobic culture tubes (18 by 160 mm; Bellco Glass, Inc., Vineland, N.J.) or 150-ml serum bottles (Wheaton, Millville, N.J.) sealed with Teflon-lined butyl stoppers (The West Co., Lionville, Pa.) that were secured with aluminum crimp seals (Wheaton).
Primary sediment enrichment cultures were generated in culture tubes by adding 2 ml of BH sediment to 8 ml of sterile E-Cl medium (approximately 5%, wt/vol [dry weight], sediment concentration), plus a mixture of sodium acetate, propionate, and butyrate to final concentrations of 2.5 mM each. Congener 2,3,5,6-tetrachlorobiphenyl (2,3,5,6-CB) was solubilized in acetone and added to each culture to a final concentration of 173 μM (50 ppm), and this resulted in a 0.1% (vol/vol) concentration of acetone. Cultures were incubated under strict anaerobic conditions at 30°C in the dark. Killed-cell controls were sterilized in an autoclave at 121°C for a total of 3 h (two 1.5-h treatments). Sequential transfers of sediment-containing cultures were made as follows. The entire sediment-containing culture was made into a suspension by shaking, and then the particulate matter was allowed to settle for approximately 1 min. Supernatant material was then transferred in order to minimize the amount of sediment passed to the next vessel. Sequential transfers (10% [vol/vol]) from primary enrichment cultures were made into E-Cl medium with dried BH sediment (0.1%, wt/vol [dry weight], unless stated otherwise) that was then sterilized in an autoclave at 121°C for a total of 3 h (two 1.5-h treatments). Subsequent transfers were made under identical conditions every 2 to 5 months. Sequential transfers (10% [vol/vol]) for the establishment of sediment-free cultures were made every 2 to 5 months into identical media without sediment. Following the first two transfers, the amount of sediment passed was negligible.
Spectrophotometric analysis.
Growth in sediment-free cultures was monitored by measuring the increase in optical density at 600 nm (OD600) with a Spectronic 20D spectrophotometer (Milton Roy, Rochester, N.Y.).
Sampling and PCB analysis.
Aliquots were withdrawn anaerobically once at each time point from shaken cultures by using the reverse end of a 5-ml glass pipette (front end for sediment-free samples). Samples were extracted in ethyl acetate and passed over a Florisil-copper column as previously described by Berkaw et al. (2). Analysis was conducted with a Hewlett-Packard 5890A gas chromatograph (GC) equipped with an electron capture detector (ECD) and an RTX-1 capillary column as previously described (2). Standards for 2-, 3-, 4-, 2,3-, 2,5-, 2,6-, 3,5-, 2,3,5-, 2,3,6- and 2,3,5,6-CB were purchased from AccuStandard (New Haven, Conn.). PCB congeners were identified by retention time and quantified with a 16-point calibration curve for each congener according to the method of Berkaw et al. (2).
RESULTS AND DISCUSSION
Selection of ortho dechlorination.
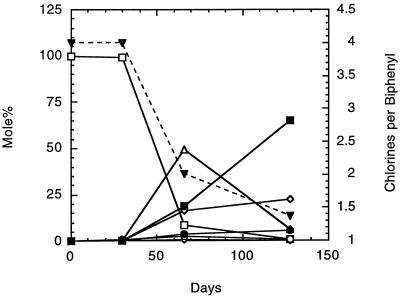
Reductive dechlorination of 2,3,5,6-CB was observed to occur in primary enrichment cultures incubated with BH sediment under anaerobic conditions. GC-ECD analysis revealed various meta and ortho dechlorination products. Large amounts of transient 3,5-CB, along with smaller amounts of 2,5-CB and 2,6-CB, were observed, while 3-CB eventually became the dominant product (Fig. 1). No dechlorination products were ever observed in killed-cell controls (sterilized sediment and media) or no-inoculum controls.
FIG. 1.
Dechlorination of 2,3,5,6-CB by a primary enrichment culture with BH sediment (5.0%, wt/vol [dry weight]). Mole percent and chlorines-per-biphenyl data are from a single culture. Symbols: ■, mole percent for 3-CB; ◊, 2,5-CB; •, 2,6-CB; ▵, 3,5-CB; ○, 2,3,5-CB; ⧫, 2,3,6-CB; and □, 2,3,5,6-CB. ▾, chlorines per biphenyl.
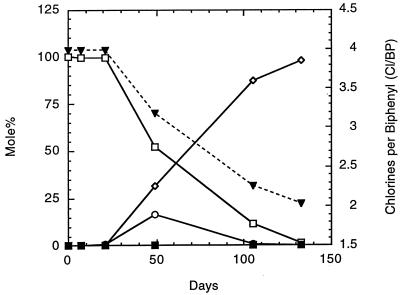
Sequential transfers (10% [vol/vol]) from the primary culture were made into E-Cl medium containing 0.1% (wt/vol [dry weight]) sterile BH sediment. Selection of ortho dechlorination reactions with a loss of meta reactions was observed after the first transfer. Further transfer resulted in cultures that exclusively ortho dechlorinated 2,3,5,6-CB to 3,5-CB within 50 days, with 2,3,5-CB as the only intermediate detected by GC-ECD analysis (Fig. 2). In contrast to the large accumulation of 3-CB observed in primary cultures, virtually all of the 2,3,5,6-CB was transformed to 3,5-CB in the transferred cultures, with no other end products observed. This pattern of strict ortho dechlorination of the 2,3,5,6-CB remained the same through five sequential transfers (made once every 3 to 5 months) beyond the primary enrichment cultures. In addition, no monochlorobiphenyl arose, even after extensive incubation lasting up to a year.
FIG. 2.
Dechlorination of 2,3,5,6-CB after the first transfer of the supernatant from the primary enrichment culture into E-Cl medium with BH sediment (0.1% wt/vol [dry weight]). Mole percent and chlorines-per-biphenyl data are from a single culture. Symbols: ■, mole percent for 3-CB; ◊, 3,5-CB; ○, 2,3,5-CB; and □, 2,3,5,6-CB. ▾, chlorines per biphenyl.
Microbial dechlorination of 2,3,5,6-CB in the absence of sediment.
Sequential transfers (10% [vol/vol]) from the primary cultures containing 5% (wt/vol [dry weight]) BH sediment were made into E-Cl medium with no added sediment. By the second transfer, the sediment was no longer visible to the naked eye and a low rate of dechlorination was observed. A mixed culture dominated by blunt-end rod-shaped cells and small vibrio-shaped cells developed. Each transfer culture was started at an OD600 between 0.01 and 0.05 and was transferred after the OD600 was >0.1, regardless of the degree of dechlorinating activity. Congener 2,3,5,6-CB was added to a final concentration of 173 μM, which is significantly above its aqueous solubility limit (3). However, reasonable recoveries of the PCB, 80% on average, could be made by vigorously mixing the culture with a pipette before sampling. It was assumed that the PCBs were sorbing to or partitioning into the biomass.
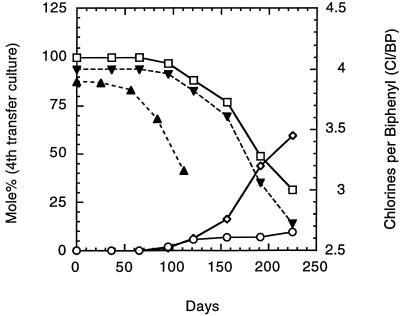
The objective at this point was to detect a significant amount of dechlorination product in the sediment-free culture series. Significant amounts (mole percent) of 2,3,5-CB and 3,5,-CB began to accumulate in the fourth-sequential-transfer cultures following extensive incubation (Fig. 3). After more than 200 days, when ortho dechlorination had been clearly established and the culture had become more turbid (OD600 > 0.2), these sediment-free cultures were transferred again. This fifth-sequential-transfer culture had a shortened lag time of less than 50 days (Fig. 3). No dechlorination was observed with sterile (killed-cell) or no-inoculum controls. Only ortho dechlorination of the 2,3,5,6-CB to 2,3,5-CB and 3,5-CB was observed at all times with all of these cultures. Since the establishment of stable dechlorination, we have been able to routinely transfer these cultures into identical sediment-free media and still maintain dechlorinating activity.
FIG. 3.
Chlorines-per-biphenyl data for fourth- and fifth-sequential-transfer cultures without sediment. Mole percent data are given for the fourth-transfer culture. All data are given as the averages from duplicate cultures. Symbols: ◊, mole percent for 3,5-CB; ○, 2,3,5-CB; and □, 2,3,5,6-CB. ▾, chlorines per biphenyl of fourth-sequential-transfer culture; ▴, chlorines per biphenyl of fifth-sequential-transfer culture.
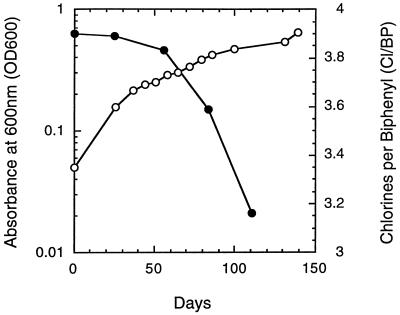
The appearance of dechlorination at the fourth transfer of the sediment-free cultures after an incubation period exceeding that of earlier cultures in the transfer series suggests that the transfers were made too quickly (at low cell density) during the early part of the enrichment process. OD data for a later set of active sediment-free cultures (Fig. 4) revealed that significant dechlorination does not occur until the OD600 exceeds 0.2. This observation supports our conclusion that the ability to maintain good dechlorination earlier on in the sediment-free enrichment series was hindered by premature transfer of the cultures at low turbidity. Perhaps the earlier transfers at lower turbidity had prevented the development of hearty dechlorinating cultures and sustainability was simply an issue of low numbers of dechlorinators among the total population. The possibility that the organisms responsible for the dechlorination needed an extensive amount of time to adjust to the altered conditions (lack of sediment) before being able to carry out the dechlorination also exists. This latter possibility may be associated with the uptake (availability) of the PCB or supply of a nutrient. It is also possible that during this lengthy process we enriched for a prototroph that no longer requires a component of the sediment in order to dechlorinate a PCB.
FIG. 4.
OD (○) and chlorines-per-biphenyl (•) data from duplicate fifth-sequential-transfer cultures without sediment.
Sediment stimulation of ortho dechlorination.
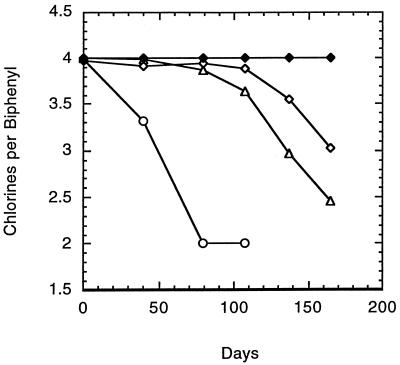
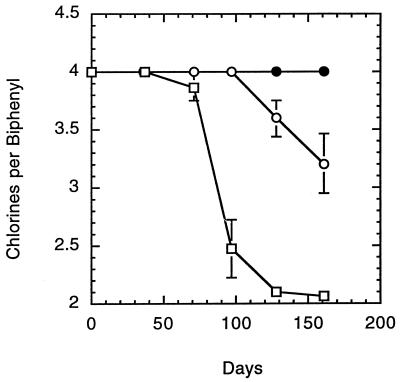
The above results demonstrate that ortho dechlorination is independent of the sediment. However, several results show the sediment to have a stimulatory effect. The first suggestion of this was observed with the decrease in the rate and extent of ortho dechlorination that accompanied the shift from meta-and-ortho to strictly ortho dechlorination (Fig. 1 and 2). This occurred after a primary culture had been transferred to a medium with far less sediment (5.0 to 0.1%, wt/vol [dry weight]). This change in activity could have been due to the decrease in the amount of sediment present. To examine this, a range of sediment concentrations was tested under the conditions described above. In order to be certain of the sediment concentration, the supernatant from the primary culture was transferred (10% [vol/vol]) into vessels containing E-Cl medium with the different amounts of BH sediment to be tested. After 4 months of incubation, transfers were made from these cultures into identical medium and the results of this second set of cultures are presented in Fig. 5. While dechlorinating activity could be maintained regardless of the sediment concentration, the lag preceding dechlorination increased to more than 100 days when the sediment concentration was lowered to 0.05% (dry wt). The cultures incubated with 1.0% (dry wt) sediment exhibited a higher rate of dechlorination and a shorter lag time than did those incubated with lesser amounts of sediment. Killed-cell controls (sterilized sediment cultures) exhibited no dechlorination. From a qualitative perspective, dechlorination did not change with sediment concentration and remained strictly ortho. Additional experiments with sediment-free cultures also demonstrated that the sediment could be stimulatory. Pre-dechlorination sediment-free cultures (in this case the fourth sequential sediment-free transfer cultures before the onset of dechlorination) were transferred into E-Cl medium with and without 1.0% (dry weight) BH sediment (sterile). The pre-dechlorination transfer cultures with sediment showed a quicker recovery of dechlorination than did transfer cultures maintained without sediment (Fig. 6). Once again, no dechlorination was observed with killed-cell controls. This confirmed the existence of a factor(s) in the sediment that was stimulatory but not required for dechlorination.
FIG. 5.
Cultures with 2,3,5,6-CB and 1.0% (○), 0.1% (▵), and 0.05% (◊) (wt/vol [dry weight]) sterilized BH sediment. Supernatant from a 5.0% sediment culture was sequentially transferred with 1.0, 0.1, and 0.05% (wt/vol [dry weight]) BH sediment in E-Cl medium, incubated for 4 months, and transferred again under identical conditions. The data presented represent the second set of transferred cultures. The chlorines-per-biphenyl data for the killed-cell control with 1.0% sterilized BH sediment are for a single culture (⧫). The data from the live BH cultures are the average of duplicates.
FIG. 6.
Effect of sediment added to a pre-dechlorination sediment-free culture. The fourth-sequential-transfer sediment-free cultures (pre-dechlorination) were transferred to medium with (□) and without (○) 1.0% sterilized BH sediment. •, killed-cell control. All data are for triplicate cultures. Error bars indicate standard deviations.
The mode of action of the sediment stimulation of PCB dechlorination has not been determined. Humic acids (Aldrich Chemical Co., Milwaukee, Wis., and other sources) and anthraquinone-2,6-disulfonic acid (AQDS) have been shown to act as intermediate electron acceptors in the facilitation of biological Fe3+ reduction (4). Similarly, humic substances or AQDS might stimulate ortho PCB dechlorination. However, substitution of two different commercial humic acids at 0.1% (wt/vol [dry weight]) (Burlington Chemical Co., Long Island, N.Y., and Aldrich Chemical Co.) or 3 mM AQDS (Aldrich Chemical Co.) did not provide the same degree of stimulation of PCB dechlorination to the cultures as BH sediment (data not shown). In fact, the Aldrich humic acids and the AQDS completely inhibited dechlorination. The results from these experiments do not define the stimulatory role of the sediment, but they do demonstrate the utility of the sediment-free cultures in addressing such questions. Other possible roles for the BH sediment include stimulation due to additional carbon and energy or micronutrients, facilitation of the availability of the PCB to the microorganisms (this could also prevent toxicity due to PCBs), supply of a more suitable attachment site for microbial colonization, or supply of an extracellular catalytic intermediate similar to AQDS which may facilitate the dechlorination.
Concluding remarks.
PCB dechlorination can occur in the absence of sediment, albeit more slowly than with sediment, indicating that whatever is contributed by the sediment is not essential or irreplaceable. We have now been able to sequentially transfer the ortho-dechlorinating culture eight times over a 33-month period in the minimal, sediment-free medium described here. Further, with the use of nearly identical enrichment procedures and patience, we have recently established enrichment cultures which actively para dechlorinate 2,3,4,5-CB in the absence of sediment. These cultures are incapable of dechlorinating 2,3,5,6-CB. We are still pursuing a meta-dechlorinating sediment-free enrichment culture.
Perhaps the most significant contribution of the findings presented here is that the sediment-free cultures offer opportunities to address questions of mechanism, cell structure, and identity that were not approachable in the past. For example, with the ability to make microscopic observation and determine OD, etc., questions concerning growth and whether it can be coupled to PCB dechlorination can now be more easily addressed. Isolation and characterization of the microorganisms present in these cultures can now proceed at a faster pace since the organisms are in a defined medium. We are taking advantage of this by investigating a broader group of electron donors and acceptors, individually, without interference from unknown substances in the sediment while monitoring the microbial population through molecular identification.
The development of the actively dechlorinating sediment-free cultures provides a unique opportunity for further experimentation concerning the identification of the stimulating factors in the sediment. In addition, the sediment-free cultures can act as a model system to investigate biochemical and geochemical mechanisms internal and external to the cell which may contribute to PCB dechlorination. Finally, studies of how to grow these organisms free of soil or sediment may lead to the ability to mass culture such organisms. This is needed in order to advance investigation of the biochemical mechanism of PCB dechlorination and could also be important for bioaugmentation studies of PCB-contaminated sediments.
ACKNOWLEDGMENTS
This work was supported by grants from the Office of Naval Research (ONR), U.S. Department of Defense (DOD), to H.M. (grant N00014-96-1-0116) and to K.S. (grant N00014-96-1-0115). L.C. was supported by a DOD award (Augmentation Awards for Science and Engineering Research Training) from ONR to H.M. (grant N00014-96-1-1033).
REFERENCES
- 1.Baker J, Mason R, Cornwell J, Ashley J, Halka J, Hill J. Spatial mapping of sedimentary contaminants in the Baltimore Harbor, Patapsco River, Back River System. Report UMCES [CBL] 97-142. Solomon, Md: Chesapeake Biological Laboratory; 1997. [Google Scholar]
- 1a.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 2.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes D A, Harrison B K, Dolfing J. Estimation of Gibbs free energies of formation for polychlorinated biphenyls. Environ Sci Technol. 1993;27:725–731. [Google Scholar]
- 4.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 5.Maryland Department of the Environment. Toxics regional action plan for Baltimore Harbor. Baltimore: Maryland Department of the Environment; 1996. [Google Scholar]
- 6.Morris P J, Mohn W W, Quensen III J F, Boyd S A, Tiedje J M. General Electric Company Research and Development Program for the Destruction of PCBs, Ninth progress report. Schenectady, N.Y: General Electric Corporate Research and Development; 1990. Establishment and characterization of an anaerobic, Aroclor 1242-dechlorinating culture. [Google Scholar]
- 7.Wolin E A, Wolin M J, Wolfe R S. Formation of methane by bacterial extracts. J Biol Chem. 1963;238:2882–2886. [PubMed] [Google Scholar]
- 8.Wu Q, Sowers K R, May H D. Microbial reductive dechlorination of Aroclor 1260 in anaerobic slurries of estuarine sediments. Appl Environ Microbiol. 1998;64:1052–1058. doi: 10.1128/aem.64.3.1052-1058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q, Wiegel J. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl Environ Microbiol. 1997;63:4826–4832. doi: 10.1128/aem.63.12.4826-4832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]