Abstract
BACKGROUND
Although several experimental therapeutics for Ebola virus disease (EVD) have been developed, the safety and efficacy of the most promising therapies need to be assessed in the context of a randomized, controlled trial.
METHODS
We conducted a trial of four investigational therapies for EVD in the Democratic Republic of Congo, where an outbreak began in August 2018. Patients of any age who had a positive result for Ebola virus RNA on reverse-transcriptase–polymerase-chain-reaction assay were enrolled. All patients received standard care and were randomly assigned in a 1:1:1:1 ratio to intravenous administration of the triple monoclonal antibody ZMapp (the control group), the antiviral agent remdesivir, the single monoclonal antibody MAb114, or the triple monoclonal antibody REGN-EB3. The REGN-EB3 group was added in a later version of the protocol, so data from these patients were compared with those of patients in the ZMapp group who were enrolled at or after the time the REGN-EB3 group was added (the ZMapp subgroup). The primary end point was death at 28 days.
RESULTS
A total of 681 patients were enrolled from November 20, 2018, to August 9, 2019, at which time the data and safety monitoring board recommended that patients be assigned only to the MAb114 and REGN-EB3 groups for the remainder of the trial; the recommendation was based on the results of an interim analysis that showed superiority of these groups to ZMapp and remdesivir with respect to mortality. At 28 days, death had occurred in 61 of 174 patients (35.1%) in the MAb114 group, as compared with 84 of 169 (49.7%) in the ZMapp group (P = 0.007), and in 52 of 155 (33.5%) in the REGN-EB3 group, as compared with 79 of 154 (51.3%) in the ZMapp subgroup (P = 0.002). A shorter duration of symptoms before admission and lower baseline values for viral load and for serum creatinine and aminotransferase levels each correlated with improved survival. Four serious adverse events were judged to be potentially related to the trial drugs.
CONCLUSIONS
Both MAb114 and REGN-EB3 were superior to ZMapp in reducing mortality from EVD. Scientifically and ethically sound clinical research can be conducted during disease out-breaks and can help inform the outbreak response.
In August 2018, an outbreak of Ebola virus disease (EVD) began in the provinces of North Kivu and Ituri in the Democratic Republic of Congo (DRC); it was the tenth known outbreak of EVD in that country.1,2 The outbreak became the second largest that has been recorded since the first description of Zaire ebolavirus infection in 1976, and it is surpassed only by the 2013–2016 outbreak in West Africa that resulted in more than 11,000 deaths.
After the end of the outbreak in West Africa, the World Health Organization (WHO) initiated a series of discussions to develop an R&D Blueprint for EVD research that included a working group focused on how experimental therapeutics should be assessed in the context of the next EVD outbreak.3 These and other discussions led to a consensus that when a new outbreak occurred, the most promising experimental therapeutics should be studied in the context of a randomized, controlled trial, if possible.4 This groundwork facilitated the uniting of the international community and DRC leadership to develop and implement the trial described in this report.
Methods
Trial Design
The Pamoja Tulinde Maisha (PALM [“Together Save Lives” in the Kiswahili language]) trial compared ZMapp with three newer investigational agents.5 Patients were assigned in a 1:1:1:1 ratio to receive ZMapp (a triple monoclonal antibody agent), remdesivir (a nucleotide analogue RNA polymerase inhibitor6), MAb114 (a single human monoclonal antibody derived from an Ebola survivor7,8), or REGN-EB3 (a coformulated mixture of three human IgG1 monoclonal antibodies9,10). ZMapp was chosen as the control on the basis of data from the Partnership for Research on Ebola Virus in Liberia II (PREVAIL II) trial.11 The current trial was originally designed in November 2018 as a three-group trial, and the protocol was updated in January 2019 to add REGN-EB3 as a fourth group; data from this group were compared with those of patients in the ZMapp group who were enrolled on or after the time the REGN-EB3 group was added (the ZMapp subgroup). The primary end point was death at 28 days.
Trial Oversight
The trial was jointly approved by the ethics board at the University of Kinshasa and the institutional review board at the National Institute of Allergy and Infectious Diseases (NIAID) and was overseen by an independent data and safety monitoring board. Trial staff at participating Ebola treatment centers included staff from the Alliance for International Medical Action (ALIMA), International Medical Corps (IMC), Médecins sans Frontières (MSF), and the DRC Ministry of Health. Written informed consent was obtained from all patients or their legal guardians, and assent forms were obtained for children according to local standards and requirements. Full details about the trial design, conduct, oversight, and analyses are provided in the protocol and the Supplementary Appendix, both available with the full text of this article at NEJM.org. The PALM Writing Group performed the primary data analyses, wrote the manuscript, and, on behalf of the PALM Study Group, vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The Office of Clinical Research Policy and Regulatory Operations of the Division of Clinical Research of the NIAID is the holder of the Investigational New Drug application (125530) from the Food and Drug Administration. The Biomedical and Advanced Research and Development Authority of the U.S. Department of Health and Human Services provided financial support for the production of ZMapp and REGN-EB3. NIAID and the Defense Advanced Research Projects Agency of the U.S. Department of Defense provided financial support for the production and provision of MAb114.
Screening and Randomization
Patients were assessed for eligibility on the basis of a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay to detect the RNA of the nucleoprotein of Ebola virus (EBOV). Patients of any age, including pregnant women, were eligible if they had a positive result on RT-PCR within 3 days before screening and if they had not received other investigational agents (except experimental vaccines) within the previous 30 days. Neonates who were 7 days of age or younger were eligible if the mother had documented EVD. Randomization was stratified according to baseline nucleoprotein cycle-threshold (Ct) value (≤22.0 or >22.0, corresponding to higher and lower viral loads, respectively, as determined by quantitative RT-PCR) and Ebola treatment center. Trial-group assignments were placed in sequentially numbered envelopes, which were distributed to trial sites to be opened at the time of enrollment. Data were recorded on bar-coded paper case-report forms that were transmitted from the site to a server, where they were digitally sorted into electronic patient folders with the use of software developed by the University of Minnesota and were then entered by trial staff at the Institut National de Recherche Biomédicale (INRB) Coordinating Center (Kinshasa, DRC) and NIAID (Bethesda, MD) into the Web-based REDCap database.
Trial Procedures
All patients received standard care, which consisted of administration of intravenous fluids, daily clinical laboratory testing, correction of hypoglycemia and electrolyte imbalances, and administration of broad-spectrum antibiotic agents and antimalarial agents as indicated. All four trial agents were administered intravenously. Patients in the ZMapp group received a dose of 50 mg per kilogram of body weight every third day beginning on day 1 (for a total of three doses). Patients in the remdesivir group received a loading dose on day 1 (200 mg in adults, and adjusted for body weight in pediatric patients), followed by a daily maintenance dose (100 mg in adults) starting on day 2 and continuing for 9 to 13 days, depending on viral load. Patients in the MAb114 group received a dose of 50 mg per kilogram, administered as a single infusion on day 1. Patients in the REGN-EB3 group received a dose of 150 mg per kilogram, administered as a single infusion on day 1.
The Xpert Ebola Assay (Cepheid) was used for detection of the EBOV RNAs encoding surface glycoprotein and nucleoprotein.12–14 Clinical chemical analyses of plasma samples that had been separated from whole blood were performed with the use of the Piccolo Xpress Chemistry Analyzer (Abbott).
Statistical Analysis
The primary end point (death at 28 days) was assessed with the use of a modified Boschloo’s test for hypothesis testing.15 We estimated that 145 patients would need to be enrolled in each group to give the trial approximately 80% power, at a type I error rate of 5%, to show that mortality would be 50% lower in each of the groups than in the ZMapp group (15% vs. 30%). Each of the primary comparisons of remdesivir, MAb114, and REGN-EB3 with ZMapp was tested at a two-sided type I error rate of 5%, without adjustment for multiplicity (as prespecified in the statistical analysis plan). After an assessment that was conducted in a blinded manner, the protocol was amended in July 2019 to increase the sample size to 725 to improve the power of the trial while taking into account the availability of ZMapp. The sample size was revised to 185 patients each in the ZMapp, remdesivir, and MAb114 groups and 170 in the REGN-EB3 group. Comparisons were restricted to patients who were enrolled in the trial concurrently.15,16 Interim data and safety monitoring included four analyses of efficacy that were performed on the basis of prespecified enrollment targets (Table S1 in the Supplementary Appendix). Additional details are provided in the statistical analysis plan, which is included with the protocol.
Results
Patients
From November 20, 2018, to August 9, 2019, a total of 681 patients were enrolled and underwent randomization at Ebola treatment centers in Beni (335 patients), Butembo (243 patients), Katwa (46 patients), and Mangina (57 patients). Eight patients were excluded from the final analysis: 1 patient was later found to have been ineligible because of a false positive EVD result on RT-PCR assay, and 7 patients underwent randomization during a 2-week period when ZMapp was unavailable because of compromised cold-chain conditions. Of the remaining 673 participants, 169 were assigned to receive ZMapp, 175 to receive remdesivir, 174 to receive MAb114, and 155 to receive REGN-EB3. A total of 154 patients were assigned to the ZMapp group after the REGN-EB3 group had been added (the ZMapp subgroup), and data from these patients were used in the comparison of REGN-EB3 with ZMapp (Fig. S1).
Most patients (74.4%) were 18 years of age or older, 12.8% were 6 to 17 years of age, and 12.8% were 5 years of age or younger, of whom 0.7% were neonates (≤7 days old). A total of 55.6% patients were female, of whom 6.1% were pregnant at the time of EVD diagnosis (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of the Trial Population.*
| Characteristic | All Patients (N = 673) | ZMapp (N = 169) | Remdesivir (N = 175) | MAb114 (N = 174) | REGN-EB3 (N = 155) | ZMapp Subgroup† (N =154) |
|---|---|---|---|---|---|---|
| Age — yr | 28.8±17.6 | 29.7±16.8 | 29.6±17.2 | 27.4±18.5 | 28.2±18.2 | 30.2±16.7 |
| Age group — no. (%) | ||||||
| ≤5 yr | 86 (12.8) | 20 (11.8) | 16 (9.1) | 26 (14.9) | 24 (15.5) | 17 (11.0) |
| ≤7 days | 5 (0.7) | 2 (1.2) | 2(1.1) | 1 (0.6) | 0 | 2(1.3) |
| >5 yr to <18 yr | 86 (12.8) | 14 (8.3) | 25 (14.3) | 29 (16.7) | 18 (11.6) | 13 (8.4) |
| ≥18 yr | 501 (74.4) | 135 (79.9) | 134 (76.6) | 119 (68.4) | 113 (72.9) | 124 (80.5) |
| Female sex— no. (%) | 374 (55.6) | 87 (51.5) | 98 (56.0) | 98 (56.3) | 91 (58.7) | 80 (51.9) |
| Positive result on pregnancy test— no./total no. (%) | 17/277 (6.1) | 4/63 (6.3) | 6/77 (7.8) | 5/69 (7.2) | 2/68 (2.9) | 4/61 (6.6) |
| Weight — kg (% with missing data) | 47.0±19.3 (0.1) | 49.2±19.2 (0) | 47.8±17.7 (0.6) | 44.8±19.8 (0) | 46.1±20.4 (0) | 49.6±18.8 (0) |
| Patient-reported vaccination with rVSVΔG-ZEBOV-GP — no./total no. (%)‡ | 155/620 (25.0) | 41/154 (26.6) | 43/156 (27.6) | 36/157 (22.9) | 35/153 (22.9) | 41/154 (26.6) |
| <10 days before admission to the Ebola treatment center | 80/155 (51.6) | 21/41 (51.2) | 18/43 (41.9) | 21/36 (58.3) | 20/35 (57.1) | 21/41 (51.2) |
| ≥10 days before admission to the Ebola treatment center | 60/155 (38.7) | 18/41 (43.9) | 21/43 (48.8) | 10/36 (27.8) | 11/35 (31.4) | 18/41 (43.9) |
| Timing not reported | 15/155 (9.7) | 2/41 (4.9) | 4/43 (9.3) | 5/36 (13.9) | 4/35 (11.4) | 2/41 (4.9) |
| Current illness§ | ||||||
| Nucleoprotein Ct value ≤22 — no./total no. (%) | 282/670 (42.1) | 70/168 (41.7) | 73/173 (42.2) | 73/174 (42.0) | 66/155 (42.6) | 64/153 (41.8) |
| Nucleoprotein Ct value (% with missing data)¶ | 24.0±5.6 (0.4) | 23.4±5.2 (0.6) | 23.8±5.3 (1.1) | 24.6±6.4 (0) | 24.1±5.3 (0) | 23.3±5.1 (0.7) |
| Glycoprotein Ct value (% with missing data) | 28.5±4.9 (2.4) | 28.3±4.7 (1.2) | 28.4±4.8 (2.3) | 28.5±5.1 (5.2) | 28.7±4.9 (0.6) | 28.0±4.6 (1.3) |
| Days since onset of symptoms (% with missing data) | 5.5±3.5 (1.2) | 5.6±3.6 (1.2) | 5.4±3.4 (2.3) | 5.5±3.6 (0.6) | 5.4±3.2 (0.6) | 5.5±3.6 (1.3) |
| Positive result for malaria — no./total no. (%) | 57/557 (10.2) | 12/140 (8.6) | 15/139 (10.8) | 13/140 (9.3) | 17/138 (12.3) | 12/140 (8.6) |
| Serum chemical values (% with missing data) | ||||||
| Creatinine — mg/dl¶ | 2.5±2.9 (18.6) | 2.9±3.3 (22.5) | 2.7±3.0 (17.7) | 2.1±2.6 (17.2) | 2.5±2.8 (16.8) | 2.7±3.0 (22.7) |
| Potassium — mmol/liter | 4.4±1.1 (30.5) | 4.3±1.1 (34.9) | 4.3±1.1 (26.9) | 4.4±1.3 (28.7) | 4.4±1.0 (31.6) | 4.3±1.1 (33.8) |
| AST — U/liter¶ | 668±700 (40.6) | 767±745 (43.2) | 713±702 (47.2) | 546±617 (42.0) | 648±726 (38.1) | 775±749 (42.9) |
| ALT — U/liter | 379±464 (18.1) | 404±475 (21.3) | 385±471 (18.3) | 358±433 (17.8) | 368±483 (14.8) | 390±445 (21.4) |
| Vital signs (% with missing data) | ||||||
| Blood pressure — mm Hg | ||||||
| Systolic | 106.9±17.5 (13.7) | 106.1±14.9 (8.9) | 107.2±18.5 (13.1) | 106.7±17.6 (17.2) | 107.6±19.0 (15.5) | 105.9±14.8 (9.1) |
| Diastolic | 70.3±15.0 (13.7) | 71.0±14.1 (8.9) | 70.7±14.4 (13.1) | 69.7±14.7 (17.2) | 70.0±17.1 (15.5) | 70.2±14.0 (9.1) |
| Pulse — beats/min | 98.2±20.8 (2.2) | 97.2±21.1 (2.4) | 97.2±20.0 (1.7) | 98.5±21.5 (1.7) | 100.0±20.6 (3.2) | 97.4±21.4 (2.6) |
| Body temperature — °C | 37.4±1.2 (1.0) | 37.5±1.2 (0.6) | 37.3±1.3 (1.1) | 37.4±1.2 (1.1) | 37.4±1.2 (1.3) | 37.5±1.2 (0.6) |
| Respiratory rate — breaths/min | 25.1±7.5 (4.6) | 24.8±7.0 (5.9) | 24.6±6.9 (2.3) | 25.1±7.8 (4.6) | 25.8±8.2 (5.8) | 24.8±7.3 (5.8) |
| Oxygen saturation — % | 95.8±4.2 (5.2) | 95.7±3.1 (5.3) | 96.4±3.9 (2.9) | 95.5±5.4 (6.9) | 95.8±4.1 (5.2) | 95.6±3.2 (5.8) |
Plus–minus values are means ± SD. The term “% with missing data” refers to the percentage of patients with missing data. All participants received standard care in addition to the assigned treatment. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for potassium to milligrams per deciliter, divide by 0.2558. Percentages may not total 100 because of rounding. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and RT-PCR reverse-transcriptase–polymerase-chain-reaction.
The ZMapp subgroup consisted of patients who were enrolled in the ZMapp group on or after the time the REGN-EB3 group was added.
Information on vaccination status during screening was not collected until January 26, 2019, with a revision to the protocol. The total number of patients reflects this.
The nucleoprotein and glycoprotein of Ebola virus RNA were detected with the use of quantitative reverse-transcriptase–polymerase-chain-reaction assay, and the levels are expressed as cycle-threshold (Ct) values.
Figure S2 provides the distributions according to group of nucleoprotein Ct values, creatinine levels, AST levels, and the median values for each group.
The mean (±SD) baseline nucleoprotein Ct value was 24.0±5.6, and 42.1% of patients had a baseline value of 22.0 or lower. Patients were enrolled within an average of 5.5 days after the onset of symptoms. The most commonly reported baseline symptoms were diarrhea (in 53.8% of the patients), fever (in 51.4%), abdominal pain (in 46.4%), headache (in 44.4%), and vomiting (in 39.4%) (Table S2). Malaria coinfection was identified in 57 of 557 patients (10.2%). Patient-reported information regarding vaccination status (i.e., whether the patient had received the rVSVΔG-ZEBOV-GP vaccine) was available for 620 patients; of these, 155 (25.0%) reported that they received the vaccine. Among patients who reported that they had been vaccinated, 38.7% reported that they had received the vaccination at least 10 days before enrollment.
The mean baseline serum creatinine level was 2.5±2.9 mg per deciliter (221±256 μmol per liter), the mean aspartate aminotransferase level was 668±700 U per liter, and the mean alanine aminotransferase level was 379±464 U per liter. The mean baseline creatinine and aspartate aminotransferase values were higher in the ZMapp and remdesivir groups than in the other two groups. However, the baseline creatinine level was not recorded in 18.6% of patients, aspartate aminotransferase level was not recorded in 40.6%, and alanine aminotransferase level was not recorded in 18.1%. In addition, 70.1% of the available baseline samples indicated some degree of hemolysis.
Mortality
On August 9, 2019, when 681 patients had been enrolled, the data and safety monitoring board conducted an interim analysis on data from 499 patients and, on the basis of two observations, recommended terminating random assignment to ZMapp and remdesivir. First, results in the REGN-EB3 group crossed an interim boundary for efficacy with respect to a surrogate end point for death at 28 days that took into account outcomes in all patients with at least 10 days of follow-up (Fig. S3). Second, an analysis of mortality showed that there was a clear separation between the MAb114 and REGN-EB3 groups and the ZMapp and remdesivir groups (Fig. S4).
A total of 673 patients were included in the primary analyses. At 28 days, death had occurred in 290 patients (43.1%) overall, in 18.8% of patients with a low viral load (Ct value >22.0), and in 76.1% with a high viral load (Ct value ≤22.0) (Table 2).
Table 2.
Comparison of Death at 28 Days According to Treatment Group.
| Population | ZMapp | Remdesivir | Difference, Remdesivir vs. ZMapp | MAb114 | Difference, MAb114 vs. ZMapp | REGN-EB3 | ZMapp Subgroup | Difference, REGN-EB3 vs. ZMapp Subgroup |
|---|---|---|---|---|---|---|---|---|
| no. of deaths/total no. (%) | no. of deaths/total no. (%) | percentage points (95% Cl) | no. of deaths/total no. (%) | percentage points (95% Cl) | no. of deaths/total no. (%) | no. of deaths/total no. (%) | percentage points (95%, Cl) | |
| Overall | 84/169 (49.7) | 93/175 (53.1) | 3.4 (−7.2 to 14.0) | 61/174 (35.1) | −14.6 (−25.2 to −1.7)* | 52/155 (33.5) | 79/154 (51.3) | −17.8 (−28.9 to −2.9)* |
| Patients with high viral load† | 60/71 (84.5) | 64/75 (85.3) | 0.8 (−15.3 to 17.2) | 51/73 (69.9) | −14.6 (−33.0 to −0.5) | 42/66 (63.6) | 56/65 (86.2) | −22.5 (−41.8 to −5.1) |
| Patients with low viral load† | 24/98 (24.5) | 29/100 (29.0) | 4.5 (−9.1 to 19.1) | 10/101 (9.9) | −14.6 (−32.4 to −2.6) | 10/89 (11.2) | 23/89 (25.8) | −14.6 (−32.6 to −2.3) |
The result is significant according to the interim stopping boundary of P<0.035 for the MAb114 group and P<0.028 for the REGN-EB3 group.
Patients with a high viral load had an EBOV nucleoprotein Ct value of 22.0 or less. Patients with a low viral load had an EBOV nucleoprotein Ct value of more than 22.0. The total number is the total number of patients in this category for each group.
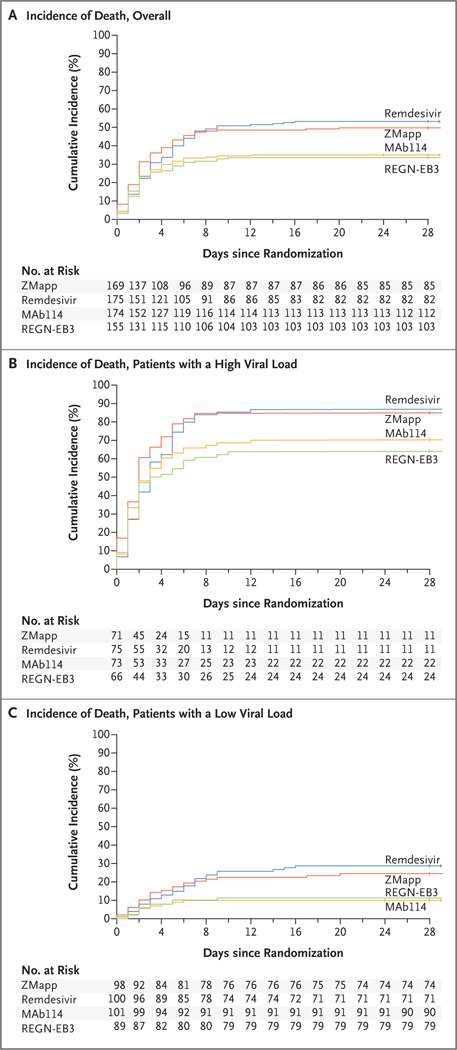
The percentage of patients who died was lower in the MAb114 group and in the REGN-EB3 group than in the ZMapp group (Fig. 1 and Table 2). The difference between the MAb114 and the ZMapp groups was −14.6 percentage points (95% confidence interval [CI], −25.2 to −1.7; P = 0.007); the difference between the REGN-EB3 group and the ZMapp subgroup was −17.8 percentage points (95% CI, −28.9 to −2.9; P = 0.002); and the difference between the remdesivir and ZMapp groups was 3.4 percentage points (95% CI, −7.2 to 14.0). (Fig. S5 shows the differences in mortality in the remdesivir, MAb114, and REGN-EB3 groups relative to the ZMapp group according to Ct value, age, sex, and site.) The survival benefits seen in the MAb114 and REGN-EB3 groups were also seen in sensitivity analyses adjusted for potential baseline imbalances (Tables 3 and 4 and Table S3).
Figure 1. Cumulative Incidence of Death.
Shown are Kaplan–Meier estimates of the cumulative incidence of death. Panel A shows the estimates in the overall population, Panel B the estimates in patients who had a nucleoprotein cycle-threshold (Ct) value of 22 or less at baseline (corresponding to a high viral load), and Panel C the estimates in patients who had a Ct value of more than 22 at baseline (corresponding to a low viral load).
Table 3.
Logistic-Regression Analyses for Death at 28 Days.
| Variable | No. of Patients in Analysis* | Odds Ratio (95% confidence interval)† | |||
|---|---|---|---|---|---|
| For Each Variable | Remdesivir vs. ZMapp | MAb114 vs. ZMapp | REGN-EB3 vs. ZMapp | ||
| Duration of symptoms | 615 | 1.11 (1.05–1.16) per day of symptoms‡ | 1.04 (0.66–1.64) | 0.49 (0.31–0.78) | 0.45 (0.28–0.73) |
| Nucleoprotein Ct value | 620 | 0.66 (0.62–0.71) per 1 unit increase | 1.29 (0.71–2.34) | 0.39 (0.21–0.73) | 0.37 (0.20–0.68) |
| Years of age | 623 | 1.00 (1.00–1.01) per 1 yr increase | 1.07 (0.68–1.66) | 0.52 (0.33–0.82) | 0.48 (0.31–0.77) |
| Creatinine level§ | 507 | 1.43 (1.31–1.56) per 1 mg/dl increase | 0.93 (0.54–1.59) | 0.48 (0.27–0.84) | 0.38 (0.21–0.67) |
| AST level§ | 380 | 1.15 (1.11–1.20) per 100 U/liter increase | 1.06 (0.54–2.05) | 0.31 (0.14–0.67) | 0.29 (0.14–0.63) |
| ALT level§ | 511 | 1.43 (1.33–1.54) per 100 U/liter increase | 0.95 (0.54–1.68) | 0.37 (0.20–0.69) | 0.36 (0.20–0.66) |
| Patient-reported vaccination§ | 620 | 0.37 (0.24–0.55) yes vs. no | 1.06 (0.67–1.68) | 0.48 (0.30–0.77) | 0.44 (0.28–0.71) |
Model estimates include data from patients who were enrolled after the REGN-EB3 group was added. The number of patients in the analysis reflects the number enrolled after the REGN-EB3 group was added for whom data were available for each variable.
Each row shows the odds ratios derived from a multivariate logistic-regression model that included the variable listed plus the four treatment groups.
The variable reflects each additional day of symptoms before admission to the treatment center.
Because of its clinical significance, the variable was added after the statistical analysis plan was finalized but before analysis of the data.
Table 4.
Multivariate Logistic-Regression Analyses for Death at 28 Days in the 371 Patients Who Had Data Available for All Variables.
| Variable | Odds Ratio (95% CI) |
|---|---|
| Assignment to remdesivir vs. ZMapp | 0.99 (0.46–2.14) |
| Assignment to MAb114 vs. ZMapp | 0.24 (0.10–0.61) |
| Assignment to REGN-EB3 vs. ZMapp | 0.21 (0.08–0.53) |
| Duration of symptoms before admission to treatment center, per each additional day | 1.12 (1.00–1.24) |
| Baseline nucleoprotein Ct value per 1-unit increase | 0.67 (0.59–0.76) |
| Years of age per 1 yr increase | 1.02 (1.00–1.04) |
| Creatinine level per 1 mg/dl increase | 1.36 (1.18–1.58) |
| AST level per 100 U/liter increase | 1.00 (0.92–1.07) |
| ALT level per 100 U/liter increase | 0.96 (0.79–1.17) |
| Patient-reported vaccination, yes vs. no | 0.47 (0.21–1.01) |
Secondary Efficacy End Points
In an analysis of the time to the first negative result on RT-PCR assay for EBOV nucleoprotein, in which patients who had died were considered as not having had viral clearance, the time to the first negative result was shorter in the MAb114 and REGN-EB3 groups than in the ZMapp group (median in the MAb114 group, 16 days; median in the REGN-EB3 group, 15 days; median in the ZMapp group, 27 days) (Fig. 2). Among patients in the remdesivir group, the estimated median time was more than 28 days because mortality exceeded 50%.
Figure 2. Time to Viral Clearance.
Panel A shows the time to the first negative result for Ebola virus (EBOV) nucleoprotein on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay in all groups, with deaths imputed as the worst time. The dots indicate individual patients, the triangles indicate patients who were enrolled before January 2019 when the protocol was revised to add the REGN-EB3 group, and the horizontal bars indicate the group means. The black hashed bar in the remdesivir group indicates that the median time was not observed because more than 50% of patients in this group died before the first negative result. Data are not shown for one patient in the ZMapp group and one patient in the REGN-EB3 group who did not have a first negative result before day 28 but who had a negative result at days 48 and 41, respectively. Panel B shows the values for EBOV nucleoprotein as determined on RT-PCR, according to day of the trial. The symbols indicate the median, and the vertical bars indicate the interquartile range.
Prognostic Variables
A longer duration of symptoms before treatment was associated with significantly worse outcomes. Of note, 19% of patients who arrived at the treatment center within 1 day after the reported onset of symptoms died, as compared with 47% of patients who arrived after they had had symptoms for 5 days (Table S4). The odds of death increased by 11% (95% CI, 5 to 16) for each day after the onset of symptoms that the patient did not present to the treatment center (Table 3).
The odds of death were lower among patients with lower viral loads (odds ratio per unit increase in Ct value, 0.66; 95% CI, 0.62 to 0.71) and higher among patients with higher levels of creatinine (odds ratio per 1 mg per deciliter increase, 1.43; 95% CI, 1.31 to 1.56), aspartate aminotransferase (odds ratio per 100 U per liter increase, 1.15; 95% CI, 1.11 to 1.20), and alanine aminotransferase (odds ratio per 100 U per liter increase, 1.43; 95% CI, 1.33 to 1.54). A multivariate logistic-regression analysis showed that the duration of symptoms at enrollment, baseline nucleoprotein Ct value, and serum creatinine level all remained significant prognostic indicators of death (Table 4). Across all models, the effect estimates of treatment with MAb114 and REGN-EB3 remained significant (Table 3 and 4).
The percentage of patients who died was lower among those who reported that they had received the rVSVΔG-ZEBOV-GP vaccine than among those who reported no vaccination (27.1% [42 of 155 patients] vs. 48.4% [225 of 465]). However, patients who reported vaccination were also more likely to have had fewer days of illness before enrollment, higher baseline nucleoprotein Ct values, and lower levels of alanine aminotransferase (Table S5).
Safety
At least 98% of the patients received the infusions according to protocol (Table S6). A total of 29 serious adverse events were determined by trial investigators to be potentially related to the trial drugs (Table S7). However, after adjudication by an independent pharmacovigilance committee, four events in three patients, all of which resulted in death, were determined to be possibly related to a trial drug: one patient in the ZMapp group had worsening of gastrointestinal symptoms; one patient in the ZMapp group had periinfusional hypotension and hypoxia that responded to resuscitation after treatment interruption but that resulted in death within 24 hours; and one patient in the remdesivir group had hypotension that resulted in cessation of a loading dose of remdesivir and that was followed rapidly by cardiac arrest. However, even in these cases, the deaths could not readily be distinguished from underlying fulminant EVD itself.
Delays in Treatment Administration
The mean time from randomization to administration of the first infusion was somewhat longer in the ZMapp and remdesivir groups than in the MAb114 and REGN-EB3 groups. (Table S8 and Fig. S6 provide a summary of the time from randomization to the first infusion according to trial group and site, and Table S9 provides the results of a sensitivity analysis of outcomes that excluded data from patients with delays of more than 6 hours.) Twelve patients were enrolled but died before receiving the first infusion: one in the ZMapp group, three in the remdesivir group, three in the MAb114 group, and five in the REGN-EB3 group.
Discussion
In this trial of four promising experimental treatments against Z. ebolavirus, the combination of standard care plus either MAb114 or REGN-EB3 was superior to standard care plus ZMapp against the Ituri EBOV variant currently circulating in the DRC. Survival benefits were seen both in patients with high viral loads and in those with low viral loads at presentation. The reason that mortality among patients who received ZMapp was 22% in the PREVAIL II trial (conducted during the outbreak in West Africa) and 50% in our trial (conducted during the current outbreak in the DRC) is unclear. Potential differences in virulence, the relevant viral epitopes,14 patient populations, duration of symptoms, and standard-of-care practices are being explored.
In addition to differential effects of the four trial agents with respect to mortality, the results showed the importance of early diagnosis and treatment. We observed an 11% increase in the odds of death for each day that symptoms persisted before enrollment. These data highlight the need for community awareness that earlier diagnosis and treatment are associated with increased survival. Similarly, there was an effect of baseline viral load with respect to death at 28 days with each trial drug: mortality among patients who had a nucleoprotein Ct value of 22 or less at screening (i.e., high viral load) was 4 times as high as mortality among patients with a nucleoprotein Ct value of greater than 22 (i.e., low viral load). As described previously, the degree of baseline renal dysfunction was also a strong adverse prognostic indicator of survival, despite the use of medical countermeasures,17,18 with higher creatinine levels at presentation correlating with a higher risk of death.
Given that 97% of deaths in this trial occurred within 10 days after enrollment, the efficacy of MAb114 and REGN-EB3 as compared with that of ZMapp and remdesivir might be partly attributable to the fact that the full treatment courses of MAb114 and REGN-EB3 were administered in a single dose, whereas ZMapp and remdesivir were administered in multiple infusions. Differences in the time to appearance of the first negative nucleoprotein Ct result among trial groups support this observation; patients in the MAb114 and REGN-EB3 groups had faster rates of viral clearance than patients in the ZMapp and remdesivir groups. With ZMapp, the longer preparation time and the recommendation to allot up to 4 hours for the infusion of the first dose led to some delays in initiating therapy until the following day for patients who arrived later in the day to their respective treatment centers. However, in a sensitivity analysis, mortality was only slightly lower when ZMapp recipients with delayed therapy were excluded.
Although most characteristics at baseline were balanced across the four groups, values for serum creatinine and aminotransferases were higher in the ZMapp and remdesivir groups than in the MAb114 and REGN-EB3 groups; patients in the latter groups had better outcomes, despite similar durations of illness before enrollment. This suggests that enrolled patients might, on average, have been somewhat sicker in the ZMapp and the remdesivir groups, which could potentially account for some of the differences in outcomes. A high percentage of missing baseline data complicates this analysis. Nevertheless, sensitivity analyses confirm the persistence of benefits of treatment with MAb114 and REGN-EB3 despite these potential imbalances.
Of the 620 patients for whom information on vaccination with rVSVΔG-ZEBOV-GP was available, 155 patients (25.0%) reported that they had received the vaccine; of these, 38.7% reported that they had received the vaccine at least 10 days before the onset of clinical symptoms. Patients who reported vaccination were more likely to enroll sooner after the onset of symptoms and generally had more favorable prognostic profiles at baseline, suggesting a possible relationship between vaccination and health-seeking behaviors associated with improved outcomes. Alternatively, the less severe clinical status of these persons at presentation could be the result of a direct effect of the vaccine on outcomes. A limitation of these results is that vaccination status was reported by the patient; efforts to confirm vaccination status are under way. Given that vaccination status was not a randomization factor in this trial, it is not possible to draw firm conclusions about its effect on mortality.
With few exceptions, the safety profiles of all four trial drugs were generally consistent with either their limited previous investigational use in EBOV-infected humans, published phase 1 data in healthy volunteers, or both. Twenty-nine serious adverse events were reported by the investigators as possibly related to the experimental treatments — not all of which occurred during the treatment period. On review, four were thought to be possibly related to the trial-drug infusions. It is difficult to distinguish adverse events associated with the trial drug from those related to underlying EVD, so the assessment of relatedness is challenging. These favorable safety profiles support the notion that relative efficacy rather than safety considerations will most likely provide the major rationale for the future use of these drugs.
Although the observed treatment benefits of MAb114 and REGN-EB3 were striking, 34% of all patients and 67% of patients who presented with higher viral loads died despite receiving one of these agents. Exploration of more efficacious interventions — such as further improvements in aggressive supportive-care measures and combination strategies that use agents with potentially complementary mechanisms of action — is needed. It is worth noting, however, that all the treatments chosen for this trial had shown comparatively high survival rates in nonhuman primate EBOV challenge models with the use of a non-Ituri EBOV variant (Kikwit), which illustrates a potential limitation of these models in evaluating single-drug and (future) combination-drug strategies.
We encountered numerous challenges in the performance of this trial. It was conducted in a region of the DRC in which there is regional violence, mistrust of government, mistrust of the Ebola response, an unstable electrical power grid, transportation difficulties, and a history of high morbidity from other infectious diseases. Missing results from laboratory tests make the logistic-regression analyses difficult to interpret. Continual oversight of staffing and supply-chain issues by the DRC Ministry of Health, the INRB, the WHO, ALIMA, IMC, and MSF was essential to maintaining an appropriate standard of supportive care in the trial centers. The trial was interrupted temporarily in two participating centers that had to be evacuated because of violence directed against those units by local community or paramilitary groups who were reportedly suspicious of the activities under way in those facilities.
Reaching a successful conclusion to this challenging trial required careful planning as well as the cooperation, support, and coordination of national and international health agencies, government leaders, pharmaceutical companies, dedicated oversight boards, scientists, and nongovernmental organizations. This trial showed that it is possible to conduct scientifically rigorous and ethically sound research during an outbreak, even in a conflict zone. Although it is important to recognize the collective strength of this partnership in ensuring the completion of the trial, the single greatest factor that ensured its success was the commitment of the staff in the field and at the sites (the physicians, nurses, pharmacists, hygienists, the gardes-malades [guardians of the sick], and the numerous other support staff) who worked under highly challenging circumstances at the front lines of this effort in the Ebola treatment centers, as well as that of the patients themselves.
Supplementary Material
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Supported primarily by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). In-kind support and cosponsorship were provided by the national government of the Democratic Republic of Congo (DRC) and the African Coalition for Epidemic Research, Response, and Training. Logistic support was provided by the World Health Organization (WHO). Some funding for NIAID was provided by the National Cancer Institute through a contract (HHSN261200800001E) with Leidos Biomedical Research and subcontracts to the Mitchell Group. The Biomedical and Advanced Research and Development Authority of the U.S. Department of Health and Human Services provided financial support for the production of ZMapp (contract number, HHSO100201400009C) and REGN-EB3 (contract number, HHSO100201700016C). NIAID and the Defense Advanced Research Projects Agency of the U.S. Department of Defense provided financial support for the production and provision of MAb114. Mapp Biopharmaceutical provided ZMapp, Gilead Sciences provided remdesivir, and NIAID provided MAb114 to the project. Regeneron Pharmaceuticals provided financial support for the provision of REGN-EB3 to the project.
(Funded by the National Institute of Allergy and Infectious Diseases and others; PALM ClinicalTrials.gov number, NCT03719586.)
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the members of the PALM Consortium Study Team (see the Supplementary Appendix) for their many contributions in conducting the trial under very challenging field conditions, the members of the data and safety monitoring board (Lisa A. Cooper, M.D., M.P.H. [chair], Scott Hammer, M.D., Dave DeMets, Ph.D., M.Sc., Ann Sarah Walker, Ph.D., M.Sc., Mesia Kahunu, Pharm.D., Ph.D., Amadou Traore, M.D., M.Sc., Albert Faye, Ph.D., Salim Abdulla, Ph.D., M.Sc., and Rebecca DerSimonian, Sc.D., and Sally Hunsberger, Ph.D. [coexecutive secretaries]) for their oversight, the WHO and their coordinating committees (see Table S11), Anthony Fauci (NIAID), Robert Redfield (Centers for Disease Control and Prevention [CDC]), and Peter Salama and Michael Ryan (WHO) for leadership guidance, CDC staff (including Raimi Ewetola, Alstead Forbes, and Laetitia Vahaviraki) and the WHO staff (including the incident manager and field coordinators, supply and logistic teams, and Global Outbreak Alert and Response Network clinician experts) for in-country support, Raoul Kamanda and colleagues from the DRC Ministry of Health for invaluable support, and, most important, the patients themselves for their extraordinary bravery and altruism in participating in a trial of these experimental countermeasures.
Appendix
The members of the PALM Writing Group are as follows: Billy Sivahera, M.D., Modet Camara, M.D., Richard Kojan, M.D., Robert Walker, M.D., Bonnie Dighero-Kemp, B.S., Huyen Cao, M.D., Philippe Mukumbayi, M.Pharm., Placide Mbala-Kingebeni, M.D., Steve Ahuka, M.D., Sarah Albert, M.P.H., Tyler Bonnett, M.S., Ian Crozier, M.D., Michael Duvenhage, N.Dip.I.T., Calvin Proffitt, M.A., Marc Teitelbaum, M.D., Thomas Moench, M.D., Jamila Aboulhab, M.D., Kevin Barrett, B.S.N., Kelly Cahill, M.S., Katherine Cone, M.S.W., Risa Eckes, M.A., Lisa Hensley, Ph.D., Betsey Herpin, M.S.N., Elizabeth Higgs, M.D., Julie Ledgerwood, D.O., Jerome Pierson, Ph.D., Mary Smolskis, M.A., Ydrissa Sow, M.D., John Tierney, M.P.M., Sumathi Sivapalasingam, M.D., Wendy Holman, B.S., Nikki Gettinger, M.P.H., David Vallée, Pharm.D., and Jacqueline Nordwall, M.S.
The affiliations of the members of the PALM Writing Group are as follows: the Alliance for International Medical Action (B.S., M.C., R.K.); the Biomedical Advanced Research and Development Authority (R.W.); Battelle (B.D.-K.); Gilead (H.C.); Institut National de Recherche Biomédicale (P.M., P.M.-K., S. Ahuka); Leidos (S. Albert, T.B., I.C., M.D., C.P., M.T.); Mapp Biopharmaceutical (T.M.); the National Institute of Allergy and Infectious Diseases (J.A., K.B., K. Cahill, K. Cone, R.E., L.H., B.H., E.H., J.L., J.P., M.S., Y.S., J.T.); Regeneron (S.S.); Ridgeback Biotherapeutics (W.H.); the Mitchell Group (N.G., D.V.); and University of Minnesota (J.N.).
Footnotes
A complete list of members of the PALM Consortium Study Team is provided in the Supplementary Appendix, available at NEJM.org.
References
- 1.Ebola situation reports: Democratic Republic of the Congo (archive). Geneva: World Health Organization; (https://www.who.int/ebola/situation-reports/drc-2018/en/). [Google Scholar]
- 2.Ebola virus disease — Democratic Republic of the Congo. Geneva: World Health Organization, September 19, 2019. (https://www.who.int/csr/don/19-september-2019-ebola-drc/en/). [Google Scholar]
- 3.Ebola/Marburg research and development (R&D) roadmap. Geneva: World Health Organization, May 2018. (https://www.who.int/blueprint/priority-diseases/key-action/Ebola-Marburg_Draft_Roadmap_publiccomment_MAY2018.pdf?ua=1). [Google Scholar]
- 4.Dodd LE, Follmann D, Proschan M, et al. On the importance of randomized controlled trials for Ebola virus disease therapeutics: a meta-analysis from the West African outbreak. Sci Transl Med (in press). [Google Scholar]
- 5.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531:3 81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corti D, Misasi J, Mulangu S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016; 351: 1339–42. [DOI] [PubMed] [Google Scholar]
- 8.Gaudinski MR, Coates EE, Novik L, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet 2019; 393:8 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascal KE, Dudgeon D, Trefry JC, et al. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in nonhuman primates. J Infect Dis 2018; 218:S uppl_5: S612–S626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivapalasingam S, Kamal M, Slim R, et al. Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect Dis 2018;1 8: 884–93. [DOI] [PubMed] [Google Scholar]
- 11.The PREVAIL II Writing Group for the Multi-National PREVAIL II Study Team. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med 2016; 375: 1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, MD: Food and Drug Administration, December 2009. (https://www.fda.gov/media/77832/download). [Google Scholar]
- 13.Xpert Ebola assay. Solna, Sweden: Cepheid, March 2015. (https://www.fda.gov/media/91944/download). [Google Scholar]
- 14.Mbala-Kingebeni P, Aziza A, Di Paola N, et al. Medical countermeasures during the 2018 Ebola virus disease outbreak in the North Kivu and Ituri Provinces of the Democratic Republic of the Congo: a rapid genomic assessment. Lancet Infect Dis 2019;1 9: 648–57. [DOI] [PubMed] [Google Scholar]
- 15.Fay MP, Proschan MA, Brittain E. Com-bining one-sample confidence procedures for inference in the two-sample case. Biometrics 2015; 71:1 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proschan MA, Dodd LE, Price D. Statistical considerations for a trial of Ebola virus disease therapeutics. Clin Trials 2016; 13: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and Ddimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis 2007; 196: Suppl 2: S364–S371. [DOI] [PubMed] [Google Scholar]
- 18.Connor MJ Jr, Kraft C, Mehta AK, et al. Successful delivery of RRT in Ebola virus disease. J Am Soc Nephrol 2015; 26: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.