Abstract
Objective:
Electroconvulsive therapy (ECT) remains the benchmark for treatment resistant depression, yet its cognitive side effects have a negative impact on treatment. A predictive safety biomarker early in ECT treatment is needed to identify patients at cognitive risk to maximize therapeutic outcomes and minimize side effects. We used ictal EEG frequency analysis from suprathreshold treatments to assess the relationships between ECT dose, ictal power across different frequency domains and cognitive outcomes.
Methods:
Seventeen subjects with treatment resistant depression received right unilateral (RUL) ECT. Structural MRI was obtained pre-ECT for electric field (E-field) modeling to assess ECT dose. Serial assessments with 24-lead EEG captured ictal activity. Clinical and cognitive assessments were performed pre- and post-ECT. The primary cognitive outcome was the change in Delis Kaplan Executive Function Verbal Fluency Letter Fluency (ΔDKEFS-VF LF).
Results:
Ictal theta (4–8 Hz) power in the Fp1/Fp2 channels was associated with both whole-brain E-field strength (t(2,12)=19.5, p = 0.007)/(t(2,10)=21.85, p=0.02) and ΔDKEFS-VF LF scores (t(2,12)=−2.05, p = .05)/(t(2,10)=−2.20, p=.01). Other frequency bands (beta, alpha, delta, and gamma) did not demonstrate this relationship.
Conclusions:
This pilot data identifies ictal theta power as a potential safety biomarker in ECT and is related to the strength of the ECT dose. Ictal theta power could prove to be a convenient and powerful tool for clinicians to identify those patients most susceptible to cognitive impairment early in the treatment series. Additional studies are needed to assess the role of longitudinal changes in ictal theta power throughout the ECT series.
INTRODUCTION
Electroconvulsive Therapy (ECT) is effective for treatment resistant depression and is widely used in the United States. The therapeutic effect of ECT is achieved by administering a stimulus substantially above the seizure threshold, yet as the stimulus increases it can worsen cognitive outcomes [1]. While most ECT-associated cognitive side effects are acute and transient, some effects can persist for 6 months or longer [2–4]. These side effects include changes in attention, verbal fluency, memory, and executive function, and can lead to suboptimal outcomes and worsening stigma towards the procedure [5, 6]. Present day dosing algorithms, such as seizure threshold titration and formula dosing based on age and sex, lack scientific rationale as they are unable to account for individual variability to electric current (head shape, skull thickness, tissue composition, etc.) [7, 8]. Furthermore, ECT dosing algorithms rely on frequency and train duration to increase total charge while amplitude, which is directly proportional to the induced electric field and offers the most direct control in the volume of tissue stimulated, remains fixed [7]. A safety biomarker rooted in individual variability is needed to identify a patient’s susceptibility to cognitive side effects so that optimal treatment settings can be identified early in the treatment course in order to maximize both clinical and cognitive safety.
Despite decades of research to define seizure adequacy, ictal electroencephalography (EEG) has been infrequently assessed as a cognitive biomarker. A recent meta-analysis on ictal EEG in ECT found that just one out of 44 studies that met inclusion criteria reported cognitive outcomes [9]. That study found no significant association between peri-ictal EEG features and cognitive outcomes [10]. Another study examined differences in ketamine and methohexital anesthesia induction during ECT and found that ketamine enhanced ictal EEG evidence of seizure intensity. The study provided preliminary evidence that ketamine may be associated with a lower level of ECT-related cognitive side effects when compared to methohexital anesthesia as measured by post-treatment time to orientation [11]. A third study with a small cohort of patients who received bitemporal (BT) ECT (n=8) found that post-ictal suppression and slow-wave amplitude positively correlated with delayed memory, while ictal slow-wave amplitude was negatively correlated with phonemic fluency [12]. To our knowledge, these three studies constitute the corpus of literature in the past twenty years relating ictal EEG measurements in pulse wave ECT to ECT-induced cognitive side effects, in domains of time to orientation, memory, and verbal fluency.
ECT dosing as measured by electric field (E-field) modeling takes into account individual variability and may be related to cognitive outcomes. Realistic computational models of the head can predict E-field distribution in the brain induced by ECT, accounting for anatomical parameters such as tissue conductivity, head and brain geometry, and also properties of the current-carrying electrodes such as size and placement on the scalp (right-unilateral, bitemporal) [7, 13, 14]. The work-flow to develop realistic head models for electric stimulation include image segmentation, tessellation of volume into a mesh, electrode placement on the scalp, and solving for the E-fields with the finite element method [13, 15]. These E-field models have been validated with motor evoked potentials and intraoperatively with cortical EEG [14]. By utilizing structural MRI (sMRI) data, E-field modeling can capture individual anatomical differences to explain variable ECT dosing [16, 17]. Prior work has shown that the E-field strength has a direct relationship with hippocampal neuroplasticity and a negative relationship with antidepressant response [18, 19]. Argyelan et al. demonstrated in patients receiving ECT that the amygdala and hippocampus had a strong relationship between E-field and volumetric change and that high E-fields were associated with robust volume changes in a dose-dependent fashion; however, neither the E-field nor volumetric change was associated with antidepressant outcomes. Fridgeirsson et al. found that a stronger E-field in the temporal lobes was associated with decreased therapeutic response in patients who received BT ECT. A common limitation to these modeling studies is that the E-field only describes the spatial extent of the direct electrical stimulation. The relationship between the E-field and seizure expression, and the ultimate clinical and neurocognitive outcome remains unknown.
The purpose of this study was to investigate ictal EEG power as a potential bridge between ECT variable dosing in the brain as measured by E-field modeling and cognitive impairment. The data is from a parent study with a primary focus of investigating the relationship between E-field and neuroplasticity. In this double-blind controlled-trial, older patients (50–80 years old) were randomized into three amplitude arms: 600, 700, and 800 mA. E-field models were generated in all patients as well as neurocognitive batteries and mood scales pre, mid, and post-ECT. In line with recent advances in cognitive testing and the results of our parent study, the Delis Kaplan Executive Function System (DKEFS) Verbal Fluency Test Category and Letter Fluency (DKEFS-VF CF and LF) tasks were found to be the most sensitive tests to amplitude-mediated cognitive impairment and were used as our primary outcome measures for cognitive impairment [20, 21]. We examined 17 subjects who received structural MRIs, 24-lead EEG, and cognitive and therapeutic assessments. Linear regression models examined the relationships between E-field models, ictal power in each frequency band, and cognitive outcomes controlling for age and premorbid intellectual function. We hypothesized that frequencies in the lower bands would demonstrate an association.
MATERIALS & METHODS
For detailed information on study inclusion/exclusion criteria, neurocognitive and clinical assessments, ECT treatment parameters and study design, we refer to the published findings of our randomized clinical trial that compared three different pulse amplitudes (600, 700, and 800 mA) and antidepressant and clinical outcomes [22]. The data presented in this analysis includes subjects who agreed to 24-lead EEG acquisitions.
Subjects:
The University of New Mexico (UNM) Human Research Protections Office (HRPO) approved this investigation. All subjects signed procedural consent or assented to the research protocol with the surrogate medical decision-maker providing consent. Of the 62 subjects who completed the parent study, 17 underwent a structural MRI to generate E-field modeling and completed 24-lead EEG capture at a RUL suprathreshold treatment. Every attempt was made to capture the earliest suprathreshold RUL treatment with 59% of captures happening at either treatment #2 or #3. 15 subjects had Fp1 EEG captures and 13 subjects had Fp2 EEG captures.
Cognitive and Therapeutic Evaluations:
Subjects were recruited through the ECT service at the University of New Mexico. Subjects were started with right unilateral (RUL) placement and randomized to three fixed amplitude arms: 600 mA, 700 mA, or 800 mA. All analysis was done on only the RUL treatments. Subjects completed a neurocognitive battery and depression symptom severity assessments pre-ECT (V1), mid-ECT (before treatment #6, V2), and post-ECT (V3). Patient’s final scores were assessed as the difference between pre- and post-ECT (V3-V1), unless they switched to bitemporal (BT) at V2 secondary due to non-response, in which case final scores were assessed as (V2-V1). Bitemporal treatments were excluded from the analysis due to the substantial difference in E-field values when modelling for bitemporal electrode placement. A separate analysis of bitemporal outcomes was not possible given the small sample size (n=4). The DKEFS-VF LF was used as our primary outcome measure for cognitive impairment [20, 21]. The DKEFS-VF LF raw score was converted into demographic-adjusted (age) scaled scores. The Hamilton Depression Rating Scale-24 items (HDRS-24) was used to measure antidepressant outcome [23]. The Montreal Cognitive Assessment (MoCA, version 7.1) and Test of Premorbid Function (TOPF) at baseline were used to screen for global cognitive function and premorbid intellectual function respectively [24, 25].
EEG:
The SMARTING 24-lead channel amplifier and mobile device was used for EEG capture (mBrainTrain, Inc., Belgrade, Serbia). The sampling rate was 500Hz. The initial single referencing node was located in the FCz position. Cleaning of data consisted of excluding all channels with an impedance > 20 kΩ. Channels were then visually inspected, and excluded if they were saturated or contained artifacts during the ictal period. The remaining good channels were re-referenced to the common average reference (CAR) [26]. Power spectral density (μV2/Hz) was calculated for each channel over the range of frequency bands; delta (1–4Hz), theta (4–8Hz), alpha (8–12Hz), beta(12–30Hz), and gamma (30–80Hz) with EEGLAB (version 2019.0) [27–29]. Ictal powers were then log10-transformed to normalize the distribution [30]. Fp1 and Fp2 channels were chosen as our primary outcome channels to align with standard ECT EEG acquisitions.
MRI:
T1 and T2 structural MRI were captured using a 3T Siemens scanner prior to ECT initiation.
T1 parameters were as followed: Repetition time (TR) = 2530 milliseconds (ms), echo time (TE) = 1.64, 3.5, 5.36, 7.22, 9.08 ms, Inversion time (TI) = 1200 ms, flip angle = 7.0 °, slices = 192, field of view = 256, matrix 256 × 256, voxel size = 1.0 × 1.0 × 1.0 millimeter (mm) and total acquisition time 6:03 (minutes: seconds).
T2 parameters were as followed: TR = 2530 ms, TE = 474 ms, flip angle = 120.0 °, slices = 192, field of view = 256, matrix 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm and total acquisition time = 5:09.
E-field Modeling:
We used Simulation of Non-Invasive Brain Stimulation (SimNIBS) (version 2.1.2) for E-field modeling [15]. SimNIBS creates a subject specific, anatomically realistic volume conductor model. The T1 and T2-weighted scans are segmented into skin, bone, eyes, cerebral spinal fluid, ventricles, and grey and white matter with a combination of FMRIB Software Library (FSL) [31] and Statistical Parametric Mapping 12 (SPM12) Computational Anatomy Toolbox [32, 33]. SimNIBS then turns this segmentation into a tetrahedral head mesh using GMSH, a three-dimensional finite element (FE) mesh generator, with unique conductivity values for each tissue type. Electrodes are added to the head mesh in either RUL orientation and simulated with corresponding current. SimNIBS then uses a finite element solver to calculate the voltages and electric fields corresponding to the stimulation throughout the head mesh. Simulations were performed using unit current, the resultant E-fields were then scaled to the assigned ECT current amplitudes (600, 700, or 800 mA). Whole-brain E-field strength, Ebrain, was calculated at 90th percentile of E-field magnitudes as an estimate of peak induced field strength, while avoiding the influence of tissue boundary effects that could bias the absolute maximum E-field values.
Statistical Analysis:
Our data passed normality testing. Linear regression models comparing the relationships between Ebrain, ictal frequency power and cognitive outcomes were performed using R (version 4.0.2) [34–37]. First, the relationship between ictal frequency power and Ebrain controlling for age was assessed. Second, the relationship between the ΔDKEFS-VF LF scaled score and ictal frequency power controlling for premorbid intelligence (TOPF) was assessed. Third, the relationship between ΔDKEFS-VF LF scaled score and ΔDKEFS-VF CF scaled scores and Ebrain controlling for premorbid intelligence (TOPF) was assessed. An exploratory whole brain analyses on the remaining 20 EEG channels was done (the two mastoid channels were excluded from analysis) and compared Ebrain, ictal power, and antidepressant outcomes with similar regression models. Given the exploratory nature of our study, no mathematical corrections were made for multiple comparisons.
RESULTS
Clinical and Demographic Characteristics:
Demographics, clinical, and neuropsychological data are summarized in Table 1. The average age for the subjects (n = 17) was 65.1 years with a standard deviation of 8.4. Six of the 17 subjects were male. Four, six and seven subjects received 600, 700, and 800 mA RUL ECT, respectively. Thirteen of the 17 subjects remained in RUL ECT throughout the ECT series.
Table 1:
Demographic and Clinical Characteristics
| Clinical and Demographic Features | (N=17) |
|---|---|
| Age | 65.1 (8.4) |
| Sex: male/female | 6/11 |
| Test of Premorbid Function | 108.1 (12.1) |
| Baseline MOCA | 24.5 (2.6) |
| Ebrain: mean (SD) | 111.9 (22.8) |
| Baseline DKEFS Category Fluency Scaled Score | 8.2 (3.9) |
| Post RUL ECT DKEFS Category Fluency Scaled Score | 7.0 (2.9) |
| Change in DKEFS Category Fluency Scaled Score | −1.6 (2.2) |
| Baseline DKEFS Letter Fluency Scaled Score | 8.7 (3.2) |
| Post RUL ECT DKEFS Letter Fluency Scaled Score | 7.7 (3.2) |
| Change in DKEFS Letter Fluency Scaled Score | −1 (2.7) |
| Baseline Hamilton Depression Rating Scale | 36.7 (5.3) |
| Post RUL ECT Hamilton Depression Rating Scale | 19.0 (13.8) |
| Change in Hamilton Depression Rating Scale | .489 (.365) |
| Log of Fp1 Ictal Theta Power (N=15) | 37.8 (29.1) |
| Log of Fp2 Ictal Theta Power (N=13) | 46.3 (33.2) |
| Responder/Non-Responder | 3/14 |
| Remitter/Non-Remitter | 6/11 |
| RUL Only/Bitemporal Switch | 13/4 |
| RUL suprathreshold treatment captured by EEG | 4.6 (3.3) |
Notes: Format is mean (SD) or / to indicate proportion
Ebrain and ictal theta power:
Ebrain and ictal power demonstrated no statistically significant relationships in both Fp1/Fp2 channels when controlling for age in delta (t(2,12)=−27.04, p=0.17)/(t(2,10)=38.22, p=0.10), alpha (t(2,12)=11.44, p=0.27)/(t(2,10)=17.96, p=0.12), beta (t(2,12)=11.46, p=0.36)/(t(2,10)=16.97, p=0.20), and gamma (t(2,12)=9.03, p=0.62)/(t(2,10)=11.08, p=0.56) frequency bands. In contrast, Ebrain was significantly associated with ictal theta power in both Fp1 (t(2,12)=19.5, p = 0.007) and Fp2 (t(2,10)=21.85, p=0.02) channels when controlling for age (Fig. 1A and 1B). A whole-brain exploratory analysis demonstrated that Ebrain was related to ictal theta power in 75% of the remaining 20 channels (p<0.05 in green) (Figure 1C). No particular area of strength was noted, but the midline channels (Fz, Cz, and Cpz) demonstrated the weakest association (p>0.05).
Figures 1A,1B,1C:
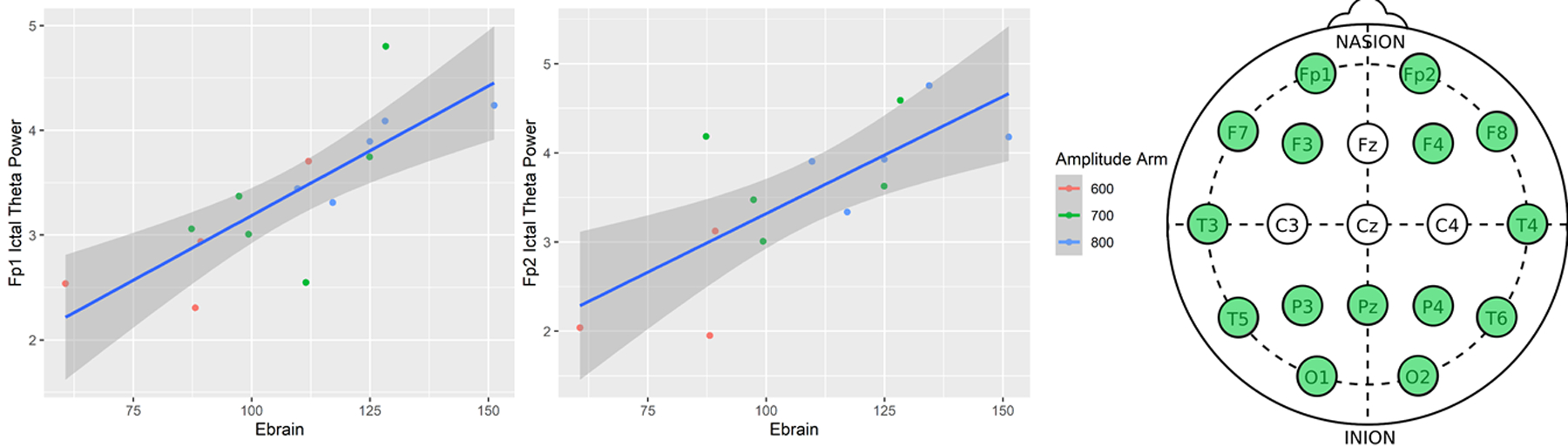
1A. Left. Ictal Theta Power vs. Ebrain in the Fp1 Channel. 1B. Middle. Theta Power vs. Ebrain in the Fp2 Channel. 1C. Right. Whole-brain exploratory analysis of ictal theta power and Ebrain with standard 19-lead EEG map shown (CPz, P7, P8 and mastoid channels not shown). Green leads denote statistical significance (p<0.05).
Ictal theta power and cognitive outcomes:
There was no statistically significant relationship between ictal power and category fluency in any bands. We focused on letter fluency as our primary cognitive outcome. Ictal power and letter fluency demonstrated no statistically significant relationships when controlling for premorbid intelligence in both Fp1/Fp2 channels in delta (t(2,12)=−0.13, p=0.78)/(t(2,10)=−0.66, p=0.15), alpha (t(2,12)=−1.07, p=0.23)/(t(2,10)=, p=0.024), beta (t(2,12)=−0.22, p=0.77)/(t(2,10)=−1.34, p=0.12), and gamma (t(2,12)=0.13, p =0.80)/(t(2,10)=−0.24, p=0.72) frequency bands. In contrast, ictal theta power was associated with cognitive outcomes in Fp1 (t(2,12)=−2.05, p = 0.05) with Fp2 demonstrating a stronger relationship (t(2,10)=−2.20, p=0.01) (Fig. 2A and 2B). A whole-brain exploratory analysis demonstrated that ictal theta power was related to cognitive outcomes when controlling for premorbid intelligence in 70% of the remaining 20 channels (p<0.05 in green) (Figure 2C). The midline and right hemisphere (Fz, Pz, POz, O2, T8) demonstrated the strongest association (p<0.01) and the left temporal-occipital area (P3, T3, T5, P7, O1) demonstrated the weakest (p>0.05)
Figures 2A,2B,2C:
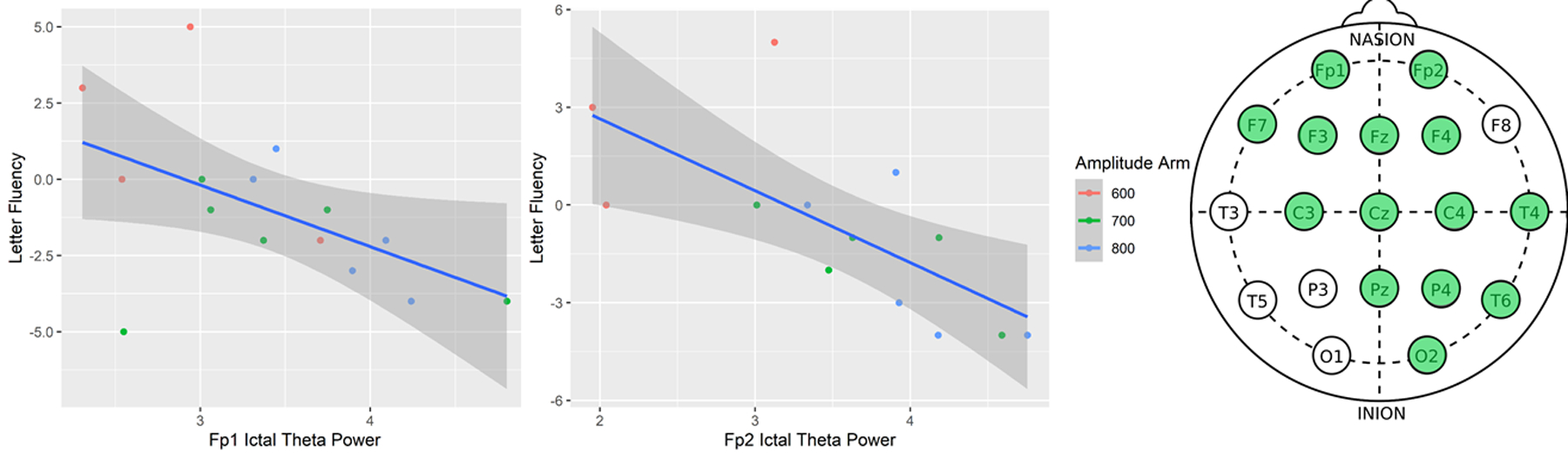
2A. Left. Letter Fluency vs. Ictal Theta Power in the Fp1 Channel 2B. Middle. Letter Fluency vs. Ictal Theta Power in the Fp2 Channel 2C. Right. Whole-brain exploratory analysis of cognitive performance and ictal theta power with standard 19-lead EEG map shown (CPz, P7, P8 and mastoid channels not shown). Green leads denote statistical significance (p<0.05).
Ebrain and cognitive outcomes:
Linear models showed no statistically significant relationship between Ebrain and letter fluency (t(2,14)=−2.06, p = .06) (Fig. 3). Linear regression results are summarized in Table 2.
Figure 3:
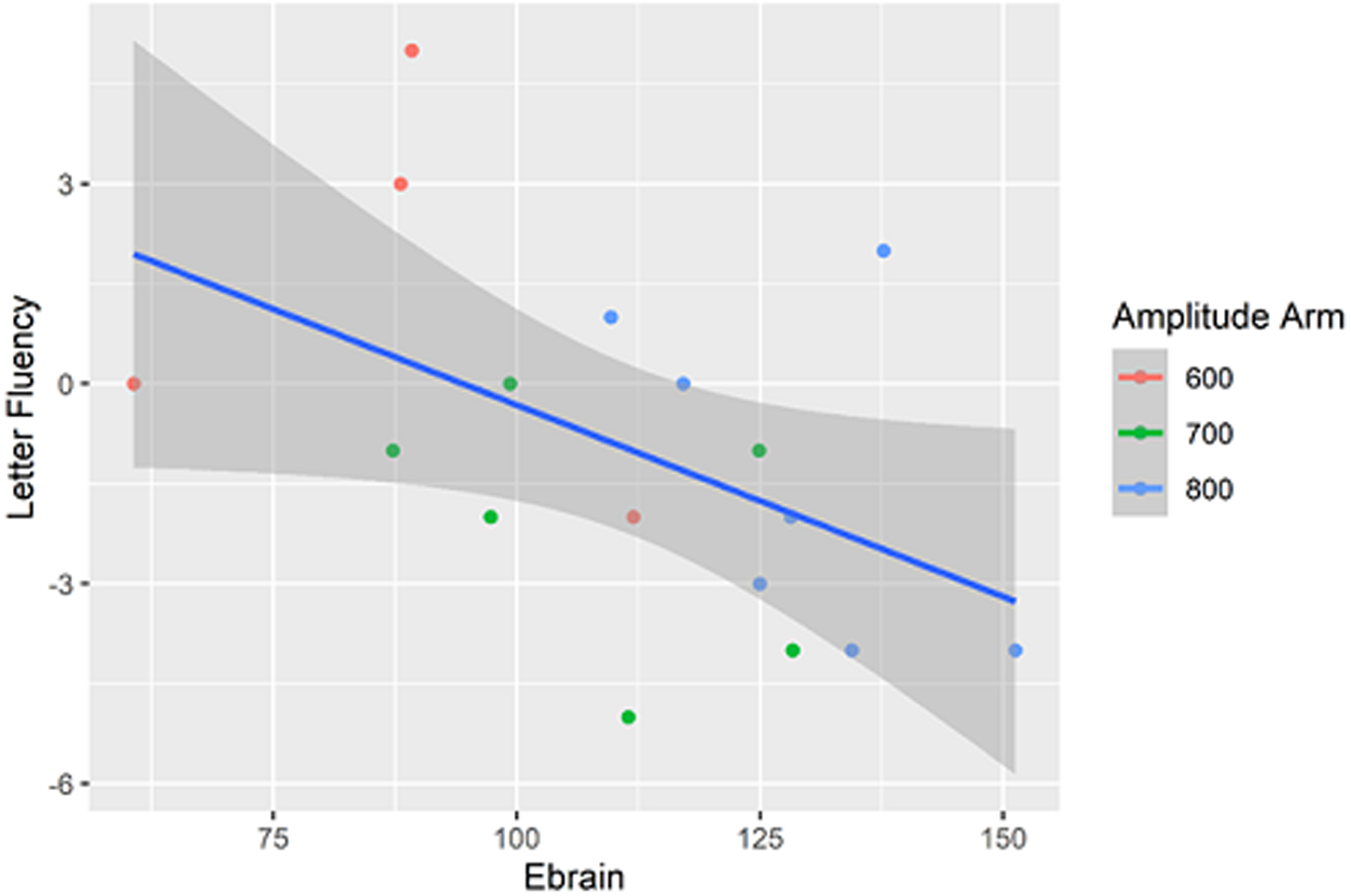
Letter Fluency versus Ebrain (p = 0.06).
Table 2:
Regression Results: See the five models presented in the rows below. The outcome variable is listed across the top. The variables and covariables are listed on the left side of the column. An example of how to read the first model under 1 is provided: ΔLetter Fluency = 3.60 – 2.05 * (Log of Fp1 Theta Power) + .02 * (Test of Premorbid Function)
| Regression Results | |||||
|---|---|---|---|---|---|
| ΔLetter Fluency | Log of Fp1 Theta Power | Log of Fp2 Theta Power | ΔLetter Fluency | ||
| OLS | OLS | OLS | OLS | ||
| 1 | 2 | 3 | 4 | 5 | |
| Constant | 3.60 (−8.70, 15.89) | 6.76 (−7.49, 21.00) | 2.27 (.16, 4.38) | 3.49* (1.12, 5.85) | 5.52 (−7.50, 18.54) |
| Log of Fp1 Theta Power | −2.05* (−3.90, −.21) | ||||
| Log of Fp2 Theta Power | −2.20* (−3.63, −.78) | ||||
| Test of Premorbid Function | .02 (−.08, .12) | 0.00 (−.11, .12) | −0.00 (−.10, .10) | ||
| Ebrain | .03*** (.02, .04) | .03*** (.02, .04) | −.06 (−.11, −0.00) | ||
| Age | −.03 (−.06, 0.00) | −.05* (−.08, −.01) | |||
| Observations | 15 | 13 | 15 | 13 | 17 |
| R2 | .29 | .48 | .70 | .74 | .23 |
| Adjusted R2 | .17 | .38 | .65 | .69 | .12 |
| Residual Std. Error | 2.44 (df = 12) | 2.16 (df = 10) | .41 (df = 12) | .48 (df = 10) | 2.54 (df = 14) |
| F Statistic | 2.41 (df = 2; 12) | 4.69* (df = 2; 10) | 14.02*** (df = 2; 12) | 14.57** (df = 2; 10) | 2.13 (df = 2; 14) |
Notes:
P < .05,
P < .01,
P < .001
OLS – Ordinary Least Squares
Ebrain, ictal theta power and antidepressant outcomes:
Ebrain showed no statistically significant association with antidepressant outcomes (% change in HDRS) when controlling for age (t(1,15)=0.005, p = 0.25). Ictal theta power in the Fp1/Fp2 (t(2,12)=0.07, p=0.65)/(t(2,10)=0.22, p=0.06) channels showed no statistically significant association with antidepressant outcomes when controlling for age. A whole-brain exploratory analysis demonstrated that ictal theta power was significantly associated with antidepressant outcomes when controlling for age in CPz and P8 (p<0.05).
DISCUSSION
We obtained baseline structural MRI and 24-lead EEG in a suprathreshold treatment in 17 older subjects with MDD who were treated with RUL ECT. We examined whether E-field strength (Ebrain) was associated with ictal power in different frequency bands. We then examined the relationship between Ebrain and ictal power and cognitive outcomes as measured by the change in letter and category fluency scores pre- and post-RUL ECT. Ictal theta power (4–8 Hz) was associated with Ebrain in 17 of 22 EEG channels (77%), including Fp1/Fp2, the two channels used to monitor seizure activity during routine ECT. Furthermore, an association was found in 16/22 EEG channels (73%), including Fp1/Fp2, between ictal theta power and the ΔDKEFS-VF LF scaled score (letter fluency). This association was noted to a much lesser extent in the alpha band, with 11/22 EEG channels (50%) reaching statistical significance, including Fp1, and were absent in the delta, beta, and gamma bands. No association was observed between ictal theta power and antidepressant outcomes in the Fp1/Fp2 channels. Neither Ebrain nor ictal power were associated with the change in category fluency. While a direct relationship between Ebrain and the change in letter fluency was insignificant (p=0.06). Our results align with a previous investigation and provide evidence that ictal theta power may act as an ECT safety biomarker by bridging ECT dosing as measured by Ebrain and cognitive impairment as measured by phonemic (letter) fluency [12].
Ictal theta power is a measure that is easily accessible on most ECT devices and could identify excessive dosing and cognitive risk early on in treatment. Unlike post-ictal recovery time, ictal theta power will not be confounded by emergent agitation and related treatments which affects approximately 10% of patients [38]. Evidence suggests that early ECT dosing impacts cognitive outcomes with a significant lag time between initial parameter selection and eventual cognitive impairment [1, 39–41]. In the context of non-response, the ECT clinician can increase the ECT dose for eventual therapeutic response without potential adverse consequences other than prolonging the ECT series. In contrast, reducing the ECT dose in the context of ECT-induced cognitive impairment may minimize though not completely eliminate cognitive impairment. Thus, early identification of cognitive risk at the first suprathreshold treatment has clinical translational implications for immediate corrective action to mitigate the onset of cognitive impairment. Based on our results, increased ictal theta power may be suggestive of excessive E-field strength. E-field strength is proportional to pulse amplitude [7, 15]. If the first suprathreshold treatments generates excessive ictal theta power, the ECT clinician may elect to decrease the E-field with a reduced amplitude in subsequent treatments to reduce cognitive risk. The specific threshold for the amount of theta-power from the first suprathreshold treatment that is associated with cognitive risk needs to be determined, but the premise could be a useful and accessible tool for the ECT clinician.
The interaction between ictal theta power and cognitive impairment remains unclear. Increases in theta oscillations in the resting state are associated with executive function and decreased vigilance [42–44]. Additionally, anatomical correlates of theta oscillation activity include deep brain structures like the hippocampus, which is thought to generate a gradation of theta frequencies across its body to coordinate brain-wide networks [45, 46]. Increases in ictal theta and alpha power, known as theta-alpha activity (TAA), are associated with seizure activity in the epilepsy literature and are thought to be caused by seizures spreading across the cortex [47]. Phonemic (letter) fluency tends to be governed by fronto/fronto-temporal circuitry and semantic (category) fluency tends to be governed by temporal circuitry. The association between ictal theta power and the change in letter fluency scores in our study along with the lack of association between ictal theta power and category fluency suggests that increased ictal theta power reflects frontal circuitry dysfunction [48–52].
The results of this study should be interpreted in the context of several limitations. First, the small sample size (n=17) in an older patient sample limits generalizability and our ability to factor in other demographic variables (e.g., sex, anesthetic agent, medications). Also, many small studies identifying biomarkers of clinical outcomes in ECT have not been replicated. The small sample also precludes the use of a sophisticated mediation analysis to determine the causal paths reflected in the context of ictal theta power that are expected to mediate effects of the E-field on cognitive outcomes. Second, while every attempt was made to capture the earliest suprathreshold treatment with 24-lead EEG, the add-on to acquire the 24-lead EEGs was not initiated until the middle of recruitment in the parent study and many of the acquisitions were completed after the third treatment (41%) secondary to poor tolerance of the EEG cap. Third, longitudinal EEG changes were not assessed. While the E-field is a static measure determined from the pre-ECT sMRI, ictal theta power is dynamic and may change across the ECT series. Fourth, we used the average reference in preprocessing the EEG signal and our results may not allow for direct comparison with the clinical 2-channel montage of Fp1/Fp2 referenced to their respective ipsilateral mastoid. Future investigations should include digital collection of two-channel EEG across all treatments with select treatments focused on the multi-channel acquisitions to assess longitudinal changes in EEG metrics.
Future investigations will address these limitations and may include additional imaging and cognitive measures to further elucidate the mechanisms of cognitive impairment. Due to the saturation of amplifiers, EEG is unable to monitor brain activity during the stimulation period. Implementing another imaging modality that can monitor continuously from stimulation to post-ictal recovery may help elucidate how ECT dosing and seizure phenomenon interact. Near infrared spectroscopy (NIRS), which measures cerebral blood flow and oxygenation, is a notable candidate. Multiple studies have used NIRS and EEG during ECT, with one showing a significant drop in cerebral blood flow and oxygenation during stimulation on the ipsilateral side of RUL ECT when compared to the contralateral side [53–55]. The authors hypothesized that this difference was due to current-induced vasoconstriction, stymying vascular autoregulation and inducing a perfusion/metabolic mismatch. They further speculated that the magnitude of this drop could be associated with therapeutic outcome. Revisiting this line of research with sophisticated cognitive testing may illuminate the interplay between electric dosing and the resulting seizure, while disentangling therapeutic and cognitive outcomes. In conclusion, ictal theta power could provide clinicians with an immediately available tool to identify early on in the ECT course those patients most at risk of cognitive impairment, resulting in measurement-based care precision ECT-dosing.
Highlights:
Ictal theta power may be a convenient and reliable safety biomarker for ECT.
Increased ictal theta power early in RUL ECT treatment may be associated with cognitive outcomes.
Ictal power with different frequency bands does not appear to be associated with ECT’s antidepressant effect.
ECT dose as measured by E-field modeling is associated with ictal theta power and cognitive outcomes.
Grant support:
This data was supported by the National Institute of Mental Health, the National Institute of General Medical Sciences, and the UNM Clinical and Translational Science Center; MH125126, MH111826, 1P30GM122734 and CTSC017-10. ZD is supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (ZIAMH002955).
Footnotes
Disclosures: Dr. McClintock is a Consultant to Pearson Assessment.
References
- [1].Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993;328(12):839–46. [DOI] [PubMed] [Google Scholar]
- [2].Obbels J, Vansteelandt K, Bouckaert F, Dols A, Stek M, Verwijk E, et al. Neurocognitive functioning after electroconvulsive therapy in late-life depression: A 4-year prospective study. Acta Psychiatr Scand 2021;143(2):141–50. [DOI] [PubMed] [Google Scholar]
- [3].Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology 2007;32(1):244–54. [DOI] [PubMed] [Google Scholar]
- [4].Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 2010;68(6):568–77. [DOI] [PubMed] [Google Scholar]
- [5].Porter RJ, Baune BT, Morris G, Hamilton A, Bassett D, Boyce P, et al. Cognitive side-effects of electroconvulsive therapy: what are they, how to monitor them and what to tell patients. BJPsych Open 2020;6(3):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Semkovska M, Landau S, Dunne R, Kolshus E, Kavanagh A, Jelovac A, et al. Bitemporal Versus High-Dose Unilateral Twice-Weekly Electroconvulsive Therapy for Depression (EFFECT-Dep): A Pragmatic, Randomized, Non-Inferiority Trial. Am J Psychiatry 2016;173(4):408–17. [DOI] [PubMed] [Google Scholar]
- [7].Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT 2010;26(3):159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ittasakul P, Likitnukul A, Pitidhrammabhorn U, Waleeprakhon P, Goldman MB. Stimulus intensity determined by dose-titration versus age-based methods in electroconvulsive therapy in Thai patients. Neuropsychiatr Dis Treat 2019;15:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Francis-Taylor R, Ophel G, Martin D, Loo C. The ictal EEG in ECT: A systematic review of the relationships between ictal features, ECT technique, seizure threshold and outcomes. Brain Stimul 2020;13(6):1644–54. [DOI] [PubMed] [Google Scholar]
- [10].Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA. Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacology 2004;29(4):813–25. [DOI] [PubMed] [Google Scholar]
- [11].Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA 3rd, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci 2003;15(1):27–34. [DOI] [PubMed] [Google Scholar]
- [12].Azuma H, Fujita A, Otsuki K, Nakano Y, Kamao T, Nakamura C, et al. Ictal electroencephalographic correlates of posttreatment neuropsychological changes in electroconvulsive therapy: a hypothesis-generation study. J ECT 2007;23(3):163–8. [DOI] [PubMed] [Google Scholar]
- [13].Huang Y, Datta A, Bikson M, Parra LC. Realistic volumetric-approach to simulate transcranial electric stimulation-ROAST-a fully automated open-source pipeline. J Neural Eng 2019;16(5):056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thielscher A, Antunes A, Saturnino GB. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? Annu Int Conf IEEE Eng Med Biol Soc 2015;2015:222–5. [DOI] [PubMed] [Google Scholar]
- [16].Lee WH, Lisanby SH, Laine AF, Peterchev AV. Comparison of electric field strength and spatial distribution of electroconvulsive therapy and magnetic seizure therapy in a realistic human head model. Eur Psychiatry 2016;36:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng 2015;23(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fridgeirsson EA, Deng ZD, Denys D, van Waarde JA, van Wingen GA. Electric field strength induced by electroconvulsive therapy is associated with clinical outcome. Neuroimage Clin 2021;30:102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Argyelan M, Oltedal L, Deng ZD, Wade B, Bikson M, Joanlanne A, et al. Electric field causes volumetric changes in the human brain. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lisanby SH, McClintock SM, Alexopoulos G, Bailine SH, Bernhardt E, Briggs MC, et al. Neurocognitive Effects of Combined Electroconvulsive Therapy (ECT) and Venlafaxine in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Geriatr Psychiatry 2020;28(3):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abbott CC, Quinn D, Miller J, Ye E, Iqbal S, Lloyd M, et al. Electroconvulsive Therapy Pulse Amplitude and Clinical Outcomes. Am J Geriatr Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abbott CC, Quinn D, Miller J, Ye E, Iqbal S, Lloyd M, et al. Electroconvulsive Therapy Pulse Amplitude and Clinical Outcomes. Am J Geriatr Psychiatry 2021;29(2):166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hamilton M Rating depressive patients. J Clin Psychiatry 1980;41(12 Pt 2):21–4. [PubMed] [Google Scholar]
- [24].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- [25].Wechsler D Test of Premorbid Functioning. The Psychological Corporation, San Antonion, TX; 2009. [Google Scholar]
- [26].Lei X, Liao K. Understanding the Influences of EEG Reference: A Large-Scale Brain Network Perspective. Front Neurosci 2017;11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Inc. TM. MATLAB 2018a. 2018a ed. Natick, Massachusetts: The MathWorks Inc.; 2018. [Google Scholar]
- [28].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- [29].Miyakoshi M How to extract EEG power of frequency bands, https://sccn.ucsd.edu/wiki/Makoto’s_useful_EEGLAB_code#How_to_extract_EEG_power_of_frequency_bands_.2806.2F06.2F2020_updated.29; 2020. [accessed November, 2020.
- [30].Gasser T, Bacher P, Mocks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 1982;53(1):119–24. [DOI] [PubMed] [Google Scholar]
- [31].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208–19. [DOI] [PubMed] [Google Scholar]
- [32].Nielsen JD, Madsen KH, Puonti O, Siebner HR, Bauer C, Madsen CG, et al. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. Neuroimage 2018;174:587–98. [DOI] [PubMed] [Google Scholar]
- [33].Friston KJ. Statistical Parametric Mapping: The analysis of funtional brain images. Amsterdam: Elsevier/Academic Press; 2007. [Google Scholar]
- [34].Team R. RStudio: Integrated Development for R. . RStudio, PBC, Boston, MA; 2020. [Google Scholar]
- [35].Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- [36].Hlavac M stargazer: Well-Formatted Regression and Summary Statistics Tables. R package version 5.2.2 ed. Bratislava, Slovakia: Central European Labour Studies Institute (CELSI); 2018. [Google Scholar]
- [37].Wickham H ggplot2: Elegant Graphics for Data Analysis. Verlag New York: Springer; 2016. [Google Scholar]
- [38].Tzabazis A, Schmitt HJ, Ihmsen H, Schmidtlein M, Zimmermann R, Wielopolski J, et al. Postictal agitation after electroconvulsive therapy: incidence, severity, and propofol as a treatment option. J ECT 2013;29(3):189–95. [DOI] [PubMed] [Google Scholar]
- [39].Sackeim HA, Portnoy S, Neeley P, Steif BL, Decina P, Malitz S. Cognitive consequences of low-dosage electroconvulsive therapy. Ann N Y Acad Sci 1986;462:326–40. [DOI] [PubMed] [Google Scholar]
- [40].Sackeim HA, Decina P, Portnoy S, Neeley P, Malitz S. Studies of dosage, seizure threshold, and seizure duration in ECT. Biol Psychiatry 1987;22(3):249–68. [DOI] [PubMed] [Google Scholar]
- [41].McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry 2000;57(5):438–44. [DOI] [PubMed] [Google Scholar]
- [42].Braboszcz C, Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage 2011;54(4):3040–7. [DOI] [PubMed] [Google Scholar]
- [43].Nigbur R, Ivanova G, Sturmer B. Theta power as a marker for cognitive interference. Clin Neurophysiol 2011;122(11):2185–94. [DOI] [PubMed] [Google Scholar]
- [44].Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology 2011;48(8):1083–7. [DOI] [PubMed] [Google Scholar]
- [45].Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci 2012;32(19):6550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Goyal A, Miller J, Qasim SE, Watrous AJ, Zhang H, Stein JM, et al. Functionally distinct high and low theta oscillations in the human hippocampus. Nat Commun 2020;11(1):2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sip V, Scholly J, Guye M, Bartolomei F, Jirsa V. Evidence for spreading seizure as a cause of theta-alpha activity electrographic pattern in stereo-EEG seizure recordings. PLoS Comput Biol 2021;17(2):e1008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 2006;12(6):896–900. [DOI] [PubMed] [Google Scholar]
- [49].Herrmann MJ, Ehlis AC, Fallgatter AJ. Frontal activation during a verbal-fluency task as measured by near-infrared spectroscopy. Brain Res Bull 2003;61(1):51–6. [DOI] [PubMed] [Google Scholar]
- [50].Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 2000;47(4):470–6. [PubMed] [Google Scholar]
- [51].Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14(2):167–77. [PubMed] [Google Scholar]
- [52].Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia 1998;36(6):499–504. [DOI] [PubMed] [Google Scholar]
- [53].Saito S, Miyoshi S, Yoshikawa D, Shimada H, Morita T, Kitani Y. Regional cerebral oxygen saturation during electroconvulsive therapy: monitoring by near-infrared spectrophotometry. Anesth Analg 1996;83(4):726–30. [DOI] [PubMed] [Google Scholar]
- [54].Fujita Y, Takebayashi M, Hisaoka K, Tsuchioka M, Morinobu S, Yamawaki S. Asymmetric alternation of the hemodynamic response at the prefrontal cortex in patients with schizophrenia during electroconvulsive therapy: a near-infrared spectroscopy study. Brain Res 2011;1410:132–40. [DOI] [PubMed] [Google Scholar]
- [55].Fabbri F, Henry ME, Renshaw PF, Nadgir S, Ehrenberg BL, Franceschini MA, et al. Bilateral near-infrared monitoring of the cerebral concentration and oxygen-saturation of hemoglobin during right unilateral electro-convulsive therapy. Brain Res 2003;992(2):193–204. [DOI] [PubMed] [Google Scholar]


