Abstract
The nitrate-tolerant organism Klebsiella oxytoca CECT 4460 tolerates nitrate at concentrations up to 1 M and is used to treat wastewater with high nitrate loads in industrial wastewater treatment plants. We studied the influence of the C source (glycerol or sucrose or both) on the growth rate and the efficiency of nitrate removal under laboratory conditions. With sucrose as the sole C source the maximum specific growth rate was 0.3 h−1, whereas with glycerol it was 0.45 h−1. In batch cultures K. oxytoca cells grown on sucrose or glycerol were able to immediately use sucrose as a sole C source, suggesting that sucrose uptake and metabolism were constitutive. In contrast, glycerol uptake occurred preferentially in glycerol-grown cells. Independent of the preculture conditions, when sucrose and glycerol were added simultaneously to batch cultures, the sucrose was used first, and once the supply of sucrose was exhausted, the glycerol was consumed. Utilization of nitrate as an N source occurred without nitrite or ammonium accumulation when glycerol was used, but nitrite accumulated when sucrose was used. In chemostat cultures K. oxytoca CECT 4460 efficiently removed nitrate without accumulation of nitrate or ammonium when sucrose, glycerol, or mixtures of these two C sources were used. The growth yields and the efficiencies of C and N utilization were determined at different growth rates in chemostat cultures. Regardless of the C source, yield carbon (YC) ranged between 1.3 and 1.0 g (dry weight) per g of sucrose C or glycerol C consumed. Regardless of the specific growth rate and the C source, yield nitrogen (YN) ranged from 17.2 to 12.5 g (dry weight) per g of nitrate N consumed. In contrast to batch cultures, in continuous cultures glycerol and sucrose were utilized simultaneously, although the specific rate of sucrose consumption was higher than the specific rate of glycerol consumption. In continuous cultures double-nutrient-limited growth appeared with respect to the C/N ratio of the feed medium and the dilution rate, so that for a C/N ratio between 10 and 30 and a growth rate of 0.1 h−1 the process led to simultaneous and efficient removal of the C and N sources used. At a growth rate of 0.2 h−1 the zone of double limitation was between 8 and 11. This suggests that the regimen of double limitation is influenced by the C/N ratio and the growth rate. The results of these experiments were validated by pulse assays.
Nitrate is the source of nitrogen most widely used by aerobic organisms, including plants, fungi, and bacteria (14, 15, 29). Assimilatory nitrate utilization involves the sequential action of assimilatory nitrate reductase, which converts nitrate to nitrite, and nitrite reductase, which converts nitrite to ammonium. Ammonium is subsequently incorporated into carbon skeletons, usually via the glutamine synthetase-glutamate synthase pathway (15, 23).
Although the ability to use nitrate is widespread in nature, nitrate is considered a ubiquitous pollutant (18) because at high concentrations it inhibits cell growth (25, 26) and because at low concentrations in drinking water it is toxic (32). High nitrate loads are commonly found in industrial effluents from wash tanks in dairy factories and are also produced during the synthesis of nitroorganic compounds in the pharmaceutical and explosives industries (4, 20, 25, 30). Reducing high nitrate loads in wastes is of interest, particularly if the wastes are processed at conventional treatment plants where futile conversion of nitrate to nitrite can occur when there are high nitrate loads (19). Wastewaters from the synthesis of nitroorganic compounds usually contain large amounts of sulfates and carbonates, which are inhibitors of denitrification (20, 30). In an attempt to remove high nitrate loads from this type of industrial waste, we isolated a strain belonging to the genus Klebsiella that tolerated nitrate at concentrations up to 1 M and was able to thrive under aerobic conditions in a culture medium containing up to 150 mM nitrate. This strain was identified as a Klebsiella oxytoca strain and was deposited in the Spanish Type Culture Collection (CECT) as strain CECT 4460 (25).
In this paper we describe nitrate removal by this strain under laboratory conditions in both batch and chemostat cultures containing sucrose or glycerol or mixtures of these compounds as the sole sources of C and energy. Glycerol was chosen because it was the C source used to isolate strain CECT 4460 (25). Several sugars are used by strain CECT 4460, including sucrose, glucose, lactose, fructose, and galactose, and sucrose was chosen for use in this study because of its low cost and because its presence in industrial wastes (e.g., sugar beet molasses and other wastes) makes it a potentially cheap source of C for biotreatment of wastes with high nitrate loads. Under optimal growth conditions nitrate elimination by K. oxytoca CECT 4460 occurred without nitrite or ammonium accumulation. In chemostat cultures the zone of double nutrient limitation (C limitation and N limitation) was found to be influenced by the growth rate. At a growth rate of 0.1 h−1, growth was limited by C and N when the C/N ratio of the influent medium was between 10 and 30, whereas at a growth rate of 0.2 h−1, growth was limited at a C/N ratio of 8 to 11. Under these conditions both C and N were completely removed; therefore, these conditions can be considered optimal for biotreatment.
MATERIALS AND METHODS
Microorganisms, growth medium, and culture conditions.
K. oxytoca CECT 4460 was grown on mineral medium M8 supplemented as suggested by Egli and Fiechter (10). The medium used contained (per liter of deionized water) 2.020 g of KNO3, 5.65 g of KH2PO4, 0.5 g of NaCl, 2.46 g of MgSO4·7H2O, 82 mg of disodium EDTA · 2H2O, 1.25 mg of ZnCl2, 0.75 mg of MnCl2 · 4H2O, 7.5 mg of H3BO3, 5 mg of CoSO4 · 7H2O, 0.25 mg of CuCl2 · 2H2O, 0.5 mg of NiCl2 · 6H2O, 0.75 mg of Na2MoO4·2H2O, and 7 mg of FeCl3 · 6H2O. Before heat sterilization, the medium was completely mixed and acidified with concentrated H2SO4 to pH 3.0. The C source (sucrose or glycerol or both) was sterilized separately and was added to the mineral medium after it was cooled so that the final total carbon concentration was 3.9 g/liter. Continuous cultivation was performed in bioreactors (working volume, 2.5 liters; MBR, Wetzikon, Switzerland). Before cells were inoculated, the pH of the medium was adjusted to 7.0, and the pH was maintained at 7.0 ± 0.1 by automatic addition of 1 M NaOH-KOH. The temperature was set at 30 ± 0.1°C. Cultures were operated in batch or continuous mode as indicated below. The aeration rate was 1 ± 0.1 volume of air per volume of culture liquid per min, and the impeller speed was 1,000 rpm.
Analytical methods.
Compounds present in the culture medium were analyzed after the cells were removed by centrifugation with a Sorvall model R5C centrifuge at 12,000 × g for 15 min. The nitrite content was determined by the method of Snell and Snell (28). The nitrate content was determined by using a Spectroquant 14773 kit from Merck (Darmstadt, Germany) under the conditions recommended by the supplier or by using a specific nitrate electrode and a potentiometer (MicropH 2002; Crison, Barcelona, Spain). The detection limits were 10 mg/liter for nitrate and 0.05 mg/liter for nitrite. The dissolved total organic carbon content was measured with a TOC-UNOR gas analyzer (H. Maihak AG, Hamburg, Germany) for values ranging from 0 to 100 mg/liter. The glycerol, glucose, and sucrose contents were determined enzymatically with commercial kits obtained from Boehringer (Mannheim, Germany). The acetate concentration was measured by high-pressure ion exclusion chromatography as described by Bally (3), and the detection limit was 1 mg of acetate C per liter. The C and N contents of the biomass were determined with an elemental carbon, hydrogen, nitrogen, and sulfur analyzer (model EA 1108; Carlo Erba, Milan, Italy).
Biomass determination.
The biomass was determined as cell dry weight by filtration through a polycarbonate membrane filter (pore size, 0.45 μm; diameter, 47 mm; Millipore Corp., Bedford, Mass.). Cells collected on filters were dried at 105°C to constant weight.
RESULTS AND DISCUSSION
Determination of the μmax in batch cultures of K. oxytoca CECT 4460.
To establish the dilution rate limits for continuous cultivation of K. oxytoca CECT 4460, the maximum specific growth rate (μmax) in batch cultures was determined. To do this, bacterial cells were grown in minimal medium containing 0.5 to 4.0 g of C from glycerol or from sucrose per liter and 20 mM NO3−. Cells were maintained for 35 generations in the exponential growth phase, and then the μmax was determined. Cells growing with glycerol as the sole C source had a μmax of 0.45 ± 0.01 h−1, whereas cultures growing with sucrose as the sole C source had a μmax of 0.3 ± 0.01 h−1.
Growth of K. oxytoca CECT 4460 in batch cultures with sucrose and glycerol.
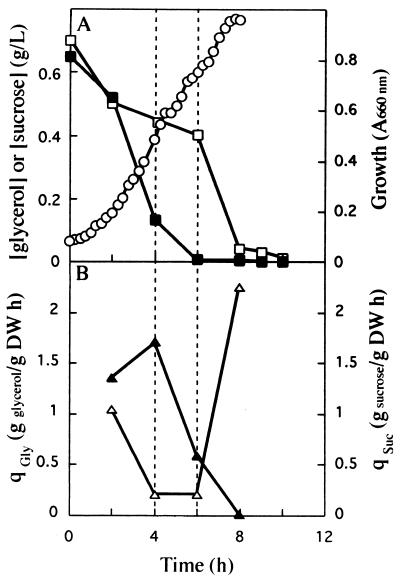
For a number of microorganisms, culture conditions noticeably influence the pattern of C utilization, particularly when mixtures of C sources are used. In these cases catabolite repression is one of the main mechanisms responsible for preferential use of a given C source (7, 9, 22). We therefore studied the response of K. oxytoca CECT 4460 to mixtures of sucrose and glycerol in detail. K. oxytoca CECT 4460 was precultured in a chemostat (dilution rate, 0.2 h−1) in which either glycerol or sucrose was the sole C source. The resulting cells were used as the inocula for batch assays performed in minimal medium containing a 1:1 mixture of sucrose and glycerol (Fig. 1 and 2). Regardless of the C source used to cultivate the inoculum, cells exposed to a mixture of glycerol and sucrose exhibited a diauxic growth curve. The diauxic phase was shorter when the inoculum was from glycerol precultures than when the inoculum was from sucrose precultures (Fig. 1A and 2A). In batch cultures both C sources were used simultaneously from the beginning of the assay, although there were considerable differences in the specific uptake rates for glycerol and sucrose utilization (Fig. 1B and 2B). When cells were precultured with glycerol, both C sources were used simultaneously during the first 2 h of growth (Fig. 1B). The specific rate of sucrose consumption was 1.35 ± 0.02 g per g (dry weight) per h, and the specific rate of glycerol consumption was 1.04 ± 0.03 g per g (dry weight) per h. After 2 h the glycerol uptake rate was markedly reduced (to 0.2 ± 0.01 g per g [dry weight] per h), whereas sucrose uptake continued at approximately the same rate. These results suggest that sucrose repressed glycerol utilization. The supply of sucrose was fully exhausted after 5 to 6 h (Fig. 1A); after this the glycerol remaining in the culture medium was used as the sole substrate for growth, and its specific rate of consumption increased to 2.25 ± 0.02 g per g (dry weight) per h.
FIG. 1.
Growth of K. oxytoca CECT 4460 in batch cultures with a 1:1 mixture of sucrose and glycerol. K. oxytoca CECT 4460 was pregrown in a C-limited chemostat with glycerol as the C source (dilution rate, 0.2 h−1) and was transferred to a culture medium containing sucrose and glycerol. (A) Growth (○) and the concentrations of sucrose (■) and glycerol (□) were determined at different times. (B) Specific consumption rates were determined for glycerol (qGly) (▵) and sucrose (qSuc) (▴). DW, dry weight; A660nm, absorbance at 660 nm.
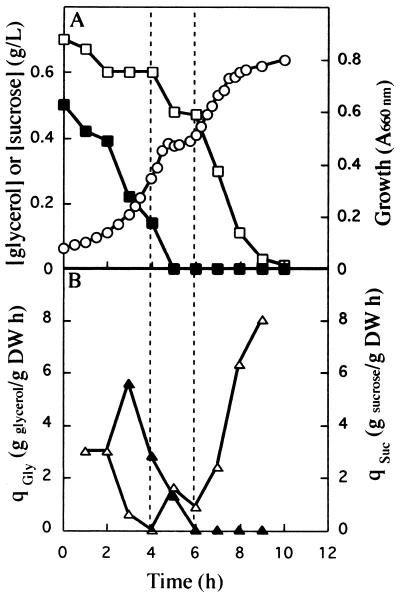
FIG. 2.
Growth of K. oxytoca CECT 4460 in batch cultures with a 1:1 mixture of sucrose and glycerol. The conditions were the same as those described in the legend to Fig. 1 except that the cells were pregrown in a chemostat with sucrose (dilution rate, 0.2 h−1).
This pattern of utilization of C sources was also reflected in the specific growth rate. Simultaneous utilization of sucrose and glycerol during the first 2 h of culture resulted in a specific growth rate (0.49 ± 0.02 h−1) that was higher than the specific growth rate when either sucrose (0.3 ± 0.01 h−1) or glycerol (0.45 ± 0.01 h−1) was the sole C source. The glycerol which remained in the culture medium after the supply of sucrose was exhausted supported a specific growth rate of 0.21 ± 0.04 h−1 in the second phase of growth (this specific growth rate was considerably lower than the μmax observed with glycerol-precultured cells). A synergistic effect due to simultaneous utilization of more than one C source by different microorganisms has been observed previously when there was an excess of substrate (2, 9, 12).
Cells that were pregrown on sucrose and transferred to medium containing sucrose plus glycerol also utilized sucrose preferentially (Fig. 2A). During the first 2 h both C sources were used at a specific rate of 3 ± 0.15 g per g (dry weight) per h (Fig. 2B), but after 2 h the consumption of sucrose increased to 5.6 ± 0.1 g per g (dry weight) per h and the rate of consumption of glycerol decreased until glycerol consumption was totally repressed. After 5 to 6 h, when the sucrose concentration fell below our detection limit, glycerol was again consumed until it became undetectable in the culture medium (Fig. 2B). These results suggest that cells pregrown in a chemostat with sucrose as the limiting growth factor exhibit constitutive utilization of sucrose and glycerol but that upon transfer to batch cultures without C source limitation sucrose is preferentially used instead of glycerol.
In diauxic cultures the substrate that supports the highest growth rate is generally utilized first, while consumption of the second substrate is repressed (16). However, with K. oxytoca CECT 4460 we observed the opposite behavior; the first substrate to be used was sucrose, which supported a lower specific growth rate than glycerol supported. When the supply of sucrose was exhausted, growth stopped, but it later resumed. This may have reflected the time needed for full induction of the glycerol utilization system. These results support the hypothesis that sucrose uptake is constitutive, whereas glycerol uptake can be repressed by sucrose.
Growth of K. oxytoca CECT 4460 in batch cultures with glucose and glycerol.
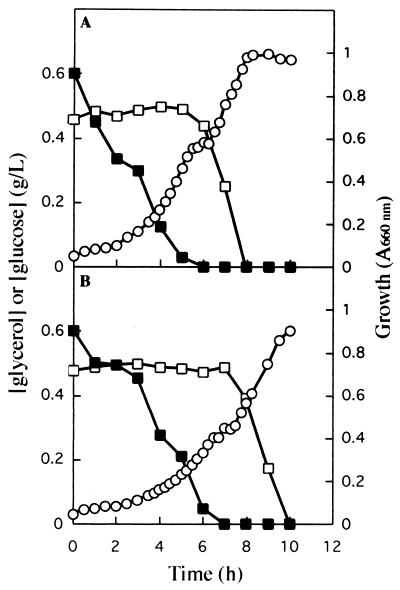
To determine whether glucose was responsible for the inhibition of glycerol utilization, K. oxytoca CECT 4460 cells were grown for 35 generations in minimal medium containing glycerol or glucose (0.5 g of C per liter) and 20 mM NO3−. Cells in the exponential growth phase were transferred to minimal medium containing a 1:1 mixture of glucose and glycerol (Fig. 3). Regardless of the C source used to grow the inoculum, cells exposed to a mixture of glycerol and glucose exhibited a diauxic growth curve. In both cases glucose was used first, and the supply of glucose was completely exhausted within 6 to 8 h; after this the glycerol remaining in the culture medium was used as the sole substrate for growth (Fig. 3A and B). During the first 6 h glycerol consumption was negligible. Thereafter and when the glucose concentration fell below our detection limit, glycerol consumption started and continued until the supply of this C source was exhausted (Fig. 3A and B). These results support the hypothesis that once sucrose is hydrolyzed to glucose plus fructose, glycerol utilization is repressed by glucose metabolism.
FIG. 3.
Growth of K. oxytoca CECT 4460 in batch cultures with a 1:1 mixture of glucose and glycerol. (A) K. oxytoca CECT 4460 was pregrown on glycerol and transferred to a culture medium containing glucose and glycerol. Growth (○) and the concentrations of glucose (■) and glycerol (□) were determined at different times. (B) Same as panel A except that the cells were pregrown on glucose. A660nm, absorbance at 660 nm.
Growth kinetics of K. oxytoca CECT 4460 in continuous cultures with glycerol as the sole C source.
Continuous cultures of K. oxytoca CECT 4460 with glycerol as the sole C source were set up at dilution rates ranging from 0.05 to 0.3 h−1. The relationships among dilution rate, dry weight of cells, culture yield, and residual concentrations of glycerol, nitrate, and nitrite were determined under steady-state conditions (Table 1). In general, the higher the growth rate, the lower the dry weight of the culture and, consequently, the lower the yield with respect to the amount of C or N consumed. The residual glycerol concentration was negligible at dilution rates between 0.05 and 0.15 h−1, and the residual concentration of nitrate was about 20 mg/liter. At a dilution rate equal to or higher than 0.2 h−1 the residual concentration of glycerol was about 0.5 g/liter, while the residual concentration of nitrate decreased to approximately 10 mg/liter. These results suggest that K. oxytoca CECT 4460 was carbon limited at growth rates equal to or lower than 0.15 h−1, whereas it was nitrogen limited at growth rates equal to or higher than 0.2 h−1. To ensure that N-limited conditions were used in subsequent assays, chemostats were run at a dilution rate of 0.2 h−1. The relationship between steady-state regimens and growth rates was similar to that described by Cocaing-Bousquet et al. (5) for chemostat cultures of Corynebacterium glutamicum; namely, the cultures were C limited at low dilution rates and N limited at high growth rates.
TABLE 1.
Relationship between growth rate and culture parameters for K. oxytoca growing with glycerol and nitratea
| Growth rate (h−1) | Dry wt (g/liter) | YN (g [dry wt]/g of N) | YC (g [dry wt]/g of C) | Residual concn (mg/liter) of:
|
|
|---|---|---|---|---|---|
| Glycerol | Nitrate | ||||
| 0.05 | 5.0 | 16.3 | 1.2 | 2 | 21.7 |
| 0.10 | 5.3 | 17.2 | 1.3 | 16 | 20.4 |
| 0.15 | 5.2 | 16.8 | 1.2 | 1 | 19.2 |
| 0.20 | 4.7 | 15.2 | 1.2 | 500 | 12.4 |
| 0.25 | 4.7 | 15.3 | 1.2 | 500 | 9.3 |
| 0.30 | 4.3 | 14.0 | 1.1 | 500 | 10.5 |
K. oxytoca CECT 4460 was grown under steady-state conditions at different growth rates. Dry weights and the concentrations of glycerol and nitrate were determined as indicated in Materials and Methods. Yields are expressed as grams (dry weight) per gram of N or C consumed. In the inflowing medium the concentrations of nitrate and glycerol were 1.24 and 10 g/liter, respectively. The values are the averages of values from at least six independent assays; the standard deviations were less than 5% of the values shown.
Growth kinetics of K. oxytoca CECT 4460 in continuous cultures with sucrose as the sole C source.
In batch cultures the elimination of nitrate when sucrose was the sole C source was as efficient as the elimination of nitrate when glycerol was the sole C source, although some nitrite did accumulate. The accumulation of nitrite was more evident under anaerobic conditions than under aerobic conditions (data not shown). Because nitrite accumulation is detrimental for the operation of a nitrate treatment plant, we tested whether this was also the case in chemostat cultures under aerobic conditions. K. oxytoca CECT 4460 was grown aerobically in chemostat cultures at growth rates between 0.05 and 0.20 h−1 (Table 2). When there was an increase in the growth rate, there was a slight decrease in the dry weight of the culture, although this decrease hardly influenced yield nitrogen (YN) and yield carbon (YC) (Table 2). However, it should be noted that the yields under aerobic conditions were 1.5- to 2-fold higher than the yields under anaerobic conditions (data not shown). For growth rates tested, the residual concentration of nitrate was between 13 and 22 mg/liter, and no nitrite or ammonium accumulated in the culture medium. The residual sucrose concentration was negligible at dilution rates between 0.05 and 0.15 h−1 (Table 2). At a dilution rate of 0.2 h−1 the residual concentration of sucrose was 0.47 ± 0.03 g/liter. These results suggest that when sucrose was the sole C source, K. oxytoca CECT 4460 also became carbon limited at growth rates equal to or lower than 0.15 h−1, as we observed when glycerol was used as the sole C source. When the dilution rate was increased to more than 0.2 h−1, the culture was washed out (data not shown), probably because this rate approached the μmax of the culture for sucrose (0.3 h−1).
TABLE 2.
Relationship between growth rate and culture parameters for K. oxytoca growing with sucrose and nitratea
| Growth rate (h−1) | Dry wt (g/liter) | YN (g [dry wt]/g of N) | YC (g [dry wt]/g of C) | Residual concn (mg/liter) of:
|
|
|---|---|---|---|---|---|
| Sucrose | Nitrate | ||||
| 0.05 | 4.0 | 15 | 1.0 | 0 | 13 |
| 0.1 | 4.0 | 15 | 1.0 | 0 | 13 |
| 0.15 | 3.9 | 15 | 1.0 | 8 | 22 |
| 0.2 | 3.8 | 14 | 1.1 | 470 | 20 |
The conditions were the same as those described in Table 1, footnote a, except that sucrose (9.3 g/liter) was used instead of glycerol.
Growth of K. oxytoca CECT 4460 in continuous cultures with mixtures of glycerol and sucrose as C sources.
K. oxytoca CECT 4460 preferentially used sucrose in batch cultures when glycerol and sucrose were present simultaneously. As mentioned above, this was unexpected; usually, when more than one C source is supplied, the one used preferentially is the one that supports the higher growth rate, which was not the case for sucrose and glycerol utilization by K. oxytoca CECT 4460. We therefore tested whether in a chemostat culture at a fixed growth rate (dilution rate, 0.2 h−1) the presence of the two C sources in the medium also resulted in preferential utilization of sucrose. For this study a chemostat culture of K. oxytoca CECT 4460 under steady-steady conditions was fed increasing concentrations of sucrose, so that the total amount of carbon in the feed was 3.9 g/liter (Table 3).
TABLE 3.
Effects of different proportions of glycerol and sucrose in the continuous culture feed at a constant dilution rate of 0.2 h−1 on dry weight and utilization of C and N
| % of:
|
Dry wt (g/liter) | YN (g [dry wt]/g of C) | YC (g [dry wt]/g of C) | Residual concn (mg/liter) of:
|
|||
|---|---|---|---|---|---|---|---|
| Glycerol | Sucrose | Nitrate | Glycerol | Sucrose | |||
| 100 | 0 | 4.7 | 15.2 | 1.2 | 12 | 500 | 0 |
| 50 | 50 | 4.7 | 14.5 | 1.3 | 8 | 468 | 4 |
| 25 | 75 | 4.4 | 13.7 | 1.1 | 16 | 0 | 5 |
| 15 | 85 | 4.3 | 13.1 | 1.1 | 14 | 0 | 8 |
| 10 | 90 | 4.1 | 12.5 | 1.0 | 13 | 1 | 6 |
| 0 | 100 | 3.8 | 14.6 | 1.1 | 20 | 0 | 470 |
K. oxytoca CECT 4460 was grown under continuous culture conditions at a dilution rate of 0.2 h−1 with different mixtures of glycerol and sucrose, so that the total C concentration in the feed was 3.9 g/liter. In the inflowing medium the concentration of nitrate was 1.24 g/liter. Other conditions were the same as the conditions described in Table 1, footnote a.
For each glycerol/sucrose ratio, time was allowed for steady-state conditions to become established, and then different parameters (dry weight and residual concentrations of C an N) were measured. The dry weight (biomass) of the culture decreased with increasing amounts of sucrose in the mixtures. Accordingly, YN and YC also decreased with increasing sucrose concentrations. This suggested that K. oxytoca CECT 4460 used sucrose less efficiently than it used glycerol, as was expected from the results obtained with a single C source (Tables 1 and 2). At the same growth rate, the YC for glycerol was slightly higher than the YC for sucrose.
The residual nitrate concentration in the medium was on the order of 10 to 20 mg/liter regardless of the sucrose concentration in the feed. No accumulation of nitrite was observed during growth with any of the mixtures.
The residual concentrations of glycerol and sucrose were also determined. When the ratio of sucrose to glycerol in the mixture was 1, the level of sucrose was negligible, whereas the glycerol concentration was about 470 ± 30 mg/liter. This suggested that there was preferential use of sucrose but at the same time indicated that there was simultaneous use of both C sources, because otherwise the glycerol concentration would have been 5.0 g/liter. Furthermore, a decrease in the initial proportion of glycerol in the sucrose-glycerol mixture resulted in lower (indeed negligible) glycerol concentrations. The residual concentration of either sucrose or glycerol during growth with the mixtures at a dilution rate of 0.2 h−1 was lower than the residual concentration during growth with the corresponding concentration of glycerol or sucrose alone in cultures (Tables 1 through 3). Similar results have been observed with other microorganisms (11, 21) growing in chemostat cultures with different mixtures. This was true for a methylotrophic yeast culture growing with glucose and methanol as the C sources and for Escherichia coli growing with mixtures of glucose and galactose. This finding probably reflects the fact that, in nature, microbes must cope with multiple C sources at low concentrations and suggests that under these conditions C sources are used more efficiently than when a single carbon source is present.
Consumption kinetics under transient-state conditions in continuous cultures with glycerol.
K. oxytoca CECT 4460 was able to simultaneously consume sucrose and glycerol under steady-state conditions in continuous cultures (Table 3). In batch cultures the rate at which these C sources were utilized was noticeably influenced by the growth conditions of the inoculum, and sucrose was used in preference to glycerol. To determine how the addition of one of the C sources influenced utilization of the other C source, pulses of one of the C sources were added to a steady-state culture of K. oxytoca that had been established with the other C source (Fig. 4).
FIG. 4.
Simultaneous utilization of sucrose and glycerol after a pulse of sucrose was added to K. oxytoca cells growing in a continuous culture with glycerol as the sole C source. An N-limited steady-state culture (dilution rate, 0.3 h−1) of K. oxytoca CECT 4460 with glycerol as the sole C source was pulsed at zero time with 7.5 g of sucrose per liter. At different times the concentrations of sucrose (○) and glycerol (•) (A) and the specific glycerol consumption rates (qgly) (□) (B) were determined. The initial concentration of glycerol was 10 g/liter. DW, dry weight.
A steady-state culture of K. oxytoca CECT 4460 growing at a dilution rate of 0.3 h−1 with glycerol as the sole C source was established. Under steady-state conditions the dry weight of the culture was 4.3 ± 0.1 g/liter. The cells used glycerol at a rate of 0.58 ± 0.02 g per g (dry weight) per h (Fig. 4B); the residual concentration of glycerol was 1.7 ± 0.1 g/liter (Fig. 4A), and nitrate or nitrite was undetectable (data not shown). This culture was pulsed with 7.5 g of sucrose per liter, and we recorded the sucrose, glycerol, nitrate, and nitrite contents and growth as a function of time. Nitrate and nitrite remained practically undetectable (data not shown) and the dry weight of the culture decreased from 4.35 ± 0.01 to 4.1 ± 0.02 g/liter during the time required for consumption of all of the sucrose (data not shown). Cells were able to utilize sucrose and glycerol simultaneously from the beginning of the assay (Fig. 4A). As sucrose was consumed, the specific rate of glycerol utilization decreased from 0.58 ± 0.02 g per g (dry weight) per h in the absence of sucrose to 0.44 ± 0.02 g per g (dry weight) per h in the presence of sucrose (Fig. 4B). This represented a decrease in the initial glycerol uptake value of about 25%. As a consequence, glycerol accumulated in the culture, reaching a concentration of 3.9 ± 0.1 g/liter (Fig. 4A). Once sucrose was consumed, the rate of glycerol utilization increased, and the residual glycerol concentration decreased accordingly.
The rate of sucrose consumption was determined by calculating the difference between the theoretical washout and the sucrose concentration present in the culture medium at a given time. The specific sucrose consumption rate was estimated to be approximately 0.16 ± 0.02 g per g (dry weight) per h, and the sucrose pulse was completely consumed within 4 h. In the glycerol steady-state chemostat the amount of C from sucrose used by the Klebsiella strain after the pulse was equivalent to the amount of C from glycerol that the microbe did not use. These results suggest that simultaneous utilization of two C sources was adjusted to satisfy the organism’s C requirements. These results also confirm that sucrose utilization is constitutive in cultures of K. oxytoca CECT 4460 growing on glycerol.
A similar assay was performed in which pulses of 10 g of glycerol per liter were added to a continuous culture containing sucrose as the sole C source at a dilution rate of 0.2 h−1. No consumption of glycerol was observed, and glycerol was removed by washout (data not shown).
Double substrate limitation in cultures grown with sucrose and nitrate as the sole sources of carbon and nitrogen, respectively.
To determine the C/N ratio that resulted in complete elimination of the C source supplied and the N source supplied, a series of assays were performed with different C/N ratios. Tables 1 and 2 show that chemostat cultures of K. oxytoca CECT 4460 became C or N limited depending on the growth rate. As zones of double nutrient limitation have been described for C and N utilization by Egli (8), it was thought that such a situation can also occur with K. oxytoca depending on the C/N ratio of the culture medium and the dilution rate. We determined the zone of double nutrient limitation (C limitation and N limitation) for K. oxytoca CECT 4460 at dilution rates of 0.1 and 0.2 h−1 by using different C/N ratios.
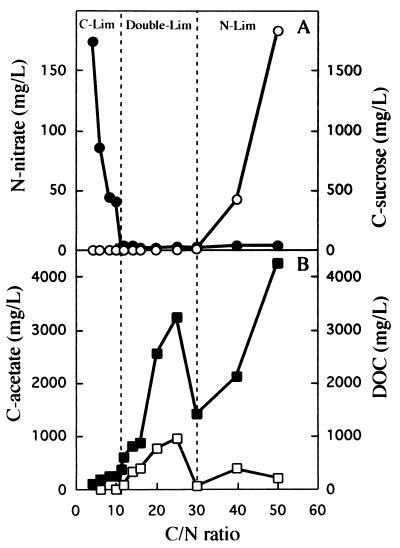
The residual concentrations of sucrose and nitrate under steady-state conditions were used to determine whether a culture was sucrose limited, nitrate limited, or sucrose and nitrate limited. Figure 5 shows the results obtained at a dilution rate of 0.1 h−1. The following three distinct growth regimens were recognized (Fig. 5A): (i) sucrose limitation, in which there was excess nitrate (this was observed at C/N ratios of <10); (ii) nitrate limitation, in which there was excess sucrose (this was observed at C/N ratios of >30); and (iii) an intermediate regimen, in which the concentrations of both sucrose and nitrate were below our detection limits (i.e., when the C/N ratio was between 10 and 30). In previous studies performed with other microorganisms (1, 6, 8, 13, 24, 27) researchers have described a transitional double substrate-limited growth regimen between two distinct single-nutrient-limited zones. We observed an increase in the total organic carbon concentration in the culture medium with the transition regimen (Fig. 5B). This increase in the total organic carbon concentration corresponded to excretion of acetate into the culture medium by K. oxytoca CECT 4460 during the double-nutrient-limited growth phase. This behavior was also observed in batch cultures of Klebsiella pneumoniae, in which approximately 5 to 10% of the metabolized C was excreted in the form of acetate (31). To determine whether K. oxytoca CECT 4460 accumulated reserve polymers in any of the three distinct growth regimens, we measured the C and N contents of the biomass at each C/N ratio (data not shown). The proportions of C and N in the biomass were constant at all C/N ratios, indicating that no reserve polymers accumulated.
FIG. 5.
Effect of the C/N ratio on sucrose limitation, nitrate limitation, and sucrose-nitrate limitation in a chemostat culture of K. oxytoca CECT 4460 at a dilution rate of 0.1 h−1. K. oxytoca CECT 4460 was grown with nitrate and sucrose at different C/N ratios. Steady-state residual concentrations of nitrate N (•) and sucrose C (○) were determined (A), and the concentrations of acetate (□) and total organic carbon (DOC) (■) in the culture medium were determined at the different C/N ratios (B).
At a dilution rate of 0.2 h−1 the double-nutrient-limited zone became narrower and shifted towards a lower C/N ratio; it was sucrose and nitrate limited when the C/N ratio was between 8.2 and 11 (data not shown). This behavior has been observed previously with other microorganisms (13, 17, 24).
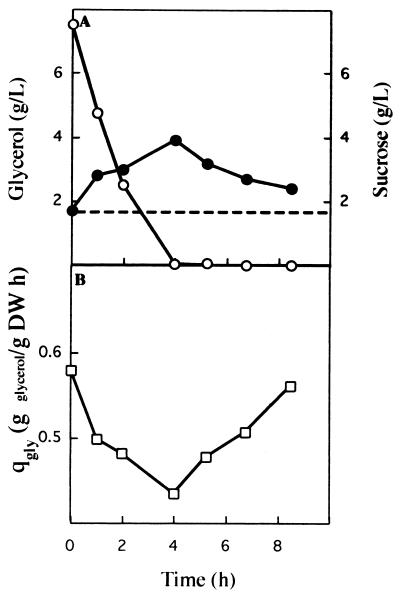
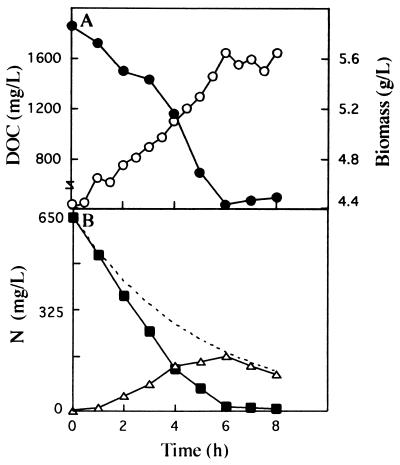
To validate these results, we determined the overcapacity of the nitrate elimination system in continuous cultures containing sucrose. A nitrate-limited chemostat culture of K. oxytoca CECT 4460 growing at a dilution rate of 0.2 h−1 with sucrose as the sole C source was established. Once steady-state conditions were reached, the culture was pulsed with 2.8 g of NO3− per liter (Fig. 6). Six hours later the residual sucrose concentration had decreased from about 1,900 to 440 mg/liter, and the biomass had increased from 4.4 ± 0.1 to 5.7 ± 0.1 g/liter (Fig. 6A). Nitrate consumption was determined by calculating the difference between the theoretical washout and the nitrate concentration present in the culture medium at a given time (Fig. 6B). In this assay the specific rate of C utilization was 0.05 ± 0.005 g per g (dry weight) per h, and the specific rate of N utilization was 0.0062 ± 0.0001 g per g (dry weight) per h, which gave a C/N ratio of 8.1. These results are in agreement with the zone in which sucrose and nitrate simultaneously became limiting growth factors at a dilution rate of 0.2 h−1.
FIG. 6.
Overcapacity of the nitrate elimination system in a continuous culture with sucrose. A nitrate-limited steady-state culture (dilution rate, 0.2 h−1) of K. oxytoca CECT 4460 with sucrose as the sole C source was pulsed at zero time with 2.8 g of KNO3 per liter. (A) Evolution of biomass (○) and concentration of sucrose measured as total organic carbon (DOC) (•). (B) Theoretical washout for nitrate (--), true concentration of nitrate in the medium (■), and consumption of nitrate (▵), which was determined by calculating the difference between the washout of nitrate and the actual nitrate concentration in the culture medium.
A similar assay was performed with a nitrate-limited chemostat culture of K. oxytoca CECT 4460 growing at a dilution rate of 0.25 h−1 with glycerol as the sole C source. Once steady-state conditions were reached, the residual glycerol concentration was about 500 mg/liter. The culture was pulsed with 0.8 g of NO3− per liter, and the specific rates of C and N utilization were determined. Two hours later the residual glycerol concentration was about 200 mg/liter, and this concentration remained constant with time. This was accompanied by an increase in the culture biomass from 4.6 ± 0.05 to 4.85 ± 0.05 g/liter. The specific rate of C utilization was 0.0165 ± 0.0005 g per g (dry weight) per h, and the specific rate of N utilization was 0.002 ± 0.0005 g per g (dry weight) per h, which gave a C/N ratio of 8.1. These results are also in agreement with the data obtained when sucrose was the sole C source.
Conclusions.
We found that in chemostat cultures containing sucrose or glycerol or mixtures of these C sources, K. oxytoca CECT 4460 efficiently removed high nitrate loads without accumulating nitrite or ammonium. In chemostat cultures containing sucrose and glycerol, K. oxytoca CECT 4460 simultaneously consumed both C sources, whereas in batch cultures consumption was sequential, with sucrose used preferentially before glycerol.
In chemostat cultures of K. oxytoca CECT 4460 at a dilution rate of 0.1 h−1, when the C/N ratio of the influent medium was in the range from 10 to 30, the culture was simultaneously sucrose and nitrate limited. At a higher growth rate (e.g., at a dilution rate of 0.2 h−1) the zone of double nutrient limitation was narrower and shifted towards lower C/N ratios (C/N ratios between 8 and 11). These results suggest that both the N source and the C source were removed so that their concentrations were negligible. Therefore, these conditions can be considered optimal for biotreatment of the industrial wastewater tested here with regard to simultaneous elimination of C and N.
ACKNOWLEDGMENTS
This work was supported by a grant from Unión Española de Explosivos and by grant PETRI 93-084 from CICYT. G.P. thanks the MAPFRE Foundation for a fellowship and EAWAG for the use of facilities in Dübendorf and for partial financial support.
We thank H. P. Füchslin for excellent technical assistance.
REFERENCES
- 1.Al-Awadhi N, Egli T, Hamer G, Mason C A. The process utility of thermotolerant methylotrophic bacteria. I. An evaluation in chemostat culture. Biotechnol Bioeng. 1990;36:816–820. doi: 10.1002/bit.260360810. [DOI] [PubMed] [Google Scholar]
- 2.Babel W. Energetische und biochemische Aspekte der Mischsubstrat-Fermentation. In: Ringpfeil M, editor. Biotechnologie. Berlin, Germany: Akademie Verlag; 1982. pp. 183–188. [Google Scholar]
- 3.Bally M. Physiology and ecology of nitriloacetate degrading bacteria in pure culture, activated sludge and surface waters. Ph.D. dissertation. Zürich, Switzerland: Swiss Federal Institute of Technology; 1994. [Google Scholar]
- 4.Clarkson W W, Ross B J B, Krishnamachari S. 45th Purdue Industrial Waste Conference Proceedings. Chelsea, Mich: Lewis Publishers; 1991. Denitrification of high-strength industrial wastewater; pp. 347–357. [Google Scholar]
- 5.Cocaing-Bousquet M, Guyonvarch A, Lindley N D. Growth rate-dependent modulation of carbon flux through central metabolism and the kinetic consequences for glucose-limited chemostat cultures of Corynebacterium glutamicum. Appl Environ Microbiol. 1996;62:429–436. doi: 10.1128/aem.62.2.429-436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchars M G, Attwood M M. The influence of the carbon:nitrogen ratio of the growth medium on the cellular composition and regulation of enzyme activity in Hyphomicrobium sp. J Gen Microbiol. 1989;135:787–793. [Google Scholar]
- 7.Duetz W A, Marqués S, De Jong C, Ramos J L, Van Andel J G. Inducibility of the TOL catabolic pathway in Pseudomonas putida (pWW0) growing on succinate in continuous culture: evidence of carbon catabolite repression control. J Bacteriol. 1994;176:2354–2361. doi: 10.1128/jb.176.8.2354-2361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egli T. On multiple-nutrient-limited growth of microorganisms, with special reference to dual limitation by carbon and nitrogen substrates. Antonie Leeuwenhoek. 1991;60:225–234. doi: 10.1007/BF00430367. [DOI] [PubMed] [Google Scholar]
- 9.Egli T. The ecological and physiological significance of the growth of heterotrophic microorganisms with mixtures of substrates. Adv Microb Ecol. 1995;14:305–386. [Google Scholar]
- 10.Egli T, Fiechter A. Theoretical analysis of media used in the growth of yeasts on methanol. J Gen Microbiol. 1981;123:365–369. [Google Scholar]
- 11.Egli T, Lendenmann U, Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie Leeuwenhoek. 1993;63:289–298. doi: 10.1007/BF00871224. [DOI] [PubMed] [Google Scholar]
- 12.Egli T, Lindley N D, Quayle J R. Regulation of enzyme synthesis and variation of residual methanol concentration during carbon-limited growth of Kloeckera sp. 2201 on mixtures of methanol and glucose. J Gen Microbiol. 1983;129:1269–1281. [Google Scholar]
- 13.Egli T, Quayle J R. Influence of the carbon:nitrogen ratio of the growth medium on the cellular composition and the ability of the methylotrophic yeast Hansenula polymorpha to utilize mixed carbon sources. J Gen Microbiol. 1986;132:1779–1788. [Google Scholar]
- 14.Goldman B S, Lin J T, Stewart V. Identification and structure of the nasR gene encoding a nitrate- and nitrite-responsive positive regulator of nasFEDCBA (nitrate assimilation) operon expression in Klebsiella pneumoniae MSal. J Bacteriol. 1994;176:5077–5085. doi: 10.1128/jb.176.16.5077-5085.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero M G, Vega J M, Losada M. The assimilatory nitrate-reducing system and its regulation. Annu Rev Plant Physiol. 1981;32:169–204. [Google Scholar]
- 16.Harder W, Dijkhuizen L. Strategies of mixed substrate utilization in microorganisms. Phil Trans R Soc London B. 1982;297:459–480. doi: 10.1098/rstb.1982.0055. [DOI] [PubMed] [Google Scholar]
- 17.Herbert D. Stoichiometric aspects of microbial growth. In: Dean A C R, Ellwood D C, Evans C G T, Melling J, editors. Continuous culture 6: applications and new fields. Chichester, United Kingdom: Ellis Hordwood; 1976. pp. 1–30. [Google Scholar]
- 18.Keith L H, Telliard W A. Priority pollutants. I. A perspective view. Environ Sci Technol. 1979;13:416–423. [Google Scholar]
- 19.Krishnamachari S, Clarkson W W. 47th Purdue Industrial Waste Conference Proceedings. Chelsea, Mich: Lewis Publishers; 1993. Nitrite accumulation in the effluents from high-strength denitrification of industrial wastewater; pp. 383–392. [Google Scholar]
- 20.Lawson C T. 35th Purdue Industrial Waste Conference Proceedings. Chelsea, Mich: Lewis Publishers; 1981. Development of a biological denitrification process for a high-strength industrial waste; pp. 882–888. [Google Scholar]
- 21.Lendenmann U, Snozzi M, Egli T. Kinetics of the simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture. Appl Environ Microbiol. 1996;62:1493–1499. doi: 10.1128/aem.62.5.1493-1499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magasanik B. Catabolite repression. Cold Spring Harbor Symp Quant Biol. 1961;26:249–262. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Magasanik B, Neidhardt F C. Regulation of carbon and nitrogen utilization. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1318–1325. [Google Scholar]
- 24.Minkevich I G, Krynitskaya A Y, Eroshin V K. A double substrate limitation zone of continuous microbial growth. In: Kyslik P, Dawes E A, Krumphanzl V, Novak M, editors. Continuous culture. London, United Kingdom: Academic Press; 1988. pp. 171–189. [Google Scholar]
- 25.Piñar G, Duque E, Haïdour A, Oliva J M, Sánchez-Barbero L, Calvo V, Ramos J L. Removal of high concentrations of nitrate from industrial wastewater by bacteria. Appl Environ Microbiol. 1997;63:2071–2073. doi: 10.1128/aem.63.5.2071-2073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos J L, Haïdour A, Duque E, Piñar G, Calvo V, Oliva J M. Metabolism of nitrate esters by a consortium of two bacteria. Nat Biotechnol. 1996;14:320–322. doi: 10.1038/nbt0396-320. [DOI] [PubMed] [Google Scholar]
- 27.Rutgers M, Balk P A, Van Dam K. Quantification of multiple-substrate controlled growth. Simultaneous ammonium and glucose limitation in chemostat cultures of Klebsiella pneumoniae. Arch Microbiol. 1990;153:478–484. doi: 10.1007/BF00248430. [DOI] [PubMed] [Google Scholar]
- 28.Snell F D, Snell C T. Colorimetric methods of analysis. Vol. 2. New York, N.Y: Van Nostrand; 1949. pp. 802–807. [Google Scholar]
- 29.Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988;52:190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker J F, Jr, Helfrich M V, Donaldson T L. Biodenitrification of uranium refinery wastewaters. Environ Prog. 1989;8:97–101. [Google Scholar]
- 31.Wanner U, Egli T. Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol Rev. 1990;75:19–44. doi: 10.1111/j.1574-6968.1990.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 32.World Heath Organization. Guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization; 1984. [Google Scholar]