Abstract
To expedite gene discovery in eye development and its associated defects, we previously developed a bioinformatics resource-tool iSyTE (integrated Systems Tool for Eye gene discovery). However, iSyTE is presently limited to lens tissue and is predominantly based on transcriptomics datasets. Therefore, to extend iSyTE to other eye tissues on the proteome level, we performed high-throughput tandem mass spectrometry (MS/MS) on mouse embryonic day (E)14.5 retina and retinal pigment epithelium combined tissue and identified an average of 3,300 proteins per sample (n=5). High-throughput expression profiling-based gene discovery approaches–involving either transcriptomics or proteomics–pose a key challenge of prioritizing candidates from thousands of RNA/proteins expressed. To address this, we used MS/MS proteome data from mouse whole embryonic body (WB) as a reference dataset and performed comparative analysis–termed “in silico WB-subtraction”–with the retina proteome dataset. In silico WB-subtraction identified 90 high-priority proteins with retina-enriched expression at stringency criteria of ≥2.5 average spectral counts, ≥2.0 fold-enrichment, False Discovery Rate <0.01. These top candidates represent a pool of retina-enriched proteins, several of which are associated with retinal biology and/or defects (e.g., Aldh1a1, Ank2, Ank3, Dcn, Dync2h1, Egfr, Ephb2, Fbln5, Fbn2, Hras, Igf2bp1, Msi1, Rbp1, Rlbp1, Tenm3, Yap1, etc.), indicating the effectiveness of this approach. Importantly, in silico WB-subtraction also identified several new high-priority candidates with potential regulatory function in retina development. Finally, proteins exhibiting expression or enriched-expression in the retina are made accessible in a user-friendly manner at iSyTE (https://research.bioinformatics.udel.edu/iSyTE/), to allow effective visualization of this information and facilitate eye gene discovery.
Keywords: Retina, Proteome, Mass spectrometry, Development, Embryogenesis, Gene discovery, iSyTE
Introduction
Identification of genes associated with ocular development and its associated defects remains a challenge even though there has been widespread application of transcriptomics and proteomics toward these goals in recent years (Anand and Lachke 2017; Ahmad et al. 2018; Wolf et al. 2022). This is because high-throughput approaches such as RNA-sequencing or mass spectrometry identify thousands of candidates (RNA or protein) and the principal challenge lies in the application of effective downstream analyses for prioritizing candidates that are relevant to the development, homeostasis or pathology of the specific tissue/cell type of interest. Furthermore, prioritization strategies based solely on the extent of expression (i.e., absolute expression) may result in missing important regulatory candidates that are not necessarily among the most highly expressed, but may nevertheless have a key role in development of the tissue/cell type.
We developed a user-friendly web-based resource-tool called iSyTE (integrated Systems Tool for Eye gene discovery) to address these challenges in the eye; however, the earlier versions were focused on the lens (Lachke et al. 2012b; Kakrana et al. 2018; Anand et al. 2018; Aryal et al. 2020a). In addition to absolute expression in a tissue/cell type, iSyTE prioritizes candidate genes by comparing the gene expression profiles – in this case, of the lens – to that of a reference dataset, namely, mouse embryonic whole body (WB), a process termed “in silico WB-subtraction”. This results in prioritization of candidates based on their “enriched-expression” in the lens as opposed to solely based on their absolute expression. Application of the iSyTE approach has effectively identified many new genes (e.g., Caprin2, Celf1, Mafg, Mafk, Rbm24, Tdrd7, etc.) as well as led to the characterization of several regulatory pathways associated with eye/lens development and defects (Lachke et al. 2011, 2012a, b; Kasaikina et al. 2011; Wolf et al. 2013; Manthey et al. 2014; Agrawal et al. 2015; Dash et al. 2015, 2020; Audette et al. 2016; Patel et al. 2017, 2022; Anand and Lachke 2017; Cavalheiro et al. 2017; Siddam et al. 2018, 2023; Krall et al. 2018; Padula et al. 2019; Barnum et al. 2020; Aryal et al. 2020b; Anand et al. 2021; Choquet et al. 2021; Lachke 2022).
The present version of iSyTE is restricted to data on the lens, and furthermore, it is predominantly based on transcriptomic data in the form of microarrays or RNA-seq (Lachke et al. 2012b; Kakrana et al. 2018; Anand et al. 2018), with limited data on the proteome (Aryal et al. 2020a). Moreover, it is known that the correlation between RNA profiles and protein profiles is complex and not necessarily linear (Maier et al. 2009; Liu et al. 2016; Buccitelli and Selbach 2020). Contributing to this is regulation at the post-transcriptional level – that involves non-coding RNA or RNA-binding protein (RBP)-mediated control over mRNA splicing, transport, stability and translation – all of which determines the cellular proteome (Brinegar and Cooper 2016; Hentze et al. 2018; Gebauer et al. 2021). Indeed, recent findings have demonstrated that RBPs play a conserved role in mediating post-transcriptional control in eye and lens development (Lachke et al. 2011; Lachke and Maas 2011; Dash et al. 2015, 2016, 2020; Siddam et al. 2018; Shao et al. 2020; Barnum et al. 2020; Nakazawa et al. 2020; Sundar et al. 2020; Aryal et al. 2020b; Lachke 2022; Matalkah et al. 2022). For example, deficiency of the RBPs Caprin2, Celf1, Rbm24 and Tdrd7 are linked to lens defects and/or cataract in various animal models or human patients (Lachke 2022). In particular, in Celf1 conditional knockout lenses, while the bulk mRNA levels for p27Kip1, Pax6 and Prox1 are not significantly changed, their encoded proteins levels are profoundly altered, indicating post-transcriptional control mediated by this RBP at the translational level (Siddam et al. 2018; Aryal et al. 2020b).
Together, these findings suggest that along with the transcriptome, characterization of the proteome is important to determine the factors that are important in a specific cell/tissue of interest. While there are currently many independent transcriptomics studies on the retina (Ratnapriya et al. 2019; Clark et al. 2019; Lukowski et al. 2019; Liang et al. 2019), studies on the retina proteome, especially in embryonic development, are limited (Zhao et al. 2007; Mizukami et al. 2008; Finnegan et al. 2008; Balasubramani et al. 2010). Indeed, there is no proteome dataset of wild-type mouse embryonic day (E) 14.5, when eye development has progressed to the formation of the neural retina (which at this stage comprises of uncommitted neuroblasts and newly differentiated neurons) (Cepko et al. 1996) and the retinal pigment epithelium (RPE). Such a dataset would identify new candidates that may advance the understanding of retina development. To address these knowledge-gaps in the context of eye development, we report here a tandem mass spectrometry (MS/MS)-based protein profiling of the mouse E14.5 neural retina and RPE combined tissue (termed henceforth as “retina” in this manuscript) and its comparative analysis with in silico WB-subtraction. We demonstrate that while retina protein expression alone (i.e., retina proteome not subjected to in silico subtraction) can identify several genes linked to retina biology and defects and is in itself helpful, in silico WB-subtraction provides another effective approach in prioritizing key candidates that are not necessarily among the highest expressed proteins in the retina. We generated new expression tracks at the University of California at Santa Cruz (UCSC) Genome Browser and make this new data accessible through iSyTE.
Methods
Animals
Mice of the background C57BL/6J, obtained from The Jackson Laboratory, were used as wild-type animals in this study, and were bred and maintained at the University of Delaware Center for Animal research facility. The Institutional Animal Care and Use Committee (IACUC) approved the animal protocol (AUP#1226). All the animal experiments described in this study were performed in adherence to the guidelines in the Association of Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
Tissue Collection
Mice were bred and pregnant females were euthanized for obtaining embryos for collection of retina tissue. The day on which a vaginal plug was detected was designated as embryonic day (E) 0.5, and tissues was collected at E14.5. Whole retina tissue (retina + retinal pigment epithelium (RPE), henceforth referred to as “retina”) were micro-dissected from E14.5 mouse embryos and stored at −80°C until further processing. Whole embryonic tissue minus the eye at E14.5 was considered as “whole embryonic body” (WB). Five biological replicates with each replicate consisting of two retinas isolated from the same embryo were collected. Tissues were processed as previously described (Aryal et al. 2020a). Briefly, tissue samples were suspended in 120 μl of TEAB buffer (167 mM triethyl ammonium bicarbonate buffer) and subjected to probe-sonication in a Fisher Scientific 60 Sonic Dismembrator. To these lysed samples, 40 μl of 20% SDS, 1% DCA and 40 μl of water were added to bring the total volume of each sample to 200 μl (final concentration: 4% SDS, 0.2% DCA, 100 mM TEAB), which were next centrifuged (16000 × g, 2 min., room temperature) followed by heating (90°C for 15 min.). Sample protein quantification was estimated by BCA protein assay kit (Thermo Fisher Cat. No. 23225). For each biological replicate (n = 5 biological replicates), 55 μg of protein/sample was subjected to trypsinization as previously described (Erde et al. 2017). Briefly, a modified enhanced filter-aided digestion protocol (e-FASP) using Amicon 30 kDa ultracentrifugation devices was executed. Samples were subjected to TCEP (Tris Carboxy Ethyl Phosphene) reducing reagent at 90°C for 10 min, followed by transferring to an Amicon filter. Samples were then buffer exchanged into 8 M Urea, 0.2% deoxycholic acid (DCA), 100 mM TEAB. Next, samples were subjected to alkylation with iodoacetamide, exchanged into 0.2% DCA, 50 mM TEAB (pH 8.0) digestion buffer, and subjected to overnight digestion by trypsin (1:20 enzyme:substrate concentration). After overnight trypsin digestion, samples were subjected to centrifugation and the filtrate, which contained the peptides, was subjected to extraction with ethyl acetate, which served to remove DCA. A SpeedVac vacuum concentrator (Thermo Fisher Scientific) was then used to dry the samples which were then resuspended in 100 μl of HPLC-grade water. Next, a Pierce Quantitative Colorimetric Peptide Assay Kit was used to perform a peptide assay on the samples and the average peptide recovery from mouse E14.5 retina samples was estimated to be ~45 μg/sample. Whole embryonic body (WB) tissue (eye removed) sample processing was performed as previously described (Aryal et al. 2020a).
Mass Spectrometry
Mass spectrometry (MS) was performed as previously described (Aryal et al. 2020a). Briefly, protein samples (concentration: 4 μg in 5% Formic acid) were loaded for 5 min on an Acclaim PepMap 0.1 × 20 mm NanoViper C18 peptide trap (Thermo Fisher Scientific)(flow rate: 10 μl/min; mobile phase: in a 2% acetonitrile, 0.1% formic acid). PepMap RSLC C18 2 μm particle, 75 μm × 50 cm EasySpray column (Thermo Fisher Scientific) was used for separating peptides over 205 min on a 7.5–30% acetonitrile gradient (mobile phase: 0.1% formic acid, 300 nl/min flow rate) with Dionex NCS-3500RS UltiMate RSLC nano UPLC system. An Orbitrap Fusion mass spectrometer configured with an EasySpray NanoSource (Thermo Fisher Scientific) was used to collect tandem MS data, using data dependent analysis (DDA) configuration and a MS/DD-MS/MS instrument method (full MS resolutions: 120,000 at m/z 200, mass range 375–1500, charge state 2–7; full MS AGC target: 400,000; intensity threshold: 5,000; max inject time: 50 ms and 10 ppm dynamic exclusion for 60 s; AGC target value for fragment spectra: 5,000; isolation mode: quadrupole; isolation width: 1.6 m/z; isolation offset: off; activation type: CID; collision energy: fixed 35%; maximum injection time: 300 ms; detector type: IonTrap). Centroid mode using positive polarity was used to acquire data. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the Proteomics IDEntifications (PRIDE) database (https://www.ebi.ac.uk/pride/) (Perez-Riverol et al. 2022) partner repository with the dataset identifier PXD039490.
Raw File Conversion and Database Search
MSConvert (Proteowizard toolkit) was used to convert RAW files to MS2 format for the samples as described (Chambers et al. 2012; Aryal et al. 2020a). The retina samples had ~74K MS2 scans per run. A software (available at https://github.com/pwilmart/fasta_utilities.git) was used to download a canonical mouse reference proteome (version 2019.04; 22,287 sequences) from UniProt. To this, a concatenated sequence-reversed decoy database was added along with common contaminants (179 sequences) to obtain 44,932 entries. Peptide sequences were assigned to the MS2 spectra (PSMs) using the search engine Comet (Eng et al. 2013), which was configured as previously described (Aryal et al. 2020a) and were as follows: tryptic cleavage (maximum of two missed cleavages); monoisotopic parent ion mass tolerance of 1.25 Da; monoisotopic fragment ion tolerance of 1.0005 Da; fragment bin offset of 0.4; b-, y-, and neutral loss ions were used in scoring (flanking peaks were not used); variable modification of oxidation (+15.9949 Da) on methionine was specified; static modification of alkylation (+57.0215 Da) of cysteines was specified.
PSM Error Control
The PAW pipeline (https://github.com/pwilmart/PAW_pipeline.git) and the target/decoy method described previously (Elias and Gygi 2007; Wilmarth et al. 2009) was used for post-processing the highest scoring matches for individual PSMs (obtained from Comet) using false discovery rate (FDR) error control. Peptides of different charge states (2+, 3+, and 4+ were considered) and modification state (unmodified or oxidized) were processed to derive accurate delta mass conditional score histograms. FDR values were estimated based on target and decoy score histograms as a function of a Peptide-Prophet-like discriminant score to set thresholds for experiment-wide PSM FDR of 1% as described previously (Keller et al. 2002; Aryal et al. 2020a). A minimum length of 7 amino acids-length were considered for peptide matches. The number of confidently identified (1% FDR) PSMs per sample was 35.4K and the identification rate was 48%.
Protein Inference
The expressed proteins were inferred, using basic parsimony principles, based on the filtered PSM sequences (Nesvizhskii and Aebersold 2005). Protein identification required two distinct peptides per protein, in at least one sample. Homologous protein family members were grouped using an extended parsimony algorithm when evidence to distinguish family members was insufficient. In total, 3,963 proteins were detected after grouping (excluding common contaminant proteins) with 37 decoy matches, for a protein FDR of about 0.9%. The average number of proteins identified per sample was 3,296.
Quantitative Analysis
For the retina and the WB samples, equal amounts of protein were digested and the total spectral counts (SpC, a robust semi-quantitative measure) were measured. Prior to protein inference, the SpC for individual samples were tallied and they independently validated the peptide assay results. Next, the retina and the WB samples were matched by subjecting the individual samples to be scaled to the average total spectral count per sample. Both the retina and the WB samples had about 3,300 protein identifications per sample. Next, the proteins with enriched expression in the retina compared to WB were determined as follows: For individual proteins, the average SpC for all samples was computed from the scaled data, and only values greater than 2.5 (2,675 proteins) were considered in the differential expressed enrichment analysis between the retina and WB. SpC has been used for estimating relative protein abundance in previous studies (Liu et al. 2004). The Bioconductor package, edgeR, was used for the differential gene expression analysis (Robinson and Oshlack 2010; Robinson et al. 2010). The default Benjamini-Hochberg multiple testing corrections and the exact test in edgeR were applied in R (version 3.5.3). The application of edgeR and TMM normalization for spectral counting is established (Fei et al. 2011; Bharadwaj et al. 2013).
Gene Ontology Analysis
For functional annotation by gene ontology (GO) categories, a cluster-based analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID v6 .8) (Huang et al. 2009) was performed on candidate proteins with retina-expression and retina-enriched expression that were identified by in silico WB-subtraction (the cut-offs were: ≥2.5 average spectral counts, ≥2.0 fold-enrichment, FDR <0.01). Benjamini corrected significant p-values were considered for prioritization of the pathways and GO categories identified from this analysis as previously described (Aryal et al. 2020a).
Results and Discussion
Embryonic retina proteome generation and quality assessment
We designed an experimental workflow to isolate mouse E14.5 retina, generate its proteome and perform in silico WB-subtraction (Fig. 1A). Retina tissue was micro-dissected from mouse E14.5 eyes and processed for protein preparation and proteome analysis. Mouse WB preparation was performed as previously described (Aryal et al. 2020a). Further, proteome downstream analyses were performed according to the outlined workflow (Fig. 1B). From individual retina and WB samples (n = 5 biological replicates), 55 μg of protein were subjected to eFASP (enhanced filter-aided sample preparation) and digestion by trypsin. After digestion, equal amounts of peptides were used for high-throughput tandem mass spectrometry (MS/MS) and spectral count (SpC) data were generated. Application of stringent criteria (≥2 distinct peptides per protein in at least one sample, ≥2.5 average SpC in the retina) to the resulting data led to enrichment analysis of 2,675 proteins in the E14.5 retina (Supplementary Table S1). Across the samples, on average ~35K SpC were detected. Total average SpC was subjected to TMM (trimmed mean of M-values) normalization using edgeR package (Robinson et al. 2010) to account for differences in SpC between retina and WB (Fig. 2A). Next, the quality of data was assessed by boxplots for the normalized SpC datasets that demonstrated that the median expression levels were similar between all the retina and the WB samples (Fig. 2A). Further, multidimensional scaling-based cluster analysis was performed to examine the quality of TMM normalized SpC proteome data. Cluster analysis demonstrated that all five biological replicates of the retina clustered closer to each other and distinctly away from WB samples, which themselves clustered together (Fig. 2B). Sample to sample correlation within the retina and within the WB samples was examined by scatter plot comparisons in all combinations for retina and WB samples, which demonstrated high correlation between samples of the same type (Fig. 2C, D). While the five WB samples correlated with each other at r value >0.98, all the five retina samples correlated with each other at r value >0.97. Finally, comparing the correlation between the average SpC of the retina and that of WB shows that the correlation is much lower (r = 0.81) between the retina and WB (Fig. 2E).
Fig. 1. Workflow of the experimental strategy to generate MS/MS protein profile of the mouse embryonic retina and retinal pigment epithelium combined tissue.
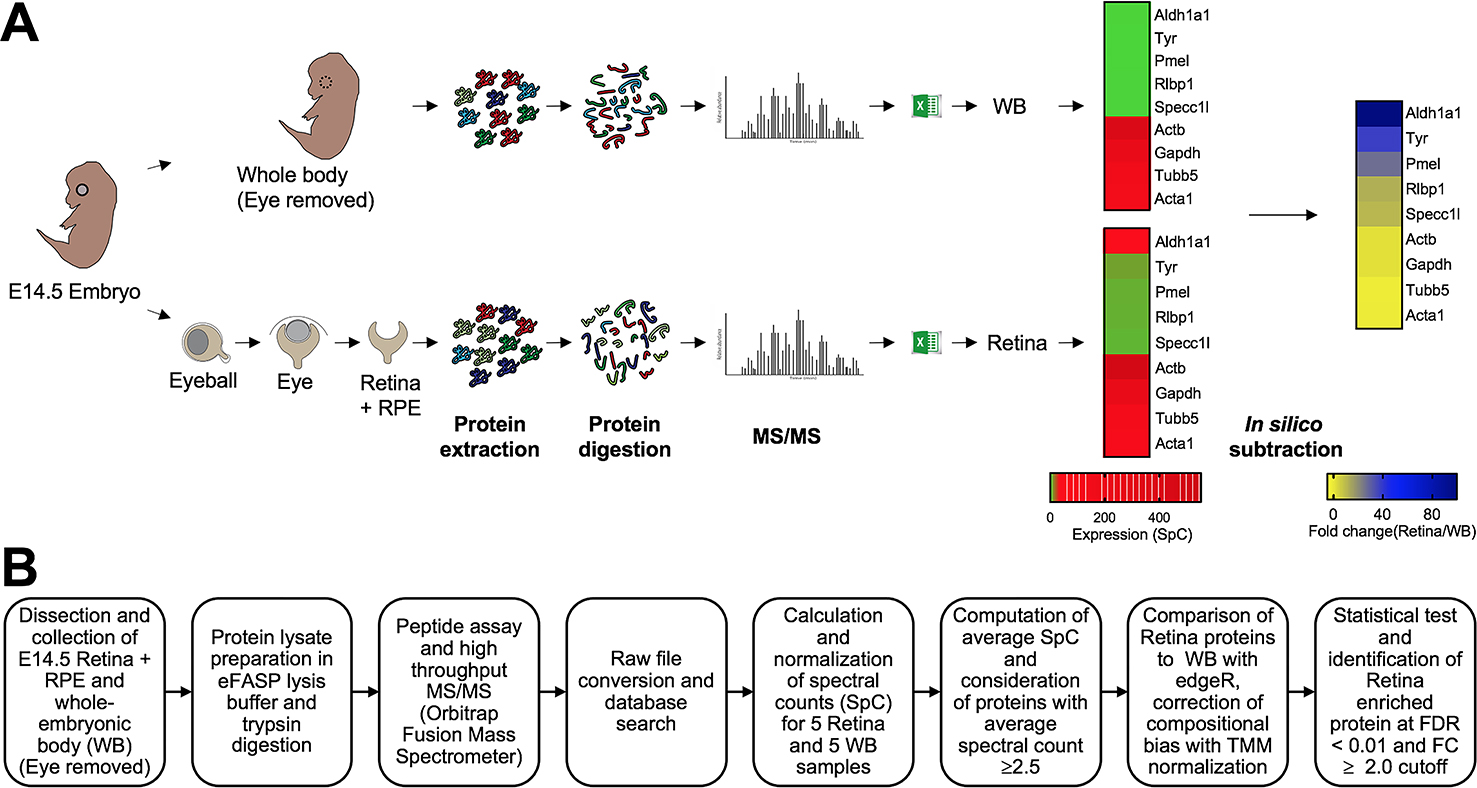
(A) Mouse eyes at embryonic day (E)14.5 were isolated, and the retina and retinal pigment epithelium combined tissue (termed retina) was micro-dissected. The whole body (WB) with eye tissue removed was processed similarly and used as reference for differential protein expression analysis. Retina and WB samples (n = 5 for each sample type, 55 μg protein per sample) were subjected to high-throughput tandem mass spectrometry (MS/MS). (B) The workflow for differential protein expression analysis is outlined. The edgeR pipeline was used to determine differential protein expression using normalized spectral counts. Proteins passing stringency criteria of ≥2.5 average spectral counts, ≥2.0 fold-change (in retina, compared to WB), False Discovery Rate <0.01 were considered to have enriched expression in the retina.
Fig. 2. Quality assessment of MS/MS data.
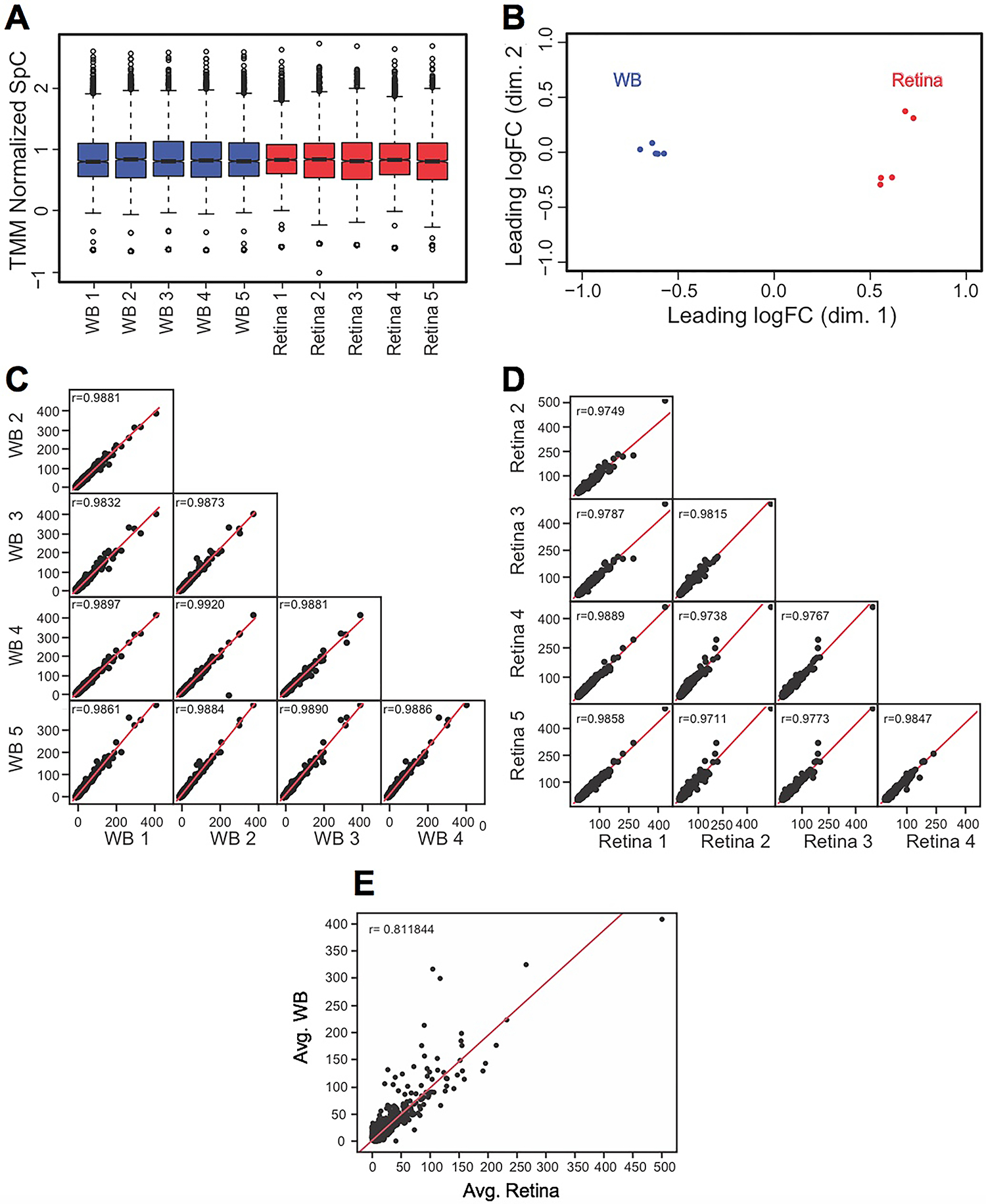
(A) TMM (trimmed mean of M-values) normalization of spectral counts in WB and retina samples using edgeR to correct for the dramatic compositional differences. The retina and the WB samples showed comparable median SpCs in boxplots (TMM normalized SpC are shown in y-axis). (B) Individual biological replicates of the retina and WB samples clustered together while the overall retina and WB samples clustered separately from each other in Multidimensional scaling analysis (leading dimensions 1 and 2 are represented by the axes). (C) Sample-to-sample consistency was examined by generating a scatter matrix for the five WB samples and (D) the five retina samples. (E) A scatter plot with regression analysis shows no correlation (r = 0.81) between the average retina and average WB samples.
Gene Ontology (GO) analysis of proteins expressed in the E14.5 mouse
Before performing other downstream analysis, we characterized the E14.5 proteins that were identified. Many proteins previously linked to retinal development and disease were identified in the proteome analysis, based solely on expression (Supplementary Table S1 for all proteins and Supplementary Table S2 for the top 150). To examine whether specific pathways relevant to retina biology were enriched in this dataset, a cluster-based analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID v6 .8) for functional annotation by gene ontology (GO) categories. This analysis identified several interesting pathways. These were related to post-transcriptional control of gene expression, e.g., “GO:0003723 RNA-binding”, “GO:0030529 intracellular ribonucleoprotein complex”, “GO:0006397 mRNA processing” and “GO:0051028 mRNA transport” (Fig. 3)(Supplementary Table S3). Proteins involved in other molecular pathways and processes e.g., “GO:0015031 protein transport”, “GO:0055114 oxidation-reduction process” were also identified in the dataset. Finally, pathways in basic cell biological processes were also enriched, e.g., “GO:0007049 cell cycle”, “GO:0098641 cadherin binding involved in cell-cell adhesion”, and “GO:0003779 actin binding” in the total proteins expressed in E14.5 mouse retina. Together, these represent promising new candidates for future investigations in the retina.
Fig. 3. Gene ontology (GO) analysis of proteins expressed in the E14.5 retina and retinal pigment epithelium combined tissue.
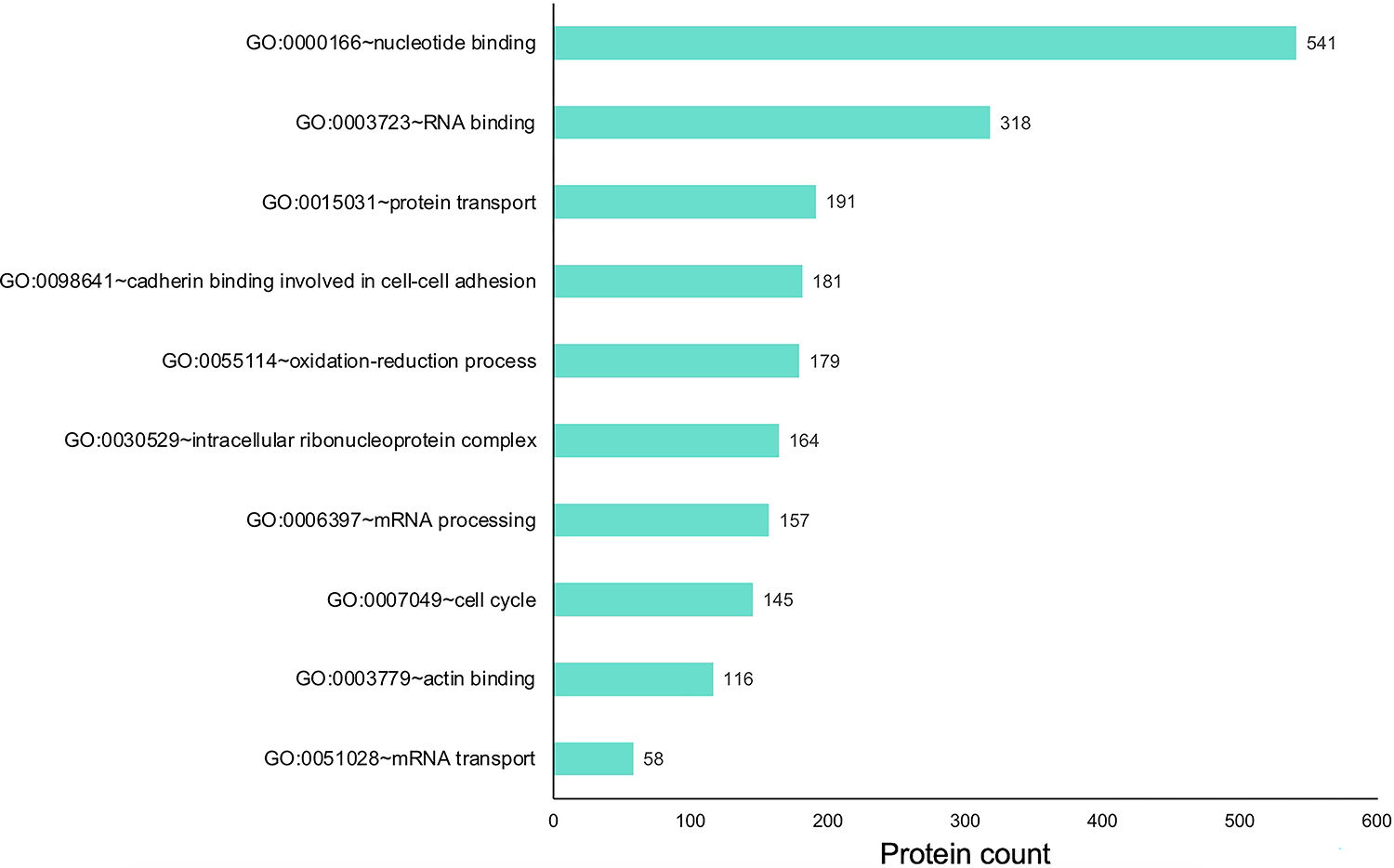
Proteins expressed in the retina were subjected to cluster-based analysis using the Database for Annotation, Visualization, and Integrated Discovery (DAVID v6 .8) for functional annotation by gene ontology (GO) categories. This analysis identified candidates representing several GO terms that may be relevant to retinal biology, including those involved in various molecular, cellular and physiological processes. The x-axis represents the number of protein candidates identified in the specific GO term shown on the y-axis.
MS/MS in silico WB-subtraction identifies proteins exhibiting retina-enriched expression
While GO analysis of total expressed proteins were helpful, to further prioritize the candidates from the E14.5 retina proteome, the “in silico WB-subtraction” approach, which has been effectively applied for prioritizing cataract-linked genes in the lens, was applied to this dataset. To do so, we computed the average SpC for all samples and scaled (normalized) data for each protein. Those peptides that passed the filtration criteria of ≥2.5 SpC were considered in the analysis. This approach identified 2675 proteins that could be tested for differential expression between the retina and WB samples. At ≥2.0 fold-enrichment and FDR <0.01 cut-off, 90 proteins had enriched expression in the retina compared to WB (Table 1). These “retina-enriched” proteins identified many proteins linked to retinal defects and revealed several new promising candidates (Fig. 4) demonstrating that the in silico WB-subtraction approach can be effectively applied to the retina. Further, compared to absolute expression of proteins, in silico WB-subtraction could more effectively prioritize key proteins associated with retina biology and disease. For example, the top 30 proteins ranked on relative abundance in the retina (i.e., not subjected to in silico WB-subtraction) did not contain a single protein that has been associated with retina development or defects/disease (Fig. 5A). Indeed, candidates in this list, termed “retina expression” list, were representative of general housekeeping/structural proteins such as Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), Actins (Acta1, Actb), Myosins (Myh3, Myh9, Myh10), Tubulin (Tubb5), Collagen (Col12a1) and several others, not exclusively associated with retina biology. In sharp contrast, the list of the top 30 candidates identified by in silico WB-subtraction, termed “retina enriched” list of candidates, contained 1/3rd (10 out of 30) candidates that have been associated with retinal biology and/or defects/disease (Fig. 5B). These are Aldehyde dehydrogenase family 1, subfamily A1 (Aldh1a1), Tyrosinase (Tyr), Keratocan (Kera), Melanocyte protein PMEL (Pmel), Hemicentin-1 (Hmcn1), Retinaldehyde-binding protein 1 (Rlbp1), Harvey rat sarcoma virus oncogene (Hras), Laminin subunit alpha-5 (Lama5), Epidermal growth factor receptor (Egfr), Hephaestin (Heph) and Teneurin-3 (Tenm3). Importantly, the top candidates identified by the in silico WB-subtraction approach contained regulatory proteins that are not necessarily among the top highly expressed proteins in the retina. In contrast, no regulatory proteins were detected in the top 30 retina expression list. Finally, the significant differences in SpC levels for different proteins between the retina and WB serves to explain the basis for the effectiveness of the in silico WB-subtraction strategy in prioritization of candidates relevant to the retina and its associated defects (Fig. 5B).
Table 1.
Top 90 proteins with enriched expression in mouse E14.5 retina and retinal pigment epithelium combined tissue as compared to WB
| Rank | UniProt Gene Name | Uniprot Accession | Primary Protein Name | Associated Retina or Eye Defects | References |
|---|---|---|---|---|---|
| 1 | Aldh1a1 | P24549 | Retinal dehydrogenase 1 (RALDH 1; RalDH1) | Expressed throughout development in dorsal retina; Choroidal hypoplasia in mice | (Fan et al. 2003; Goto et al. 2018; Duester 2022) |
| 2 | Tyr | P11344 | Tyrosinase | Retinal defects; optic chiasmatic abnormality in mice | (Jeffery et al. 1994, 1997) |
| 3 | Kera | O35367 | Keratocan (KTN) | - | |
| 4 | Pmel | Q60696 | Premelanosome protein | RPE melanosome shape defect in mice | (Hellström et al. 2011) |
| 5 | Cryge | Q03740 | Crystallin γE | - | |
| 6 | Crybb3 | Q9JJU9 | Crystallin βB3, N-terminally processed | - | |
| 7 | Fras1 | Q80T14 | Extracellular matrix protein FRAS1 | - | |
| 8 | Hmcn1 | D3YXG0 | Hemicentin-1 | AMD (age-related macular degeneration) in human | (Pras et al. 2015) |
| 9 | Crybb1 | Q9WVJ5 | Cystallin βB1 | - | |
| 10 | Nfatc4 | Q8K120 | Nuclear factor of activated T-cells, cytoplasmic 4 (NF-ATc4; NFATc4) | - | |
| 11 | Mylk | Q6PDN3 | Myosin light chain kinase, smooth muscle, deglutamylated form | - | |
| 12 | Rlbp1 | Q9Z275 | Retinaldehyde-binding protein 1 | Retinitis punctatta albescens in human | (Morimura et al. 1999) |
| 13 | Specc1l | Q2KN98 | Cytospin-A | - | |
| 14 | Igdcc4 | Q9EQS9 | Immunoglobulin superfamily DCC subclass member 4 | - | |
| 15 | Crym | O54983 | Ketimine reductase mu-crystallin | - | |
| 16 | Epb41l5 | Q8BGS1 | Band 4.1-like protein 5 | - | |
| 17 | Lig3 | P97386 | DNA ligase 3 | - | |
| 18 | Champ1 | Q8K327 | Chromosome alignment-maintaining phosphoprotein 1 | - | |
| 19 | Tes | P47226 | Testin | - | |
| 20 | Mdc1 | Q5PSV9 | Mediator of DNA damage checkpoint 1 | - | |
| 21 | Hras | Q61411 | GTPase HRas, N-terminally processed | Retinal dystrophy in human | (Pierpont et al. 2017) |
| 22 | Lama5 | Q61001 | Laminin subunit α-5 | Retinal defects; malformation of retina morphology in mice | (Jones et al. 2020) |
| 23 | Frem2 | Q6NVD0 | FRAS1-related extracellular matrix protein 2 | Detected in outer plexiform layer of the retina; mutations associated with Cryptophthalmos in human | (Zhang et al. 2019) |
| 24 | Mfap2 | P55002 | Microfibrillar-associated protein 2 (MFAP-2) | - | |
| 25 | Smarcd1 | Q61466 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 1 | - | |
| 26 | Egfr | Q01279 | Epidermal growth factor receptor | Retinal cell fate determination | (Lillien 1995) |
| 27 | Heph | Q9Z0Z4 | Hephaestin | Retinal defects; retinal degeneration in mice | (Hahn et al. 2004) |
| 28 | Rai14 | Q9EP71 | Ankycorbin | - | |
| 29 | Tenm3 | Q9WTS6 | Teneurin-3 (Ten-3) | Retinal defects in mouse and zebrafish | (Leamey et al. 2007) (Antinucci et al. 2013) |
| 30 | Nelfe | P19426 | Negative elongation factor E (NELF-E) | - | |
| 31 | Znf326 | O88291 | Zinc finger protein 326 | - | |
| 32 | Crocc | Q8CJ40 | Rootletin | Retinal defects; retinal degeneration in mice | (Yang et al. 2005) |
| 33 | Cadm1 | Q8R5M8 | Cell adhesion molecule 1 | Retinal defects; retinal photoreceptor synapse defects in mice | (Ribic et al. 2014) |
| 34 | Fbln5 | Q9WVH9 | Fibulin-5 (FIBL-5) | Retinal defects; AMD and cutis laxa in human | (Lotery et al. 2006) (Stone et al. 2004) |
| 35 | Wiz | O88286 | Widely-interspaced zinc finger motifs | - | |
| 36 | Cryaa | P24622 | Crystallin αA chain | Retinal neovascularization defects in mice | (Xu et al. 2015) |
| 37 | Znf219 | Q6IQX8 | Zinc finger protein 219 | - | |
| 38 | Gldc | Q91W43 | Glycine dehydrogenase (decarboxylating), mitochondrial | - | |
| 39 | Mex3a | G3UYU0 | Mex3 RNA-binding family member A | - | |
| 40 | Nub1 | P54729 | NEDD8 ultimate buster 1 | - | |
| 41 | Uncharacterized protein FLJ45252 homolog | Q6PIU9 | Uncharacterized protein FLJ45252 homolog | - | |
| 42 | Kif1a | P33173 | Kinesin-like protein KIF1A | - | |
| 43 | Dync2h1 | Q45VK7 | Cytoplasmic dynein 2 heavy chain 1 | Retinal defects; non-syndromic inherited retinal disease in human | (Vig et al. 2020) |
| 44 | Gja1 | P23242 | Gap junction alpha-1 protein | Retinal defects (optic nerve and retinal dysplasia) and other eye defects in human | (Gabriel et al. 2011) |
| 45 | Fbn2 | Q61555 | Fibrillin-2 C-terminal peptide | AMD | (Ratnapriya et al. 2014) |
| 46 | Pcca | Q91ZA3 | Propionyl-CoA carboxylase alpha chain, mitochondrial (PCCase subunit alpha) | - | |
| 47 | Zmym4 | A2A791 | Zinc finger MYM-type protein 4 | - | |
| 48 | Emilin3 | P59900 | EMILIN-3 | - | |
| 49 | Tbl1x | Q9QXE7 | Transducin (beta)-like 1 X-linked | - | |
| 50 | Bcat1 | P24288 | Branched-chain-amino-acid aminotransferase, cytosolic (BCAT(c)) | - | |
| 51 | Niban1 | Q3UW53 | Protein Niban 1 | - | |
| 52 | Ehmt2 | Q9Z148 | Histone-lysine N-methyltransferase EHMT2 | - | |
| 53 | Brd7 | O88665 | Bromodomain-containing protein 7 | - | |
| 54 | Taf15 | Q8BQ46 | TAF15 RNA polymerase II, TATA box binding protein (TBP)-associated factor | - | |
| 55 | Hspa4l | P48722 | Heat shock 70 kDa protein 4L | - | |
| 56 | Msi1 | Q61474 | RNA-binding protein Musashi homolog 1 (Musashi-1) | Retinal defects; defects in photoreceptor morphogenesis in mice | (Sundar et al. 2020) |
| 57 | Aldh5a1 | Q8BWF0 | Succinate-semialdehyde dehydrogenase, mitochondrial | - | |
| 58 | Strn | O55106 | Striatin | - | |
| 59 | Dido1 | Q8C9B9 | Death-inducer obliterator 1 (DIO-1) | - | |
| 60 | Ctcf | Q61164 | Transcriptional repressor CTCF | Retinal defects; loss of anterior retina in mice; involved in control of chromosome structure | (Watson et al. 2014) |
| 61 | Sptbn2 | Q68FG2 | Spectrin beta chain | - | |
| 62 | Rnmt | Q9D0L8 | mRNA cap guanine-N7 methyltransferase | - | |
| 63 | Cux1 | P53564 | Homeobox protein cut-like 1 | Controls RPGRIP1L, which is associated with loss of photoreceptors and is a modifier of retinal degeneration in cilipathies | (Khanna et al. 2009; Stratigopoulos et al. 2011) |
| 64 | Ephb2 | P54763 | EphB2/CTF2 | Retinal defects; loss of axons in retina/optic nerve in mice | (Fu and Sretavan 2012) |
| 65 | Golga5 | Q9QYE6 | Golgin subfamily A member 5 | - | |
| 66 | Golgb1 | E9PVZ8 | Golgi autoantigen, golgin subfamily b, macrogolgin 1 | - | |
| 67 | Ank2 | Q8C8R3 | Ankyrin-2 (ANK-2) | Retinal defects; defects in the Na/K-ATPase and Na/Ca exchanger in the inner segment of rod photoreceptors in mice | (Kizhatil et al. 2009b) |
| 68 | Snx6 | Q6P8X1 | Sorting nexin-6, N-terminally processed | - | |
| 69 | Srsf2 | Q62093 | Serine/arginine-rich splicing factor 2 | Upregulated in the vitreous in human cases of glaucoma; mutations in 5% patients with Uveal melanoma | (Mirzaei et al. 2017; Akin-Bali 2021) |
| 70 | Smarce1 | O54941 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 | - | |
| 71 | Hmgn1 | P18608 | Non-histone chromosomal protein HMG-14 | - | |
| 72 | Ptprf | A2A8L5 | Receptor-type tyrosine-protein phosphatase F | - | |
| 73 | Dnajc7 | Q9QYI3 | DnaJ homolog subfamily C member 7 | - | |
| 74 | Pnn | O35691 | Pinin | - | |
| 75 | Mccc2 | Q3ULD5 | Methylcrotonoyl-CoA carboxylase beta chain, mitochondrial (MCCase subunit beta) | - | |
| 76 | Nipbl | Q6KCD5 | Nipped-B-like protein | - | |
| 77 | Ank3 | G5E8K5 | Ankyrin-3 (ANK-3) | Retinal defects; transport of cyclic nucleotide-gated channels to the plasma membrane of rod outer segments in frog | (Kizhatil et al. 2009a) |
| 78 | Rbp1 | Q00915 | Retinol-binding protein 1 | Retinal defects; defects in organization of nascent outer segment membranes in frog | (Wang et al. 2010) |
| 79 | Yap1 | P46938 | Transcriptional coactivator YAP1 (Yes-associated protein 1) | Retinal defects; retinal pigment epithelium differentiation defects in mice | (Lu et al. 2020) |
| 80 | Smarcc1 | P97496 | SWI/SNF complex subunit SMARCC1 | - | |
| 81 | Utrn | E9Q6R7 | Utrophin | - | |
| 82 | Cald1 | E9QA15 | Caldesmon 1 | - | |
| 83 | Ccar1 | Q8CH18 | Cell division cycle and apoptosis regulator protein 1 | - | |
| 84 | Cttn | Q60598 | Src substrate cortactin | - | |
| 85 | Prrc2c | Q3TLH4 | Protein PRRC2C | - | |
| 86 | Arid1a | A2BH40 | AT-rich interactive domain-containing protein 1A (ARID domain-containing protein 1A) | - | |
| 87 | Cbx1 | P83917 | Chromobox protein homolog 1 | - | |
| 88 | Dcn | P28654 | Decorin | Retinal defects; retinal angiogenic disease including neuronal and vascular defects in mice | (Lim et al. 2018) |
| 89 | Mif | P34884 | Macrophage migration inhibitory factor (MIF) | Associated with proliferative retinopathy in mice | (Wang et al. 2017) |
| 90 | Igf2bp1 | O88477 | Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2 mRNA-binding protein 1; IMP-1) | Retinal defects; retinal ganglion axonal outgrowth and tectal coverage, and retinal cell survival defects in Zebrafish | (Gaynes et al. 2015) |
Fig. 4. In silico WB-subtraction identifies candidates with enriched expression in the mouse embryonic retina and retinal pigment epithelium combined tissue.
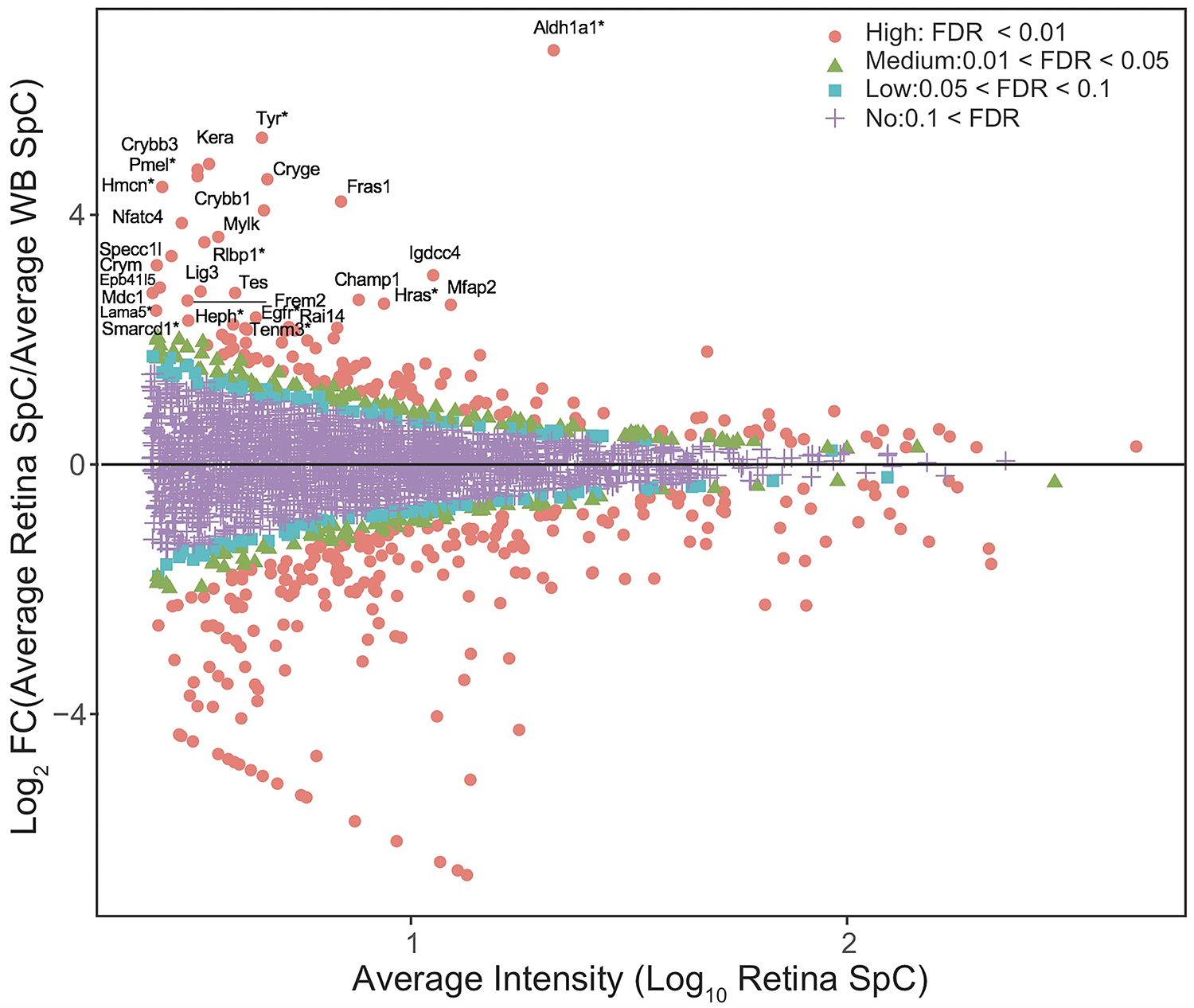
(A) Proteins with the average SpC ≥ 2.5 between retina and WB samples (n=2675) were further processed for identifying differentially expressed candidates. This analysis showed that 90 proteins were enriched in retina compared to WB ( ≥ 2.0 fold-change, FDR <0.01 cut-off). (B) MA plot (M = log ratio of retina to WB, A = average intensity) representation of differential protein expression profiling wherein the “high” (red, circle, FDR < 0.01), “medium” (green, triangle, 0.01 < FDR < 0.05), “low” probability retina-enriched (blue, square, 0.05 < FDR < 0.1) and non-enriched candidates (magenta, cross, 0.1 < FDR) are indicated. Several candidates associated with retinal defects (*) can be identified in this plot.
Fig. 5. Prioritization of candidates associated with retina defects by in silico WB-subtraction.
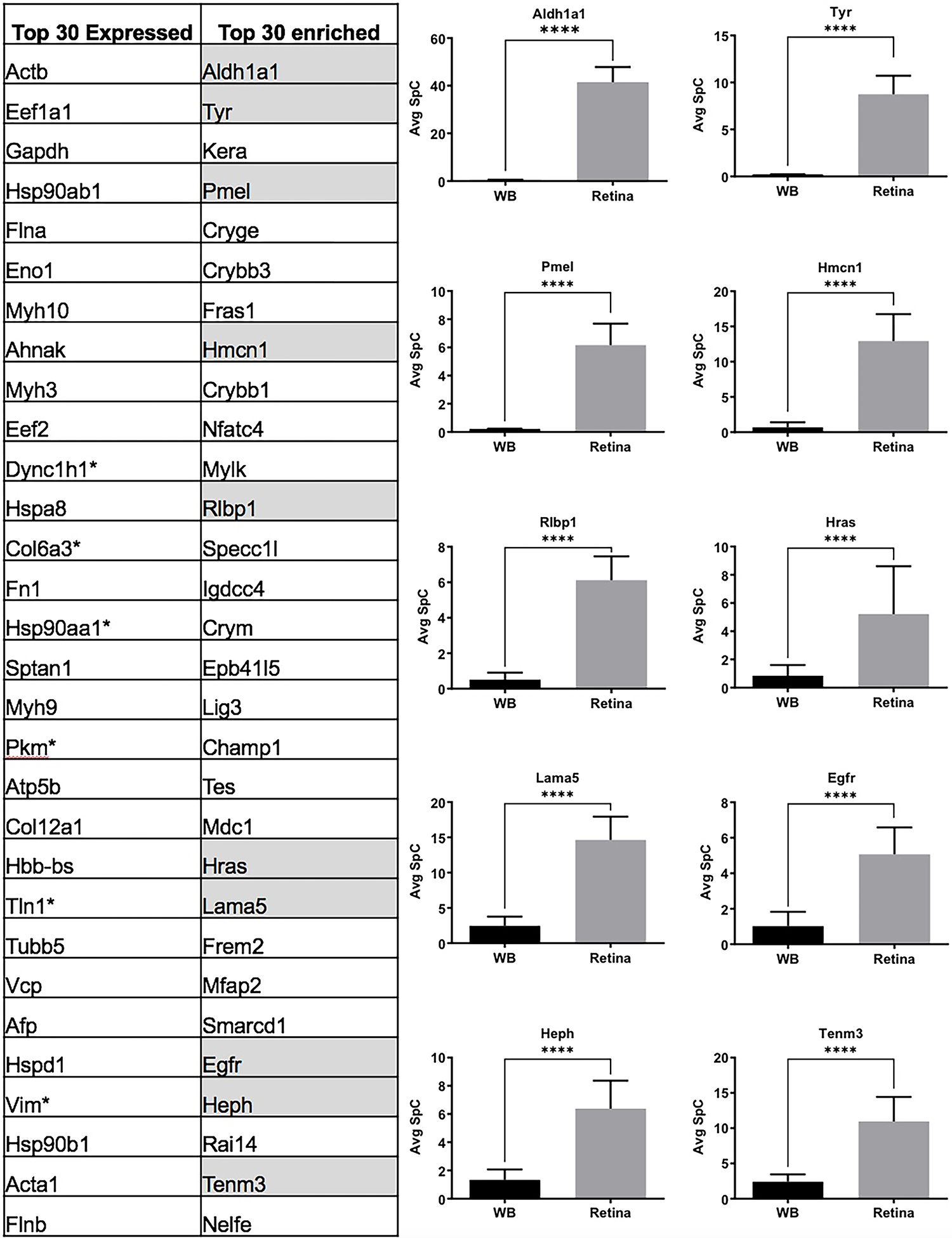
(A) Comparison of top 30 retina expressed proteins with the top 30 retina “enriched-expression” proteins shows that 1/3rd of the candidates in the “enriched-expression” category are associated with the retinal defects (highlighted in grey) and are not amongst the top proteins expressed in the retina. (B) Aldh1a1, Tyr, Pmel, Hmcn1, Rlbp1, Hras, Lama5, Egfr, Heph and Tenm3 associated with retinal defects that were not present in the top 30 “expressed” candidates show significant (p<0.001) enrichment in the retina compared to WB, demonstrating the effectiveness of the in silico WB-subtraction strategy in identifying these candidates from 2,675 expressed proteins. The average SpC of individual proteins are shown in y-axis.
Biological and disease relevance of top retina-enriched proteins
Next, we conducted a detailed analysis of all 90 retina-enriched candidates in the context of the published literature to determine their potential relevance to retina biology and defects. Application of this evidence-based curation identified 30 of the 90 (~33%) retina-enriched proteins prioritized by the in silico WB-subtraction strategy to be associated with retina biology and/or defects (Table 1). The topmost enriched gene Aldehyde dehydrogenase family 1, subfamily A1 (Aldh1a1; also known as Raldh1 (Retinal dehydrogenase 1)) is shown to regulate dorsal choroidal vasculature development via Sox9 upregulation in retinal pigmented epithelium in mice (Goto et al. 2018). The enriched-expression list also independently identified Tyrosinase (Tyr) protein which is essential for melanin biosynthesis and therefore critical for RPE (retinal pigment cells) and other retinal cells (Jeffery et al. 1994, 1997). Among the candidates is the premelanosome (Pmel) protein, whose deficiency in mice results in cell shape changes, e.g., the normally “oblong” shaped melanosomes turn spherical in RPE cells (Hellström et al. 2011). Further, whole exome sequencing of patients with early-onset age-related macular degeneration (AMD) revealed a single base deletion in another candidate with retina-enriched expression, the hemicentin-1 (HMCN1) gene (Pras et al. 2015).
Several proteins involved in signaling pathways were identified among the candidates with retina-enriched expression. Mutations in the candidate, Harvey rat sarcoma virus oncogene (Hras), are associated with retinal dystrophy in two patients with Costello syndrome (Pierpont et al. 2017). Another factor prioritized by in silico-WB subtraction is the epidermal growth factor receptor (Egfr), which is associated with retinal cell fate determination (Lillien 1995). Additionally, eph receptor B2 (Ephb2) was identified and its deletion in mouse is associated with axonal degeneration in retinal ganglion cell (Fu and Sretavan 2012). The receptor-type tyrosine-protein phosphatase F (Ptprf) is known to be expressed in retinal ganglion cells in mice (Lorber et al. 2005). Further, mutation in Fibulin-5 (FBLN5) is reported in human patients with AMD (Stone et al. 2004; Lotery et al. 2006). Morpholino-based reduction in the retinol-binding protein 1 (Rbp1) has been shown to result in misfolding of outer segments of retina in Xenopus (Wang et al. 2010). Recessive mutations in another top candidate, retinaldehyde-binding protein 1 (RLBP1), cause Retinitis punctatta albescens in humans (Morimura et al. 1999).
Some RNA-binding proteins (RBPs) involved in post-transcriptional gene expression control that are linked to retina development and differentiation were also among the prioritized proteins. Musashi homolog 1 (Msi1) is an RBP whose deficiency causes photoreceptor morphogenesis defect in mice (Sundar et al. 2020). Another RBP, the insulin-like growth factor 2 mRNA-binding protein 1 (Igf2bp1) is required for retinal ganglion cell axon outgrowth in zebrafish (Gaynes et al. 2015). Mex3 RNA-binding family member A (Mex3a) is expressed in the ciliary marginal zone in zebrafish (Naef et al. 2020). The mRNA cap guanine-N7 methyltransferase (Rnmt) is known to be expressed in Xenopus retina (Lokapally et al. 2016). Among other proteins with a regulatory function that were identified, deletion in mouse of the transcriptional coactivator Yes-associated protein 1 (Yap1) shows that Yap1 is essential for maintaining retinal pigmented epithelium differentiation (Lu et al. 2020).
Several proteins associated with the cell membrane and/or cytoskeleton were identified. Patients with mutation in the gap junction alpha-1 protein (Gja1) exhibit optic nerve and retinal dysplasia (Gabriel et al. 2011). The ankyrin proteins identified here, ankyrin-2 (Ank2) and ankyrin-3 (Ank3), have been shown to be essential for development of rod photoreceptors in mice (Kizhatil et al. 2009a, b). Knockout mice for another candidate, the cell adhesion molecule 1 (Cadm1), exhibited impaired response to light stimulation and for structural integrity of rod synapses (Ribic et al. 2014). Deficiency of the transmembrane protein, teneurin-3 (Tenm3) in zebrafish causes abnormal retinal ganglion cell morphology and the lack of Tenm3 in mice lead to defects in binocular visual coordination (Leamey et al. 2007; Antinucci et al. 2013).
Similarly, extracellular proteins as well as other cellular proteins with relevance to retinal biology were identified. Genetic variants in fibrillin-2 C-terminal peptide (FBN2) are reported to contribute to AMD (Ratnapriya et al. 2014). Cytoplasmic dynein 2 heavy chain 1 (Dync2h1) mutations in humans have been associated with non-syndromic inherited retinal disease (Vig et al. 2020). Deficiency in mice of the macrophage migration inhibitory factor (Mif) is associated with reduction in proliferation and inhibition of preretinal angiogenesis (Wang et al. 2017). Further, deficiency in mice of the small leucine-rich proteoglycan family protein Decorin (Dcn) results in structural and microvascular defects in retina (Lim et al. 2018). Finally, deficiency of rootletin (Crocc), recognized as a major component of the ciliary rootlet, is reported to cause retinal degeneration in mice (Yang et al. 2005).
Some genes that function in early retina development were also identified. For example, the transcriptional repressor CTCF (Ctcf) was among these candidates, and its deletion in mouse forebrain at E8.5 causes apoptosis and reduced retinal tissue by E13.5 (Watson et al. 2014). Live-cell fluorescence imaging has demonstrated that Ctcf, along with Cohesin, functions to control chromosome structure, including chromosome looping, potentially impacting long-range transcriptional regulation (Mach et al. 2022). Interestingly, while the chromatin modeler, SMARCA4 (BRG1), previously linked with Coffin-Siris syndrome (Kosho et al. 2014) and with retinal dystrophy in an individual human case (Cappuccio et al. 2019) was not found in the present study, several other related proteins from the SWI/SNF family were detected. These are Smarc1 (Baf57), Smarcd1 (Baf60a) and Smarcc1 (Baf155). It will be interesting to explore these proteins in the context of retina biology. Further, the transcription factor Cux1, identified in this study, is known to control the expression of the cilia-associated protein RPGRIP1L (retinitis pigmentosa GTPase regulator-interacting protein-1 like) (Stratigopoulos et al. 2011). Interestingly, mutations in RPGRIP1L are associated with Meckel-Gruber and Joubert syndromes and a variant in this gene is associated with loss of photoreceptors and is recognized as a modifier of retinal degeneration in humans (Khanna et al. 2009). Further, a point mutation identified in the mouse Laminin subunit alpha-5 (Lama5) showed defective retinal cup morphology as early as at E15.5 (Jones et al. 2020). Proteins involved in homeostasis were also identified in this study. For example, hephaestin (Heph) is reported to be essential for iron homeostasis in mice and its deficiency is associated with retinal degeneration (Hahn et al. 2004).
Several other proteins were identified that are independently reported to be expressed in retina, but their function has not been examined in detail. We identified several crystallins (Crybb1, Crybb3, Cryge, and Crym) that are known to be expressed in the retina and have been associated with retinal ganglion cell survival and regeneration (Xi et al. 2003; Piri et al. 2013). Interestingly, knockout mice for another crystallin prioritized in this study, alpha-crystallin A chain (Cryaa), exhibit retinal neovascularization defect (Xu et al. 2015). Similarly, a binding partner (NEDD8 ultimate buster 1 (NUB1)) of the retinal defect-associated protein, Aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1), identified here, is shown to be expressed in developing and adult human retina (Akey et al. 2002). The non-histone chromosomal protein, HMG-14 (Hmgn1), is expressed throughout retina in adult mouse (Lucey et al. 2008). The multi-functional serine and arginine-rich (SR) and desmosome associated protein Pinin (Pnn) is independently reported to be expressed photoreceptors of developing mouse retina (Leu and Ouyang 2006).
A few other proteins identified here have been reported to be associated with eye defects or disease, but their specific function in the retina has not been explored in detail. For example, FRAS1-related extracellular matrix protein 2 (Frem2) is associated with Cryptophthalmos (Yu et al. 2018). Interestingly, keratocan (Kera) deficiency in mice is associated with corneal defects, but its role in the retina has not been examined (Liu et al. 2003). Further, the splicing factor Srsf2 protein, independently identified in this study, has also been found to be upregulated in the vitreous in human cases of glaucoma (Mirzaei et al. 2017), and its mutations are observed in ~5% of patients with Uveal melanoma (Akin-Bali 2021). Finally, kinesin-like protein 1A (Kif1a) has been associated with optic nerve hyperplasia but its mechanistic role is not known in detail (Raffa et al. 2017).
Together, this documented association to retina biology of nearly 1/3rd of the top 90 proteins identified by in silico-WB subtraction, renders confidence that other candidates may also have key roles in the retina and may be linked to its defects.
Gene Ontology analysis of retina-enriched proteins
To gain insights into the relevance of the 90 candidates identified by in silico WB-subtraction to retina biology, a cluster-based analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID v6 .8) for functional annotation by gene ontology (GO) categories (Fig. 6) (Supplementary Table S4). This analysis assigned 90 retina enriched proteins into several annotation clusters. These are proteins involved in regulatory processes such as chromatin remodeling, e.g., “GO:0006338 chromatin remodeling,” “GO:0016569 covalent chromatin modification”, “GO:0071564 npBAF complex”, “GO:0016514 SWI/SNF complex”, “GO:0090544 BAF-type complex”, “GO:0071565 nBAF complex”, “GO:0006337 nucleosome disassembly”, “GO:0043044 ATP-dependent chromatin remodeling”, as well as those involved in signaling pathways, e.g., “GO:0043406 positive regulation of MAP kinase activity”. Proteins involved in basic cellular processes, e.g., “GO:0008283 cell proliferation”, “GO:0007155 cell adhesion” were also identified. Additionally, proteins involved in extracellular matrix were identified, e.g., “GO:0005604 basement membrane”, “GO:0005578 proteinaceous extracellular matrix”. Finally, proteins with roles in nervous system development were identified (Fig. 6) (Supplementary Table S4). Thus, this analysis identifies key candidates in specific processes relevant to retina biology, which can be functionally characterized in future studies.
Fig. 6. Gene ontology (GO) analysis of proteins with enriched expression in the E14.5 retina and retinal pigment epithelium combined tissue.
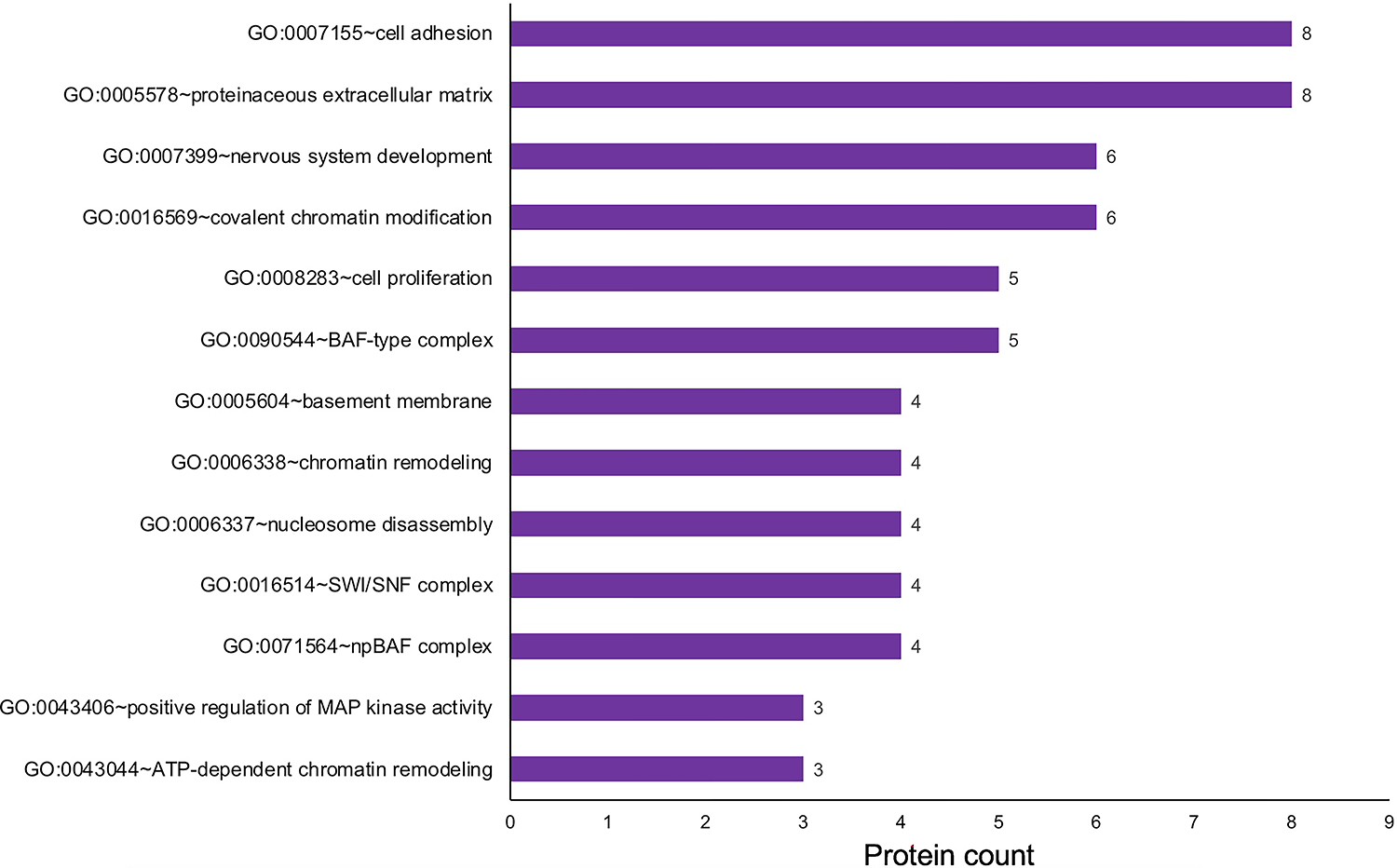
The 90 proteins identified to exhibit “enriched expression” in the mouse retina and retinal pigment epithelium combined tissue were analyzed by the Database for Annotation, Visualization and Integrated Discovery (DAVID v6 .8) for functional clustering and annotation based on gene ontology (GO) categories. This analysis identified candidates representing several GO terms that are relevant to retina biology, including “nervous system development”, “positive regulation of MAP kinase activity”, “chromatin remodeling” and “cell adhesion”. The x-axis represent the number of protein candidates identified in the specific GO term shown on the y-axis.
Visualization and access of retina-enriched and retina-expressed proteins in iSyTE
Next, we wanted to make this rich proteome information freely available to the research community. Thus, we developed new custom annotation-tracks on the UCSC Genome Browser that provide a heat-map representation of proteins based on their absolute expression or enriched-expression in the E14.5 mouse retina. These tracks are publicly accessible through the web-based resource-tool iSyTE (https://research.bioinformatics.udel.edu/iSyTE/). As examples, the enrichment and expression in the retina, of proteins previously linked to retina defects, e.g., Hras and Tyr, are shown as visualized in iSyTE (Fig. 7A, B). This web-based resource-tool will allow ready and user-friendly visualization of proteins in the E14.5 mouse retina.
Fig. 7. iSyTE allows ready visualization of proteins expressed or enriched-expressed in the retina and retinal pigment epithelium combined tissue.
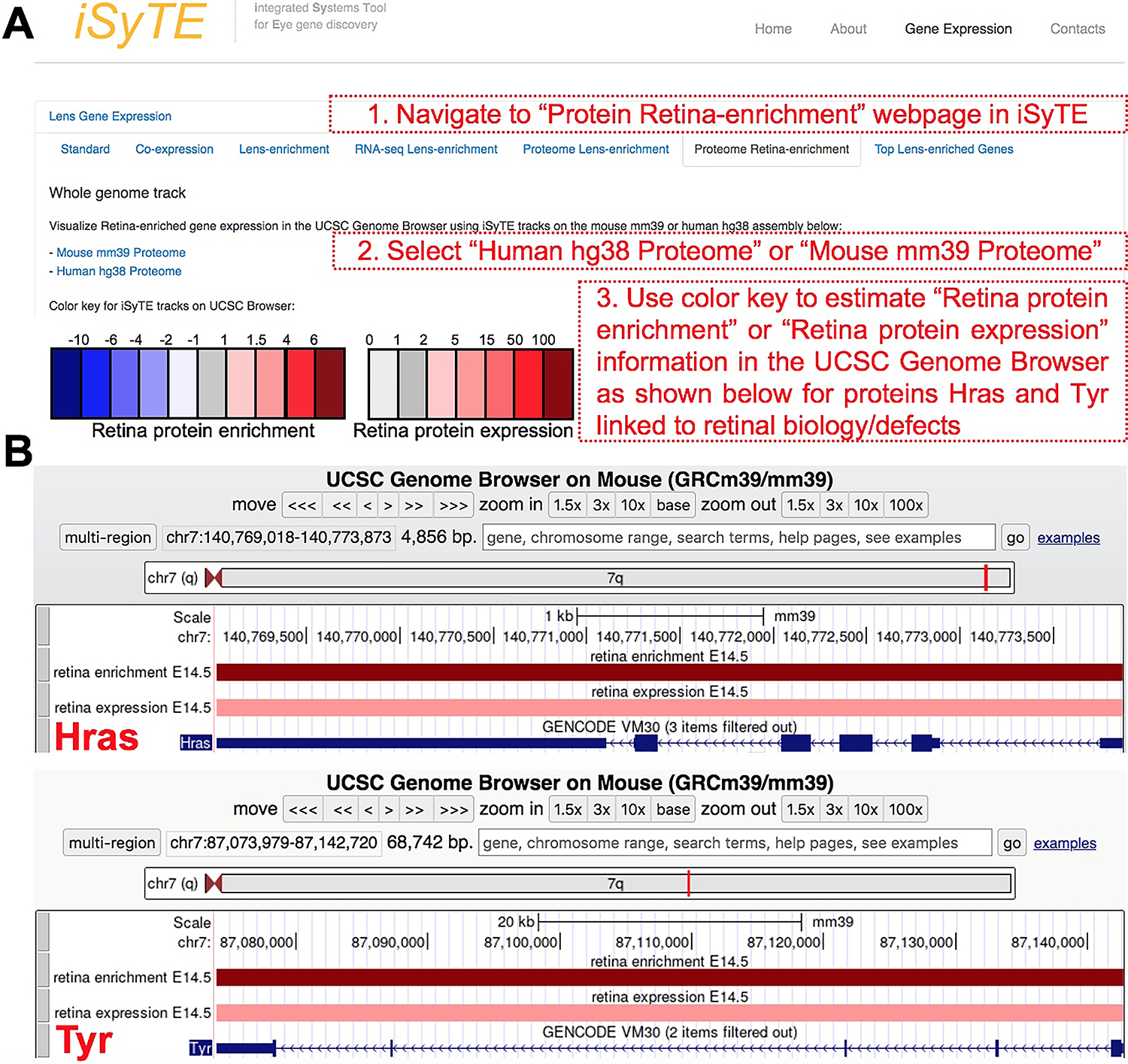
The retina protein expression and enriched-expression data can be visualized through the iSyTE web-resource tool at https://research.bioinformatics.udel.edu/iSyTE/. (A) On the iSyTE main webpage, navigate to “Proteome Retina-enrichment”, select the Human hg38 or Mouse mm39 assembly on the UCSC Genome Browser using iSyTE tracks, and input the protein candidate of interest to visualize its expression or enriched expression in the retina. The heat-map color key can be used to estimate the retina protein expression or enriched-expression. (B) As example, visualization of the expression and enriched expression of Hras and Tyr proteins in the retina are shown.
Conclusion
Recent studies have highlighted that post-transcriptional regulation of gene expression plays a key role in determining the cellular proteome in eye development. Therefore, it is important to include ocular proteome data to the existing RNA-based profiling datasets to gain new insights into eye development. As a proof-of-principal we previously generated proteomic profiles for the mouse lens and the embryonic whole body (WB) and effectively applied in silico WB-subtraction strategy to identify proteins with lens-enriched abundance, which – in addition to consideration of absolute expression scores – allows a prioritized list of proteins for further study (Aryal et al. 2020). In the present study, we expanded this approach to the mouse embryonic retina. We identified 90 proteins with retina-enriched expression. Nearly 1/3rd of these candidates have been previously reported to be associated with retinal defects. This suggests that in silico WB-subtraction was effective in prioritizing select candidates from over 2,600 identified proteins and of the top-prioritized 90 proteins, about 2/3rd represent an unexplored pool of candidates for future characterization of their function in the retina. Indeed, there exist independent evidence in the literature for several of these candidates to be expressed in the retina, in agreement with the proteome data reported in the present study. Further, in addition to these “retina-enriched” candidates, nearly 4,000 proteins were found to be present in the mouse E14.5 retina proteome. It should be noted that while many proteins linked to retina biology and pathology were identified in this study, transcription factors (TFs) such as Otx2, Sox2 and Vsx2 with key roles in the retina were not detected. This may be due to the following reasons. While they may be enriched in tissues, TFs are often in lower abundance compared to other expressed proteins (Tacheny et al. 2013). Furthermore, their levels are often spatiotemporally restricted in specific cells within the tissue, information that is compromised when using bulk tissue (as is the case in the present study). In the present study, we measured static protein relative abundances and did not attempt dynamic system measurements (e.g., those informing on protein turnover). Although 2675 quantifiable proteins (from the total 4680 proteins detected, which is generally considered a deep proteome) were identified in the present study, since the above mentioned TFs were not among these proteins, this suggests that more sensitive methods would be needed to detect these proteins in future studies. Together, these datasets and their ready accessibility through the web-based ocular gene discovery tool iSyTE represent a rich resource for prioritizing candidates for future hypothesis-driven studies in retina development. Finally, this study serves as a proof of the principle that in silico subtraction can also be applied to the retina and RPE to identify promising new candidates in these tissues. In the future, this approach will be expanded to prioritize candidates in other developmental stages of the retina.
Supplementary Material
Funding
This work was supported by National Institutes of Health [R01 EY021505 and R01 EY029770] to S.L. S.A. was supported by a Dissertation Fellows Award from the University of Delaware, a Fight For Sight Summer Research Fellowship and a Sigma Xi Grant-in-Aid Research Award. D.A. was supported by a Knights Templar Pediatric Ophthalmology Career Starter Grant Award. Support from the University of Delaware Proteomics and Mass Spectrometry Facility was made possible through funding from the State of Delaware and National Institutes of Health / National Institute of General Medical Sciences INBRE Program Grant [P20 GM103446]. Mass spectrometric analysis was performed by the OHSU Proteomics Shared Resource with partial support from NIH core grants [P30 EY010572 and P30 CA069533] and shared instrument grant [S10 OD012246].
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
Supplementary Material in the form of four tables is submitted along with the manuscript.
References
- Agrawal SA, Anand D, Siddam AD, et al. (2015) Compound mouse mutants of bZIP transcription factors Mafg and Mafk reveal a regulatory network of non-crystallin genes associated with cataract. Hum Genet 134:717–735. 10.1007/s00439-015-1554-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad MT, Zhang P, Dufresne C, et al. (2018) The Human Eye Proteome Project: Updates on an Emerging Proteome. Proteomics 18:e1700394. 10.1002/pmic.201700394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey DT, Zhu X, Dyer M, et al. (2002) The inherited blindness associated protein AIPL1 interacts with the cell cycle regulator protein NUB1. Hum Mol Genet 11:2723–2733. 10.1093/hmg/11.22.2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin-Bali DF (2021) Bioinformatics analysis of GNAQ, GNA11, BAP1, SF3B1,SRSF2, EIF1AX, PLCB4, and CYSLTR2 genes and their role in the pathogenesis of Uveal Melanoma. Ophthalmic Genet 42:732–743. 10.1080/13816810.2021.1961280 [DOI] [PubMed] [Google Scholar]
- Anand D, Al Saai S, Shrestha SK, et al. (2021) Genome-Wide Analysis of Differentially Expressed miRNAs and Their Associated Regulatory Networks in Lenses Deficient for the Congenital Cataract-Linked Tudor Domain Containing Protein TDRD7. Front Cell Dev Biol 9:615761. 10.3389/fcell.2021.615761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand D, Kakrana A, Siddam AD, et al. (2018) RNA sequencing-based transcriptomic profiles of embryonic lens development for cataract gene discovery. Hum Genet 137:941–954. 10.1007/s00439-018-1958-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand D, Lachke SA (2017) Systems biology of lens development: A paradigm for disease gene discovery in the eye. Exp Eye Res 156:22–33. 10.1016/j.exer.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinucci P, Nikolaou N, Meyer MP, Hindges R (2013) Teneurin-3 specifies morphological and functional connectivity of retinal ganglion cells in the vertebrate visual system. Cell Rep 5:582–592. 10.1016/j.celrep.2013.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal S, Anand D, Hernandez FG, et al. (2020a) MS/MS in silico subtraction-based proteomic profiling as an approach to facilitate disease gene discovery: application to lens development and cataract. Hum Genet 139:151–184. 10.1007/s00439-019-02095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal S, Viet J, Weatherbee BAT, et al. (2020b) The cataract-linked RNA-binding protein Celf1 post-transcriptionally controls the spatiotemporal expression of the key homeodomain transcription factors Pax6 and Prox1 in lens development. Hum Genet 139:1541–1554. 10.1007/s00439-020-02195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette DS, Anand D, So T, et al. (2016) Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 143:318–328. 10.1242/dev.127860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani M, Schreiber EM, Candiello J, et al. (2010) Molecular interactions in the retinal basement membrane system: a proteomic approach. Matrix Biol 29:471–483. 10.1016/j.matbio.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Barnum CE, Al Saai S, Patel SD, et al. (2020) The Tudor-domain protein TDRD7, mutated in congenital cataract, controls the heat shock protein HSPB1 (HSP27) and lens fiber cell morphology. Hum Mol Genet 29:2076–2097. 10.1093/hmg/ddaa096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. (2013) Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res 32:102–180. 10.1016/j.preteyeres.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinegar AE, Cooper TA (2016) Roles for RNA-binding proteins in development and disease. Brain Res 1647:1–8. 10.1016/j.brainres.2016.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccitelli C, Selbach M (2020) mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet 21:630–644. 10.1038/s41576-020-0258-4 [DOI] [PubMed] [Google Scholar]
- Cappuccio G, Brunetti-Pierri R, Torella A, et al. (2019) Retinal dystrophy in an individual carrying a de novo missense variant of SMARCA4. Mol Genet Genomic Med 7:e682. 10.1002/mgg3.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro GR, Matos-Rodrigues GE, Zhao Y, et al. (2017) N-myc regulates growth and fiber cell differentiation in lens development. Dev Biol 429:105–117. 10.1016/j.ydbio.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, et al. (1996) Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A 93:589–595. 10.1073/pnas.93.2.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, et al. (2012) A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30:918–920. 10.1038/nbt.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet H, Melles RB, Anand D, et al. (2021) A large multiethnic GWAS meta-analysis of cataract identifies new risk loci and sex-specific effects. Nat Commun 12:3595. 10.1038/s41467-021-23873-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BS, Stein-O’Brien GL, Shiau F, et al. (2019) Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 102:1111–1126.e5. 10.1016/j.neuron.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Brastrom LK, Patel SD, et al. (2020) The master transcription factor SOX2, mutated in anophthalmia/microphthalmia, is post-transcriptionally regulated by the conserved RNA-binding protein RBM24 in vertebrate eye development. Hum Mol Genet 29:591–604. 10.1093/hmg/ddz278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Dang CA, Beebe DC, Lachke SA (2015) Deficiency of the RNA binding protein caprin2 causes lens defects and features of peters anomaly. Dev Dyn 244:1313–1327. 10.1002/dvdy.24303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S, Siddam AD, Barnum CE, et al. (2016) RNA-binding proteins in eye development and disease: implication of conserved RNA granule components. Wiley Interdiscip Rev RNA 7:527–557. 10.1002/wrna.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G (2022) Towards a Better Vision of Retinoic Acid Signaling during Eye Development. Cells 11:322. 10.3390/cells11030322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JE, Gygi SP (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4:207–214. 10.1038/nmeth1019 [DOI] [PubMed] [Google Scholar]
- Eng JK, Jahan TA, Hoopmann MR (2013) Comet: an open-source MS/MS sequence database search tool. Proteomics 13:22–24. 10.1002/pmic.201200439 [DOI] [PubMed] [Google Scholar]
- Erde J, Loo RRO, Loo JA (2017) Improving Proteome Coverage and Sample Recovery with Enhanced FASP (eFASP) for Quantitative Proteomic Experiments. Methods Mol Biol 1550:11–18. 10.1007/978-1-4939-6747-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S-I, et al. (2003) Targeted Disruption of Aldh1a1 (Raldh1) Provides Evidence for a Complex Mechanism of Retinoic Acid Synthesis in the Developing Retina. Mol Cell Biol 23:4637–4648. 10.1128/MCB.23.13.4637-4648.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei SS, Wilmarth PA, Hitzemann RJ, et al. (2011) Protein database and quantitative analysis considerations when integrating genetics and proteomics to compare mouse strains. J Proteome Res 10:2905–2912. 10.1021/pr200133p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan S, Robson JL, Wylie M, et al. (2008) Protein expression profiling during chick retinal maturation: a proteomics-based approach. Proteome Sci 6:34. 10.1186/1477-5956-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CT, Sretavan D (2012) Involvement of EphB/Ephrin-B signaling in axonal survival in mouse experimental glaucoma. Invest Ophthalmol Vis Sci 53:76–84. 10.1167/iovs.11-8546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel LAR, Sachdeva R, Marcotty A, et al. (2011) Oculodentodigital dysplasia: new ocular findings and a novel connexin 43 mutation. Arch Ophthalmol 129:781–784. 10.1001/archophthalmol.2011.113 [DOI] [PubMed] [Google Scholar]
- Gaynes JA, Otsuna H, Campbell DS, et al. (2015) The RNA Binding Protein Igf2bp1 Is Required for Zebrafish RGC Axon Outgrowth In Vivo. PLoS ONE 10:e0134751. 10.1371/journal.pone.0134751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Schwarzl T, Valcárcel J, Hentze MW (2021) RNA-binding proteins in human genetic disease. Nat Rev Genet 22:185–198. 10.1038/s41576-020-00302-y [DOI] [PubMed] [Google Scholar]
- Goto S, Onishi A, Misaki K, et al. (2018) Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells. Elife 7:. 10.7554/eLife.32358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, et al. (2004) Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A 101:13850–13855. 10.1073/pnas.0405146101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström AR, Watt B, Fard SS, et al. (2011) Inactivation of Pmel alters melanosome shape but has only a subtle effect on visible pigmentation. PLoS Genet 7:e1002285. 10.1371/journal.pgen.1002285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Castello A, Schwarzl T, Preiss T (2018) A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 10.1038/nrm.2017.130 [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Jeffery G, Brem G, Montoliu L (1997) Correction of retinal abnormalities found in albinism by introduction of a functional tyrosinase gene in transgenic mice and rabbits. Brain Res Dev Brain Res 99:95–102. 10.1016/s0165-3806(96)00211-8 [DOI] [PubMed] [Google Scholar]
- Jeffery G, Schütz G, Montoliu L (1994) Correction of abnormal retinal pathways found with albinism by introduction of a functional tyrosinase gene in transgenic mice. Dev Biol 166:460–464. 10.1006/dbio.1994.1329 [DOI] [PubMed] [Google Scholar]
- Jones LK, Lam R, McKee KK, et al. (2020) A mutation affecting laminin alpha 5 polymerisation gives rise to a syndromic developmental disorder. Development 147:. 10.1242/dev.189183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakrana A, Yang A, Anand D, et al. (2018) iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res 46:D875–D885. 10.1093/nar/gkx837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaikina MV, Fomenko DE, Labunskyy VM, et al. (2011) Roles of the 15-kDa selenoprotein (Sep15) in redox homeostasis and cataract development revealed by the analysis of Sep 15 knockout mice. J Biol Chem 286:33203–33212. 10.1074/jbc.M111.259218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392. 10.1021/ac025747h [DOI] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, et al. (2009) A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 41:739–745. 10.1038/ng.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Baker SA, Arshavsky VY, Bennett V (2009a) Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 323:1614–1617. 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Sandhu NK, Peachey NS, Bennett V (2009b) Ankyrin-B is required for coordinated expression of beta-2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Exp Eye Res 88:57–64. 10.1016/j.exer.2008.09.022 [DOI] [PubMed] [Google Scholar]
- Kosho T, Miyake N, Carey JC (2014) Coffin-Siris syndrome and related disorders involving components of the BAF (mSWI/SNF) complex: historical review and recent advances using next generation sequencing. Am J Med Genet C Semin Med Genet 166C:241–251. 10.1002/ajmg.c.31415 [DOI] [PubMed] [Google Scholar]
- Krall M, Htun S, Anand D, et al. (2018) A zebrafish model of foxe3 deficiency demonstrates lens and eye defects with dysregulation of key genes involved in cataract formation in humans. Hum Genet 137:315–328. 10.1007/s00439-018-1884-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA (2022) RNA-binding proteins and post-transcriptional regulation in lens biology and cataract: Mediating spatiotemporal expression of key factors that control the cell cycle, transcription, cytoskeleton and transparency. Exp Eye Res 214:108889. 10.1016/j.exer.2021.108889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, et al. (2011) Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331:1571–1576. 10.1126/science.1195970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Higgins AW, Inagaki M, et al. (2012a) The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet 131:235–250. 10.1007/s00439-011-1064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Ho JWK, Kryukov GV, et al. (2012b) iSyTE: integrated Systems Tool for Eye gene discovery. Invest Ophthalmol Vis Sci 53:1617–1627. 10.1167/iovs.11-8839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Maas RL (2011) RNA Granules and Cataract. Expert Rev Ophthalmol 6:497–500. 10.1586/eop.11.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamey CA, Merlin S, Lattouf P, et al. (2007) Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS Biol 5:e241. 10.1371/journal.pbio.0050241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu S, Ouyang P (2006) Spatial and temporal expression profile of pinin during mouse development. Gene Expr Patterns 6:620–631. 10.1016/j.modgep.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Liang Q, Dharmat R, Owen L, et al. (2019) Single-nuclei RNA-seq on human retinal tissue provides improved transcriptome profiling. Nat Commun 10:5743. 10.1038/s41467-019-12917-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien L (1995) Changes in retinal cell fate induced by overexpression of EGF receptor. Nature 377:158–162. 10.1038/377158a0 [DOI] [PubMed] [Google Scholar]
- Lim RR, Gupta S, Grant DG, et al. (2018) Retinal Ultrastructural and Microvascular Defects in Decorin Deficient (Dcn −/−) Mice. Microscopy and Microanalysis 24:1264–1265. 10.1017/S1431927618006803 [DOI] [Google Scholar]
- Liu C-Y, Birk DE, Hassell JR, et al. (2003) Keratocan-deficient mice display alterations in corneal structure. J Biol Chem 278:21672–21677. 10.1074/jbc.M301169200 [DOI] [PubMed] [Google Scholar]
- Liu H, Sadygov RG, Yates JR (2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 76:4193–4201. 10.1021/ac0498563 [DOI] [PubMed] [Google Scholar]
- Liu Y, Beyer A, Aebersold R (2016) On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 165:535–550. 10.1016/j.cell.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Lokapally A, Metikala S, Hollemann T (2016) Expressional characterization of mRNA (guanine-7) methyltransferase (rnmt) during early development of Xenopus laevis. Int J Dev Biol 60:65–69. 10.1387/ijdb.150409th [DOI] [PubMed] [Google Scholar]
- Lorber B, Hendriks WJAJ, Van der Zee CEEM, et al. (2005) Effects of LAR and PTP-BL phosphatase deficiency on adult mouse retinal cells activated by lens injury. Eur J Neurosci 21:2375–2383. 10.1111/j.1460-9568.2005.04065.x [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Baas D, Ridley C, et al. (2006) Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat 27:568–574. 10.1002/humu.20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Scott PA, Vukmanic EV, et al. (2020) Yap1 is required for maintenance of adult RPE differentiation. FASEB J 34:6757–6768. 10.1096/fj.201903234R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey MM, Wang Y, Bustin M, Duncan MK (2008) Differential expression of the HMGN family of chromatin proteins during ocular development. Gene Expr Patterns 8:433–437. 10.1016/j.gep.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski SW, Lo CY, Sharov AA, et al. (2019) A single-cell transcriptome atlas of the adult human retina. The EMBO Journal 38:e100811. 10.15252/embj.2018100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach P, Kos PI, Zhan Y, et al. (2022) Cohesin and CTCF control the dynamics of chromosome folding. Nat Genet 54:1907–1918. 10.1038/s41588-022-01232-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T, Güell M, Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Letters 583:3966–3973. 10.1016/j.febslet.2009.10.036 [DOI] [PubMed] [Google Scholar]
- Manthey AL, Lachke SA, FitzGerald PG, et al. (2014) Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development. Mech Dev 131:86–110. 10.1016/j.mod.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalkah F, Jeong B, Sheridan M, et al. (2022) The Musashi proteins direct post-transcriptional control of protein expression and alternate exon splicing in vertebrate photoreceptors. Commun Biol 5:1–15. 10.1038/s42003-022-03990-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei M, Gupta VB, Chick JM, et al. (2017) Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci Rep 7:12685. 10.1038/s41598-017-12858-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami M, Kanamoto T, Souchelnytskyi N, Kiuchi Y (2008) Proteome profiling of embryo chick retina. Proteome Sci 6:3. 10.1186/1477-5956-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura H, Berson EL, Dryja TP (1999) Recessive mutations in the RLBP1 gene encoding cellular retinaldehyde-binding protein in a form of retinitis punctata albescens. Invest Ophthalmol Vis Sci 40:1000–1004 [PubMed] [Google Scholar]
- Naef V, De Sarlo M, Testa G, et al. (2020) The Stemness Gene Mex3A Is a Key Regulator of Neuroblast Proliferation During Neurogenesis. Front Cell Dev Biol 8:549533. 10.3389/fcell.2020.549533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Shichino Y, Iwasaki S, Shiina N (2020) Implications of RNG140 (caprin2)-mediated translational regulation in eye lens differentiation. J Biol Chem 295:15029–15044. 10.1074/jbc.RA120.012715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Aebersold R (2005) Interpretation of shotgun proteomic data: the protein inference problem. Mol Cell Proteomics 4:1419–1440. 10.1074/mcp.R500012-MCP200 [DOI] [PubMed] [Google Scholar]
- Padula SL, Anand D, Hoang TV, et al. (2019) High-throughput transcriptome analysis reveals that the loss of Pten activates a novel NKX6–1/RASGRP1 regulatory module to rescue microphthalmia caused by Fgfr2-deficient lenses. Hum Genet 138:1391–1407. 10.1007/s00439-019-02084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Anand D, Monies D, et al. (2017) Novel phenotypes and loci identified through clinical genomics approaches to pediatric cataract. Hum Genet 136:205–225. 10.1007/s00439-016-1747-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Anand D, Motohashi H, et al. (2022) Deficiency of the bZIP transcription factors Mafg and Mafk causes misexpression of genes in distinct pathways and results in lens embryonic developmental defects. Front Cell Dev Biol 10:981893. 10.3389/fcell.2022.981893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y, Bai J, Bandla C, et al. (2022) The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 50:D543–D552. 10.1093/nar/gkab1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Richards M, Engel WK, et al. (2017) Retinal dystrophy in two boys with Costello syndrome due to the HRAS p.Gly13Cys mutation. Am J Med Genet A 173:1342–1347. 10.1002/ajmg.a.38110 [DOI] [PubMed] [Google Scholar]
- Piri N, Kwong JMK, Caprioli J (2013) Crystallins in retinal ganglion cell survival and regeneration. Mol Neurobiol 48:819–828. 10.1007/s12035-013-8470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras E, Kristal D, Shoshany N, et al. (2015) Rare genetic variants in Tunisian Jewish patients suffering from age-related macular degeneration. J Med Genet 52:484–492. 10.1136/jmedgenet-2015-103130 [DOI] [PubMed] [Google Scholar]
- Raffa L, Matton M-P, Michaud J, et al. (2017) Optic nerve hypoplasia in a patient with a de novo KIF1A heterozygous mutation. Can J Ophthalmol 52:e169–e171. 10.1016/j.jcjo.2017.02.021 [DOI] [PubMed] [Google Scholar]
- Ratnapriya R, Sosina OA, Starostik MR, et al. (2019) Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat Genet 51:606–610. 10.1038/s41588-019-0351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapriya R, Zhan X, Fariss RN, et al. (2014) Rare and common variants in extracellular matrix gene Fibrillin 2 (FBN2) are associated with macular degeneration. Hum Mol Genet 23:5827–5837. 10.1093/hmg/ddu276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribic A, Liu X, Crair MC, Biederer T (2014) Structural organization and function of mouse photoreceptor ribbon synapses involve the immunoglobulin protein synaptic cell adhesion molecule 1. J Comp Neurol 522:900–920. 10.1002/cne.23452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A (2010) A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11:R25. 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Lu T, Zhang C, et al. (2020) Rbm24 controls poly(A) tail length and translation efficiency of crystallin mRNAs in the lens via cytoplasmic polyadenylation. Proc Natl Acad Sci USA 117:7245–7254. 10.1073/pnas.1917922117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddam AD, Duot M, Coomson SY, et al. (2023) High-Throughput Transcriptomics of Celf1 Conditional Knockout Lens Identifies Downstream Networks Linked to Cataract Pathology. Cells 12:1070. 10.3390/cells12071070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddam AD, Gautier-Courteille C, Perez-Campos L, et al. (2018) The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development. PLoS Genet 14:e1007278. 10.1371/journal.pgen.1007278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, et al. (2004) Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med 351:346–353. 10.1056/NEJMoa040833 [DOI] [PubMed] [Google Scholar]
- Stratigopoulos G, LeDuc CA, Cremona ML, et al. (2011) Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem 286:2155–2170. 10.1074/jbc.M110.188482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar J, Matalkah F, Jeong B, et al. (2020) The Musashi proteins MSI1 and MSI2 are required for photoreceptor morphogenesis and vision in mice. J Biol Chem 296:100048. 10.1074/jbc.RA120.015714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacheny A, Dieu M, Arnould T, Renard P (2013) Mass spectrometry-based identification of proteins interacting with nucleic acids. J Proteomics 94:89–109. 10.1016/j.jprot.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Vig A, Poulter JA, Ottaviani D, et al. (2020) DYNC2H1 hypomorphic or retina-predominant variants cause nonsyndromic retinal degeneration. Genet Med 22:2041–2051. 10.1038/s41436-020-0915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin J, Kaiser U, et al. (2017) Absence of macrophage migration inhibitory factor reduces proliferative retinopathy in a mouse model. Acta Diabetol 54:383–392. 10.1007/s00592-016-0956-8 [DOI] [PubMed] [Google Scholar]
- Wang X, Tong Y, Giorgianni F, et al. (2010) Cellular retinol binding protein 1 modulates photoreceptor outer segment folding in the isolated eye. Dev Neurobiol 70:623–635. 10.1002/dneu.20798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LA, Wang X, Elbert A, et al. (2014) Dual effect of CTCF loss on neuroprogenitor differentiation and survival. J Neurosci 34:2860–2870. 10.1523/JNEUROSCI.3769-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, David LL (2009) Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Infor 2:223–234. 10.1007/s12177-009-9042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Boneva S, Schlecht A, et al. (2022) The Human Eye Transcriptome Atlas: A searchable comparative transcriptome database for healthy and diseased human eye tissue. Genomics 114:110286. 10.1016/j.ygeno.2022.110286 [DOI] [PubMed] [Google Scholar]
- Wolf L, Harrison W, Huang J, et al. (2013) Histone posttranslational modifications and cell fate determination: lens induction requires the lysine acetyltransferases CBP and p300. Nucleic Acids Res 41:10199–10214. 10.1093/nar/gkt824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Farjo R, Yoshida S, et al. (2003) A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis 9:410–419 [PubMed] [Google Scholar]
- Xu Q, Bai Y, Huang L, et al. (2015) Knockout of αA-crystallin inhibits ocular neovascularization. Invest Ophthalmol Vis Sci 56:816–826. 10.1167/iovs.14-14734 [DOI] [PubMed] [Google Scholar]
- Yang J, Gao J, Adamian M, et al. (2005) The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol 25:4129–4137. 10.1128/MCB.25.10.4129-4137.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Lin B, Xie S, et al. (2018) A homozygous mutation p.Arg2167Trp in FREM2 causes isolated cryptophthalmos. Hum Mol Genet 27:2357–2366. 10.1093/hmg/ddy144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang D, Dongye M, et al. (2019) Loss-of-function mutations in FREM2 disrupt eye morphogenesis. Exp Eye Res 181:302–312. 10.1016/j.exer.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Zhao J, Izumi T, Nunomura K, et al. (2007) MARCKS-like protein, a membrane protein identified for its expression in developing neural retina, plays a role in regulating retinal cell proliferation. Biochem J 408:51–59. 10.1042/BJ20070826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


