Abstract
The replacement of benzene rings with sp3-hybridized bioisosteres in drug candidates generally improves pharmacokinetic properties while retaining biological activity1–5. Rigid, strained frameworks such as bicyclo[1.1.1]pentane and cubane are particularly well-suited since the ring strain imparts high bond strength and thus metabolic stability on its C–H bonds. Cubane is the ideal bioisostere since it provides the closest geometric match to benzene6,7. At present, however, all cubanes in drug design, like almost all benzene bioisosteres, act solely as substitutes for mono- or para-substituted benzene rings1–7. This is due to the difficulty of accessing 1,3- and 1,2-disubstituted cubane precursors. The adoption of cubane in drug design has been further hindered by the poor compatibility of cross-coupling reactions with the cubane scaffold, owing to a competing metal-catalyzed valence isomerization8–11. Herein, we disclose expedient routes to 1,3- and 1,2-disubstituted cubane building blocks using a convenient cyclobutadiene precursor and a photolytic C–H carboxylation reaction, respectively. Moreover, we leverage the slow oxidative addition and rapid reductive elimination of copper to develop C–N, C–C(sp3), C–C(sp2), and C–CF3 cross-coupling protocols12,13. Our research enables facile elaboration of all cubane isomers into drug candidates thus enabling ideal bioisosteric replacement of ortho-, meta-, and para-substituted benzenes.
The substitution of a benzene group with an sp3-hybridized bioisostere can produce drug candidates with improved compound properties1,2. Suitable bioisosteres emulate the size and the rigid steric relationship between substituents in the parent benzene unit thus maintaining the activity, while reducing the overall C(sp2)-character, which generally improves key pharmacokinetic properties like solubility and metabolic stability14. Bicyclo[1.1.1]pentanes (BCPs) and cubanes are particularly privileged, since they are rigid, and because their strained nature imparts high s-character and thus bond strength on their C–H bonds15. While BCPs are now routinely employed in drug discovery, cubanes remain less explored despite being a better geometric match to benzene (Fig. 1a)6,7,16. Furthermore, all cubanes in drug candidates, like most benzene bioisosteres, are either mono-substituted or bear linear exit vectors 180° apart, acting solely as substitutes for terminal or para-substituted phenyl rings. While several bicycloalkanes have recently been explored as nonlinear benzene isosteres17–24, their scarcity is a severe limitation for drug design given that over 170 approved drugs contain ortho- or meta-substituted benzene rings. 1,2- and 1,3-disubstituted cubanes are ideally suited to bridge this gap since they most closely emulate the size and spatial arrangement of the substituents in the parent benzenes.
Figure 1 |. Cubanes in medicinal chemistry.
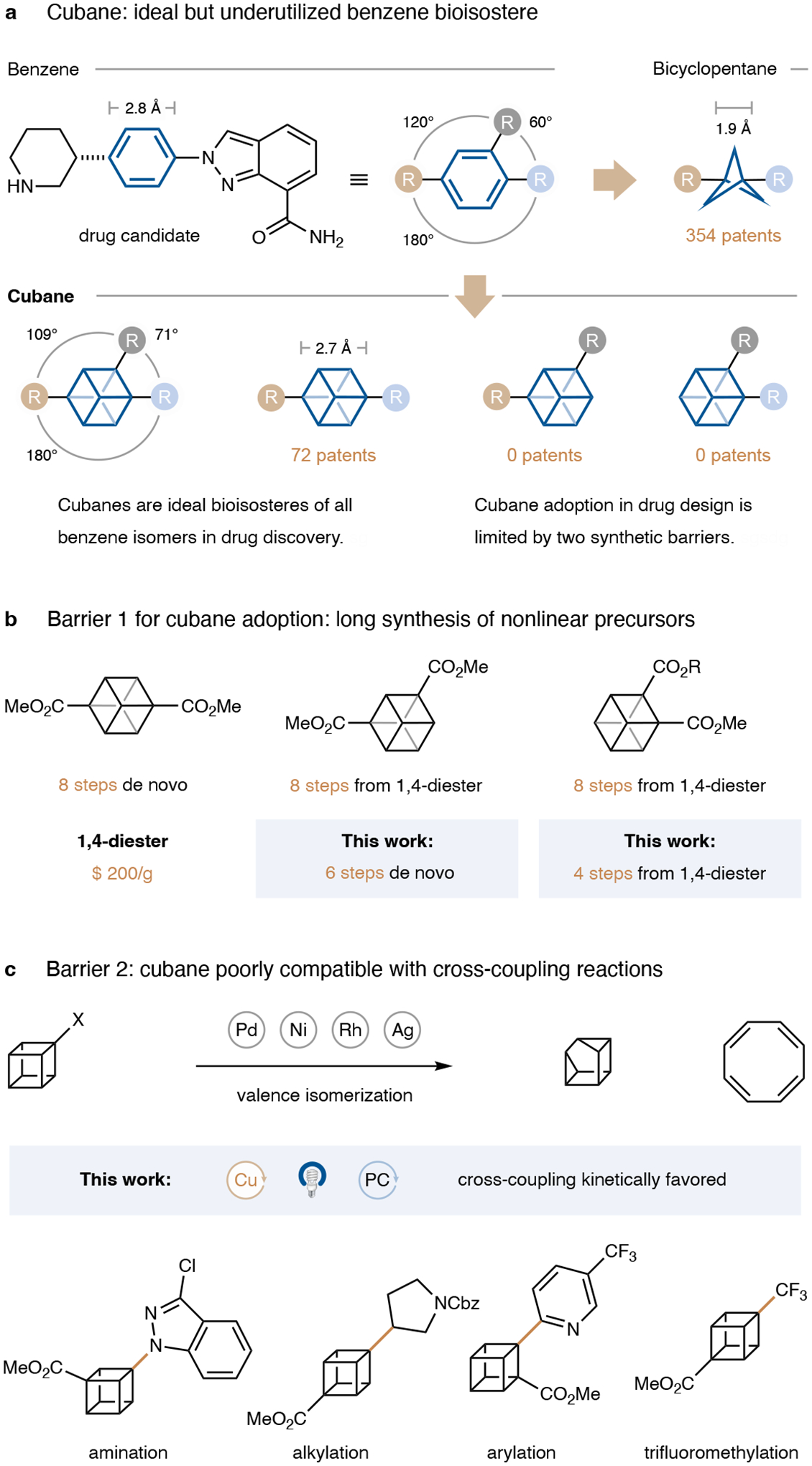
a, Cubane closely resembles benzene in spacer size and exit vector orientation but remains underutilized in drug discovery – especially as bioisosteres of nonlinear benzenes. b, c, Our concise syntheses of 1,3- and 1,2-cubane precursors and copper photoredox-catalyzed cross-coupling reactions remove the major barriers for cubane adoption in medicinal chemistry. Me, methyl; Cbz, carbobenzyloxy.
Access to these nonlinear cubanes is hampered by protracted sequences towards the respective precursors (Fig. 1b). Eaton’s linear dimethyl cubane-1,4-dicarboxylate, the most commercially available cubane-containing fragment, can be synthesized in 8 steps on a laboratory scale and has been scaled up to a kilogram scale via a flow photoreactor25–27. By contrast, the nonlinear 1,3- and 1,2-dicarboxylate isomers both require 8 additional steps, starting from the expensive 1,4-diester28.
Moreover, the adoption of cubanes in medicinal chemistry is limited by the lack of cross-coupling reactions. All cubane-containing drug candidates have been synthesized by traditional carboxylic acid reactivity, such as amide couplings and heterocycle syntheses29–31. Despite recent progress on the arylation of cubane11,32,33, a general, fragment-based cross-coupling of cubanes and challenging bond formations such as cubane–N, cubane–C(sp3) and cubane–CF3 remain elusive due to the metal-catalyzed strain-releasing valence isomerization of cubanes (Fig. 1c)8–11. With the goal of increasing the adoption of cubanes in medicinal chemistry, we set out to develop expedient routes to cubane-1,3- and 1,2-diesters and a general platform for cubane cross-coupling.
Results and discussion
Cubane-1,3-dicarboxylate ester synthesis
We were inspired by an isolated report on the synthesis of cubane-1,3-dicarboxylic acid published by the Pettit group in 196634. Under this protocol, a Diels-Alder reaction between cyclobutadiene, generated in situ from cyclobutadieneiron tricarbonyl, and 2,5-dibromobenzoquinone, served to construct the cubane framework in only 3 steps. To date, this synthesis has seen no application in medicinal chemistry, likely owing to the arduous synthesis of the cyclobutadiene precursor cyclobutadieneiron tricarbonyl, which involves 4 steps (9% overall yield35,36) and requires inconvenient reagents such as chlorine gas, benzene, and highly toxic diiron nonacarbonyl. Convenient access to 1,3-cubane precursors would thus require the development of a new, readily accessible cyclobutadiene precursor.
As a key design principle, this new precursor would be required to liberate cyclobutadiene under mild, oxidative conditions, since the quinone coupling partner is itself an oxidant, and because the key bisalkene intermediate 2 is unstable (Fig. 2, a). Drawing inspiration from a 1975 study by the Masamune group37, we reasoned that 1,2-dihydropyridazine 1, which is readily available from commercial material (75% yield over 2 steps)38, could be a suitable candidate. We developed an improved route to cyclobutadiene (7) commencing with light-mediated, endocyclic 4-π-cyclization of dihydropyridazine 1 followed by deprotection to generate diazetidine 539,40. Oxidation to diazine 6 by the mild oxidant 2,5-dibromoquinone followed by nitrogen extrusion releases cyclobutadiene, which can undergo [4+2] cycloaddition with a second equivalent of the quinone to form bisalkene 2 thus intercepting the key intermediate of Pettit’s synthesis34. Following optimization, the sequence was telescoped and proceeded in 80% analytical yield. The quinone was removed by a reductive workup prior to the internal [2+2] cycloaddition to diketone 3 to prevent decomposition by sensitization. This workup enabled us to circumvent the highly challenging recrystallization of the unstable bisalkene 2 used in Pettit’s synthesis. Finally, Favorskii ring contraction and esterification provided dimethyl cubane-1,3-dicarboxylate (4). The entire synthesis requires 4 steps from dihydropyridazine 1 (6 from commercial material), proceeds in 35% isolated yield on a 1 mmol scale (26% from commercial material), requires only one chromatographic purification, and can be conducted in 3 days, thus rapidly providing sufficient quantities for medicinal chemistry projects (see SI for details).
Figure 2 |. Synthetic strategies towards non-linear cubane precursors.
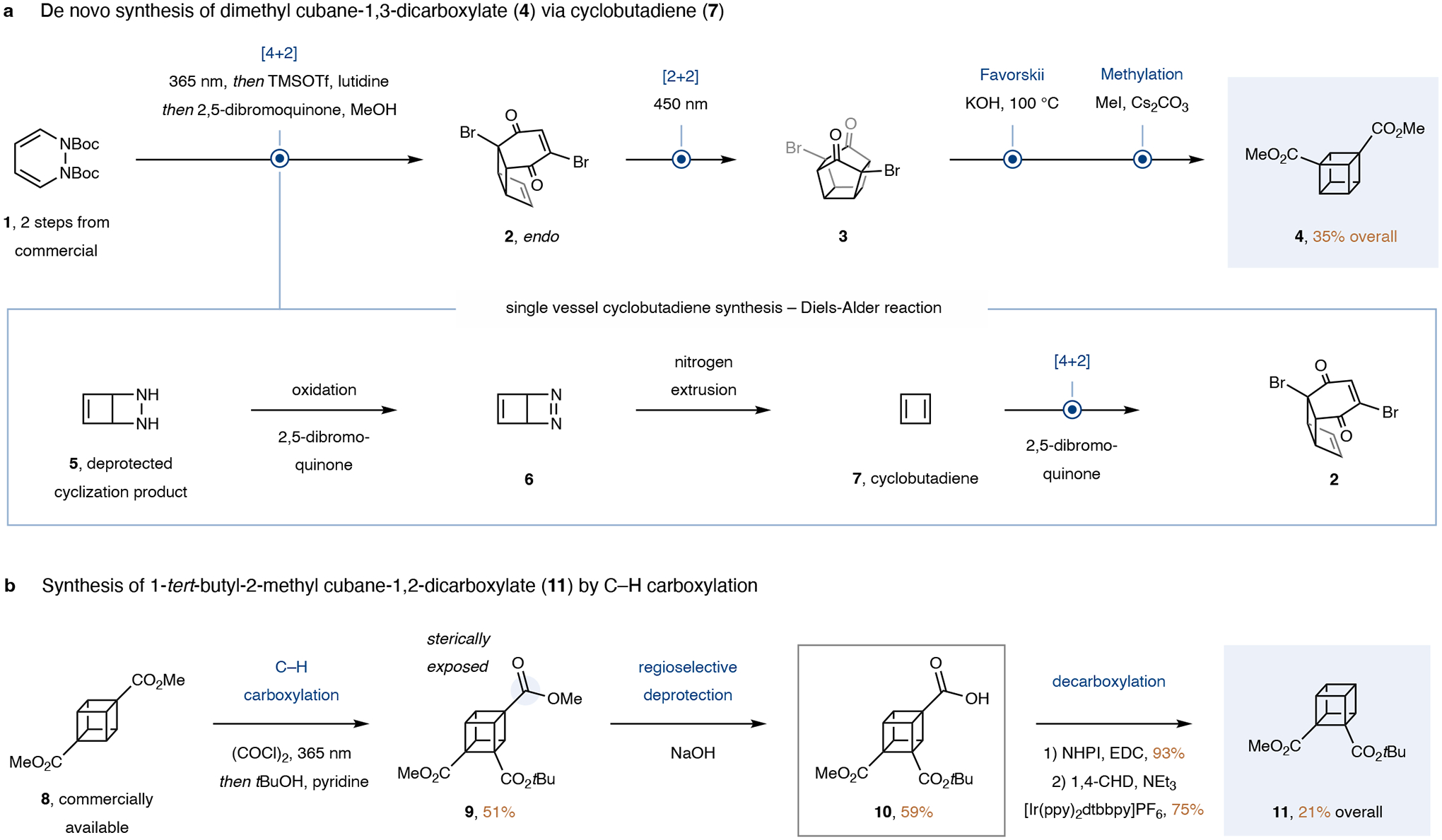
a, De novo-synthesis of dimethyl 1,3-cubanedicarboxylate (4) using dihydropyridazine 1 as precursor of cyclobutadiene (7). b, Synthesis of tert-butylmethyl 1,2-cubanedicarboxylate (11) by C–H carboxylation. Me, methyl; Boc, tert-butylcarbonyl; TMS, trimethylsilyl; Tf, trifluoromethylsulfonyl; tBu, tert-butyl; NHPI, N-hydroyphthalimide; EDC, 1-ethyl-3-(−3-dimethylaminopropyl) carbodiimide hydrochloride; CHD, cyclohexadiene; Et, ethyl; ppy, 2-phenylpyridine; dtbbpy, 4,4’-ditert-butyl-2,2’-bipyridine.
Cubane-1,2-dicarboxylate ester synthesis
However, a similar strategy proved unsuitable for the synthesis of the corresponding cubane-1,2-diester due to a competing Haller-Bauer cleavage (see SI for details). We speculated that C–H functionalization could provide a workaround to access this valuable substitution pattern (Fig. 2b). In order to avoid laborious installation and removal of a directing group, we decided to start with the symmetrical, commercially available dimethyl cubane-1,4-dicarboxylate (8). Inspired by a cubane C–H carboxylation reported by Bashir-Hashemi41,42, we utilized a light-mediated one-pot C–H carboxylation/ esterification sequence. Deprotection of the sterically exposed methyl ester of 9 yielded the monoacid 10. Photoredox-mediated decarboxylation in presence of 1,4-cyclohexadiene was achieved via the redox-active ester. Overall, cubane 1,2-diester 11 was obtained in 21% isolated yield over 4 steps from commercially available cubane 8.
Copper-mediated amination of cubanes
With facile routes to the cubane diesters in hand, we set out to develop a general and modular cross-coupling platform en route to a wide array of functionalized cubane isomers. We intended to utilize the carboxylic acid handles introduced by the cubane-forming Favorskii reaction for metallaphotoredox-mediated decarboxylative cross-coupling43,44. Crucially, the metal must be compatible with the highly strained cubane framework. Typical cross-coupling catalysts, such as nickel and palladium complexes, are known to facilitate cubane decomposition by strain-releasing valence bond isomerization to generate products such as cuneane (12) and cyclooctatetraene (13)8–11. Several decomposition pathways have been proposed, including oxidative insertion into the cubane framework (to 14, Fig. 3)9 and decomposition of metal-cubane complexes (15)10. We realized that both of these undesirable pathways would be suppressed under a copper catalytic manifold since copper is known to undergo slow oxidative addition and rapid reductive elimination12. The former property should prevent it from decomposing cubane via oxidative insertion while the latter should ensure that reductive elimination outcompetes valence isomerization.
Figure 3 |. Copper-mediated cross-coupling of cubane.
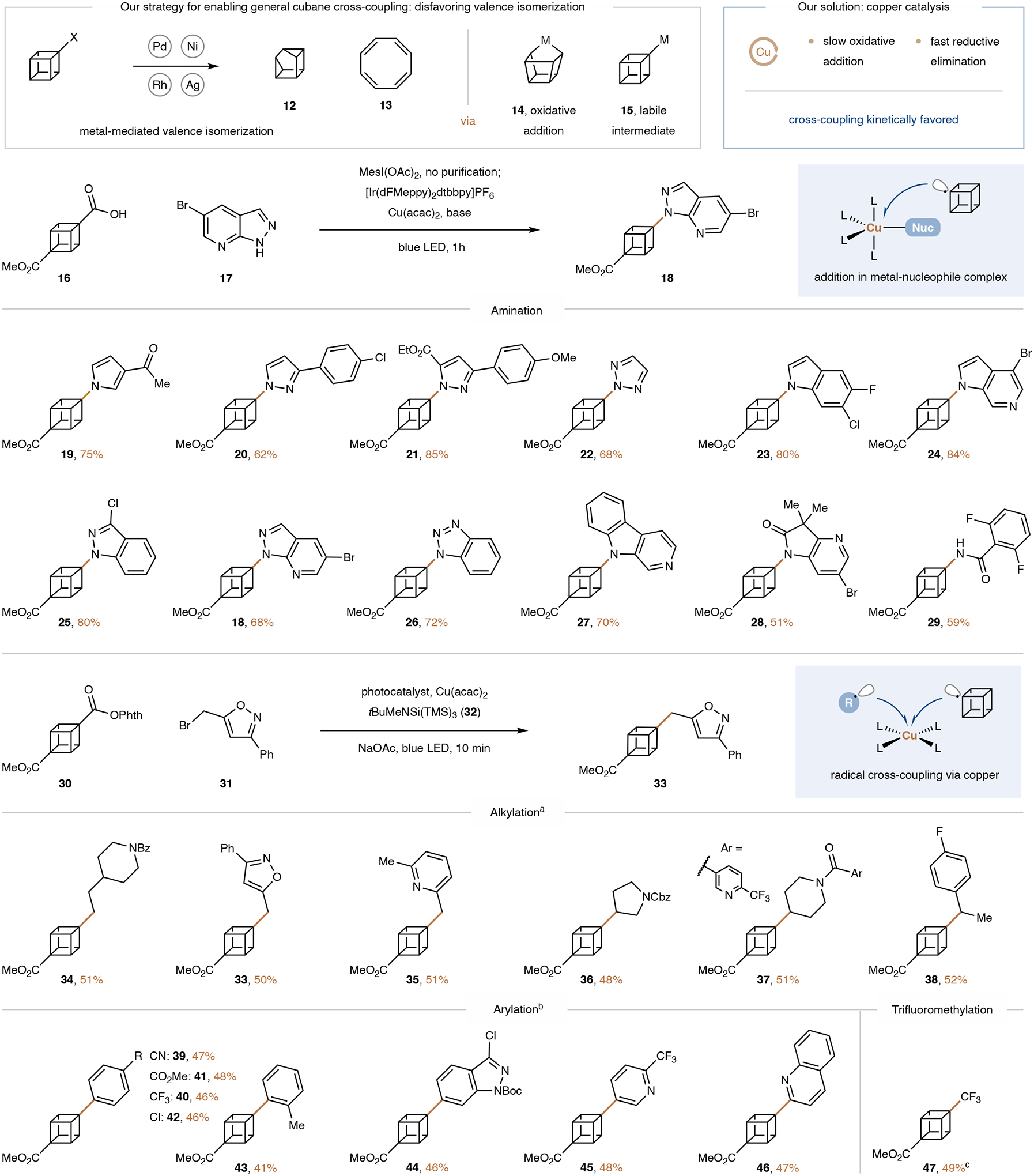
See SI sections 4 and 5 for additional examples. Isolated yields. aPhotocatalyst: 4-CzIPN. bTetrachlorophthalimidyl used instead of Phth. Photocatalyst: [Ru(4,4’-dClbpy)3](PF6)2 for aryl bromides and [Ir(dFCF3ppy)2(4,4’-d(CF3)bpy)]PF6 for heteroaryl bromides. c19F NMR yield vs 1,4-difluorobenzene. Me, methyl; Mes, 1,3,5-trimethylphenyl; Ac, acetyl; dFMebpy, 2-(2,4-difluorophenyl)-5-methylpyridine; dtbbpy, 4,4’-ditert-butyl-2,2’-bipyridine; acac, acetylacetone; LED, light-emitting diode; Nuc, nucleophile tBu, tert-butyl; TMS: trimethylsilyl; Bz, benzoyl; Ph, phenyl, Phth, phthalimidyl; Cbz, carbobenzyloxy; Boc, tert-butylcarboxyl; SI, supplementary information; 4-CzIPN, 2,4,5,6-Tetrakis(9H-carbazol-9-yl) isophthalonitrile.
Given the importance of C–N cross-coupling reactions in pharmaceutical research45,46, we tested our hypothesis by adopting our laboratory’s protocol for the decarboxylative amination of alkyl carboxylic acids13 to cubane functionalization starting from commercially available 1,4-disubstituted cubane 16 (Fig. 3). To our delight, an optimized procedure enabled C(sp3)–N Bond formation for a wide range of products in good yields. The scope included heteroaromatic amines (18–27) as well as amide functionalities (28, 29). Multifunctional substrates such as triazole and benzotriazole were alkylated with complete regioselectivity (22, 26). Many functional groups including ketones (19), aryl halides (e.g., 24), esters (18–29), and ethers (21) were tolerated, thus enabling orthogonal functionalization of the products. This method is therefore a direct, convenient, and general alternative to the Curtius rearrangement47 for the synthesis of aminated cubanes. Such motifs are desirable since they can act as bioisosteres of anilines, which are structural alerts for drug discovery due to their tendency for oxidative arene metabolism leading to adverse idiosyncratic drug reactions48,49.
C–C cross coupling of cubanes
In order to develop C–C cross-coupling reaction of cubanes, we designed a new, unified mechanistic platform that utilizes copper to couple cubyl radicals with alkyl and aryl radicals. Prior to our work, no general copper-mediated alkylation or arylation reactions of alkyl radicals were known. In order to be able to utilize the widely available alkyl and aryl halides as coupling partners, we planned to utilize silyl radical-mediated halide abstraction from the broadly available bromides to generate alkyl and aryl radicals12,50. We sought to pair this oxidative activation mode with a reductively generated cubyl radical derived from a redox-active ester (30). Both radicals could then undergo radical cross-coupling via copper catalysis (see SI for a detailed design plan). To prevent decomposition of the electrophilic redox-active ester, we developed a new, non-nucleophilic tertiary aminosilane (32, see SI for details). Under the optimized conditions, primary, secondary, and benzylic alkyl bromides were coupled with the tertiary cubane in good yields (Fig. 3, 33–38). Moreover, many functional groups, including the metal-sensitive isoxazole-moiety (33), were tolerated, demonstrating the mildness of the reaction.
Under slightly modified conditions, aryl and heteroaryl bromides were also coupled with cubane (39–46) and synthetically useful functional groups such as cyanides (39) and aryl chlorides (42, 44) were preserved. Of note is the tolerance of an ortho-substituent in the coupling with the sterically hindered, tertiary cubyl radical (43).
Given the prevalence of trifluoromethylated benzenes in drug candidates, we next sought to extend our new copper-mediated radical-radical coupling manifold to the trifluoromethylation of cubanes. We intended to generate trifluoromethyl radical reductively using a modified version of Umemoto’s reagent previously developed by our group12, while generating the cubyl radical oxidatively starting directly from the cubane carboxylic acid 16. We were pleased to find that this challenging tertiary C(sp3)–CF3 cross-coupling proceeded in good yield (to 47). We have thus achieved a general platform for the amination, arylation, alkylation, and trifluoromethylation of cubanes.
Cross coupling of new cubane isomers
We proceeded to explore the cross-coupling of the newly synthesized 1,3- and 1,2- disubstituted cubane isomers (see SI for the synthesis of the free acids and redox-active esters). Both cubane isomers underwent amination and alkylation in yields comparable to the 1,4-isomers (Fig. 4a, 48–51). The arylation reactions proceeded in lower, yet still synthetically useful yields (52, 53).
Figure 4 |. Synthetic and medicinal applications of novel cubane isosteres.
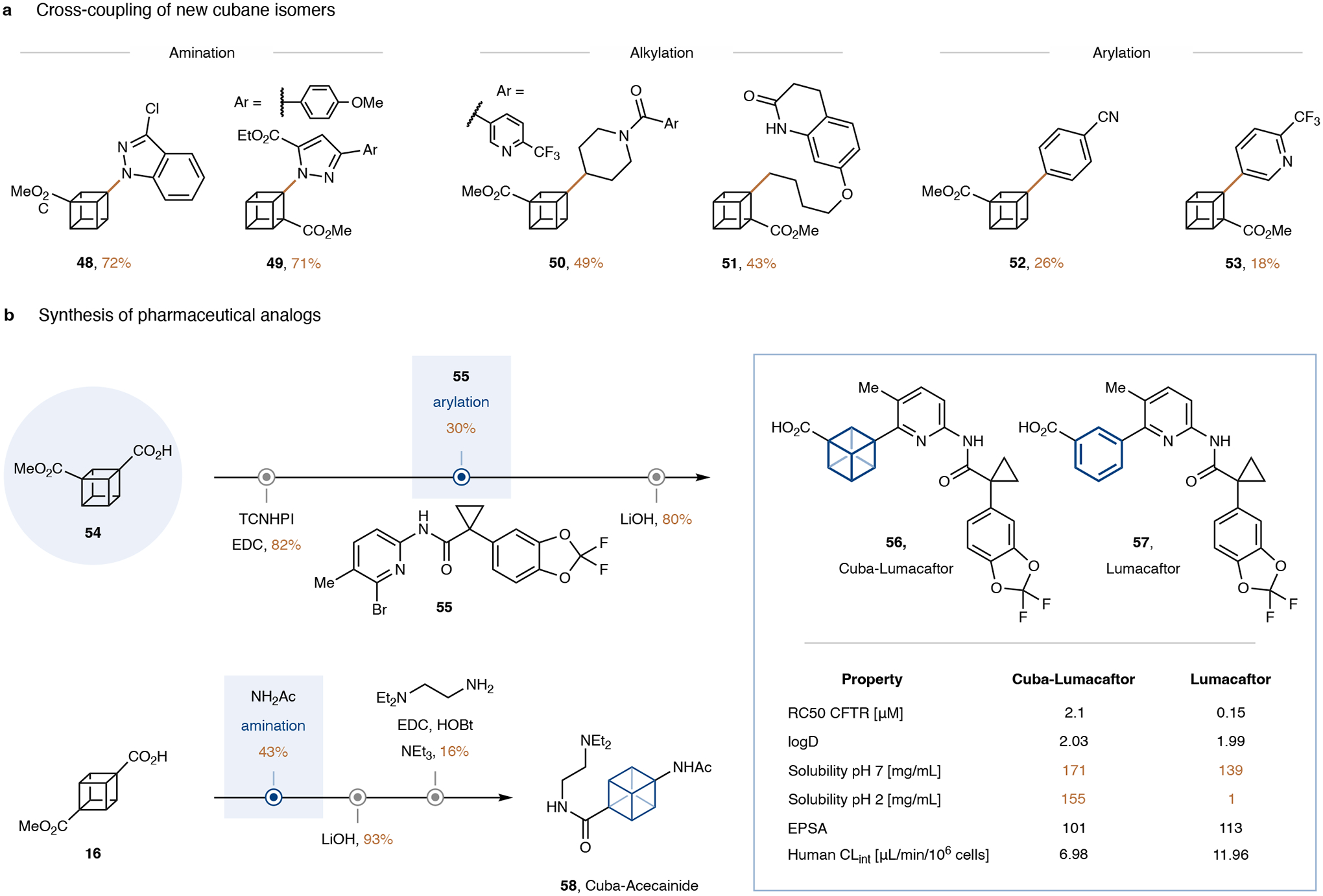
a, Cross-couplings of 1,3- and 1,2-cubane isomers. b, Synthesis of cubane-containing analogues of drug candidates via the newly developed protocols. See supplementary information for experimental details. Me, methyl; Ac, acetyl; EDC, 1-ethyl-3-(−3-dimethylaminopropyl) carbodiimide hydrochloride; HOBt, hydroxybenzotriazole; Et, ethyl; TCNHPI, tetrachloro N-hydroyphthalimide; RC50, half-maximal rescue concentration; CFTR, cystic fibrosis transmembrane conductance regulator; CLint, in vitro intrinsic clearance.
Synthesis of pharmaceutical analogs
Finally, we demonstrated that our streamlined synthetic routes and cross-coupling protocols enable the rapid synthesis of cubane analogs of benzene containing drugs (Fig. 4b, 56, 58). Cuba-Lumacaftor (56) was synthesized starting from our new, monohydrolyzed 1,3-disubstituted cubane precursor 54, with our new copper-mediated arylation reaction with a complex aryl bromide as the key step. Cuba-Acecainide (58) was synthesized from 1,4-monoacid 16 using our copper-catalyzed cubane amination. Biological studies were conducted for the cubane-containing drug analogs 56 and 58, and both compounds were found to be metabolically stable (in vitro intrinsic clearance (CLint) <7 μL/min/106 cells, see SI for details). Furthermore, Cuba-Lumacaftor (56) still showed high activity (see half-maximal rescue concentration (RC50)) despite the structural change near a binding moiety of the original, optimized drug (57). Interestingly, the Cuba-Lumacaftor (58) has an improved solubility compared to the parent benzene-containing drug at all measured pH values. This pH-independent high solubility would allow for improved compound absorption throughout the gastrointestinal tract. Moreover, bioisosteric replacement of the benzene ring with a cubane showed increased metabolic stability (CLint = 6.98 μL/min/106 cells) compared to the parent benzene-containing drug (CLint = 11.96 μL/min/106 cells) thus further demonstrating the positive influence of bioisosteric replacement on the physicochemical and pharmacokinetic properties.
Conclusions
In conclusion, we disclose laboratory-scale syntheses of sought-after 1,3- and 1,2-disubstituted cubanes. Furthermore, we demonstrate general copper photoredox-catalyzed decarboxylative amination, arylation, alkylation, and trifluoromethylation reactions of cubanes. In the process, we developed a practical means to access the highly reactive cyclobutadiene in situ and a copper-mediated alkyl radical cross-coupling manifold. Altogether, we expect that this work will expedite the use of cubanes as bioisosteres of ortho-, meta-, and para-substituted benzenes in drug design. Moreover, we anticipate that our strategy of accessing cyclobutadiene by mild oxidation of a readily accessible dihydropyridazine and the new cross-coupling manifold will find further application in synthetic organic chemistry.
Supplementary Material
Acknowledgements
We thank Z. Dong, P. Sarver, Y. Liang, C. Oswood, W. Liu, and M. Heilmann for helpful discussions, I Pelcer and K. Conover for assistance with NMR spectroscopy, and R. Lambert for assistance with the preparation of this manuscript. We also thank J. Piesvaux, J. P. Imredy, R. L. Kraus, and B. Lacey for help with biological profiling and A. Beard, M. Darlak, S. McMinn, L. Nogle, M. Pietrafitta, D. Smith, and Y. Ye (all Merck & Co., Inc.) for help with reverse phase chromatography. The research was supported by the NIH National Institute of General Medical Sciences (NIGMS), the NIH (R35GM134897-03), the Princeton Catalysis Initiative, and kind gifts from Merck & Co., Inc., Bristol-Myers Squibb (BMS), Celgene, Genentech, Janssen Research and Development LLC, and Pfizer. M.P.W. was supported by the Deutsche Akademie der Naturforscher Leopoldina (LPDS 2018-16). F.B. was funded by the German Research Foundation (DFG) – 421436809, and J.D. was supported by an SNSF Early Postdoc.Mobility fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of a peer-reviewed paper that has been accepted for publication. Although unedited, the content has been subjected to preliminary formatting. Nature is providing this early version of the typeset paper as a service to our authors and readers. The text and figures will undergo copyediting and a proof review before the paper is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers apply.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature
Competing interests D.W.C.M. declares an ownership interest in the Penn PhD photoreactor, which is used to irradiate reactions in this work. The remaining authors declare no competing interests.
Data availability
All data are available in the main text or in the supplementary information.
References
- 1.Subbaiah MAM & Meanwell NA Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem 64, 14046–14128 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Mykhailiuk PK Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem 17, 2839–2849 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Stepan AF et al. Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem 55, 3414–3424 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Gianatassio R et al. Strain-release amination. Science 351, 241–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton PE Cubanes: Starting Materials for the Chemistry of the 1990s and the New Century. Angew. Chem. Int. Ed 31, 1421–1436 (1992). [Google Scholar]
- 7.Reekie TA, Williams CM, Rendina LM & Kassiou M Cubanes in Medicinal Chemistry. J. Med. Chem 62, 1078–1095 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Cassar L Eaton PE & Halpern J Silver(I)- and Palladium(II)-Catalyzed Isomerizations of Cubane. Synthesis and Characterization of Cuneane. J. Am. Chem. Soc 92, 6366–6368 (1970). [Google Scholar]
- 9.Cassar L, Eaton PE & Halpern J Catalysis of Symmetry-Restricted Reactions by Transition Metal Compounds. The Valence Isomerization of Cubane. J. Am. Chem. Soc 92, 3515–3518 (1970). [Google Scholar]
- 10.Plunkett S, Flanagan KJ, Twamley B & Senge MO Highly Strained Tertiary sp3 Scaffolds: Synthesis of Functionalized Cubanes and Exploration of Their Reactivity under Pd(II) Catalysis. Organometallics 34, 1408–1414 (2015). [Google Scholar]
- 11.Toriyama F et al. Redox-Active Esters in Fe-Catalyzed C–C Coupling. J. Am. Chem. Soc 138, 11132–11135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le C, Chen TQ, Liang T, Zhang P & MacMillan DWC A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Y, Zhang X & MacMillan DWC Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovering F, Bikker J, Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Liu L, Wang J-T, Zhao S-W & Guo Q-X Homolytic C–H and N–H Bond Dissociation Energies of Strained Organic Compounds. J. Org. Chem 69, 3129–3138 (2004). [DOI] [PubMed] [Google Scholar]
- 16. The number of patents was determined by a scifinder search conducted on January 12 2023 at 1 pm CET. All patents with at least one spectroscopically characterized drug candidate were counted. See SI for details.
- 17.Levterov VV, Panasyuk Y, Pivnytska VO & Mykhailiuk PK Water-Soluble Non-Classical Benzene Mimetics. Angew. Chem. Int. Ed 59, 7161–7167 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Denisenko A, Garbuz P, Shishkina SV, Voloshchuk NM & Mykhailiuk PK Saturated Bioisosteres of ortho-Substituted Benzenes. Angew. Chem. Int. Ed 59, 20515–20521 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Zhao J-X et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl. Acad. Sci. U. S. A 118, No. e2108881118. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epplin RC et al. [2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes. Nat. Commun 13, No. 6056 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iida T et al. , Practical and Facile Access to Bicyclo[3.1.1]heptanes: Potent Bioisosteres of meta-Substituted Benzenes. J. Am. Chem. Soc 144, 21848–21852 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Kleinmans R et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Frank N et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Rigotti T & Bach T Bicyclo[2.1.1]hexanes by Visible Light-Driven Intramolecular Crossed [2 + 2] Photocycloadditions. Org. Lett 24, 8821–8825 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Eaton PE & Cole TW Cubane. J. Am. Chem. Soc 86, 3157–3158 (1964). [Google Scholar]
- 26.Falkiner MJ, Littler SW, McRae KJ, Savage GP & Tsanaktsidis J Pilot-Scale Production of Dimethyl 1,4-Cubanedicarboxylate. Org. Process Res. Dev 17, 1503–1509 (2013). [Google Scholar]
- 27.Biegasiewicz KF, Griffiths JR, Savage GP, Tsanaktsidis J, Priefer R Cubane: 50 Years Later. Chem. Rev 115, 6719–6745 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Kassiou M, Coster M & Gunosewoyo H Polycyclic molecular compounds (2008).
- 29.Wlochal J, Davies RDM, Burton J Cubanes in Medicinal Chemistry: Synthesis of Functionalized Building Blocks. Org. Lett 16, 4094–4097 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Chalmers BA et al. Validating Eaton’s Hypothesis: Cubane as a Benzene Bioisostere. Angew. Chem. Int. Ed 55, 3580–3585 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Houston SD et al. The cubane paradigm in bioactive molecule discovery: further scope, limitations and the cyclooctatetraene complement. Org. Biomol. Chem 17, 6790–6798 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Bernhard SSR et al. Cubane Cross-Coupling and Cubane–Porphyrin Arrays. Chem. Eur. J 24, 1026–1030 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Okude R, Mori G, Yagi A & Itami K Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation. Chem. Sci 11, 7672–7675 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barborak JC, Watts L & Pettit R A Convenient Synthesis of the Cubane System. J. Am. Chem. Soc 88, 1328–1329 (1966). [Google Scholar]
- 35.Brewer CR, Sheehan NC, Herrera J, Walker AV & McElwee-White L Photochemistry of (η4-diene)Ru(CO)3 Complexes as Precursor Candidates for Photoassisted Chemical Vapor Deposition. Organometallics 41, 761–775 (2022). [Google Scholar]
- 36.Pettit R & Henery J Cyclobutadieneiron Tricarbonyl. Org. Synth 50, 57–59 (1970). [Google Scholar]
- 37.Masamune S, Nakamura N & Sapadaro J 1,2-Bis(β-tosylethoxycarbonyl)diazene. Its Application to the 2,3-Diazabicyclo[2.2.0]hexene System. J. Am. Chem. Soc 97, 918–919 (1975). [Google Scholar]
- 38.Britten TK, Akien GR, Kemmitt PD, Halcovitch NR & Coote SC An efficient preparation of 1,2-dihydropyridazines through a Diels-Alder/palladium-catalysed elimination sequence. Tetrahedron Lett 60, 1498–1500 (2019). [Google Scholar]
- 39.Altman LJ, Semmelhack MF, Hornby RB, Vederas JC Photochemical Isomerisation of Dimethyl 1,2-dihydropyridazine-1,2-dicarboxylate. Chem. Commun 1968, 686–687 (1968). [Google Scholar]
- 40.Britten TK, Kemmitt PD, Halcovitch NR & Coote SC 4-π-Photocyclization of 1,2-Dihydropyridazines: An Approach to Bicyclic 1,2-Diazetidines with Rich Synthetic Potential. Org. Lett 21, 9232–9235 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Bashir-Hashemi A Photochemical Carboxylation of Cubanes, Angew. Chem. Int. Ed 32, 612–613 (1993). [Google Scholar]
- 42.Collin DE, Kovacic K, Light ME & Linclau B Synthesis of Ortho-Functionalized 1,4-Cubanedicarboxylate Derivatives through Photochemical Chlorocarbonylation. Org. Lett 23, 5164–5169 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Chan AY et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev 122, 1485–1542 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez N & Gooßen LJ Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev 40, 5030–5048 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Castillo P & Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev 116, 12564–12649 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartwig JF Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res 41, 1534–1544 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W; Wurz RP; Peters JC & Fu GC Photoinduced, Copper-Catalyzed Decarboxylative C−N Coupling to Generate Protected Amines: An Alternative to the Curtius Rearrangement. J. Am. Chem. Soc 139, 12153–12156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodano TM, Combee LA & Stephenson CRJ Recent Advances and Outlook for the Isosteric Replacement of Anilines. ACS Med. Chem. Lett 11, 1785–1788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sklyarova AS, Rodionov VN, Parsons CG, Quack G, Schreiner PR, Fokin AA Preparation and testing of homocubyl amines as therapeutic NMDA receptor antagonists. Med Chem Res 22, 360–366 (2013). [Google Scholar]
- 50.Sakai HA, Liu W, Le C & MacMillan DWC Cross-Electrophile Coupling of Unactivated Alkyl Chlorides. J. Am. Chem. Soc 142, 11691–11697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or in the supplementary information.


