Summary
Rett syndrome (RTT) is a rare genetic neurological disorder that primarily affects girls and is caused by mainly mutations in the methyl-CpG-binding protein 2 (MECP2) gene, leading to critical issues in normal brain function. The condition has a global prevalence of 5 to 10 cases per 100,000 females, and there is currently no cure for RTT. However, therapy is available to manage the symptoms and improve quality of life. Trofinetide, an insulin-like growth factor 1, was originally developed as a stroke medication and progressed to Phase II clinical trials, where it exhibited favorable safety and efficacy profiles by improving several core RTT symptoms. Recently, Trofinetide received the US Food and Drug Administration (FDA) approval and orphan drug designation for the treatment of RTT, making it the first approved drug for this rare genetic disorder. It has also shown to be safe, well-tolerated and with no known drug interactions. These findings suggest that Trofinetide is a promising treatment option for individuals with RTT.
Keywords: Rett syndrome, RTT, MECP2 gene, Trofinetide
1. Introduction
Rett syndrome (RTT) is a rare genetic neurological disorder that primarily affects girls and is mainly caused by mutations in the methyl-CpG-binding protein 2 (MECP2) gene. The MECP2 gene leads to synthesis of a protein i.e., MECP2, which is critical for normal brain function (1). MECP2 gene has different functional domains i.e., N-terminal domain (NTD), methyl binding domain (MBD), intervening domain (ID), transcription repression (TRD) and C-terminal. RTT develops due to various types of mutations i.e., missense, nonsense, frame shift, deletions and others. Structurally, mutations can involve NTD, MBD, other parts of gene. The mutations in different locations give rise to variable phenotypes with varying severity (1).
RTT starts developing between 6-18 months of age following apparently normal development in initial life. In the early onset or stagnation stage, the child may experience a slowing of head growth, delays in motor milestones, and loss of muscle tone (2). The rapid destructive stage is characterized by increased irritability, crying, decreased interest in social interaction, and repetitive hand movements (2). The plateau or pseudo-stationary stage may last for several years and is marked by stabilization of behavior and slight improvement in some skills. In the late motor deterioration stage, the child may experience a decline in motor function, including loss of mobility, scoliosis, and muscle weakness (3). Furthermore, many patients do not present with all the symptoms of RTT and termed as atypical or variant RTT. Important variants of RTT are Hanefeld, Zappella, and Rolando and each variant is characterized by specific phenotypic properties (4). Other common symptoms of RTT include seizures, sleep disturbances, digestive problems, and problems with heart and lung function. Around two-thirds of patients with RTT claimed to have associated epilepsy. The most common seizures encountered in RTT are complex partial and generalized tonic‐clonic seizures (4). The patients present with early onset of seizures in Hanefeld variant which is characterized by normal MECP2 gene expression. The concomitant presence of epilepsy makes the RTT worse. The electroencephalogram (EEG) is almost always abnormal in beyond age three in RTT, however abnormal EEG is not an indication for anti-seizure drugs per se (5).
The prevalence of RTT remains 5 to 10 cases per 100,000 females globally without regional variability (6). There is no cure for RTT, but treatment includes physical, occupational, and speech therapy, as well as behavioral therapy and medication to manage seizures and other symptoms (7). Currently multimodal treatment approaches for management of RTT are symptomatic and supportive. It includes physical therapy to maintain mobility, balance, weight-bearing in scoliosis. Occupational therapy aims to improve/maintain adequate use of hands, reduce stereotypic movements and help in day to day activities. Furthermore, speech and language therapy help to maintain social interactions. The patients also require feeding and physical assistance. The drugs are mainly useful in controlling seizures, constipation, and arrhythmias (7). Even before the identification of MECP gene mutations, several trials have been conducted. The opioid antagonist, naltrexone was assessed as a treatment for periodic breathing. Another study revealed that intravenous naloxone caused slowing of EEG. When the MECP mutations were identified, a study was conducted with folate-betaine as a potential treatment. Though the parent reported scoring was better with folate-betaine, no concrete evidence was made (8).
Research into potential treatments for RTT is ongoing, and scientists are exploring a variety of approaches, including gene therapy, stem cell therapy, and drugs that target specific aspects of the disease. Gene therapy involves delivering a functional copy of the MECP2 gene to affected cells. Stem cell therapy aims to repair or replace damaged cells in the brain. Drugs that target specific aspects of the disease include compounds that increase MeCP2 protein levels or target pathways that are disrupted in RTT (9). Presently there is no definitive management of RTT hence, there is a dire need of drug(s) which can address the underlying genetic cause of the disorder.
Trofinetide which is an analog of insulin-like growth factor-1 (IGF1) was originally developed as a stroke medication and progressed to Phase II clinical trials, where it exhibited favorable safety and efficacy profile by showing improvement in several core RTT symptoms at its highest dose (10). Trofinetide, under the brand name DaybueTM, has been given approval by the US Food and Drug Administration (FDA) as a treatment for RTT in patients aged two years and above (11).
2. Mechanism of action
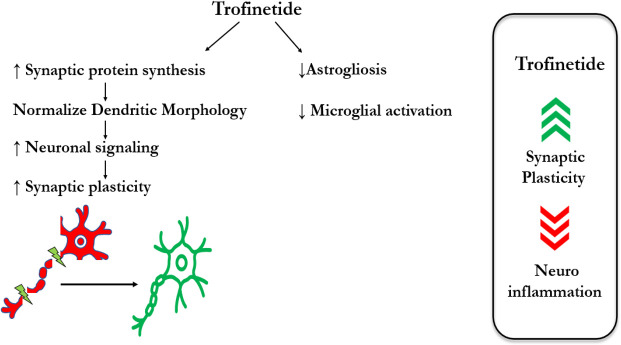
Trofinetide, a synthetic peptide being explored as a potential treatment for RTT, is believed to have multiple effects on the brain, although its mechanism of action is not yet fully understood. One potential mechanism of action is the promotion of synaptic maturation and function. The development and function of synapses, including their ability to change in strength and number in response to experience, are critical roles played by IGF-1. Studies have revealed that Trofinetide can increase the expression of genes that contribute to synaptic function and enhance synaptic plasticity in animal models of RTT (12,13). This is significant as neuroinflammation is believed to play a role in the pathophysiology of RTT and other neurodevelopmental disorders. The drug exerts its effects by normalizing abnormal neuronal and glial function, resulting in anti-inflammatory and trophic effects. It achieves this by inhibiting astrogliosis and pathologic microglial activation, which are the primary contributors to brain inflammation and neuronal damage in RTT. Trofinetide also normalizes synaptic protein synthesis, dendritic morphology, and neuronal signaling, all of which are vital for proper neuronal function. Additionally, it enhances the antioxidant response, thus protecting neurons from oxidative stress-induced damage, which is a common feature of neurodegenerative disorders, including RTT (14,15). By reducing the levels of pro-inflammatory cytokines in the brain, Trofinetide helps reduce neuroinflammation, which may protect neurons from damage (15) (Figure 1).
Figure 1.
Proposed mechanism of action of Trofinetide.
3. Pharmacokinetics
Trofinetide is administered orally and the time taken to achieve maximum drug concentration (Tmax) is about ≅3 hours. The apparent volume of distribution and half-life of Trofinetide is 80L and ≅1.5 hours respectively. About 80% of the administered drug gets excreted unchanged by the renal route and, to some extent, by feces. Trofinetide is not recommended in patients with moderate or severe renal impairment (16).
4. Clinical trials
In an exploratory double-blind, placebo-controlled Phase II clinical trial, the safety, and efficacy of Trofinetide in adolescent and adult females with RTT were evaluated (17). The study enrolled 56 patients who were randomly assigned to receive either Trofinetide or placebo and divided into three cohorts, i.e., 35mg/kg of Trofinetide twice daily for 14 days or placebo, 35 mg/kg Trofinetide twice daily for 28 days of placebo, and 70 mg/kg of Trofinetide twice daily for 28 days or placebo. The study's primary objective was to assess the efficacy, safety, and tolerability of Trofinetide in patients with RTT, and a p-value < 0.2 was considered indicative of efficacy. Out of three cohorts, the cohort with Trofinetide (70 mg/kg) showed significant improvement in three core parameters, i.e., clinician-completed CGI-I, MBA change index, and caregiver-completed top-3 concerns. The results indicated that the 35 mg/kg and 70 mg/kg dose levels of Trofinetide were generally safe and well-tolerated, with no serious adverse events observed. The estimates are presented in Table 1. The study's conclusion suggests that Trofinetide is a safe and potentially effective treatment option for RTT patients (17).
Table 1. Salient features of clinical trials of Trofinetide.
| Trial description (Ref.) | Age group, years | Comparators | Efficacy | Safety |
|---|---|---|---|---|
| Glaze DJ, et al. (17) | 15.9–44.2 | Trofinetide 70 mg/kg and 35 mg/kg twice daily or placebo | MBA change index: -2.01 vs. -0.62 (p = 0.146) CGI-I score: 3.24 vs. 3.64 (p = 0.164) Caregiver Top 3 Concerns total VAS score: -62.59 vs. -23.71 (p = 0.076) In this trial p <0.2 was considered significant. |
• The most common encountered TEAE was diarrhoea (39% -35 mg/kg group vs. 15% - placebo group), irritability (22% -35 mg/kg group vs. 15% - placebo group), and somnolence (17% -70 mg/kg group vs. 5% - placebo group) • Most AEs were mild or moderate in intensity and most events were considered not related to study drug SAE: Three subjects experienced serious adverse events, 2 subjects in the 35 mg/kg cohort and 1 subject in the 70 mg/kg cohort. All SAEs were resolved by the end of the study. |
| Glaze DJ, et al (18) | 5–15 | Trofinetide 200 mg/kg twice daily vs. placebo. | RSBQ total score: -6.7 vs. -2.3; p = 0.042 CGI-I: 3 vs. 3.5; p = 0.029) RTT-DSC: -76 vs. 25.85; p = 0.025) |
• The tolerability of Trofinetide was very good at all 3 dose levels • Diarrhoea: 200 mg (56%); 100 mg (13%), 50 mg (27%), placebo (4%) • Vomiting: 200 mg (22%); 100 mg (13%), 50 mg (7%), placebo (13%) • Upper respiratory tract infection: 200 mg (19%); 100 mg (0%), 50 mg (7%), placebo (13%) • Pyrexia: 200mg (11%); 100mg (19%), 50mg (0%), placebo (8%) SAE: Four SAEs occurred in 3 participants. All the SAEs were deemed not related to study medication and resolved by the end of the study. |
| Neul LJ, et al (19) | 5–20 | Trofinetide oral solution (1 g Trofinetide per 5 mL, calculated as per the weight) vs. placebo | RSBQ: -4.9 vs.-1.7; p = 0.018 CGI-I: 3.5 vs. 3.8; p = 0.003 CSBS-DP-IT Social total score: -0.1 vs.-1.1; p = 0.006 |
• Common treatment-emergent adverse events included diarrhea 80.6% for Trofinetide vs. 19.1% for placebo and vomiting 26.88% for Trofinetide vs. 9.57% for placebo • All the TEAE were of mild to moderate in severity SAE: 1 case of Bacteremia, bronchiolitis, covid-19 pneumonia, urinary tract infection, seizure was reported. |
RSBQ: Rett Syndrome Behavior Questionnaire; CGI-I: Clinical Global Impression Scale-Improvement; RTT-DSC: RTT-Clinician Domain Specific Concerns; MBA: Motor Behavior Assessment scale; CSBS-DP-IT: Social Communication and Symbolic Behavior Scales Developmental Profile Infant-Toddler Checklist - Social Composite Score; TEAE: Treatment Emergent Adverse Event.
Another double-blind, placebo-controlled, parallel-group trial evaluated the safety, tolerability, pharmacokinetics, and efficacy of Trofinetide in female children and adolescents with RTT (18). A total of 82 participants aged 5-15 years received a placebo twice daily for 14 days, followed by either placebo or one of three doses of Trofinetide (50, 100, or 200 mg/kg) twice daily for 42 days. The important efficacy endpoints were the Rett Syndrome Behavior Questionnaire (RSBQ), the Clinical Global Impression of Improvement (CGI-I), and the Rett Syndrome Domain Specific Concerns Questionnaire (RTT-DSC). Trofinetide was safe and well-tolerated at all three doses, with no deaths occurring during the study. Four serious adverse events (SAEs) occurred in three participants, with only one participant receiving Trofinetide, and all SAEs were considered unrelated to the study medication and resolved by the end of the study. The 200 mg/kg bid dose improved core neurobehavioral RTT symptoms, overall clinical status, and the most concerning aspects of RTT identified by clinicians. Details are presented in Table 1. Trofinetide also improved clinically significant symptom areas core to RTT, including the RTT-DSC domains of ambulation and seizures. These results prove that Trofinetide is safe, well-tolerated, and effective in improving RTT symptoms in children and adolescents (18).
In a pivotal phase III 12-week, randomized, double-blind, placebo-controlled clinical trial LAVENDER study, 187 girls and young women with RTT were included. In this study, Trofinetide demonstrated statistically significant and clinically meaningful results over placebo and was effective and safe in treating RTT. Details are presented in Table 1. The findings from this trial have significant implications for the medical community. They could lead to the approval of Trofinetide as a treatment for RTT, addressing an unmet medical need for individuals with this debilitating disorder (19). The efficacy and safety endpoints of the various clinical trials of Trofinetide have been depicted in Table 1.
5. Place of Trofinetide in therapeutics and conclusion
RTT is a progressive neurodevelopmental disorder that affects females, and there is no specific therapy for RTT; managing associated conditions and addressing psychosocial function, including that of the family and caregivers, can improve the quality of life of RTT patients (20). A multidisciplinary approach is crucial to address nutritional, gastrointestinal, and motor problems, as well as nonepileptic behaviors and seizures.
Trofinetide is the first drug approved for this rare genetic disorder. It is approved for both adults and pediatric patients aged > 2 years. Trofinetide should be administered twice daily as per the weight band, i.e., 5,000 mg (9-12 kg), 6,000 mg (12-20 kg), 8,000 mg (20-35kg), 10,000 mg (35-50 kg),12,000 mg (≥ 50 kg). Trofinetide has been generally well-tolerated in clinical trials. Gastrointestinal symptoms, i.e., diarrhea and vomiting, are the most common adverse events associated with Trofinetide (17-19). Trofinetide should be discontinued in case of severe diarrhea. Trofinetide can also cause weight loss and should be stopped in case of significant weight loss. Other common adverse events were irritability, somnolence, and pyrexia. These symptoms usually occur early in treatment and can be managed with dose adjustments or symptomatic treatment. To the best of the available evidence, there are minimal drug-drug interactions that are likely to increase acceptability.
Various trials showed that Trofinetide demonstrated statistically significant clinical improvement in symptom areas core to RTT, and all the trials included patients with confirmed MeCP2 gene mutation. Presently it is challenging to comment upon the efficacy of Trofinetide on various types of mutations giving rise to RTT. Furthermore, the trials have not presented the results per the different mutations. The exact place of Trofinetide managing RTT is awaited as the drug was recently approved. Future real-world studies will only tell us about the exact effectiveness and safety of Trofinetide. These findings suggest that Trofinetide may be an effective and safe treatment option for individuals with RTT. However, careful monitoring and individualized treatment plans are necessary to ensure the best possible outcomes.
Funding
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Good KV, Vincent JB, Ausió J. MeCP2: The genetic driver of Rett syndrome epigenetics. Front Genet. 2021; 12:620859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983; 14:471-479. [DOI] [PubMed] [Google Scholar]
- 3. Mount RH, Charman T, Hastings RP, Reilly S, Cass H. The Rett Syndrome Behaviour Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J Child Psychol Psychiatry. 2002; 43:1099-1110. [DOI] [PubMed] [Google Scholar]
- 4. Operto FF, Mazza R, Pastorino GMG, Verrotti A, Coppola G. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019; 9:e01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarquinio DC, Hou W, Berg A, Kaufmann WE, Lane JB, Skinner SA, Motil KJ, Neul JL, Percy AK, Glaze DG. Longitudinal course of epilepsy in Rett syndrome and related disorders. Brain. 2017; 140:306-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petriti U, Dudman DC, Scosyrev E, Lopez-Leon S. Global prevalence of Rett syndrome: Systematic review and meta-analysis. Syst Rev. 2023; 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institutes of Health. Rett Syndrome. https://www.nichd.nih.gov/health/topics/rett (accessed June 28, 2023).
- 8. Percy AK. Progress in Rett syndrome: From discovery to clinical trials. Wien Med Wochenschr. 2016; 166:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannaj AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome- -Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008; 27:3342-3350. [DOI] [PubMed] [Google Scholar]
- 10. Gogliotti RG, Niswender CM. A coordinated attack: Rett syndrome therapeutic development. Trends Pharmacol Sci. 2019; 40:233-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris E. Trofinetide receives FDA approval as first drug for Rett syndrome. JAMA. 2023; 329:1142. [DOI] [PubMed] [Google Scholar]
- 12. Castro J, Mellios N, Sur M. Mechanisms and therapeutic challenges in autism spectrum disorders: Insights from Rett syndrome. Curr Opin Neurol. 2013; 26:154-159. [DOI] [PubMed] [Google Scholar]
- 13. Cartagena CM, Phillips KL, Williams GL, Konopko M, Tortella FC, Dave JR, Schmid KE. Mechanism of action for NNZ-2566 anti-inflammatory effects following PBBI involves upregulation of immunomodulator ATF3. Neuromolecular Med. 2013; 15:504-514. [DOI] [PubMed] [Google Scholar]
- 14. Wei HH, Lu XC, Shear DA, Waghray A, Yao C, Tortella FC, Dave JR. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J Neuroinflammation. 2009; 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012; 484:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Highlights of prescribing information. DAYBUE™ (trofinetide) oral solution. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217026s000lbl.pdf (accessed July 12, 2023).
- 17. Glaze DG, Neul JL, Percy A, Feyma T, Beisang A, Yaroshinsky A, Stoms G, Zuchero D, Horrigan J, Glass L, Jone NE. A double-blind, randomized, placebo-controlled clinical study of Trofinetide in the treatment of Rett syndrome. Pediatr Neurol. 2017; 76:37-46. [DOI] [PubMed] [Google Scholar]
- 18. Glaze DG, Neul JL, Kaufmann WE, Berry-Kravis E, Condon S, Stoms G, Oosterholt S, Della Pasqua O, Glass L, Jones NE, Percy AK; Rett 002 Study Group. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019; 92:e1912-e1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neul JL, Percy AK, Benke TA, Berry-Kravis EM, Glaze DG, Marsh ED, Lin T, Stankovic S, Bishop KM, Youakim JM. Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat Med. 2023; 29:1468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu C, Armstrong D, Marsh E, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020; 4:e000717. [DOI] [PMC free article] [PubMed] [Google Scholar]