Abstract
Objectives
To investigate the characteristics and prognostic value of fecal lactoferrin trajectories in ulcerative colitis (UC).
Methods
This study used data from the UNIFI trial (ClinicalTrials.gov, NCT02407236) and included patients who received ustekinumab during induction for trajectory modeling (n = 637). Patients who received ustekinumab during maintenance therapy were used for 1-year outcome analyses (n = 403). The levels of fecal lactoferrin, fecal calprotectin, and serum C-reactive protein were measured at weeks 0, 2, 4, and 8. The trajectories of these biomarkers were developed using a latent class growth mixed model.
Results
The trajectories of fecal lactoferrin, fecal calprotectin, and serum C-reactive protein were distinct, but all were associated with prior exposure to anti-tumor necrosis factor agents and vedolizumab. Furthermore, the fecal lactoferrin trajectory was the most valuable predictor of endoscopic, clinical, and histological remission. Compared to the high/moderate-rapid decrease trajectory group, the moderate-slow decrease, high-slow decrease, and high-stable groups had adjusted odds ratios (95% confidence interval) of 0.38 (0.18, 0.78; P = 0.010), 0.47 (0.23, 0.93; P = 0.032), and 0.33 (0.17, 0.63; P = 0.001), respectively, of 1-year endoscopic remission. Patients with high/moderate-rapid decrease trajectories also had the highest likelihood of achieving clinical and histological remission. Finally, we developed a patient-stratification scheme based on fecal lactoferrin trajectories and concentrations. Patients with good, moderate, and poor prognoses in the scheme had a distinct probability of achieving 1-year endoscopic remission (52.7%, 30.9%, and 12.8%, respectively).
Conclusions
The trajectory of fecal lactoferrin is a valuable prognostic factor for 1-year remission in UC.
Keywords: ulcerative colitis, fecal lactoferrin, trajectory
Introduction
Ulcerative colitis (UC) is a chronic and disabling inflammatory bowel disease that affects ∼0.2%–0.5% of the population in Europe and North America.1 Moreover, the incidence and prevalence rates of UC are rapidly increasing in newly industrialized countries.2 UC causes a huge and gradually increasing burden worldwide; however, the patient management strategies are far from optimal.
Predicting prognosis is one of the most important aspects of patient management. Inflammatory biomarkers, particularly fecal calprotectin (FC), fecal lactoferrin (FL), and serum C-reactive protein (CRP), have been shown to provide prognostic information in patients with UC.3, 4 For example, Dulai et al. revealed that the concentration of FC at the end of induction therapy could predict 1-year endoscopic and histological remission. FC is also associated with hospitalization and colectomy.5 Furthermore, FL, which is primarily secreted by neutrophils at the site of intestinal inflammation, could reflect disease severity in patients with UC.6 Our recent research has suggested that FL concentration could be an early predictor of long-term disease remission, as well as risk of colectomy in UC.7 Nevertheless, previous studies have focused on a single measurement of these inflammatory biomarkers, overlooking the prognostic value of longitudinal trajectories. Trajectories can capture the evolving patterns of biomarkers over time and hold great promise for predicting the prognosis of various diseases, such as colorectal cancer and hepatocellular carcinoma.8, 9 Therefore, we hypothesize that the trajectory of these inflammatory biomarkers is a critical prognostic factor in UC.
This study aimed to (1) demonstrate the trajectories of FL, FC, and CRP during induction therapy, (2) investigate the prognostic value of biomarker trajectories, and (3) ascertain whether these trajectories provide additional predictive value beyond a single biomarker measurement. We anticipate that the results of this study will assist clinicians in improving the prognosis of patients with UC.
Methods
Study population
This study used data from the UNIFI trial (ClinicalTrials.gov, NCT02407236).10 The UNIFI trial is a randomized, double-blind, placebo-controlled, phase-three trial that recruited adult patients with moderate-to-severe UC and aimed to assess the efficacy of ustekinumab.10 Other information regarding the UNIFI trial can be found in the original article published by Sands et al.10 Our study recruited patients who received ustekinumab during the induction phase of the UNIFI trial. Patients with fewer than two measurements of FL, FC, and CRP were excluded from this study. Eligible patients were included to develop trajectory models and assess the associations between trajectories and week-8 outcomes. Patients who entered the maintenance phase and received ustekinumab were utilized to assess the prognostic value of the biomarker trajectories for 1-year outcomes. Patients who did not undergo efficacy assessment at the end of the maintenance phase were excluded. Supplementary Fig. 1, see online supplementary material, presents a patient flow diagram.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The data for this study were obtained from the Yale University Open Data Access Project (No. 2022–5104), which is in agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C. Additional ethical approval and informed consent were not required for this study because the data were collected previously and presented anonymously.
Inflammatory biomarkers
Inflammatory biomarkers, including FL, FC, and CRP, were detected at weeks 0, 2, 4, and 8, if available. The lowest detectable levels of FL, FC, and CRP were 0.82 μg/ml, 30 μg/g, and 0.2 mg/l, respectively. If the concentrations of these biomarkers were lower than their lowest detectable level, we set the concentration to 0.82 μg/ml for FL, 30 μg/g for FC, and 0.2 mg/l for CRP. Owing to the skewed distribution of inflammatory biomarkers, we performed log-transformation of FL, FC, and CRP for trajectory modeling.
Covariates
In this study, we collected various covariates, including sex, age, disease duration, body mass index (BMI), smoking history, baseline concomitant medications, history of antitumor necrosis factor (TNF) agent exposure, history of vedolizumab exposure, baseline partial Mayo score, endoscopic Mayo score (EMS), and baseline concentrations of FL, FC, and CRP. For patients who failed to provide the exact date of disease diagnosis (e.g. only reported the year or year and month of diagnosis), we imputed the median date of the reported year (July 1) or month (15th day) to calculate the disease duration.
Outcomes
Week-8 and 1-year (at the end of the maintenance phase) outcomes were assessed. The primary outcomes were 1-year endoscopic remission. Endoscopic remission was defined as an EMS of zero. Secondary outcomes included histological and clinical remission at 1 year. Histological remission was defined as a highest Geboes score of < 2.0. Patients with a partial Mayo score ≤ 2 and all subscores ≤ 1 were considered to have clinical remission. Additional secondary outcomes included week-8 endoscopic response. Endoscopic response was defined as a reduction of ≥1 point in the EMS score from baseline. Patients who exited the clinical trial early were considered unable to achieve the desired outcome.
Statistical analyses
A latent class growth mixed model (LCGMM) was employed to develop the FL, FC, and CRP trajectories. LCGMM is a validated approach used to analyze longitudinal data and identify subgroups with distinct trajectories. It combines two powerful techniques: latent class analysis and growth mixture modelling. We developed the LCGMM using the lcmm package in R software.11 To determine the optimal trajectory, we performed LCGMM using a linear, quadratic, or cubic polynomial function with different class numbers ranging from 2 to 5.12 The optimal trajectory was selected based on (1) the lowest Bayesian information criterion, (2) a minimum of 5% of patients in each class, and (3) the posterior probability of assignments being >0.7 in each class.8, 12, 13 Ultimately, a quadratic function with three classes, a cubic function with five classes, and a cubic function with three classes fit the optimal trajectories of FL, FC, and CRP, respectively (supplementary Table 1, see online supplementary material).
The median [interquartile range (IQR)] and frequency (%) were used to describe continuous and categorical variables, respectively. Kruskal–Wallis and chi-square tests were performed to compare the differences in continuous and categorical variables, respectively, among the different trajectories. The correlation among trjactories of FL, FC, and CRP was calculated using Spearman correlation analysis. Statistical significance was set at P-value < 0.05. Associations between outcomes and trajectories were assessed using a multivariate logistic regression model after adjusting for potential confounders. Model 1 was adjusted for sex and age, while Model 2 was adjusted for variables from Model 1, as well as a history of anti-TNF exposure, history of vedolizumab exposure, baseline concomitant corticosteroid, baseline EMS, and treatment allocation. Subgroup analyses were performed among patients of different sex, age (<40 or ≥40 years old), disease duration (<18 or ≥18 months), and history of TNF exposure to explore whether there was any interaction. Moreover, we performed a receiver operating characteristic (ROC) curve analysis to evaluate the predictive ability of the biomarker trajectories for outcomes. The area under the ROC curve (AUC) and maximum Youden index were calculated.
We conducted a sensitivity analysis to demonstrate the consistency of our results. First, patients who withdrew prematurely from the study were excluded. Second, we further adjusted for disease duration, body mass index, and variables in Model 2.
All the statistical analyses were performed using R version 3.6.3.
Results
Baseline characteristics
A total of 637 patients were eligible for the trajectory model development (supplementary Fig. 1). As shown in Table 1, 384 (60.3%) patients were male, with a median age and disease duration of 30.0 (IQR: 30.0, 45.0) years and 6.01 (IQR: 2.78, 11.02) years, respectively. Regarding biological exposure, 345 (54.2%) patients had a history of exposure to anti-TNF agents, while 121 (19.0%) had exposure to vedolizumab. The median (IQR) baseline partial Mayo score and EMS were 6.00 (5.00, 7.00) and 3.00 (2.00, 3.00), respectively. For 1-year outcome analyses, 405 patients were included (supplementary Table 2, see online supplementary material); 240 (59.3%) were male, 204 (50.4%) had a history of anti-TNF exposure, and 64 (15.8%) had a history of vedolizumab exposure. The other baseline characteristics are described in supplementary Table 2.
Table 1.
Baseline characteristics.
| Variablea | N = 637 |
|---|---|
| Male | 384 (60.3%) |
| Age, years | 30.00 (30.00, 45.00) |
| Disease duration, years | 6.01 (2.78, 11.02) |
| BMI, kg/m2 | 24.40 (21.21, 27.55) |
| Smoking history | 208 (32.7%) |
| Concomitant medications | |
| Corticosteroids | 352 (55.3%) |
| 5-Aminosalicylic acid | 456 (71.6%) |
| Immunomodulators | 192 (30.1%) |
| History of anti-TNF exposure | 345 (54.2%) |
| History of vedolizumab exposure | 121 (19.0%) |
| Partial Mayo score | 6.00 (5.00, 7.00) |
| Endoscopic Mayo score | 3.00 (2.00, 3.00) |
| CRP, mg/l | 4.69 (1.62, 12.40) |
| FC, µg/g | 1486.00 (603.50, 2904.25) |
| FL, µg/ml | 202.79 (74.12, 443.82) |
aContinuous and categorical variables are presented as median (IQR) and frequency (%), respectively.
Characteristics of inflammatory biomarker trajectories
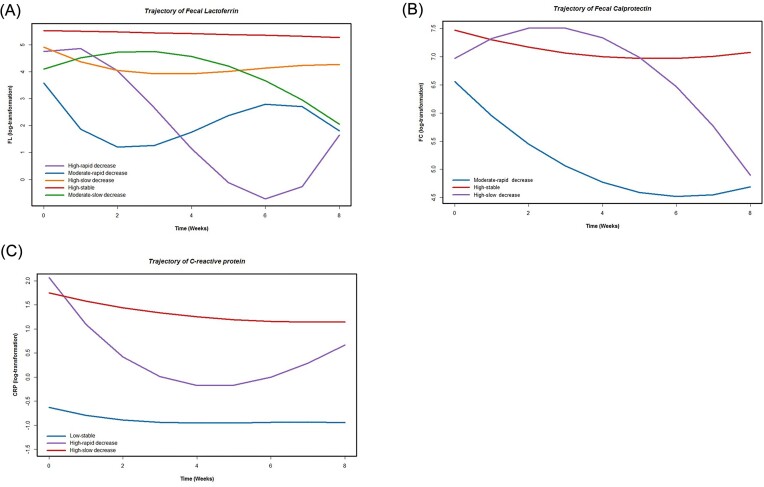
FL, FC, and CRP levels showed distinct trajectories during the induction therapy (Fig. 1). The biomarker trajectories were named based on their initial concentrations (low, moderate, and high) and variation tendencies (rapid decrease, slow decrease, and stable). The initial concentrations and variation speed of each biomarker trajectory are presented in supplementary Table 3, see online supplementary material.
Figure 1.
Trajectory of inflammatory biomarkers: (A) fecal lactoferrin; (B) fecal calprotectin; and (C) C-reactive protein.
There were five groups of FL trajectories: moderate-rapid decrease (n = 46), high-rapid decrease (n = 57), moderate-slow decrease (n = 103), high-slow decrease (n = 150), and high-stable (n = 259). In the rapid decrease group (moderate/high-rapid decrease), patients had a lower history of exposure to anti-TNF agents (P = 0.001) and vedolizumab (P < 0.001) when compared to the slow decrease and high-stable groups (supplementary Table 4, see online supplementary material). For FC, three trajectories were determined: moderate-rapid decrease (n = 124), high-slow decrease (n = 72), and high-stable (n = 435). Patients in the moderate-rapid decrease and high-slow decrease groups were less likely to have anti-TNF (P < 0.001) or vedolizumab (P < 0.001) exposure than those in the high-stable group (supplementary Table 5, see online supplementary material). For CRP, the three trajectories were labeled as slow-stable (n = 99), high-rapid decrease (n = 68), and high-slow decrease (n = 470). Patients with a slow-stable trajectory were younger, had a shorter disease duration, and had a lower proportion of anti-TNF and vedolizumab exposure (supplementary Table 6, see online supplementary material). Spearman correlation analyses showed that FL and FC trajectories (ρ = 0.161, P < 0.001), and FL and CRP trajectories (ρ < 0.001, P = 0.005) had weak correlations, while no significant correlation was observed between FC and CRP trajectories (ρ = 0.067, P = 0.132).
Moreover, we assessed the association between biomarker trajectories and endoscopic response at week 8. Moderate-slow decrease, high-slow decrease, and high-stable groups in FL had an adjusted odds ratio (OR) of 0.27 (95% confidence interval (CI): (0.14, 0.50); P < 0.001), 0.14 (95%CI: (0.07, 0.25); P < 0.001), and 0.12 (95%CI: (0.07, 0.22); P < 0.001), respectively, when compared to the rapid decrease group (Table 2). The moderate-rapid decrease group in FC had the largest proportion of patients (79.8%; n = 99) with week-8 endoscopic response, whereas the slow decrease (OR (95%CI): 0.27 (0.14, 0.53); P < 0.001) and high-stable groups (OR (95%CI): 0.12 (0.07, 0.20); P < 0.001) were less likely to achieve endoscopic response. According to the CRP trajectory, the high-slow decrease group (OR (95%CI): 0.53 (0.33, 0.86); P = 0.010), rather than the high-rapid decrease group (OR (95%CI): 1.16 (0.59, 2.29); P = 0.673), was significantly associated with a lower likelihood of an endoscopic response than the low-stability group (Table 2). Additionally, we found that combining FC and FL trajectories, as well as CRP concentration, had the highest AUC (0.7594 (95%CI: (0.7213, 0.7975)) for identifying endoscopic responses (supplementary Table 7, see online supplementary material).
Table 2.
The association between biomarker trajectories and week-8 endoscopic response.
| Univariate analysis | Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|---|
| n/N | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Trajectory of FL | |||||||
| Moderate/high-rapid decrease | 82/103 | Reference | − | Reference | − | Reference | − |
| Moderate-slow decrease | 57/103 | 0.32 (0.17, 0.58) | <0.001 | 0.32 (0.17, 0.58) | <0.001 | 0.27 (0.14, 0.50) | <0.001 |
| High-slow decrease | 60/149 | 0.17 (0.09, 0.30) | <0.001 | 0.18 (0.10, 0.31) | <0.001 | 0.14 (0.07, 0.25) | <0.001 |
| High-stable | 106/259 | 0.18 (0.10, 0.30) | <0.001 | 0.18 (0.10, 0.30) | <0.001 | 0.12 (0.07, 0.22) | <0.001 |
| Trajectory of FC | |||||||
| Moderate-rapid decrease | 99/124 | Reference | − | Reference | − | Reference | − |
| High-slow decrease | 40/72 | 0.32 (0.17, 0.60) | <0.001 | 0.31 (0.16, 0.59) | <0.001 | 0.27 (0.14, 0.53) | <0.001 |
| High-stable | 171/434 | 0.16 (0.10, 0.26) | <0.001 | 0.16 (0.10, 0.26) | <0.001 | 0.12 (0.07, 0.20) | <0.001 |
| Trajectory of CRP | |||||||
| Low-stable | 58/99 | Reference | − | Reference | − | Reference | - |
| High-rapid decrease | 45/68 | 1.38 (0.73, 2.65) | 0.322 | 1.40 (0.74, 2.69) | 0.306 | 1.16 (0.59, 2.29) | 0.673 |
| High-slow decrease | 212/469 | 0.58 (0.37, 0.90) | 0.016 | 0.59 (0.38, 0.92) | 0.021 | 0.53 (0.33, 0.86) | 0.010 |
Model 1 was adjusted for sex and age.
Model 2 was adjusted for variables from Model 1 as well as history of anti-TNF exposure, history of vedolizumab exposure, baseline concomitant corticosteroid, baseline EMS and treatment allocation.
Prognostic value of inflammatory biomarker trajectories
FL trajectory could predict endoscopic, histological, and clinical remission. For endoscopic remission, moderate-slow decrease, high-slow decrease, and high-stable had an OR (95%CI) of 0.38 (0.18, 0.78; P = 0.010), 0.47 (0.23, 0.93; P = 0.032), and 0.33 (0.17, 0.63; P = 0.001), respectively, when compared with the rapid decrease group (Table 3). For histological remission, moderate-slow decrease (OR (95%CI): 0.37 (0.15, 0.87); P = 0.005), high-slow decrease (OR (95%CI): 0.22 (0.08, 0.55); P = 0.001), and high-stable (OR (95%CI): 0.25 (0.11, 0.55); P = 0.001) were all less likely to achieve the 1-year outcome (Table 3). Furthermore, the rapid decrease trajectory group had the highest likelihood for 1-year clinical remission, followed by the moderate-slow decrease (OR (95%CI): 0.40 (0.16, 0.96); P = 0.046), high-slow decrease (OR (95%CI): 0.35 (0.14, 0.82); P = 0.020), and high-stable (OR (95%CI): 0.23 (0.10, 0.50); P < 0.001) trajectory groups (Table 3). The subgroup analyses showed no significant interaction between FL trajectories and sex, age, disease duration, or anti-TNF exposure (supplementary Tables 8–11, see online supplementary material). Furthermore, we performed sensitivity analyses and found consistent results (supplementary Tables 12 and 13, see online supplementary material). However, FC and CRP trajectories were not associated with endoscopic or clinical remission (Table 4). We further assessed the predictive ability of the biomarkers and biomarker trajectories for all 1-year outcomes. The results showed that the FL trajectory had the largest AUCs for predicting endoscopic (0.7054, 95%CI: (0.6516, 0.7592)), histological (0.7442, 95%CI: (0.678, 0.8103)), and clinical (0.7188, 95%CI: (0.6661,0.7715)) remission among all the biomarkers and biomarker trajectories (supplementary Tables 14–16, see online supplementary material).
Table 3.
The prognostic value of inflammatory biomarker trajectories for 1-year remission.
| Univariate analysis | Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|---|
| n/N | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Endoscopic remission | |||||||
| Trajectory of FL | |||||||
| Moderate/high-rapid decrease | 36/65 | Reference | − | Reference | − | Reference | − |
| Moderate-slow decrease | 22/72 | 0.35 (0.17, 0.71) | 0.004 | 0.36 (0.18, 0.73) | 0.005 | 0.38 (0.18, 0.78) | 0.010 |
| High-slow decrease | 31/91 | 0.42 (0.21, 0.80) | 0.009 | 0.46 (0.23, 0.88) | 0.020 | 0.47 (0.23, 0.93) | 0.032 |
| High-stable | 40/163 | 0.26 (0.14, 0.48) | <0.001 | 0.26 (0.14, 0.47) | <0.001 | 0.33 (0.17, 0.63) | 0.001 |
| Trajectory of FC | |||||||
| Moderate-rapid decrease | 32/73 | Reference | − | Reference | − | Reference | - |
| High-slow decrease | 18/48 | 0.77 (0.36, 1.61) | 0.489 | 0.77 (0.36, 1.62) | 0.488 | 0.87 (0.39, 1.92) | 0.728 |
| High-stable | 82/278 | 0.54 (0.32, 0.91) | 0.021 | 0.54 (0.32, 0.92) | 0.022 | 0.68 (0.38, 1.22) | 0.189 |
| Trajectory of CRP | |||||||
| Low-stable | 25/59 | Reference | − | Reference | − | Reference | − |
| High-rapid decrease | 23/53 | 1.04 (0.49, 2.21) | 0.913 | 1.13 (0.53, 2.42) | 0.751 | 1.28 (0.58, 2.87) | 0.541 |
| High-slow decrease | 84/291 | 0.55 (0.31, 0.99) | 0.043 | 0.59 (0.33, 1.07) | 0.077 | 0.71 (0.38, 1.35) | 0.289 |
| Histological remission | |||||||
| Trajectory of FL | |||||||
| Moderate/high-rapid decrease | 24/63 | Reference | − | Reference | − | Reference | − |
| Moderate-slow decrease | 12/67 | 0.35 (0.15, 0.78) | 0.012 | 0.35 (0.15, 0.79) | 0.012 | 0.37 (0.15, 0.87) | 0.025 |
| High-slow decrease | 9/83 | 0.20 (0.08, 0.45) | <0.001 | 0.22 (0.09, 0.50) | 0.001 | 0.22 (0.08, 0.55) | 0.001 |
| High-stable | 18/153 | 0.22 (0.11, 0.44) | <0.001 | 0.21 (0.10, 0.43) | <0.001 | 0.25 (0.11, 0.55) | 0.001 |
| Trajectory of FC | |||||||
| Moderate-rapid decrease | 24/69 | Reference | − | Reference | − | Reference | − |
| High-slow decrease | 8/45 | 0.41 (0.15, 0.98) | 0.052 | 0.38 (0.14, 0.93) | 0.041 | 0.38 (0.13, 0.98) | 0.053 |
| High-stable | 32/260 | 0.26 (0.14, 0.49) | <0.001 | 0.26 (0.14, 0.49) | <0.001 | 0.29 (0.14, 0.57) | <0.001 |
| Trajectory of CRP | |||||||
| Low-stable | 17/57 | Reference | − | Reference | − | Reference | − |
| High-rapid decrease | 6/50 | 0.32 (0.11, 0.86) | 0.030 | 0.34 (0.11, 0.91) | 0.039 | 0.32 (0.10, 0.91) | 0.039 |
| High-slow decrease | 41/270 | 0.42 (0.22, 0.83) | 0.010 | 0.45 (0.23, 0.88) | 0.018 | 0.48 (0.24, 1.02) | 0.050 |
| Clinical remission | |||||||
| Trajectory of FL | |||||||
| Moderate/high-rapid decrease | 57/66 | Reference | − | Reference | − | Reference | − |
| Moderate-slow decrease | 50/72 | 0.36 (0.14, 0.83) | 0.020 | 0.35 (0.14, 0.82) | 0.019 | 0.40 (0.16, 0.96) | 0.046 |
| High-slow decrease | 62/91 | 0.34 (0.14, 0.75) | 0.010 | 0.34 (0.14, 0.75) | 0.011 | 0.35 (0.14, 0.82) | 0.020 |
| High-stable | 90/162 | 0.20 (0.09, 0.41) | <0.001 | 0.20 (0.09, 0.41) | <0.001 | 0.23 (0.10, 0.50) | <0.001 |
| Trajectory of FC | |||||||
| Moderate-rapid decrease | 55/74 | Reference | − | Reference | − | Reference | - |
| High-slow decrease | 32/48 | 0.69 (0.31, 1.54) | 0.362 | 0.68 (0.30, 1.51) | 0.334 | 0.84 (0.36, 1.97) | 0.687 |
| High-stable | 180/277 | 0.64 (0.35, 1.12) | 0.131 | 0.64 (0.35, 1.13) | 0.133 | 0.80 (0.42, 1.48) | 0.481 |
| Trajectory of CRP | |||||||
| Low-stable | 46/60 | Reference | − | Reference | − | Reference | − |
| High-rapid decrease | 37/54 | 0.66 (0.29, 1.51) | 0.330 | 0.66 (0.28, 1.52) | 0.329 | 0.68 (0.28, 1.65) | 0.402 |
| High-slow decrease | 185/289 | 0.54 (0.28, 1.01) | 0.062 | 0.54 (0.27, 1.01) | 0.062 | 0.62 (0.30, 1.22) | 0.177 |
Model 1 was adjusted for sex and age.
Model 2 was adjusted for variables from Model 1 as well as history of anti-TNF exposure, history of vedolizumab exposure, baseline concomitant corticosteroid, baseline EMS and treatment allocation.
Patient stratification based on FL and its trajectory
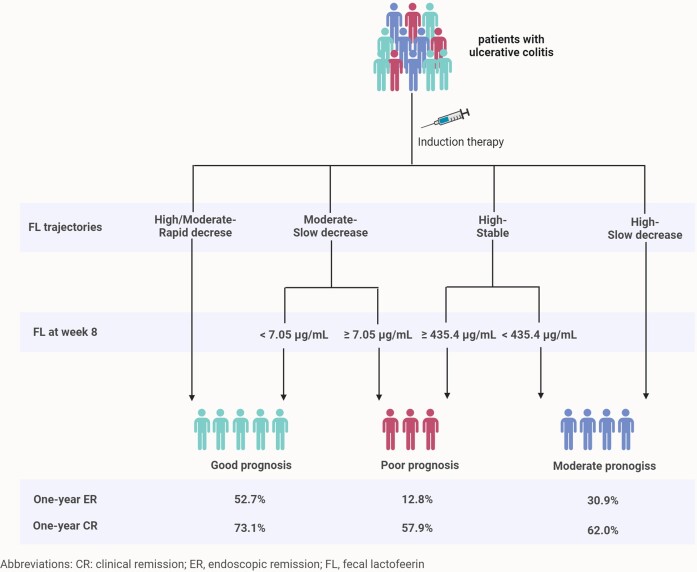
We further explored whether FL trajectories could add value to a single FL measurement (at week eight) for stratifying patients with distinct prognoses. First, we assessed the prognostic value of week-8 FL in each FL trajectory group. We performed ROC analysis to determine the optimal cut-off value for FL and assessed its association with 1-year endoscopic remission. The results showed that FL < 7.05 µg/ml in the moderate-slow decrease trajectory (OR (95%CI): 8.13 (1.76, 50.62); P = 0.013) was more likely to achieve endoscopic remission, while FL < 435.40 µg/ml in the high-stable group (OR (95%CI): 3.37 (1.11, 12.89); P = 0.047) indicated better outcome (supplementary Table 17, see online supplementary material). Second, we developed a scheme for patient stratification based on FL trajectory and week-8 FL level (Fig. 2). Patients were divided into three groups: good (rapid decrease trajectory or moderate-slow decrease trajectory plus week-8 FL < 7.05 µg/ml), moderate (high-slow decrease trajectory or high-stable trajectory plus week-8 FL < 435.40 µg/ml), and poor (moderate-slow decrease trajectory plus FL ≥ 7.05 µg/ml or high-stable trajectory plus week-8 FL ≥ 435.40 µg/ml) prognosis. Patients with good, moderate, and poor prognoses had distinct probabilities of achieving 1-year endoscopic (52.7%, 30.9%, and 12.8%, respectively) and clinical (73.1%, 62.0%, and 57.9%, respectively) remission (Fig. 2; supplementary Table 18, see online supplementary material).
Figure 2.
Scheme for patient stratification based on FL trajectory and week-8 FL level.
Discussion
We performed a post hoc analysis of the UNIFI trial to demonstrate the trajectory of the three most widely used biomarkers and assessed their predictive ability for 1-year outcomes. We discovered that FC, FL, and CRP levels had distinct trajectories during induction therapy with ustekinumab, and their trajectory characteristics were associated with a history of exposure to anti-TNF agents and vedolizumab. Among these biomarker trajectories, the FL trajectory was the most valuable for predicting clinical, endoscopic, and histological remission. Furthermore, a simple approach based on the FL trajectory and FL concentration could stratify patients with different prognoses. These findings highlight the importance of trajectories in predicting prognosis and provide new insights for clinicians to utilize biomarker trajectories in patient stratification and management.
To the best of our knowledge, this is the first study to investigate inflammatory biomarker trajectories in UC. Distinct trajectories were observed in FL, FC, and CRP; however, they had some common features. For instance, trajectories with rapidly decreasing patterns were more frequently observed in biologic-naïve patients, whereas slow-decreasing or highly stable patterns were correlated with a history of exposure to anti-TNF agents. Ustekinumab has been shown to have unsatisfactory efficacy in patients already treated with anti-TNF agents, especially in those who do not respond to these agents, compared with anti-TNF-naïve patients.14, 15 The efficacy of ustekinumab is first manifested by the degree and speed of inflammation control, which could be reflected in the trajectories of inflammatory biomarkers. Therefore, it is unsurprising that biomarker trajectories are associated with a history of biological exposure. Moreover, our study showed that the FC and FL trajectories were related to the week-8 endoscopic response. The percentage of patients with endoscopic response in the rapid decrease, slow decrease, and highly stable groups was ∼80%, 50%, and 40%, respectively. As FC and FL have been demonstrated to be useful for assessing endoscopic activity in UC,16 we further assessed the predictive value of biomarkers and biomarker trajectories for identifying endoscopic response. We found that the combination of FC, FL trajectories, and CRP concentration was the most accurate for identifying the endoscopic response, with an AUC of 0.7594. Although more high-quality research is needed to validate this finding, it provides an alternative tool for clinicians to evaluate patient response status and may reduce the need for invasive endoscopic procedures in patients with UC.
Importantly, the FL trajectory could predict 1-year remission. Previous studies have demonstrated that FL is a prognostic factor for UC. Frin et al. recruited 31 UC patients who received infliximab and found that the FL level at week 14 could predict sustained response at week 52.17 Moreover, Gisbert et al. conducted a multicenter prospective cohort study and revealed that a positive FL test was a risk factor for relapse in patients with clinical remission.18 Similar results were also found in other studies.19, 20 Recently, we performed another post hoc analysis based on the data from the UNIFI trial and revealed that FL concentration could be an early predictor of long-term disease remission and risk of colectomy in UC.7 However, it is unclear whether the trajectory of FL can predict therapeutic outcomes, especially endoscopic remission, which has been recommended as the most important long-term therapeutic target in UC.21 Our study fills the gap in this field. In our study, the rapid decrease trajectory of FL suggested the highest likelihood of 1-year remission, followed by the slow decrease trajectory, whereas the highly stable trajectory indicated the worst therapeutic outcomes. Additionally, we discovered that patients with distinct FL trajectories (e.g. high-rapid decrease and moderate-slow decrease) had different prognoses but similar FL concentrations at week 8. This phenomenon highlights the need to consider the overall trend and process of FL changes rather than relying solely on FL concentration at a single time to predict further therapeutic outcomes accurately.
We further investigated whether combining the FL trajectory and single FL measurements could better predict the prognosis and could be applied to patient stratification. FL at week 8 was shown to differentiate patients with different prognoses in groups with a moderate-slow decrease and high-stable trajectories. Therefore, we developed a scheme for patient stratification based on FL trajectory and concentration. In this scheme, patients identified as having good, moderate, and poor prognosis have likelihoods of 52.7%, 30.9%, and 12.8% of achieving 1-year endoscopic remission. This patient-stratification scheme has several advantages. First, it provides high accuracy and clear stratification. Second, the assessment process is simple and does not require complex mathematical equations or scoring systems. Third, because only one biomarker is measured, it is relatively cost-effective and non-invasive. These features render this scheme promising for use in clinical practice.
Our study has some limitations. First, it included patients with moderate-to-severe UC. Therefore, the findings of our study cannot be applied to patients with mild or severe acute UC. Second, our study could not investigate the association between biomarker trajectories and colectomy because of the lack of long-term follow-up data. However, we assessed the association between biomarker trajectories and endoscopic remission, which are associated with the risk of colectomy and are recommended as the principal long-term treatment targets in UC.21 Third, this study only included patients who received ustekinumab, instead of other biologics, such as infliximab, adalimumab, and vedolizumab. Thus, whether the present study findings could be applied to UC patinets using other biologics is still unclear and necessitates more evidence. Finally, we did not perform an external validation of the patient stratification scheme. Therefore, the validity and generalizability of our scheme require further research. Soon, we plan to perform a real-world cohort study to validate the predictive ability of our patient stratification scheme.
In conclusion, this study described the characteristics of biomarker trajectories during induction therapy and found that the FL could predict 1-year remission in patients with UC. Furthermore, a scheme based on FL trajectories and concentrations can better stratify patients. However, further studies are required to validate these findings.
Supplementary Material
Acknowledgement
This study was supported by the National Natural Science Foundation of China (Grant No. 82000520) and the China Crohn's & Colitis Foundation (Grant No. CCCF-QF-2022B36-7). This study, carried out under YODA Project #2022-5104, used data obtained from the Yale University Open Data Access Project, which is in agreement with the JANSSEN RESEARCH & DEVELOPMENT, L.L.C. The interpretation and reporting of research using these data are solely the responsibility of the authors and do not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C. We thank the Biorender (https://biorender.com/) for assisting with the figure drawing.
Contributor Information
Rirong Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, China.
Li Li, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, China.
Yizhe Tie, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, China.
Minhu Chen, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, China.
Shenghong Zhang, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, China.
Author contributions
S.Z. and M.C.: conceptualization; funding acquisition; writing—review and editing; supervision. R.C.: data curation; formal analysis; methodology; writing—original draft preparation; project administration. L.L.: formal analysis; methodology; writing—original draft preparation; project administration. Y.T.: supervision; methodology.
Conflict of interest
All authors declare that there is no conflict of interest.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet North Am Ed. 2017;390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2. Zhang S, Chen B, Wang B, et al. . Effect of induction therapy with Olamkicept vs placebo on clinical response in patients with active ulcerative colitis: A randomized clinical trial. JAMA. 2023;329(9):725–34. doi: 10.1001/jama.2023.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu D, Saikam V, Skrada KA, et al. . Inflammatory bowel disease biomarkers. Med Res Rev. 2022;42(5):1856–87. doi: 10.1002/med.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ungaro R, Colombel JF, Lissoos T, et al. . A treat-to-target update in ulcerative colitis: A systematic review. Am J Gastroenterol. 2019;114(6):874–83. doi: 10.14309/ajg.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulai PS, Feagan BG, Sands BE, et al. . Prognostic value of fecal calprotectin to inform treat-to-target monitoring in ulcerative colitis. Clin Gastroenterol Hepatol. 2023;21(2):456–66. doi: 10.1016/j.cgh.2022.07.027. [DOI] [PubMed] [Google Scholar]
- 6. Sienkiewicz M, Jaskiewicz A, Tarasiuk A, et al. . Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit Rev Food Sci Nutr. 2022;62(22):6016–33. doi: 10.1080/10408398.2021.1895063. [DOI] [PubMed] [Google Scholar]
- 7. Chen R, Tie Y, Zhang X, et al. . Fecal lactoferrin early predicts long-term outcomes in ulcerative colitis: A post-hoc analysis of the UNIFI and PURSUIT trials. UEG Journal. 2023;11(6):542–50. doi: 10.1002/ueg2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu L, Shen L, Wu Z, et al. . Trajectories of serum alpha-fetoprotein and intermediate-stage hepatocellular carcinoma outcomes after transarterial chemoembolization: A longitudinal, retrospective, multicentre, cohort study. EClinicalMedicine. 2022;47:101391. doi: 10.1016/j.eclinm.2022.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li C, Zhang D, Pang X, et al. . Trajectories of perioperative serum tumor markers and colorectal cancer outcomes: A retrospective, multicenter longitudinal cohort study. EBioMedicine. 2021;74:103706. doi: 10.1016/j.ebiom.2021.103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sands BE, Sandborn WJ, Panaccione R, et al. . Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- 11. Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: The R Package lcmm. J Stat Soft. 2017;78(2):1–56. doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- 12. Lennon H, Kelly S, Sperrin M, et al. . Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8(7):e020683. doi: 10.1136/bmjopen-2017-020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirza SS, Wolters FJ, Swanson SA, et al. . 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. Lancet Psychiatry. 2016;3(7):628–35. doi: 10.1016/S2215-0366(16)00097-3. [DOI] [PubMed] [Google Scholar]
- 14. Gisbert JP, Parody-Rua E, Chaparro M. Efficacy, effectiveness, and safety of Ustekinumab for the treatment of ulcerative colitis: A systematic review. Inflamm Bowel Dis. 2023. doi: 10.1093/ibd/izac275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh S, George J, Boland BS, et al. . Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis. J Crohns Colitis. 2018;12(6):635–43. doi: 10.1093/ecco-jcc/jjy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mosli MH, Zou G, Garg SK, et al. . C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–19.; quiz 820. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 17. Frin AC, Filippi J, Boschetti G, et al. . Accuracies of fecal calprotectin, lactoferrin, M2-pyruvate kinase, neopterin and zonulin to predict the response to infliximab in ulcerative colitis. Dig Liver Dis. 2017;49(1):11–6. doi: 10.1016/j.dld.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 18. Gisbert JP, Bermejo F, Perez-Calle JL, et al. . Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15(8):1190–8. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto T, Shimoyama T, Umegae S, et al. . Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol. 2018;9(3):e136. doi: 10.1038/s41424-018-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto T, Shiraki M, Bamba T, et al. . Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int J Colorectal Dis. 2014;29(4):485–91. doi: 10.1007/s00384-013-1817-3. [DOI] [PubMed] [Google Scholar]
- 21. Turner D, Ricciuto A, Lewis A, et al. . STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.