Abstract
Background
Improving physical activity, especially in combination with optimizing protein intake, after surgery has a potential positive effect on recovery of physical functioning in patients after gastrointestinal and lung cancer surgery. The aim of this randomized controlled trial is to evaluate the efficacy of a blended intervention to improve physical activity and protein intake after hospital discharge on recovery of physical functioning in these patients.
Methods
In this multicenter single-blinded randomized controlled trial, 161 adult patients scheduled for elective gastrointestinal or lung cancer surgery will be randomly assigned to the intervention or control group. The purpose of the Optimal Physical Recovery After Hospitalization (OPRAH) intervention is to encourage self-management of patients in their functional recovery, by using a smartphone application and corresponding accelerometer in combination with coaching by a physiotherapist and dietician during three months after hospital discharge. Study outcomes will be measured prior to surgery (baseline) and one, four, eight, and twelve weeks and six months after hospital discharge. The primary outcome is recovery in physical functioning six months after surgery, and the most important secondary outcome is physical activity. Other outcomes include lean body mass, muscle mass, protein intake, symptoms, physical performance, self-reported limitations in activities and participation, self-efficacy, hospital readmissions and adverse events.
Discussion
The results of this study will demonstrate whether a blended intervention to support patients increasing their level of physical activity and protein intake after hospital discharge improves recovery in physical functioning in patients after gastrointestinal and lung cancer surgery.
Trial registration
The trial has been registered at the International Clinical Trials Registry Platform at 14–10-2021 with registration number NL9793. Trial registration data are presented in Table 1.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-023-07705-2.
Keywords: Cancer, Surgery, Supportive care, Physical functioning, Physical activity, Protein intake
Introduction
Major surgical procedures for gastrointestinal (GI) and lung cancer frequently result in significant loss of muscle mass, caused by increased catabolism due to the surgical stress response. This has major implications for postoperative physical function and has been associated with postoperative morbidity, mortality and quality of life [1–6]. Adequate physical activity and nutrition are important to prevent loss of muscle mass [7–10]. A combination of both is even more important, as adequate protein intake is needed to optimally benefit from the physical training stimuli [11]. This has also been reflected in the results from a systematic review showing that a combination of adequate protein intake and sufficient physical activity facilitates muscle gain in sarcopenia [12]. Therefore, to minimize the postoperative loss of muscle mass and restore physical function, it is important for patients with cancer undergoing surgery to maintain or enhance their physical activity and nutritional status in the postoperative phase (Table 1).
Table 1.
Trial registration data set
| Data category | Information |
|---|---|
| Primary registry and trial identifying number |
International Clinical Trials Registry Platform NL9793 |
| Date of registration in primary registry | 14 October, 2021 |
| Secondary identifying numbers | NL78840.029.21 |
| Source(s) of monetary or material support | Amsterdam UMC, location VUmc, department of Rehabilitation |
| Primary sponsor | Amsterdam UMC, location VUmc, department of Rehabilitation |
| Secondary sponsor(s) | Amsterdam Movement Sciences Institute |
| Contact for public queries | MdL, m.e.deleeuwerk@amsteramumc.nl |
| Contact for scientific queries |
MdL, m.e.deleeuwerk@amsteramumc.nl Amsterdam UMC, location VUmc, department of Rehabilitation, Amsterdam, The Netherlands |
| Public title | Optimal Physical Recovery After Hospitalization (OPRAH study) |
| Scientific title | The efficacy of a blended intervention to improve physical activity and protein intake for optimal physical recovery after oncological gastrointestinal and lung cancer surgery: study protocol for a randomized controlled multicenter trial |
| Countries of recruitment | The Netherlands |
| Health condition(s) or problem(s) studied | Rehabilitation after oncological surgery |
| Intervention(s) | Intervention: Smartphone application and corresponding accelerometer in combination with coaching by a physiotherapist and dietician during three months after hospital discharge |
| Control: Usual care | |
| Key inclusion and exclusion criteria |
Ages eligible for study: ≥ 18 years Sexes eligible for study: both Accepts healthy volunteers: no |
| Inclusion criteria: adult patient (≥ 18 years), scheduled for curative intent surgery for gastrointestinal cancer, including esophageal and stomach cancer (upper GI), colorectal and hepato-pancreato-biliary (HPB) cancer, or lung cancer with a planned hospital stay of ≥ 2 nights, able to fill in online questionnaires in Dutch and give informed consent | |
| Exclusion criteria: pulmonary wedge resection, surgery with open/close procedure, having no access to a mobile device compatible for applications, less than 5 days between inclusion and surgery, patients who are wheelchair dependent, a Mini-Mental State Examination (MMSE) ≤ 24 and already participating in a conflicting study | |
| Study type | Multicenter randomized controlled intervention trial with allocation at level of the individual |
| Allocation: randomized | |
| Primary purpose: treatment | |
| Phase III trial | |
| Date of first enrolment | June 2022 |
| Target sample size | 161 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Recovery in physical functioning six months after hospital discharge |
| Key secondary outcomes | Physical activity, lean body mass, pain, fatigue, muscle mass, protein intake, physical performance, patient-specific activity limitations, self-efficacy, participation in social roles and activities, generic quality of life, global perceived effect, hospital readmission and adverse events |
However, patients often experience barriers to being physically active after surgery, e.g. due to physical symptoms, such as pain and fatigue, and lack of motivation or social support [13–16]. In addition, previous studies found that many surgical patients were unable to meet their protein requirements after surgery despite the advices of a dietician, e.g. due to a loss of appetite or feelings of worry [17]. Patients emphasize the need for more supportive care interventions after discharge to facilitate return to normal activities after cancer surgery [18, 19]. Therefore, additional support in promoting physical activity and protein intake is needed to improve recovery of physical functioning after surgery in these patients [20].
Self-monitoring of physical activity with the use of accelerometers is an often used strategy to increase physical activity in patients, by giving patients insight in their daily physical activity level [21]. A recent meta-analysis showed that interventions combining accelerometers with feedback using different behavioral change techniques (BCTs) and coaching by a health care professional are more effective in increasing physical activity than the use of an accelerometer alone [22]. In this study, it is suggested that this is due to the fact that incorporating coaching by a health professional to the intervention gives the opportunity to provide targeted advice and interventions for a specific population group with a more personal touch. Also, more BCTs can be used when a health care professional is involved, e.g. problem solving, social reward. In addition, findings of a review on patients with a colorectal adenoma indicate that behavioral interventions can encourage these patients to improve their diet [23]. The effect of a combined intervention, using eHealth and remote coaching by a dietician and physiotherapist in patients after GI or lung cancer is unknown.
We therefore developed a blended intervention to support patients in increasing their level of physical activity and protein intake after hospital discharge: The Optimal Physical Recovery After Hospitalization (OPRAH) intervention. The purpose of the OPRAH intervention is to encourage self-management of patients in their recovery in physical functioning, by using a smartphone application and corresponding accelerometer in combination with coaching by a physiotherapist and dietician. To investigate the potential effect of providing ongoing support on physical activity and protein intake after hospital discharge on recovery in physical functioning, the intervention will be compared to usual care. Therefore, the aim of this randomized controlled multicenter trial is to investigate the effectiveness of the OPRAH intervention on recovery of physical functioning, compared to usual care, in patients who have undergone elective GI and lung cancer surgery.
Objective and hypothesis
Objective: to evaluate the efficacy of the OPRAH intervention on recovery of physical functioning (compared with usual care) in patients who have undergone elective GI and lung cancer surgery.
Hypothesis: The aim of the intervention is to encourage self-management of patients by the use of self-monitoring on physical activity and protein intake. Patients will also be monitored in their recovery of physical activity and protein intake by a physiotherapist and dietician. If this recovery stagnates and goals are not achieved, the physiotherapist or dietician can contact the patient to identify barriers in their recovery. The use of multiple BCTs can help to reduce or eliminate these barriers and to increase the patient’s level of physical activity and protein intake. Higher levels of physical activity and achieving protein requirements are expected to have a positive effect on the recovery in physical functioning after discharge. Therefore, it is hypothesized that patients in the intervention group will have a faster and better recovery in physical functioning during the first 6 months after hospital discharge compared to patients receiving usual care.
Methods
Study design
The proposed study is a multicenter, single-blinded two-arm randomized controlled study comparing a blended intervention delivered alongside usual care, with a control arm (usual care) in patients after hospital discharge who have undergone GI or lung cancer surgery. Baseline measurements (T0) will be conducted prior to surgery and follow-up measurements take place at hospital discharge (T1) and 1 (T2), 4 (T3), 8 (T4) and 12 (T5) weeks and 6 months (T6) after hospital discharge. The trial will be conducted at two hospitals in the Netherlands: Amsterdam UMC, location VUmc and St. Antonius, location Nieuwegein. The OPRAH trial has been designed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement [24]. The Standard Protocol Items Recommendations for Interventional Trial (SPIRIT) checklist is provided as additional file. See Fig. 1 for the flowchart of the study and Fig. 2 for the SPIRIT schedule of enrollment, intervention and assessments.
Fig. 1.
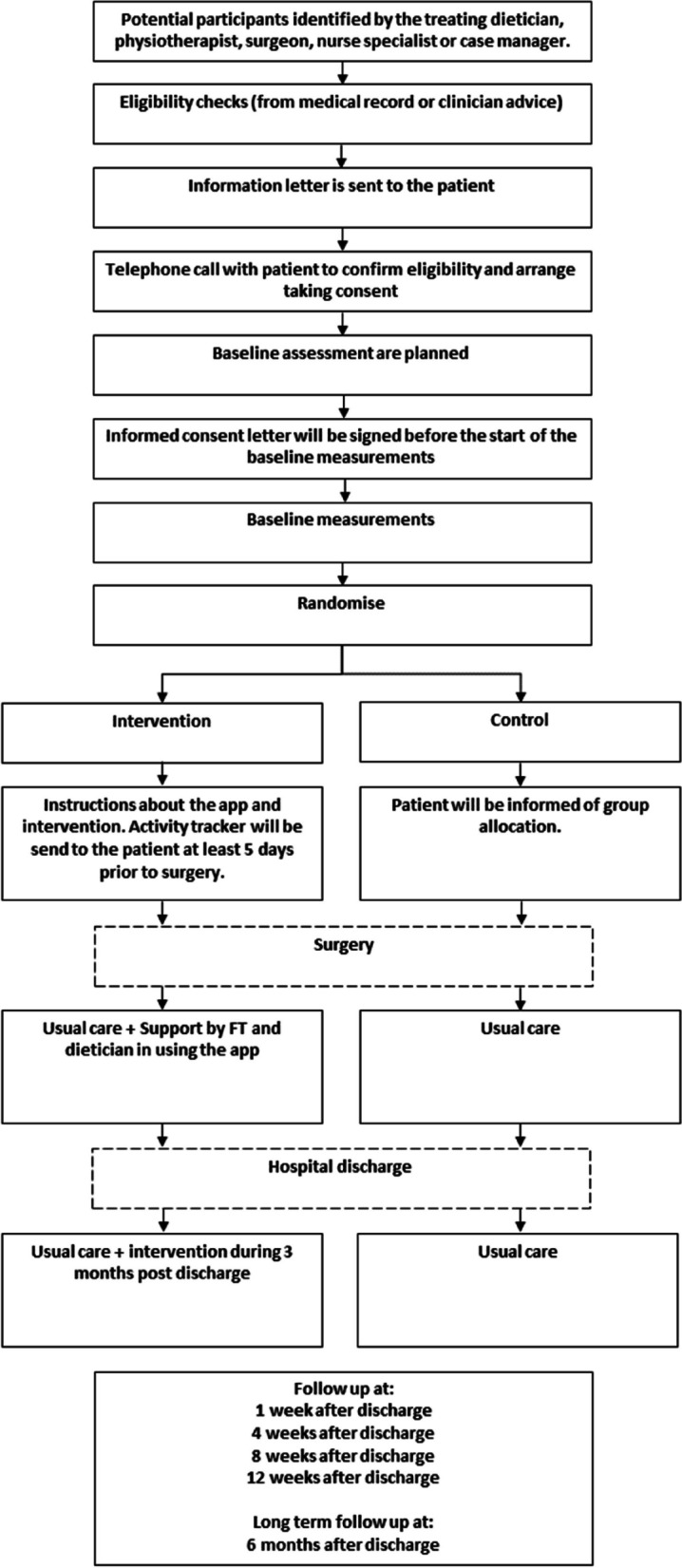
Flow chart
Fig. 2.
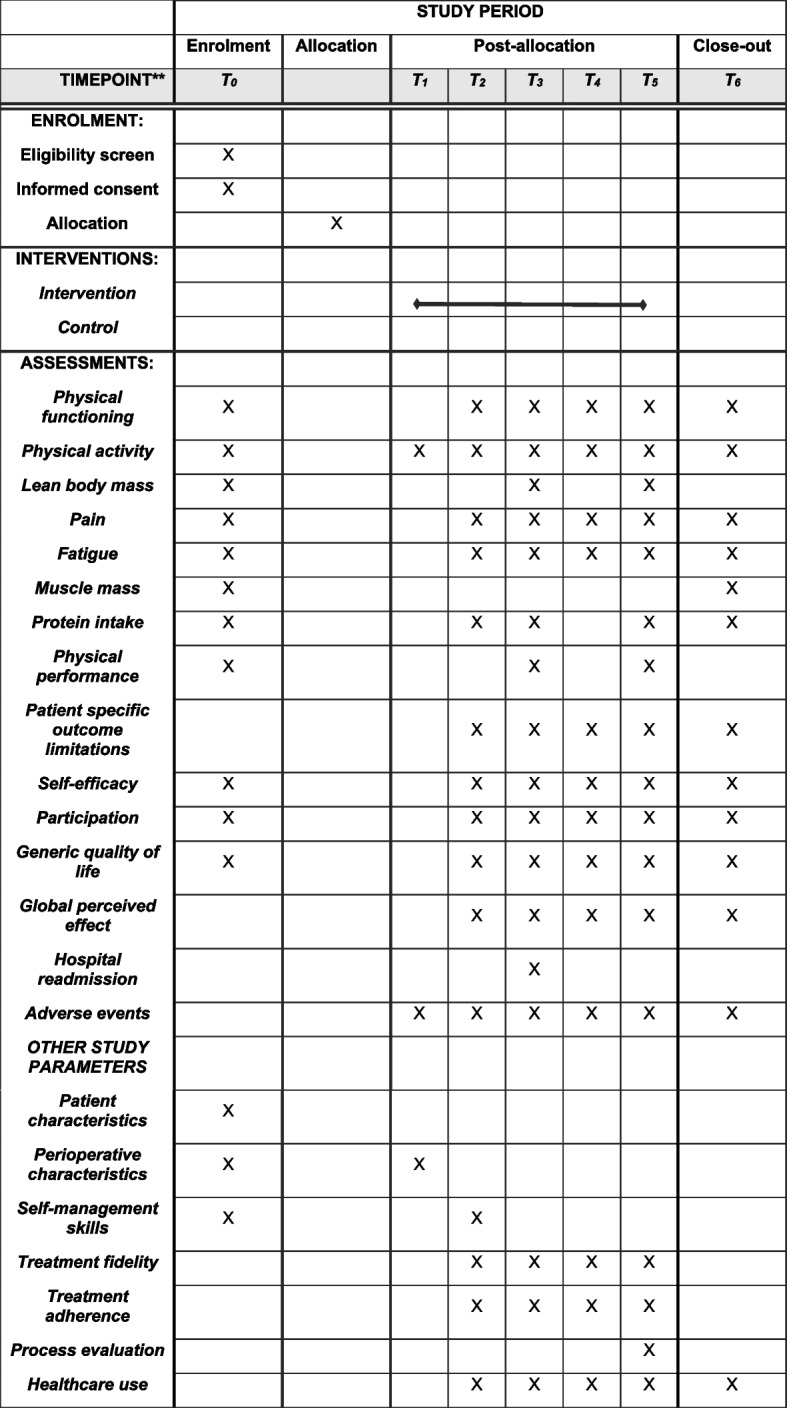
SPIRIT schedule of enrolment, intervention, and assessments
Participants
Eligibility criteria
Patients are eligible to participate when scheduled for curative intent surgery for gastrointestinal cancer, including esophageal and stomach cancer (upper GI), colorectal and hepato-pancreato-biliary (HPB) cancer, or lung cancer with a planned hospital stay of ≥ 2 nights, are aged 18 years or older, and whether they are able to fill in online questionnaires in Dutch and give informed consent.
Exclusion criteria are the following: pulmonary wedge resection, surgery with open/close procedure, having no access to a mobile device compatible for applications, less than 5 days between inclusion and surgery, patients who are wheelchair dependent, a Mini-Mental State Examination (MMSE) ≤ 24 and already participating in a conflicting study.
Recruitment
Potentially eligible patients will be informed about the study by the treating dietician, physiotherapist, nurse specialist or case-manager during a preoperative consultation. When the patient is interested in participation, the researcher will contact the patient after 24 h to further explain the study procedures and to answer questions of the patient. If the patient is eligible and willing to participate, the informed consent letter will be signed before the start of the baseline measurements. During the informed consent procedure, participants are also asked to confirm if their data may be used to support other research in the future. Participants will also indicate whether they would be willing to be contacted about future-related research and if they give consent to the making, use and retention of audio recordings of conversations with the dietitian and physiotherapist. In case there are doubts about the patients’ cognition, the MMSE will be administered before final inclusion. The patient receives a copy of the signed informed consent. The recruitment period is 18 months, with a target of approximately 10 included participants per month.
Randomization and blinding
After the baseline measurements, patients will be randomly assigned to the control or intervention group with a 1:1 allocation ratio using the randomization tool of Castor Electronic Data Capture (EDC) [25]. Randomization will be stratified per center by type of surgery (lung, HPB, upper GI, colorectal) and ASA score (1–2 or ≥ 3). The randomization tool of Castor ensures concealment of allocation. The researcher informs the participant by e-mail which group he has been assigned to. Assessments will be conducted by a blinded research assistant. Neither the patient nor the therapist will be blinded.
Sample size
For the present sample size analysis, a conservative estimate of 0.40 as the between-group effect size on the outcome physical functioning is used. This estimate is based on reported effect sizes on patient-reported outcomes of physical functioning in other studies using technology and coaching on physical activity [26, 27]. Based on alpha = 0.05, power (1 − β) = 0.80, a two-sided test for repeated measures with an expected within-subject correlation coefficient of 0.6 and 5 follow-up measurements the minimum number of 67 subjects per group is required, with a total sample size of 134 (see Formula 1). Allowing for a drop-out rate of 20% during the study, this study should include 161 patients.
The most important secondary outcome of this study is objectively measured physical activity. Therefore, a sample size calculation was also made on this outcome measure. Based on the effect sizes found in our recent systematic review and meta-analysis of interventions using activity trackers in patients during or after inpatient stay on the outcome physical activity, an effect size on physical activity of 0.50 is expected [22]. Based on alpha = 0.05, power (1 − beta) = 0.80 and a two-sided test the minimum number of subjects required is n = 128 (64 in each group). Allowing a drop-out rate of 20% during the study, a total of 154 patients should be included.
Formula 1:
Intervention
The intervention is described according to the template for intervention description and replication (TIDIER) checklist (See Supplementary File 1). The main purpose of the OPRAH intervention is to facilitate faster and better recovery in physical functioning by stimulating patients’ self-management regarding their level of physical activity and protein intake after hospital discharge.
Development of the intervention
The Medical Research Council (MRC) framework for the development and evaluation of complex intervention was used [28]. Supplementary File 3 shows the stages of the MRC framework alongside with our activities of the development process and the activities that are described in this paper. The intervention development process was guided by findings from a feasibility study [29], systematic review of literature on the effectiveness of intervention components [22], a literature search about barriers and facilitators to the targeted behavior and expert meetings with researchers and health professionals (OPRAH consortium, consisting of physiotherapists, dieticians, surgeons, researchers and a specialist in behavioral change). The behavioral change wheel was used as theoretical underpinning of the intervention [30]; with the use of this theory, we have been able to substantiate how the intervention causes change, what the active ingredients of the intervention are and how they can exert their effect. A feasibility study was conducted to evaluate the practical effectiveness.
The basis of the intervention was an existing app with a self-monitoring function of physical activity, which had been investigated in the postoperative period of oncological patients through a feasibility study [29]. Self-monitoring appeared to be feasible in this population. However, some patients emphasized the need for more support in addition to self-monitoring. This finding was strengthened by the results from our systematic review, because interventions with activity trackers in combination with coaching by a health professional seemed to be more effective in increasing physical activity during and after hospitalization [22]. In addition, the use of more behavioral change techniques (BCT’s) within the intervention was also suggested to be more effective.
Because of the important synergy between protein intake and physical activity after major oncological surgery, the app has been expanded with a self-monitoring tool for protein intake. By conducting a comprehensive literature search, barriers and facilitators to the targeted behaviors, improving physical activity and protein intake, were identified. Based on the behavioral change wheel and with input from the OPRAH consortium, a total of 15 behavioral change techniques, following the BCT taxonomy of Michie et al. [31], were identified and linked to a mode of delivery in order to target the desired behavior. (See Table 2) In Supplementary File 4, the BCTs are linked to the Capability, Opportunity, Motivation and Behavior (COM-B) model [30], intervention functions and mode of delivery. In order to improve the motivation of patients for behavioral change, motivational interviewing (MI) and shared decision making (SDM) was incorporated in the intervention. Based on initial feedback from the OPRAH consortium, the intervention was refined in preparation for evaluation.
Table 2.
Intervention components based on the BCT taxonomy (v1) of 93 hierarchically cluster techniques from Michie et al. [31]
| Behavioral change technique | Description |
|---|---|
| 1.1 Goal setting (behavior) |
Patients are able to set goals on the amount of physical activity per day. Patients will be supported by the physiotherapist to set realistic goals Goals on requirements of protein intake will be set based on advice of the dietician |
| 1.2 Problem solving | The physiotherapist/dietician analysis factors influencing the behavior and select strategies for overcoming barriers/increasing facilitator to perform behavior |
| 1.4 Action planning | Patients are able to set in-app tasks. Patients will be encouraged by the physiotherapist/dietician to plan the performance (when, what time etc.) |
| 1.5 Review behavioral goals | The physiotherapist/dietician will review the behavioral goals and consider modifying goals based on their achievement |
| 1.6 Discrepancy between current behavior and goal | Visual in-app presentation of behavior and targeted goals |
| 2.2 Feedback on behavior |
The amount of minutes patients have to be active to achieve their goal is presented in the app. The number of points remaining to achieve protein requirements is shown In addition, the physiotherapist/dietician will give the patient feedback about their activity/intake |
| 2.3 Self-monitoring of behavior | Patients are able to monitor their daily level of physical activity and protein intake via the app |
| 3.1 Social support |
Patients are able to request contact with the physiotherapist/dietician via the app The physiotherapist/dietician will contact the patient (how often is determined in consultation with the patient) |
| 4.1 Instructions on how to perform the behavior | Tailored in-app information and personalized instructions by physiotherapist/dietician |
| 5.1 Instructions about health consequences | Tailored in-app information and personalized instructions by physiotherapist/dietician |
| 7.1 Prompts/cues | Patients can have the opportunity to receive in-app reminders to reach their daily goal |
| 8.7 Graded tasks | The physiotherapists stimulate patients to set easy-to-perform tasks |
| 10.4 Social reward | Physiotherapist/dietician reward the patients if there has been effort in performing the behavior |
| 10.5 Social incentive | Patients receive in-app rewards if they achieved their goal |
| 12.5 Adding objects to the environment | Wearing the PAM sensor |
Coaching by health professionals
Coaching is an important part of the intervention, as the use of self-monitoring has proven to be more effective when combined with coaching by a healthcare professional and is considered important to improve the synergy and collaboration between physiotherapy and dietetics. Through the use of coaching, the intervention can be tailored to the clinical status of the patient. In addition, potential barriers to the desired behavior can be identified and, if they are within the scope of physiotherapist and dietician, addressed in collaboration with the patient. To support the physiotherapists and dieticians in coaching, the choice has been made to use motivational interviewing (MI) and shared decision making (SDM). MI increases patient autonomy, enhances intrinsic motivation and supports the patient’s self-efficacy all of which contributes to increasing the patient’s self-management [32]. A recent review indicated that MI is a powerful intervention in combination with self-monitoring using activity trackers to improve autonomous motivation and to reduce a-motivation for physical activity [33]. Furthermore, it was indicated that the delivery of the intervention can vary from telephone to real life coaching and can still be effective in impacting motivation, regardless of the delivery method [33]. SDM provides an opportunity to integrate evidence and patient preferences into a health-related decision [34, 35]. The physiotherapist and dieticians involved in this study have received a training about MI and SDM prior to the start of the study. The main purpose of this 3-day training course was to teach strategies according to the principles of MI to encourage patients to adopt healthy behavior, especially focused on physical activity and protein intake. In the first session, attention was paid to reflective listening; i.e. listening carefully to what the patient says and giving it back to the patient in different words in order to create understanding and clarity. Next, it was discussed what ambivalence is and how it can be recognized. Ambivalence means being pulled back and forth between the disadvantages and advantages of the current situation and the new situation. In the second session, the recognition of ambivalence was continued and conversation techniques were applied in order to guide the patient towards healthy behavior. During the last training day, all techniques were practiced with the help of a trained actor. Between the training days, the physiotherapists and dieticians applied the learned techniques in practice and reflected on this in duo sessions. To keep their knowledge and skills up to date during the study period, peer review meetings will be organized.
E-health technology
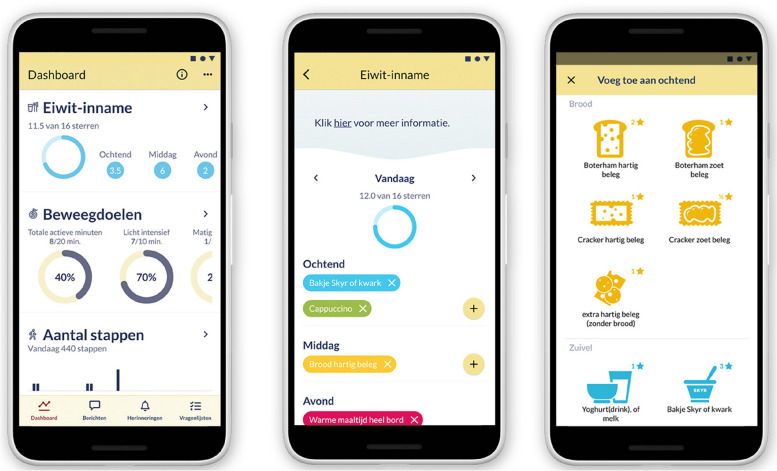
The Atris software (Peercode B.V. Geldermalsen, the Netherlands) is the investigational software used in the intervention. In combination with the ankle-worn PAM AM400 three-axis accelerometer (PAM B.V. Doorwerth, the Netherlands), the Atris software is used for self-monitoring of physical activity and protein intake. The Atris software consists of an Atris app for patients, Atris backend website for health professionals and the Atris (triggers) software. The Atris app allows patients to self-monitor their daily physical activity and protein intake through wearing the PAM and tracking daily protein intake by using a simple in-app self-registration system. See Fig. 3 for screenshots of the app and Supplementary File 2 for a subscript of the screenshots. Physical activity is represented in active minutes per day, with a distinction in low (1.4–2.99 metabolic equivalents of energy expenditure (METs)), moderate (3–7 METs), and vigorous activity (> 7 METs). The protein intake is represented in stars (★), where 1 star represents approximately 5 g of protein. The Atris app provides feedback on progress related to their goals. In addition, patients can ask questions through the app’s chat feature and receive response and information by the physiotherapist or dietician. Via the Atris backend website, the physiotherapist and dietician can interactively view patient data, send messages to the patient, and can access Atris (triggers) through the patient monitor. The software Atris (triggers) enables personalized goal setting and threshold values per patient.
Fig. 3.
Screenshots of the Atris app
Intervention description and procedures
One week prior to planned surgery, patients receive the PAM and will receive access to and get instructions about the Atris app to get familiarized with the application. Patients are asked to wear the PAM 24 h a day in a strap around the ankle, from at least 5 days prior to surgery until 3 months after surgery. During hospitalization, the treating physiotherapist and dietician guide the patients in the use of the app during their standard consultations. As the day of hospital discharge approaches, the patient will be supported by the physiotherapists and dietician using the SDM process to set goals on active minutes and protein intake for after discharge. After discharge to home, patients are coached remotely (by telephone and chat) by a physiotherapist and dietician about physical activity and protein intake during 3 months after discharge. Through a chat function in the app, patients can ask questions to the physiotherapist or dietician, the physiotherapist and dietician can also send information through the chat. The ultimate goal for physical activity is to return to pre-surgery level of physical activity. The ultimate goal for protein intake is to achieve the personal daily requirements. If the recovery stagnates and goals are not achieved, the physiotherapist or dietician will contact the patient to identify barriers in their recovery. The sub-goals and the degree of coaching will be tailored using a SDM process to the personal needs and preferences of the patient. To support patients’ self-management, MI techniques will be applied during the coaching sessions with the physiotherapist and dietician by telephone and chat [32].
Criteria for discontinuing the intervention
Patients may discontinue the intervention in the following cases:
Completion of the intervention period: Patients may discontinue the intervention once they have completed the predetermined duration of the OPRAH intervention (3 months after hospital discharge).
Adverse effects or complications: If patients experience any adverse effects or complications directly related to the intervention, it may be necessary to discontinue their participation for safety reasons.
Lack of adherence: If patients consistently fail to comply with the requirements or recommendations of the intervention, discontinuation will be considered. This can include non-engagement with wearing the PAM sensor or registration of their protein intake.
Patient’s request or withdrawal: Patients have the right to choose whether they want to continue or discontinue the intervention. If a patient decides to withdraw from the program, their participation will be discontinued.
Usual care
Both participants in the intervention and the control group receive usual care.
During hospitalization, patients are treated according to the Early Recovery After Surgery (ERAS) protocol [36]. This includes early mobilization and (nutritional) intake supported by the entire (para)medical team. In the daily consultations by the medical doctor and nursing staff, attention is paid to the improvement of mobilization and intake. The amount of consultation by the physiotherapist and dietician is determined based on the clinical assessment of the physiotherapist and dietician.
After hospital discharge, there is no usual physiotherapy care. The physiotherapist may advise the patient to continue physiotherapy in primary care, based on the clinical assessment of the physiotherapist. The usual care of the dietician differs between patient groups. In Amsterdam UMC, the dietician standard schedules postoperative consultations with patients after esophagus-, stomach-, pancreas-, biliair and Hypertherme Intraperitoneale Chemotherapy (HIPEC) cancer surgery at 2 and 4 weeks and 3 and 6 months after discharge. At St. Antonius, the dietician only schedules standard postoperative consultations at 2 weeks after discharge for patients after pancreas surgery. When necessary, more consultations can be planned. Patients after hepatic, colorectal (excl. HIPEC) or lung cancer surgery receive postoperative consultations by the dietician only on indication or the dietician may advise the patient to continue dietetic treatment in primary care. All participants are permitted to engage any form of (para)medical care during the study period.
Adverse event reporting
Adverse events (AEs)
Adverse events are defined as any undesirable experience occurring to a subject during the study, whether or not considered related to experimental intervention. All adverse events with a direct or possible link to the OPRAH trial (e.g. AEs occurring during intervention-related activities) reported spontaneously by the subject or observed by the investigator or his staff will be recorded.
Serious adverse events (SAE)
This study includes patients undergoing oncological lung or GI surgery. These types of surgery are associated with a certain risk of postoperative complications. Our intervention starts after hospital discharge; therefore, all complications during hospitalization will not be reported as SAE. We expect that our, low-risk, post-discharge intervention will not have any negative influence on the occurrence complications after discharge. Therefore, all complications after discharge which are unmistakably caused by the surgery and/or medical treatment will not be reported as SAE. When there are doubts about the relation between the intervention and the occurrence of an SAE, these SAEs will be discussed with the surgeons involved in this study per patient group. If, after discussion, there is still any doubt about the relation between the intervention and the occurrence of an SAE, the SAE will be reported.
The participating hospitals will report SAEs within 24 h to the sponsor. The sponsor will report the SAEs to the accredited medical ethical committee that approved the protocol, within 7 days of first knowledge for SAEs that result in death or are life threatening followed by a period of maximum of 8 days to complete the initial preliminary report. All other SAEs will be reported within a period of maximum 15 days after the sponsor has first knowledge of the serious adverse events.
Outcomes
The primary outcome is recovery in physical functioning 6 months after hospital discharge and the most important secondary outcome is physical activity. Other secondary outcomes are lean body mass, pain, fatigue, muscle mass, protein and energy intake, physical performance, patient-specific activity limitations, self-efficacy, participation in social roles and activities, generic quality of life, global perceived effect, hospital readmission and adverse events. See Table 3 for a detailed description of the outcome measures with the corresponding follow-up time points.
Table 3.
Outcome measures
| Construct | Measure | Abbrev | Description | Time points |
|---|---|---|---|---|
| Primary outcome | ||||
| Physical functioning | Computer Adaptive Testing (CAT) Dutch-Flemish Patient-Reported Outcome Measure Information System for Physical Functioning | CAT PROMIS-PF | The CAT PROMIS-PF is a digital questionnaire [37]. A CAT is a computer-administered measure in which a computer algorithm is used to select successive items based on responses to previous items. Using a 5-point Likert scale the questionnaire reflects the participant’ functioning in the past 7 days. The items cover a wide range of activities, from activities of daily living to more complex activities [38]. The CAT PROMIS-PF has sufficient psychometric properties in measuring the level of physical function of physiotherapy patients [39] and has been shown to be reliable and sensitive to change in surgical patients [40, 41]. | T0, T2, T3, T4, T5, T6 |
| Most important secondary outcome | ||||
| Physical activity | ActivPAL™ | The ActivPAL (PAL Technologies Ltd., Glasgow, UK) is a thigh-worn tri-axial accelerometer and uses proprietary analysis algorithms to determine posture (sedentary time, upright time) and stepping (stepping time and steps). The Activpal will be affixed to the skin with hydrogel pads on the thigh. The ActivPAL is one of the most frequently used activity trackers in clinical research and has been shown to be a valid measure of posture and stepping [42–44]. Patients in both the intervention and control group will wear the ActivPAL for 5 days | T0, T1, T3, T5, T6 | |
| Other secondary outcomes | ||||
| Lean body mass | Bioelectrical Impedance Analysis | BIA | A single frequency bio electric impedance meter (50 kHz) will be used for the BIA. These impedance meters uses 4 electrodes to measures the impedance, resistance, reactance and phase angle. A BIA is a non-invasive validated method to assess body composition, fat-free mass (FFM) and appendicular skeletal muscle mass (ASSM) patients with cancer [45]. FFM is one of the factors the dietician uses to determine patients’ personal protein requirements. FFM will be measured with the formula of Kyle [46] | T0, T3, T5 |
| Pain | Numeric Pain Rating Scale | NPRS | The NPRS is a measure of subjective intensity of pain in adult patients. The 11-point numeric scale ranges from ‘0’ (no pain) to ‘10’ (worst pain imaginable). The NPRS is a valid, reliable and usual tool to measure pain in postoperative patients [47, 48]. | T0, T2, T3, T4, T5, T6 |
| Fatigue | Dutch-Flemish Patient-Reported Outcome Measure Information System for Fatigue – Short Form | PROMIS F-SF | The PROMIS F-SF consists of seven items that measure both the experience of fatigue and the interference of fatigue on daily activities over the past week. The response scale is a 5-point Likert scale, ranging from 1 (never) to 5 (always). Total scores range from 7 to 35: a higher score indicating greater fatigue. The PROMIS F-SF showed acceptable reliability and validity and is suitable to measure fatigue across a diverse clinical population [49]. | T0, T2, T3, T4, T5, T6 |
| Muscle mass | Analyses of Computer Tomography | CT-analyses | CT images are routinely obtained in the oncological workup of lung and GI cancer patients, both pre- and postoperative. CT images obtained from whole-body PET/CT scans will be analyzed by a validated computer program AMUSE (acronym for Automatic MUscle and visceral/subcutaneous fat SEgmentor). The landmarks for the assessment of muscle mass will be the third lumbar vertebra (L3) and the fourth thoracic vertebra (Th4) | T0, T6 |
| Protein and energy intake | 48-h dietary recall | A 48-h dietary recall will be performed by trained interviewers, dietician researchers or graduate students, following a standard protocol. The intake of food items will be registered, including details of preparation methods, recipes, quantities and the size of portions, and the brands of products. The calculation of total energy intake and protein intake will be based on the database of ‘The Netherlands Nutrition Centre’. Protein intake will be calculated in gram per day. Energy intake will be calculated in kcal per day. Earlier studies suggested that a 48-h recall is superior to a single 24-h recall [50, 51]. | T0, T2, T3, T5, T6 | |
| Physical performance |
1) Hand grip strength 2) 30-s chair stand test 3) 2-min step test |
1) - 2) 30SCST 3) TMST |
1) Handgrip strength will be measured using the Jamar grip strength dynamometer as a measure of generalized muscle strength. The Jamar grip strength shows good reliability and can be used to measure changes in strength over time [52, 53]. The normative data of Dodds. et al. will be used as reference. [54] 2) The 30SCST will be used to measure functional lower extremity muscle functioning. The 30CST measures extremity strength in relation to demanding functional daily activities such as stair climbing [55]. The patient is seated in a chair and will be asked to complete as many full stands as possible in 30 s. The 30CST shows good reliability and criterion validity [56]. 3) The TMST will be used to measure exercise capacity [57]. The test requires the patients to march in place as fast as possible for 2 min while lifting the knees to a height midway between their patella and iliac crest when standing. Performance is defined as the number of steps completed in 2 min These performance tests are easy to perform at the patient’s home. The physical performance tests will be performed by trained (student) allied health professionals. Standard operating procedures will be used for all tests [58]. |
T0, T3, T5 |
| Patient-specific activity limitations | Patient-specific Functional Scale | PSFS | The Dutch PSFS includes a list of 24 activities adapted from the activity list for patients with heart failure by Beurskens et al. and patients will be asked to indicate all activities that he or she was limited in performing within the previous week. [59] Patients have also the possibility to indicate any additional activities in the “other” section. The patient will be asked to prioritize their three most important activity limitations. Patients will then be asked to rate these activities on a numeric rating scale ranging from 0 to 10, where 0 indicates no limitations in performing the activity and 10 indicates that performing the activity is impossible. The PSFS is an appropriate measure for statistical comparisons in clinical research | T0, T2, T3, T4, T5, T6 |
| Self-efficacy | General self-efficacy scale | GSES | The questionnaire contains 10 items with a 4-point response scale (1 = completely wrong, 4 = completely true). Total scores range from 10 to 40: a higher score indicating more self-efficacy. The GSES Is a valid tool to measure self-efficacy [60]. | T0, T2, T3, T4, T5, T6 |
| Participation in social roles and activities | CAT Dutch-Flemish Patient-Reported Outcome Measure Information System for participation | CAT PROMIS participation | The CAT PROMIS participation item bank including the item banks ‘Ability to participate in Social Roles and Activities’ and ‘Satisfaction with Social Roles and Activities’. The response scale is a 5-point Likert scale; a higher score indicates better participation. The CAT PROMIS item bank is a reliable and valid measurement of participation with limited administration time [61]. | T0, T2, T3, T4, T5, T6 |
| Generic Quality of Life | The 5-level EuroQol five-dimensional questionnaire | EQ-5D-5L | This EQ-5D-5L consists of five questions representing five health dimensions; mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ-5D-5L is added in order to carry out cost-effectiveness analysis. The EQ-5D-5L shows good psychometric properties and is suitable for economic evaluation studies in oncological patients [62]. | T0, T2, T3, T4, T5, T6 |
| Global perceived effect | Global Perceived Effect questionnaire | GPE | The Dutch version of the GPE will be used to measure the patient’s opinion of their recovery [63]. The questionnaire consists of two questions with a 7-point Likert scale. Patients will be asked to indicate to which extent they are recovered since the beginning of their recovery after surgery (1 = much better, 7 = much worse). In addition, patients will be asked how satisfied they are with their treatment since the beginning of their recovery after surgery (1 = very satisfied, 7 = very unsatisfied) | T0, T2, T3, T4, T5, T6 |
| Hospital readmission | Medical Record Data | Unplanned hospital readmissions within 30 days after discharge will be reported | T3 | |
| Adverse event | Medical record data and self-reported | All adverse events with a direct or possible link to the OPRAH trial (e.g. AEs occurring during intervention-related activities) reported spontaneously by the subject or observed by the investigator or his staff will be recorded. This study will include patients undergoing oncological lung or GI surgery. These types of surgery are associated with a certain risk of postoperative complications. Our intervention starts after hospital discharge; therefore, all complications during hospitalization will not be reported as SAE. We expect that our, low-risk, post-discharge intervention will not have any negative influence on the occurrence complications after discharge. Therefore, all complications after discharge which are unmistakably caused by the surgery and/or medical treatment will not be reported as SAE. When there are doubts about the relation between the intervention and the occurrence of an SAE, these SAE’s will be discussed with the surgeons involved in this study | T1 t/m T6 | |
| Other study parameters | ||||
| Patient characteristics | Self-reported and Medical Record Data |
Age (years), gender (male/female), body mass index (BMI), marital status (living together/alone), comorbidities, Mini-Mental State Examination (MMSE)*, The American Society of Anaesthesiologists Classification of physical health (ASA classification), tumor location (lung, upper GI, HPB, colorectal), stage of cancer (1–4), unintentional weight loss (% over de last 6 months), nutritional intake (% of nutritional requirements) *The MMSE will only be measured when there are doubts about the patient’s cognition. The MMSE is an instrument used for cognitive impairment in the elderly [64]. A low score on the MMSE corresponds to a low cognitive level. A score < 24 (out of 30) is usually considered deviant [65]. |
T0 | |
| Perioperative characteristics | Medical Record Data | Operation technique (open/laparoscopic), pre- or post-treatment with chemotherapy or radiotherapy, length of hospital stay (days) | T1 | |
| Self-management Skills | Self-Management Screening tool | SeMaS | The SeMaS is a short tool that can signal potential barriers for self-management that need to be addressed in the dialog with the patients and can facilitate personalized counselling. The following items of the SeMaS will be measured: level of education, experienced burden of disease, digital skills, locus of control, self-efficacy, social support, coping style, anxiety and depression. Based on the outcome factors of the SeMaS tool, the physiotherapist will give specific advice to the patients for the tailored intervention. The SeMaS is validated in patients with chronic condition in primary care [66]. However, this tool contains patient characteristics that are generally important in self-management and the questionnaire is not specific to a particular condition | T0 |
| Treatment fidelity | Audio records | To investigate whether the intervention techniques used in the intervention (MI and SDM) have been implemented properly, some consultations with the physiotherapist and dietician will be audio-recorded. The audio records will be made with professional recording equipment and in no case with a telephone. The consultations will only be recorded if the patient has given his or her specific consent to record their consultations. Researchers trained in the use of MI and SDM will listen to these consultations to determine if the techniques have been used appropriately. The recordings will be made at multiple times during the study as a learning effect of the health professionals is expected. In addition, the number of consultations with the | T1 t/m T5 | |
| Treatment adherence | Intervention data |
Treatment adherence will be measured to determine the extent to which the patients complied with the intervention. The following outcomes will be considered to determine treatment adherence: • Number of days of wearing the PAM sensor • Number of days of for which protein intake is recorded • Number of contact moments with the physiotherapist and dietician |
T1 t/m T5 | |
| Process evaluation | Focus groups | A qualitative process evaluation will be performed to evaluate the implementation of the intervention in order to identify possible barriers and facilitators for further implementation. Focus groups will be conducted with patients from the intervention group, involved healthcare professionals and members of the research team to evaluate the intervention and implementation process. In addition, user adherence of the smartphone application and web application will be measured during the study | NA | |
| Healthcare use | Self-reported and Medical Record Data | The number of consultations with a healthcare provider will be registered by asking the patients about their use of paramedic consultations in primary care and by screening the Medical Record data. This can be used to determine the difference in healthcare use and costs between the intervention and control group | ||
T0 = baseline (preoperative), T1 = at hospital discharge, T2 = 1 week after discharge, T3 = 4 weeks after hospital discharge, T4 = 8 weeks after hospital discharge, T5 = 12 weeks after hospital discharge, T6 = 6 months after hospital discharge, NA = not applicable
Data collection
At baseline, the questionnaires will be sent by the researcher via the OnlinePROMS platform. After registration of the date of hospital discharge, the questionnaires will be automatically sent at 1 week, 4 weeks, 8 weeks, 12 weeks, and 6 months after hospital discharge. To improve the retention rate, patients will receive a reminder automatically after 2 and 5 days.
Standard operating procedures have been established for performing the physical measurements. All research assistants are trained to perform these measurements accurately and consistently. To ensure a higher follow-up rate, researchers have the flexibility to visit patients at home to perform the physical measurements. This approach aims to enhance convenience for participants and increase the likelihood of their continued participation in the study.
Data management
Data obtained from the medical record and collected during baseline and follow-up measurements will be manually entered into Castor EDC. Castor EDC incorporates protection for data entry and validation to reduce data entry errors, and management features to facilitate audits and data quality assurance. Data from the online questionnaires will be saved at the OnlinePROMS database.
Both databases have been specifically developed to ensure the safeguarding of participant information in accordance with data protection regulations. Participants will be identified solely by a unique patient ID number, ensuring their anonymity. Trial-related documents will be securely stored and restricted to trial staff and authorized personnel only. Data will be anonymized promptly whenever feasible. All essential data that contains identifiable information will be retained for a period of 10 years, while anonymized digital data will be stored indefinitely. The Chief Investigator holds the role of the data manager, overseeing the management and security of the data.
Cleaned data sets will be made available to all Principal Investigators. These data sets will be securely stored on the research drive of Amsterdam UMC, VUmc location, and protected by passwords. Each Project Principal Investigator will have direct access to the data sets from their respective site, and access to data from other sites can be obtained upon request. To ensure confidentiality and privacy, any identifying participant information will be removed from the data sets if possible, when shared with project team members. Access to the full protocol, participant-level dataset and statistical code for non-commercial researchers outside the project team will be available from the corresponding author upon reasonable request.
Statistical analysis
Missing data will be handled using longitudinal data analysis. The differences in course of recovery between groups, measured with the CAT PROMIS-PF, will be analyzed using linear mixed model analysis, with group as independent variable and the PROMIS-PF at all postoperative measurement points (T1–T6) as dependent variable, adjusting for baseline PROMIS-PF (T0). The primary analysis will be conducted based on the full analysis set according to the intention to treat method.
Descriptive statistics will be calculated for all parameters, include mean, median, standard deviation, standard error of the mean and the interquartile range. In addition, per-protocol analysis will be performed among all participants with sufficient protocol adherence (> 80%). Continuous secondary outcomes are analyzed using linear mixed model, with group as the independent variable and outcome at all postoperative measurement points as dependent variable, adjusting for baseline scores. Dichotomous outcomes are analyzed using generalized mixed model with the same multilevel structure. A mediation analyses will be performed on the longitudinal trial data to determine if the relationship between the intervention and the primary outcome (PROMIS-PF) can be explained by improvement of PA and protein intake.
Qualitative data analysis of the focus groups will be conducted following the steps of thematic analysis by two researchers.
Dissemination policy
Research findings will be shared through publication in leading international peer-reviewed journals and through presentations at both national and international conferences. We are committed to disseminating the findings to all relevant stakeholders. In addition, a summary of the research findings will be sent to participants who have indicated that they would like to receive such information once the research findings are published. Standard authorship eligibility guidelines will be followed and professional writers will not be used.
Discussion
The aim of this RCT is to evaluate the efficacy of a blended intervention to improve physical activity and protein intake on recovery of physical functioning in patients after gastrointestinal and lung cancer surgery. The OPRAH intervention, investigated in this RCT, aims to increase the patient’s self-management in physical recovery, by using a smartphone application, accelerometer and coaching by a physiotherapist and dietician to improve the patient’s level of physical activity and protein intake after hospital discharge. In addition, the OPRAH intervention aims to improve the collaboration between physiotherapy and dietetics in order to achieve an optimal synergy between nutrition and physical activity in patients after oncological GI and lung surgery. With this RCT, the short- and longer-term changes in physical functioning, physical activity, and protein intake will be determined with the hypothesis that a higher level of physical activity and protein intake will improve the recovery in physical functioning.
To improve recovery in physical functioning after oncological surgery, multiple studies have focused on prehabilitation, i.e. the process to enhance the patient’s functional capacity prior to major surgery, in order to enhance clinical outcomes and therefore reduce postoperative complications [67, 68]. However, surgery causes surgery-related muscle loss: muscle loss caused by increased catabolism due to surgical stress [7]. A recent study showed that more than 50% of the patients had surgery-related muscle loss [69]. Important risk factors that contribute to loss of muscle mass after oncological surgery are inactivity and malnutrition [9, 69]. To counteract the adverse effects of surgery, it is important that interventions also focus on encouraging physical activity and protein intake in the period after surgery.
This study has several strengths. First, the theoretical underpinnings of the intervention, by conducting a systematic review on the use of activity trackers and by using the Behavioral Change Wheel. Second, the tailored approach, by personalizing physical activity and protein goals for each patient and allowing the health professional to monitor the patient remotely. Third, an app that combines self-monitoring of physical activity and protein. This combination is unique and can facilitate collaboration between the physical therapist and dietitian. Fourth, a strength of the trial design is the use of blinded assessors.
A limitation of the study is that the intervention is only accessible for patients who are able to understand the Dutch language and have their own smartphone. However, based on our experiences with the feasibility studies, we have found that currently very few patients do not own a smartphone. In addition, a study is ongoing to make the Atris app more inclusive and therefore more accessible to patients with low health literacy or who do not speak the Dutch language. If our RCT shows effectiveness, the adapted app can be implemented in the OPRAH intervention.
To our knowledge, this is the first multicenter, assessor-blind RCT testing the effect of a blended intervention focused on improving physical activity and protein intake after discharge in patients who have undergone elective GI, HPB, or lung cancer surgery on the outcome recovery in physical functioning. The results of this research will reveal whether the OPRAH intervention, aiming to motivate patients and to assist health professionals to provide ongoing monitoring and support after hospital discharge, is an effective intervention to increase physical activity and protein intake and improve physical functioning recovery in these patients.
Trial status
The most recent version of the protocol is version 3, updated on 5 July 2022. The recruitment started at 24 June 2022. Recruitment is expected to be completed by December 2023.
Supplementary Information
Additional file 1. Intervention description and replication (TiDieR).
Additional file 2. Subscript screenshots figure 2.
Additional file 3. Mapping activities to MRC Framework.
Additional file 4. The behavioural change wheel.
Acknowledgements
Not Applicable.
Supplementary information
Composition, roles and responsibilities of the coordinating center, OPRAH consortium, Medical Ethics Committee and ORPAH research group.
Coordinating center
Amsterdam UMC, location VUmc, is the coordinating center of this trial. Therefore, they are responsible for the participant recruitment, data collection, and data analysis. Amsterdam UMC, location VUmc, is also the owner of the medical devices used in this trial (movement sensors and associated software) and therefore maintains the management of these devices. MdL is mainly responsible for this.
OPRAH consortium
The OPRAH consortium is a multidisciplinary group that aims to provide expert advice and monitor the progress during the preparation and execution of the trial. Please refer to the author list for the members of the OPRAH consortium. Meetings are held twice a year, and more frequently if deemed necessary.
Medical Ethics Committee (MEC)
The MEC of Amsterdam UMC, VUmc location, is an independent multidisciplinary group that monitors the safety and effectiveness of the trial and oversees the overall conduct of the study. The Data Monitoring Center (DMC) supports clinical researchers in ensuring compliance with relevant laws and regulatory requirements, such as WMO, ICH-GCP, and/or ISO14155. During the trial, there will be four on-site monitoring visits at Amsterdam UMC, VUmc location, and three monitoring visits at St. Antonius Hospital, Nieuwegein. Both sites will have a close-out visit after the last subject visit.
OPRAH research group
The OPRAH research group is responsible for the management of the trial and will be led by MdL, the executive researcher of the OPRAH study. Other members of the Trial management group include MvdL (principal investigator), MvdS (principal investigator), EG (innovator and physiotherapist), StD (dietician-researcher), and HK (dietician-researcher). The OPRAH research group will meet approximately once a month to oversee the day-to-day management of the trial.
Abbreviations
- BCT
Behavioral change technique
- CAT
Computer Adaptive Testing
- CONSORT
Consolidated Standards of Reporting Trials
- EDC
Electronic Data Capture
- ERAS
Early Recovery After Surgery
- GI
Gastrointestinal
- HPB
Hepato-pancreato-biliary
- HIPEC
Hypertherme Intraperitoneale Chemotherapy
- MET
Metabolic equivalents of energy expenditure
- MI
Motivational interviewing
- MMSE
Mini-Mental State Examination
- MRC
Medical Research Council
- OPRAH
Optimal Physical Recovery After Hospitalization
- PROMIS
Patient-reported outcome measure information System
- RCT
Randomized controlled trial
- SDM
Shared decision making
Authors’ contributions
MvdL and MvdS are the principal investigators; they conceived the study. MdL is the executive investigator; she led the proposal and protocol development and drafted the work. SD, HK, PW, and EG contributed in the development of the intervention and study design. VdG substantially revised the protocol. All authors, including the authors of the OPRAH consortium, read and approved the final manuscript.
Funding
A research grant of the Amsterdam Movement Sciences Institute was received for financing 50% of a PhD project aiming to develop and evaluate an accelerometer-based feedback and coaching intervention for patients at risk for functional decline admitted to the hospital. A copy of the original funding documentation is presented in supplementary file 2. This current RCT was conducted in support of this project. The funder had no role in the study design, the data collection, analysis, writing of the article, or in the decision to submit the paper for publication.
Availability of data and materials
The datasets generated and/or analyzed are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The trial protocol has been approved by the Medical Ethical Research Committee (METC) of Amsterdam UMC, location VUmc (METC 2021.0627). This study will be conducted according to the principles of the Declaration of Helsinki (version 9, October 2013), in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO) and the Dutch Personal Data Protection Act (General Data Protection Regulation (GDPR)). The trial has been registered at the International Clinical Trials Registry Platform (NL9793) and the study description is available at the following web link: https://trialsearch.who.int/Trial2.aspx?TrialID=NL9793. All amendments of the protocol will be notified to the METC and the competent authority. Non-substantial amendments will not be notified to the accredited METC and the competent authority, but will be recorded and filed by the sponsor. Participating sites in the study will be notified of any changes to the study protocols. This will enable them to make the necessary arrangements to incorporate the changes, if necessary, and to confirm their continued support for the study after the implementation of the changes.
All patients will sign informed consent prior to participation in the trial. All participants will be informed that they are under no obligation to enter the trial and that they can withdraw at any time during the trial without having to give a reason and that this will not affect the medical care they receive.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marike van der Leeden and Marike van der Schaaf share last authorship.
Contributor Information
Marijke de Leeuwerk, Email: m.e.deleeuwerk@amsterdamumc.nl.
on behalf of the OPRAH consortium:
Chris Dickhoff, Marc G. Besselink, Jurriaan B. Tuynman, Mark I. van Berge Henegouwen, Joris I. Erdmann, Rosalie J. Huijsmans, Hidde P. van der Ploeg, Anne M. Eskes, Mirjam A. G. M. Pijnappels, Liesbeth Schuijs van Leeuwen, Anke B. Smits, Jasmijn van Dijk, and Eva Grimbergen
References
- 1.van der Leeden M, Balland C, Geleijn E, et al. In-hospital mobilization, physical fitness, and physical functioning after lung cancer surgery. Ann Thorac Surg. 2019;107:1639–1646. doi: 10.1016/j.athoracsur.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 2.van Zutphen M, Winkels RM, van Duijnhoven FJ, et al. An increase in physical activity after colorectal cancer surgery is associated with improved recovery of physical functioning: a prospective cohort study. BMC Cancer. 2017;17:74. doi: 10.1186/s12885-017-3066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmström M, Klefsgard R, Ivarsson B, et al. Quality of life measurements as an indicator for timing of support after oesophagectomy for cancer: a prospective study. BMC Health Serv Res. 2015;15:96. doi: 10.1186/s12913-015-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarinsefat A, Terjimanian MN, Sheetz KH, et al. Perioperative changes in trunk musculature and postoperative outcomes. J Surg Res. 2014;191:106–112. doi: 10.1016/j.jss.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Huang DD, Ji YB, Zhou DL, et al. Effect of surgery-induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res. 2017;218:58–66. doi: 10.1016/j.jss.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Otsuji H, Yokoyama Y, Ebata T, et al. Surgery-related muscle loss and its association with postoperative complications after major hepatectomy with extrahepatic bile duct resection. World J Surg. 2017;41:498–507. doi: 10.1007/s00268-016-3732-6. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama T, Kawabe T, Hirohito F, et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer. 2016;19:645–650. doi: 10.1007/s10120-015-0496-x. [DOI] [PubMed] [Google Scholar]
- 8.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai HF, Liu CY, Yang SH, et al. Factors related to frailty in older cancer patients undergoing colorectal surgery: a longitudinal study. Cancer Nurs. 2021 doi: 10.1097/ncc.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 10.Carli F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: implications of the stress response. Can J Anaesth. 2015;62:110–119. doi: 10.1007/s12630-014-0264-0. [DOI] [PubMed] [Google Scholar]
- 11.Joanisse S, McKendry J, Lim C, et al. Understanding the effects of nutrition and post-exercise nutrition on skeletal muscle protein turnover: insights from stable isotope studies. Clin Nutr Open Sci. 2021;36:56–77. doi: 10.1016/j.nutos.2021.01.005. [DOI] [Google Scholar]
- 12.Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown CJ, Williams BR, Woodby LL, et al. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2:305–313. doi: 10.1002/jhm.209. [DOI] [PubMed] [Google Scholar]
- 14.Hoyer EH, Brotman DJ, Chan KS, et al. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94:304–312. doi: 10.1097/phm.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenders N, Weenk M, van de Belt TH, et al. Exploring barriers to physical activity of patients at the internal medicine and surgical wards: a retrospective analysis of continuously collected data. Disabil Rehabil 2019: 1–7. 10.1080/09638288.2019.1685013. [DOI] [PubMed]
- 16.Granger CL, Connolly B, Denehy L, et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer. 2017;25:983–999. doi: 10.1007/s00520-016-3484-8. [DOI] [PubMed] [Google Scholar]
- 17.Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123:1455–1472. doi: 10.1097/aln.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman JGJ, Dekker-van Weering M, Wouters M, et al. Physical behavior and associations with health outcomes in operable NSCLC patients: a prospective study. Lung Cancer. 2018;119:91–98. doi: 10.1016/j.lungcan.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill L, Bennett AE, Guinan E, et al. Physical recovery in the first six months following oesophago-gastric cancer surgery. Identifying rehabilitative needs: a qualitative interview study. Disabil Rehabil 2019: 1–8. 10.1080/09638288.2019.1663946. [DOI] [PubMed]
- 20.Schoufour JD, Overdevest E, Weijs PJM, et al. Dietary protein, exercise, and frailty domains. Nutrients 2019; 11. 10.3390/nu11102399. [DOI] [PMC free article] [PubMed]
- 21.Castro O, Ng K, Novoradovskaya E, et al. A scoping review on interventions to promote physical activity among adults with disabilities. Disabil Health J. 2018;11:174–183. doi: 10.1016/j.dhjo.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Leeuwerk ME, Bor P, Van der Ploeg HP, et al. The effectiveness of interventions using activity trackers during or after inpatient care: a systemativ review and meta-analysis. Manuscript accepted for publication in the International Journal of Behavioural Nutrition and Physical Activity [DOI] [PMC free article] [PubMed]
- 23.McCahon D, Daley AJ, Jones J, et al. Enhancing adherence in trials promoting change in diet and physical activity in individuals with a diagnosis of colorectal adenoma; a systematic review of behavioural intervention approaches. BMC Cancer. 2015;15:505. doi: 10.1186/s12885-015-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019;13:S27–S30. doi: 10.4103/sja.SJA_559_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castor EDC, https://www.castoredc.com/.
- 26.Pol MC, Ter Riet G, van Hartingsveldt M, et al. Effectiveness of sensor monitoring in a rehabilitation programme for older patients after hip fracture: a three-arm stepped wedge randomised trial. Age Ageing. 2019;48:650–657. doi: 10.1093/ageing/afz074. [DOI] [PubMed] [Google Scholar]
- 27.van der Meij E, Anema JR, Leclercq WKG, et al. Personalised perioperative care by e-health after intermediate-grade abdominal surgery: a multicentre, single-blind, randomised, placebo-controlled trial. Lancet (London, England) 2018;392:51–59. doi: 10.1016/s0140-6736(18)31113-9. [DOI] [PubMed] [Google Scholar]
- 28.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ (Clinical research ed) 2021;374:n2061. doi: 10.1136/bmj.n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeuwerk ME, Botjes M, Van Vliet V, et al. Feasibility of self-monitoring of physical activity after hospital discharge in patients who have undergone gastrointestinal or lung cancer surgery: a mixed methods study Manuscript accepted for publication in JMIR Cancer. [DOI] [PMC free article] [PubMed]
- 30.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 32.Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325–334. doi: 10.1017/S135246580001643X. [DOI] [PubMed] [Google Scholar]
- 33.Nuss K, Moore K, Nelson T, et al. Effects of motivational interviewing and wearable fitness trackers on motivation and physical activity: a systematic review. Am J Health Prom AJHP. 2021;35:226–235. doi: 10.1177/0890117120939030. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann TC, Légaré F, Simmons MB, et al. Shared decision making: what do clinicians need to know and why should they bother? Med J Aust. 2014;201:35–39. doi: 10.5694/mja14.00002. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann TC, Lewis J, Maher CG. Shared decision making should be an integral part of physiotherapy practice. Physiotherapy. 2020;107:43–49. doi: 10.1016/j.physio.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Pędziwiatr M, Mavrikis J, Witowski J, et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. 2018;35:95. doi: 10.1007/s12032-018-1153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terwee CB, Roorda LD, de Vet HC, et al. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS) Qual Life Res. 2014;23:1733–1741. doi: 10.1007/s11136-013-0611-6. [DOI] [PubMed] [Google Scholar]
- 38.Rose M, Bjorner JB, Gandek B, et al. The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67:516–526. doi: 10.1016/j.jclinepi.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crins MHP, van der Wees PJ, Klausch T, et al. Psychometric properties of the PROMIS physical function item bank in patients receiving physical therapy. PLoS ONE. 2018;13:e0192187. doi: 10.1371/journal.pone.0192187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel AA, Dodwad SM, Boody BS, et al. Validation of Patient Reported Outcomes Measurement Information System (PROMIS) Computer Adaptive Tests (CATs) in the surgical treatment of lumbar spinal stenosis. Spine. 2018;43:1521–1528. doi: 10.1097/brs.0000000000002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Meij E, Anema JR, Huirne JAF, et al. Using PROMIS for measuring recovery after abdominal surgery: a pilot study. BMC Health Serv Res. 2018;18:128. doi: 10.1186/s12913-018-2929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellers C, Dall P, Grant M, et al. Validity and reliability of the activPAL3 for measuring posture and stepping in adults and young people. Gait Posture. 2016;43:42–47. doi: 10.1016/j.gaitpost.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Grant PM, Dall PM, Mitchell SL, et al. Activity-monitor accuracy in measuring step number and cadence in community-dwelling older adults. J Aging Phys Act. 2008;16:201–214. doi: 10.1123/japa.16.2.201. [DOI] [PubMed] [Google Scholar]
- 44.Bassett DR, Jr, John D, Conger SA, et al. Detection of lying down, sitting, standing, and stepping using two activPAL monitors. Med Sci Sports Exerc. 2014;46:2025–2029. doi: 10.1249/mss.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 45.Ræder H, Kværner AS, Henriksen C, et al. Validity of bioelectrical impedance analysis in estimation of fat-free mass in colorectal cancer patients. Clin Nutr. 2018;37:292–300. doi: 10.1016/j.clnu.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 49.Ameringer S, Elswick RK, Jr, Menzies V, et al. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res. 2016;65:279–289. doi: 10.1097/nnr.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNaughton SA, Mishra GD, Bramwell G, et al. Comparability of dietary patterns assessed by multiple dietary assessment methods: results from the 1946 British birth cohort. Eur J Clin Nutr. 2005;59:341–352. doi: 10.1038/sj.ejcn.1602079. [DOI] [PubMed] [Google Scholar]
- 51.Rossato SL, Fuchs SC. Diet data collected using 48-h dietary recall: within-and between-person variation. Front Nutr. 2021;8:667031. doi: 10.3389/fnut.2021.667031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trutschnigg B, Kilgour RD, Reinglas J, et al. Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Appl Physiol Nutr Metab. 2008;33:1232–1239. doi: 10.1139/h08-122. [DOI] [PubMed] [Google Scholar]
- 53.Peolsson A, Hedlund R, Oberg B. Intra- and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 54.Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE. 2014;9:e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millor N, Lecumberri P, Gómez M, et al. An evaluation of the 30-s chair stand test in older adults: frailty detection based on kinematic parameters from a single inertial unit. J Neuroeng Rehabil. 2013;10:86. doi: 10.1186/1743-0003-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 57.Bohannon RW, Crouch RH. Two-minute step test of exercise capacity: systematic review of procedures, performance, and clinimetric properties J. Geriatr Phys Ther. 2019;42:105–112. doi: 10.1519/jpt.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 58.Voeding & Beweging (VBNU) - functionele testen, https://voedingenbeweging.nu/functionele-testen/.
- 59.Beurskens AJ, de Vet HC, Köke AJ, et al. A patient-specific approach for measuring functional status in low back pain. J Manipulative Physiol Ther. 1999;22:144–148. doi: 10.1016/s0161-4754(99)70127-2. [DOI] [PubMed] [Google Scholar]
- 60.Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol. 2005;139:439–457. doi: 10.3200/jrlp.139.5.439-457. [DOI] [PubMed] [Google Scholar]
- 61.Terwee CB, Crins MHP, Boers M, et al. Validation of two PROMIS item banks for measuring social participation in the Dutch general population. Qual Life Res. 2019;28:211–220. doi: 10.1007/s11136-018-1995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwenkglenks M, Matter-Walstra K. Is the EQ-5D suitable for use in oncology? an overview of the literature and recent developments. Expert Rev Pharmacoecon Outcomes Res. 2016;16:207–219. doi: 10.1586/14737167.2016.1146594. [DOI] [PubMed] [Google Scholar]
- 63.Hudak PL, Wright JG. The characteristics of patient satisfaction measures. Spine. 2000;25:3167–3177. doi: 10.1097/00007632-200012150-00012. [DOI] [PubMed] [Google Scholar]
- 64.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 65.van Blijswijk S. MMSE schakel in diagnostisch traject. Huisarts en wetenschap. 2017;60:142–142. doi: 10.1007/s12445-017-0083-y. [DOI] [Google Scholar]
- 66.Eikelenboom N, Smeele I, Faber M, et al. Validation of Self-Management Screening (SeMaS), a tool to facilitate personalised counselling and support of patients with chronic diseases. BMC Fam Pract. 2015;16:165. doi: 10.1186/s12875-015-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Klerk M, van Dalen DH, Nahar-van Venrooij LMW, et al. A multimodal prehabilitation program in high-risk patients undergoing elective resection for colorectal cancer: a retrospective cohort study. Eur J Surg Oncol. 2021;47:2849–2856. doi: 10.1016/j.ejso.2021.05.033. [DOI] [PubMed] [Google Scholar]
- 68.Santa Mina D, Scheede-Bergdahl C, Gillis C, et al. Optimization of surgical outcomes with prehabilitation. Appl Physiol Nutr Metab. 2015;40:966–969. doi: 10.1139/apnm-2015-0084. [DOI] [PubMed] [Google Scholar]
- 69.van Wijk L, van Duinhoven S, Liem MSL, et al. Risk factors for surgery-related muscle quantity and muscle quality loss and their impact on outcome. Eur J Med Res. 2021;26:36. doi: 10.1186/s40001-021-00507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Intervention description and replication (TiDieR).
Additional file 2. Subscript screenshots figure 2.
Additional file 3. Mapping activities to MRC Framework.
Additional file 4. The behavioural change wheel.
Data Availability Statement
The datasets generated and/or analyzed are available from the corresponding author upon reasonable request.