Abstract
Monoamine oxidase (MAO) plays a central role in the metabolism of the neurotransmitters dopamine, norepinephrine, and serotonin. This brief review focuses on 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is the immediate product of MAO acting on cytoplasmic dopamine. DOPAL is toxic; however, normally DOPAL is converted via aldehyde dehydrogenase (ALDH) to 3,4-dihydroxyphenylacetic acid (DOPAC), which rapidly exits the neurons. In addition to vesicular uptake of dopamine via the vesicular monoamine transporter (VMAT), the two-enzyme sequence of MAO and ALDH keeps cytoplasmic dopamine levels low. Dopamine oxidizes readily to form toxic products that could threaten neuronal homeostasis. The catecholaldehyde hypothesis posits that diseases featuring catecholaminergic neurodegeneration result from harmful interactions between DOPAL and the protein alpha-synuclein, a major component of Lewy bodies in diseases such as Parkinson disease, dementia with Lewy bodies, and pure autonomic failure. DOPAL potently oligomerizes alpha-synuclein, and alpha-synuclein oligomers impede vesicular functions, shifting the fate of cytoplasmic dopamine toward MAO-catalyzed formation of DOPAL—a vicious cycle. When MAO deaminates dopamine to form DOPAL, hydrogen peroxide is generated; and DOPAL, hydrogen peroxide, and divalent metal cations react to form hydroxyl radicals, which peroxidate lipid membranes. Lipid peroxidation products in turn inhibit ALDH, causing DOPAL to accumulate—another vicious cycle. MAO inhibition decreases DOPAL formation but concurrently increases the spontaneous oxidation of dopamine, potentially trading off one form of toxicity for another. These considerations rationalize a neuroprotection strategy based on concurrent treatment with an MAO inhibitor and an anti-oxidant.
Keywords: Monoamine oxidase, Dopamine, DOPAL, Alpha-synuclein
Introduction
Monoamine oxidases (EC 1.4.3.4) are flavin-containing enzymes that use oxygen to remove amine groups from monoamines such as serotonin and the catecholamines dopamine, norepinephrine, and adrenaline. In the process hydrogen peroxide, ammonia, and monoamine aldehydes are generated. The reaction of MAO with cytoplasmic dopamine results in the formation of the catecholaldehyde 3,4-dihydroxyphenylacetaldehyde (DOPAL).
In monoaminergic neurons MAO is present in the outer mitochondrial membrane. The enzyme occurs in two isoforms, MAO-A and MAO-B (Youdim and Riederer 2004). The genes encoding these isoforms are located next to each other on the X-chromosome. Although most of MAO activity in the brain is of the B type, MAO-A figures prominently in the oxidative deamination of striatal dopamine (Demarest and Moore 1981; Wachtel and Abercrombie 1994; Dyck et al. 1993; Kumagae et al. 1991; Colzi et al. 1990); however, administration of drugs that are selective MAO-B inhibitors in vitro can inhibit MAO-A in vivo (Eisenhofer et al. 1986; Fowler et al. 2015).
MAO plays key roles in the metabolism of endogenous monoamines. This review focuses on DOPAL, the immediate product of MAO acting on cytoplasmic dopamine. Although essentially all of the metabolism of neuronal dopamine passes through DOPAL, the literature on DOPAL is scanty, with only a bit over 100 articles culled from PubMed, in contrast with more than 150,000 articles on dopamine and more than 20,000 on MAO.
DOPAL toxicity
As for all endogenously produced aldehydes, DOPAL is toxic. Probably the first report describing DOPAL toxicity was that by Mattammal et al. (1995). Incubation of rat pheochromocytoma PC12 cells with 6.5 μM DOPAL for 24 h resulted in withdrawal of neurites and cell death; and exposure of mesencephalic cultures to 33 μM DOPAL evoked loss of tyrosine hydroxylase (TH)-positive neurons and a reduced fiber network. Subsequent reports by Burke et al. (Kristal et al. 2001; Li et al. 2001; Burke et al. 2003, 2004; Panneton et al. 2010) noted substantial cytotoxicity and neurotoxicity. In energetically compromised mitochondria from PC12 cells, DOPAL was more than 1000 times as potent as dopamine in inducing the mitochondrial permeability transition pore protein, a harbinger of cell death (Kristal et al. 2001). DOPAL reacts with hydrogen peroxide (produced concurrently with DOPAL when MAO metabolizes cytoplasmic dopamine) to form hydroxyl radicals (Li et al. 2001). Hydroxyl radicals peroxidate lipid membranes, and the lipid peroxidation product 4-hydroxynonenal inhibits aldehyde dehydrogenase (ALDH), promoting DOPAL accumulation (Florang et al. 2007)—one of several potential vicious cycles that could threaten homeostasis in dopaminergic neurons. In rats, DOPAL microinjection into the substantia nigra reduces counts of TH-positive neurons and evokes rotational behavior indicating nigrostriatal dopamine deficiency (Panneton et al. 2010).
MAO and ALDH in series help keep cytoplasmic dopamine levels low
In rat striatum, endogenous DOPAL is formed from the action of MAO-A on cytoplasmic dopamine (Fornai et al. 2000). Normally DOPAL is converted via aldehyde dehydrogenase (ALDH) to the acid 3,4-dihydroxyphenylacetic acid (DOPAC), which rapidly exits the cells. In PC12 cells DOPAC is actively extruded via a sulfonylurea-sensitive transporter (Lamensdorf et al. 2000c).
Active uptake of cytoplasmic dopamine into vesicles via the vesicular monoamine transporter (VMAT) is not only required for dopaminergic neurotransmission but also serves as a detoxification mechanism (Gainetdinov et al. 1998; Fumagalli et al. 1999; Staal and Sonsalla 2000; Guillot and Miller 2009). This includes autotoxicity exerted by dopamine itself (Weingarten and Zhou 2001). Dopamine is well known to be prone to spontaneous oxidation to form a variety of oxidation products that are potentially toxic, including aminochrome (Munoz et al. 2012; Segura-Aguilar 2017) and 5-S-cysteinyldopamine (Cys-DA) (Badillo-Ramirez et al. 2019; Vauzour et al. 2010). As one would predict, animals with reduced vesicular uptake of dopamine have evidence of progressive nigrostriatal neurodegeneration (Caudle et al. 2007), while increased VMAT activity is neuroprotective (Lohr et al. 2014; Munoz et al. 2012).
One may conceptualize that MAO and ALDH act in series to keep cytoplasmic dopamine levels low (Fig. 1). MAO inhibition increases endogenous Cys-DA levels in guinea pig striatum (Fornstedt and Carlsson 1989) and in PC12 cells (Goldstein et al. 2016), consistent with a buildup of cytoplasmic dopamine.
Fig. 1.
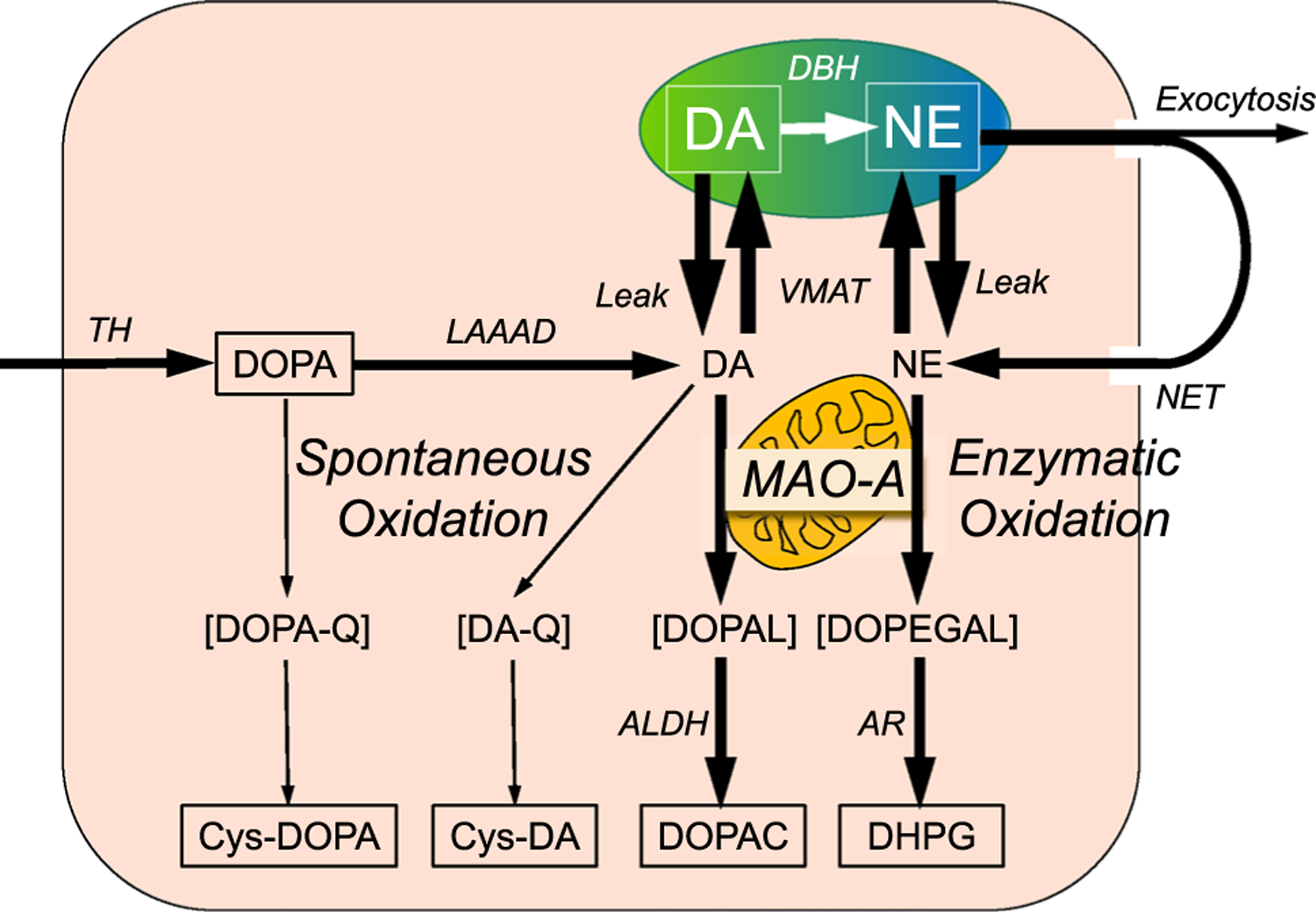
Overview of the sources and fate of intra-neuronal catecholamines, with emphasis on enzymatic oxidation catalyzed by MAO. Dopamine (DA) is produced in the cytoplasm via tyrosine hydroxylase (TH) acting on tyrosine to form 3,4-dihydroxyphenylalanine (DOPA) and then L-aromatic-amino-acid decarboxylase (LAAAD) acting on DOPA to form dopamine. Most of cytoplasmic DA is taken up into vesicles by way of the vesicular monoamine transporter (VMAT). Dopamine-beta-hydroxylase (DBH) in the vesicles catalyzes the production of norepinephrine (NE) from DA. Cytoplasmic DA is subject to oxidative deamination catalyzed by monoamine oxidase-A (MAO-A) in the outer mitochondrial membrane to form 3,4-dihydroxyphenylacetaldehyde (DOPAL), and NE is deaminated to form 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL). DOPAL is converted to 3,4-dihydroxyphenylacetic acid (DOPAC) via aldehyde dehydrogenase (ALDH), and DOPEGAL is converted to 3,4-dihydroxyphenylglycol (DHPG) via aldehyde/aldose reductase (AR). Most of vesicular DA and NE released by exocytosis is taken back up into the cytoplasm via cell membrane transporters—the NET for NE (although DA is a better substrate than NE for uptake via the NET). DOPA can undergo spontaneous oxidation to DOPA-quinone (DOPA-Q), resulting in formation of 5-S-cysteinylDOPA (Cys-DOPA), and DA can undergo spontaneous oxidation to DA-quinone (DA-Q), resulting in formation of 5-S-cysteinylDA (Cys-DA)
The catecholaldehyde hypothesis
Given the toxicity exerted by exogenously administered DOPAL and the fact that DOPAL is produced continuously in catecholaminergic neurons, one may reasonably hypothesize that endogenous DOPAL acts as an autotoxin that contributes to catecholaminergic neurodegeneration. This is the essence of the “catecholaldehyde hypothesis” (Panneton et al. 2010).
A variety of animal studies have found that genetic abnormalities or environmental exposures that increase endogenous DOPAL levels result in catecholaminergic neurodegeneration. Pharmacologic inhibition of vesicular uptake increases endogenous DOPAL levels in PC12 cells (Goldstein et al. 2012), and mice with genetically determined low VMAT activity have aging-related loss of both nigral dopaminergic and locus ceruleus noradrenergic neurons (Caudle et al. 2007; Taylor et al. 2014); and mice with knockouts of cytosolic and mitochondrial ALDH have DOPAL buildup and nigrostriatal dopaminergic neurodegeneration (Wey et al. 2012).
In humans it has been reported that decreased ALDHA1A gene expression in blood is part of a “molecular signature” that can identify early PD (Molochnikov et al. 2012). ALDH1A1 gene expression and protein are decreased in substantia nigra specimens in patients with PD (Grunblatt et al. 2018; Mandel et al. 2005).
The complex I inhibitor rotenone decreases production of NAD+, a required co-factor for ALDH. In PC12 cells rotenone increases endogenous DOPAL production (Lamensdorf et al. 2000a; Goldstein et al. 2015a), and DOPAL potentiates acute rotenone-induced cytotoxicity (Lamensdorf et al. 2000b). In the rats subacute administration of rotenone increases brain DOPAL levels and produces locomotor abnormalities resembling those in Parkinson disease (unpublished observations). The fungicide benomyl also inhibits ALDH and builds up endogenous DOPAL (Casida et al. 2014), and farm chemicals inhibiting ALDH may contribute to the incidence of PD (Fitzmaurice et al. 2013, 2014; Ritz et al. 2016).
Clinical post-mortem studies have noted DOPAL buildup in the putamen in PD (Goldstein et al. 2011, 2013) and multiple system atrophy (Goldstein et al. 2015b, 2017b), both of which feature severe putamen dopamine deficiency (Kish et al. 1988; Goldstein et al. 2017b).
An almost completely independent line of research has implicated dopamine itself as an autotoxin, based on oxidation of dopamine to dopamine-quinone and then a variety of distal oxidation products (Fig. 2) (Carlsson and Fornstedt 1991; Weingarten and Zhou 2001; Dukes et al. 2005; Khan et al. 2005; Hasegawa et al. 2006; Bisaglia et al. 2007; Chen et al. 2008; Paris et al. 2009; Leong et al. 2009; Mosharov et al. 2009; Hastings 2009; Bisaglia et al. 2010; Surmeier et al. 2011; Wu and Johnson 2011; Jana et al. 2011a; Surh and Kim 2010; Lee et al. 2011; Gautam and Zeevalk 2011; Munoz et al. 2012; Bisaglia et al. 2013; Su et al. 2013; Banerjee et al. 2014; Cai et al. 2014; Herrera et al. 2017; Burbulla et al. 2017; Mor et al. 2017; Badillo-Ramirez et al. 2019). Some of these are known to be neurotoxic, such as aminochrome (Linsenbardt et al. 2009; Paris et al. 2009; Segura-Aguilar 2019), 5-S-cysteinyldopamine (Montine et al. 1997; Badillo-Ramirez et al. 2019), and isoquinolines (Storch et al. 2002; Nagatsu 1997). These compounds have in common that they evoke mitochondrial dysfunction (Jana et al. 2011b).
Fig. 2.
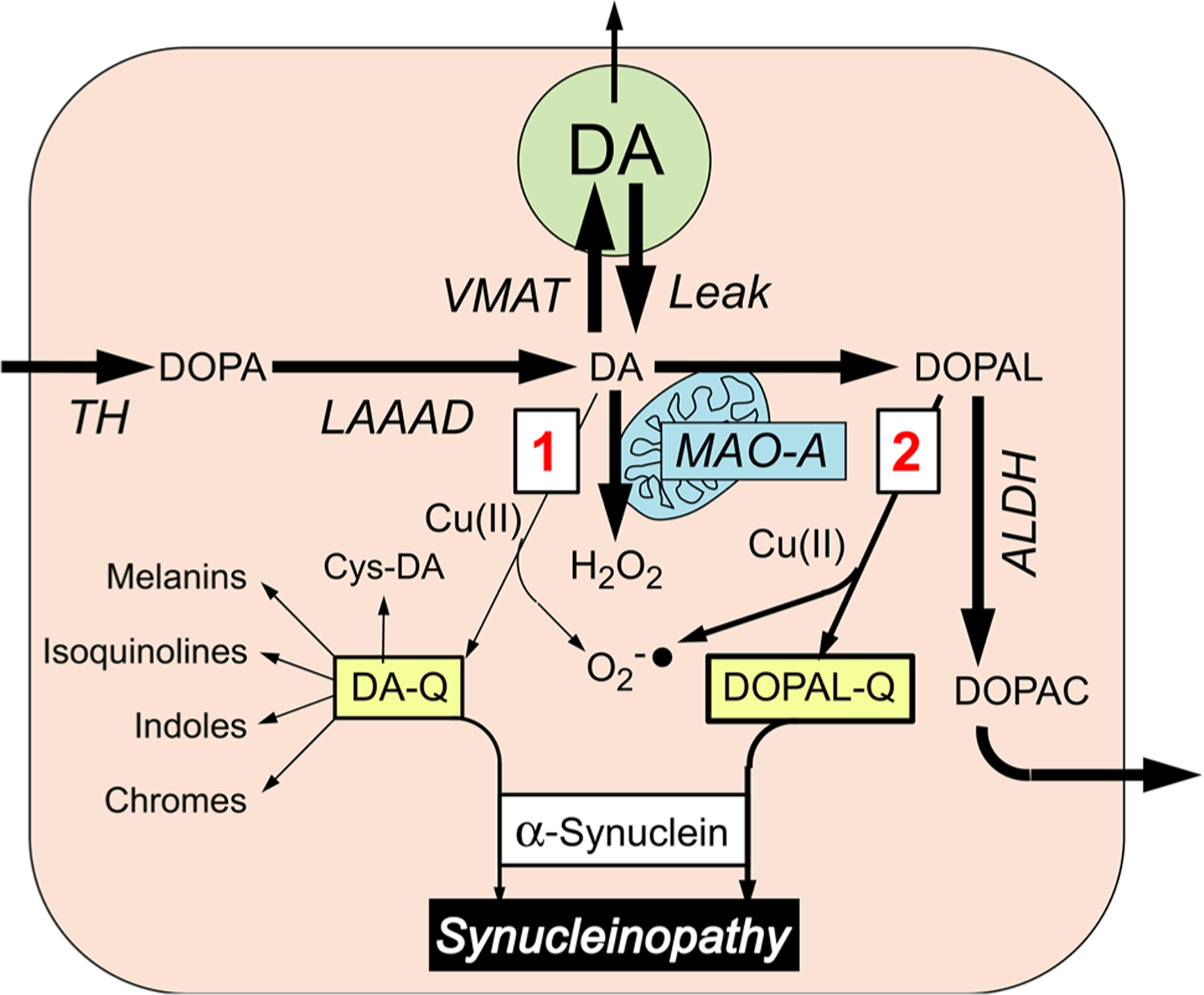
Alternative routes by which oxidation of cytoplasmic dopamine (DA) may modify alpha-synuclein. Most of cytoplasmic DA is taken up into vesicles via the vesicular monoamine transporter (VMAT); a minority undergoes oxidation, by two routes (red numbers in boxes). In route 1, DA is oxidized to form DA-quinone (DA-Q), with subsequent interactions with alpha-synuclein directly or via various further products of DA-Q, including 5-S-cysteinyldopamine (Cys-DA). In route 2, DA is oxidized enzymatically by monoamine oxidase-A (MAO-A) in the outer mitochondrial membrane to form 3,4-dihydroxyphenylacetaldehyde (DOPAL) and hydrogen peroxide (H2O2). Cu(II) promotes the oxidation of DA and DOPAL. Formation of DA-Q and DOPAL-Q is associated with generation of superoxide radicals (). DOPAL is metabolized by aldehyde dehydrogenase (ALDH) to form 3,4-dihydroxyphenylacetic acid (DOPAC), which exits the cell
DOPAL–synuclein interactions
In 1997 three reports fundamentally changed concepts about mechanisms of catecholaminergic neurodegeneration. First, in a rare Greek-Italian-American kindred in which PD was transmitted as an autosomal dominant trait, the causal genotypic abnormality was identified—A53T mutation of the gene encoding the protein alpha-synuclein (AS) (Polymeropoulos et al. 1997). Second, Lewy bodies, a histopathologic hallmark of idiopathic PD, were found to contain abundant precipitated AS (Spillantini et al. 1997). Since then the view has evolved that PD as normally encountered clinically is a form of synucleinopathy. Other synucleinopathies include multiple system atrophy (MSA), in which AS is deposited in glial cytoplasmic inclusions in the brain (Wakabayashi et al. 1998); dementia with Lewy bodies (Baba et al. 1998); and pure autonomic failure (PAF) (Arai et al. 2000; Kaufmann et al. 2001).
All these forms of synucleinopathy entail chronic autonomic failure (Appenzeller and Goss 1971; Aminoff and Wilcox 1971; Rajput and Rozdilsky 1976), manifested in particular by neurogenic orthostatic hypotension (nOH) (Micieli et al. 1987; Benarroch 2003; Bonuccelli et al. 2003; Thaisetthawatkul et al. 2004; Jain and Goldstein 2012; Velseboer et al. 2011). In 1997 we reported the first evidence that Lewy body forms of nOH involve neuroimaging evidence of cardiac noradrenergic deficiency (Goldstein et al. 1997). The deficiency involves a combination of loss of myocardial sympathetic nerves (Amino et al. 2005; Orimo et al. 2006) and functional abnormalities in residual nerves—the “sick-but-not-dead” phenomenon (Goldstein et al. 2014, 2019).
DOPAL may be a key link between synucleinopathy and catecholaminergic neurodegeneration. The catecholaldehyde potently oligomerizes AS (Burke et al. 2008), and synuclein oligomers are thought to be the toxic form of the protein (Winner et al. 2011). Importantly, DOPAL-induced synuclein oligomers impede vesicular functions (Plotegher et al. 2017), which could set the stage for another vicious cycle. Divalent metal cations—especially Cu(II)—augment DOPAL-induced oligomerization of AS (Jinsmaa et al. 2014), while anti-oxidation with reduced glutathione, ascorbic acid, or N-acetylcysteine (NAC) attenuates the oligomerization (Follmer et al. 2015; Jinsmaa et al. 2018; Anderson et al. 2016). DOPAL-induced oligomerization of AS has been proposed to reflect condensation of two DOPAL molecules in a dicatechol pyrrole lysine adduct, followed by formation of isoindole linkages (Werner-Allen et al. 2016, 2018). Superoxide radical drives this process (Werner-Allen et al. 2017). Since superoxide is also generated when DOPAL oxidizes, this is another potential vicious cycle. A recent report supported the catecholaldehyde hypothesis, in that Schiff base adducts between DOPAL and the amines rasagiline or aminoindan were found to inhibit DOPAL-induced AS aggregation and toxicity (Kumar et al. 2019).
DOPAL also evokes the formation of quinone adducts with many proteins (“quinonization”) relevant to catecholaminergic functions, including TH, L-aromatic-amino-acid-decarboxylase (LAAAD), and the type 2 VMAT—as well as AS. DOPAL is far more potent than dopamine in oligomerizing and quinonizing AS (Burke et al. 2008; Jinsmaa et al. 2018).
Oxidized dopamine can interact with AS (Hasegawa et al. 2006), promoting AS oligomerization (Lee et al. 2011; Saha et al. 2018; Leong et al. 2009). Aminochrome and 5,6-dihydroxyindole, products of dopamine oxidation, can also oligomerize AS (Huenchuguala et al. 2019; Munoz et al. 2015; Pham et al. 2009). Most investigations on this topic have not considered the possibility that dopamine-dependent AS oligomerization actually depends on production of DOPAL from dopamine via MAO (Lee et al. 2011; Leong et al. 2009; Hasegawa et al. 2006).
Recently we conducted a comprehensive assessment of the relative potencies of DOPAL and dopamine in oligomerizing and quinonizing AS (Jinsmaa et al. 2019). In both regards DOPAL was far more potent than dopamine. Even in the setting of evoked dopamine oxidation by Cu(II) or tyrosinase, dopamine did not quinonize AS. In cultured human oligodendrocytes DOPAL resulted in the formation of numerous intra-cellular quinoproteins that were visualized for the first time by near infrared microscopy. Of the two routes by which oxidation of dopamine modifies AS and other proteins, that via DOPAL therefore seems more prominent. Moreover, it stands to reason that given the alternatives of spontaneous oxidation of cytoplasmic dopamine vs. enzymatic oxidation catalyzed by MAO, the latter route would be favored.
Braak’s “gut first” concept states that “a putative environmental pathogen capable of passing the gastric epithelial lining might induce AS misfolding and aggregation in specific cell types of the submucosal plexus and reach the brain via a consecutive series of projection neurons” (Braak et al. 2006). Almost half of the synthesis and metabolism of dopamine in the body takes place in non-neuronal cells of the gut (Eisenhofer et al. 1997). One may speculate that DOPAL produced locally from abundant non-neuronal dopamine might react with AS to induce a pathogenic cascade.
Treatment implications of the catecholaldehyde hypothesis
Since MAO inhibition decreases DOPAL formation, a seemingly straightforward test of the catecholaldehyde hypothesis would be to determine whether MAO inhibitors slow the symptomatic progression of PD. Results of two large multicenter trials of the MAO-B inhibitors deprenyl (selegiline) and rasagiline, however, failed to demonstrate efficacy in this regard (Group PS 1996; Ward 1994; Fabbrini et al. 2012; Olanow et al. 2009; de la Fuente-Fernandez et al. 2010).
One can conceive of two potential explanations for this failure. First, the subjects in these clinical trials already had symptomatic PD, and the neurodegenerative process may already be advanced by the time symptoms occur. Second, MAO inhibition increases the spontaneous oxidation of cytoplasmic dopamine, as indicated by increased levels of Cys-DA (Fornstedt and Carlsson 1991; Goldstein et al. 2016)—the “MAOI tradeoff.”
The catecholaldehyde hypothesis has not yet been put to a direct test in humans. NAC does not interfere with the ability of the MAO-B inhibitor selegiline to decrease endogenous DOPAL production, while it attenuates the increase in Cys-DA induced by selegiline (Goldstein et al. 2017a), a reasonable strategy would be to combine NAC with an MAO inhibitor. A recent trial of oral and intravenous NAC alone reported retardation in the progression of symptoms of PD and of the striatal dopaminergic lesion (Monti et al. 2016, 2019).
Ideally, such a trial would involve patients with early disease or even people at risk for PD who have biomarkers of catecholaminergic neurodegeneration but without motor signs. Cardiac sympathetic neuroimaging evidence of myocardial noradrenergic deficiency and low cerebrospinal fluid levels of DOPA and DOPAC predict PD in at-risk individuals (Goldstein et al. 2018a, b). In patients with PD, the severity of the cardiac noradrenergic lesion progresses over time (Lamotte et al. 2019), and PAF can evolve into PD, DLB, or both (Kaufmann et al. 2017). By combining biomarkers of catecholaminergic dysfunction in extant neurons—the sick-but-not-dead phenomenon—with biomarkers of deposition of AS in sympathetic noradrenergic nerves (Isonaka et al. 2019), an enriched enough population may be identified for efficient testing of the catecholaldehyde hypothesis.
Acknowledgements
Research reported in this review was supported (in part) by the Intramural Research Program of the NIH, NINDS.
Abbreviations
- ALDH
Aldehyde dehydrogenase
- AS
Alpha-synuclein
- Cys-DA
5-S-Cysteinyldopamine
- DLB
Dementia with Lewy bodies
- DOPAC
3,4-Dihydroxyphenylacetic acid
- DOPAL
3,4-Dihydroxyphenylacetaldehyde
- DOPAL-Q
DOPAL-quinone
- LAAAD
L-Aromatic-amino-acid decarboxylase
- MSA
Multiple system atrophy
- NE
Norepinephrine
- NET
Cell membrane norepinephrine transporter
- nOH
Neurogenic orthostatic hypotension
- OH
Orthostatic hypotension
- PAF
Pure autonomic failure
- PD
Parkinson disease
- TH
Tyrosine hydroxylase
- VMAT
Vesicular monoamine transporter
References
- Amino T, Orimo S, Takahashi A, Uchihara T, Mizusawa H (2005) Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Path 15:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff MJ, Wilcox CS (1971) Assessment of autonomic function in patients with a Parkinsonian syndrome. Br Med J 4(779):80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DG, Florang VR, Schamp JH, Buettner GR, Doorn JA (2016) Antioxidant-mediated modulation of protein reactivity for 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite. Chem Res Toxicol 29(7):1098–1107. 10.1021/acs.chemrestox.5b00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller O, Goss JE (1971) Autonomic deficits in Parkinson’s syndrome. Arch Neurol 24:50–57 [DOI] [PubMed] [Google Scholar]
- Arai K, Kato N, Kashiwado K, Hattori T (2000) Pure autonomic failure in association with human alpha-synucleinopathy. Neurosci Lett 296:171–173 [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152(4):879–884 [PMC free article] [PubMed] [Google Scholar]
- Badillo-Ramirez I, Saniger JM, Rivas-Arancibia S (2019) 5-S-cysteinyl-dopamine, a neurotoxic endogenous metabolite of dopamine: implications for Parkinson’s disease. Neurochem Int 129:104514. 10.1016/j.neuint.2019.104514 [DOI] [PubMed] [Google Scholar]
- Banerjee K, Munshi S, Sen O, Pramanik V, Roy Mukherjee T, Chakrabarti S (2014) Dopamine cytotoxicity involves both oxidative and nonoxidative pathways in SH-SY5Y cells: potential role of alpha-synuclein overexpression and proteasomal inhibition in the etiopathogenesis of Parkinson’s disease. Parkinsons Dis 2014:878935. 10.1155/2014/878935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2003) Brainstem in multiple system atrophy: clinicopathological correlations. Cell Mol Neurobiol 23(4–5):519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 282(21):15597–15605. 10.1074/jbc.M610893200 [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Greggio E, Maric D, Miller DW, Cookson MR, Bubacco L (2010) Alpha-synuclein overexpression increases dopamine toxicity in BE2-M17 cells. BMC Neurosci 11:41. 10.1186/1471-2202-11-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Greggio E, Beltramini M, Bubacco L (2013) Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson’s disease therapies. FASEB J 27(6):2101–2110. 10.1096/fj.12-226852 [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Lucetti C, Del Dotto P, Ceravolo R, Gambaccini G, Bernardini S, Rossi G, Piaggesi A (2003) Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 60:1400–1404 [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396(1):67–72 [DOI] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Kruger R, Surmeier DJ, Krainc D (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357(6357):1255–1261. 10.1126/science.aam9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS (2003) 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res 989(2):205–213 [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25(1–2):101–115. 10.1016/s0161-813x(03)00090-1 [DOI] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE (2008) Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 115(2):193–203. 10.1007/s00401-007-0303-9 [DOI] [PubMed] [Google Scholar]
- Cai H, Liu G, Sun L, Ding J (2014) Aldehyde Dehydrogenase 1 making molecular inroads into the differential vulnerability of nigrostriatal dopaminergic neuron subtypes in Parkinson’s disease. Transl Neurodegener 3:27. 10.1186/2047-9158-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Fornstedt B (1991) Possible mechanisms underlying the special vulnerability of dopaminergic neurons. Acta Neurol Scand Suppl 136:16–18 [DOI] [PubMed] [Google Scholar]
- Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS (2014) Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem Res Toxicol 27(8):1359–1361. 10.1021/tx5002223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW (2007) Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci 27(30):8138–8148. 10.1523/jneurosci.0319-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X (2008) Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci 28(2):425–433. 10.1523/JNEUROSCI.3602-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzi A, d’Agostini F, Kettler R, Borroni E, Da Prada M (1990) Effect of selective and reversible MAO inhibitors on dopamine outflow in rat striatum: a microdialysis study. J Neural Transm Suppl 32:79–84. 10.1007/978-3-7091-9113-2_9 [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Schulzer M, Mak E, Sossi V (2010) Trials of neuroprotective therapies for Parkinson’s disease: problems and limitations. Parkinsonism Relat Disord 16(6):365–369. 10.1016/j.parkreldis.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Demarest KT, Moore KE (1981) Type A monoamine oxidase catalyzes the intraneuronal deamination of dopamine within nigrostriatal, mesolimbic, tuberoinfundibular and tuberohypophyseal neurons in the rat. J Neural Transm 52(3):175–187. 10.1007/bf01249602 [DOI] [PubMed] [Google Scholar]
- Dukes AA, Korwek KM, Hastings TG (2005) The effect of endogenous dopamine in rotenone-induced toxicity in PC12 cells. Antioxid Redox Signal 7(5–6):630–638. 10.1089/ars.2005.7.630 [DOI] [PubMed] [Google Scholar]
- Dyck LE, Durden DA, Boulton AA (1993) Effects of monoamine oxidase inhibitors on the acid metabolites of some trace amines and of dopamine in the rat striatum. Biochem Pharmacol 45(6):1317–1322. 10.1016/0006-2952(93)90285-5 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ (1986) Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 32:2030–2033 [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Friberg P, Hooper D, Fandriks L, Lonroth H, Hunyady B, Mezey E (1997) Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82(11):3864–3871 [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Abbruzzese G, Marconi S, Zappia M (2012) Selegiline: a reappraisal of its role in Parkinson disease. Clin Neuropharmacol 35(3):134–140. 10.1097/WNF.0b013e318255838b [DOI] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM (2013) Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci USA 110(2):636–641. 10.1073/pnas.1220399110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM (2014) Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology 82(5):419–426. 10.1212/WNL.0000000000000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA (2007) Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology 28(1):76–82. 10.1016/j.neuro.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, Eliezer D (2015) Oligomerization and membrane-binding properties of covalent adducts formed by the interaction of alpha-synuclein with the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL). J Biol Chem 290(46):27660–27679. 10.1074/jbc.M115.686584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Giorgi FS, Bassi L, Ferrucci M, Alessandri MG, Corsini GU (2000) Modulation of dihydroxyphenylacetaldehyde extracellular levels in vivo in the rat striatum after different kinds of pharmacological treatment. Brain Res 861(1):126–134 [DOI] [PubMed] [Google Scholar]
- Fornstedt B, Carlsson A (1989) A marked rise in 5-S-cysteinyl-dopamine levels in guinea-pig striatum following reserpine treatment. J Neural Transm 76(2):155–161 [DOI] [PubMed] [Google Scholar]
- Fornstedt B, Carlsson A (1991) Effects of inhibition of monoamine oxidase on the levels of 5-S-cysteinyl adducts of catechols in dopaminergic regions of the brain of the guinea pig. Neuropharmacology 30(5):463–468 [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Volkow ND, Shumay E, McCall-Perez F, Jayne M, Wang GJ, Alexoff DL, Apelskog-Torres K, Hubbard B, Carter P, King P, Fahn S, Gilmor M, Telang F, Shea C, Xu Y, Muench L (2015) Evidence that formulations of the selective MAO-B inhibitor, selegiline, which bypass first-pass metabolism, also inhibit MAO-A in the human brain. Neuropsychopharmacology 40(3):650–657. 10.1038/npp.2014.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG (1999) Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci 19(7):2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG (1998) Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem 70:1973–1978 [DOI] [PubMed] [Google Scholar]
- Gautam AH, Zeevalk GD (2011) Characterization of reduced and oxidized dopamine and 3,4-dihydrophenylacetic acid, on brain mitochondrial electron transport chain activities. Biochim Biophys Acta 1807(7):819–828. 10.1016/j.bbabio.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Cannon RO 3rd, Eisenhofer G, Kopin IJ (1997) Sympathetic cardioneuropathy in dysautonomias. N Engl J Med 336(10):696–702 [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC (2011) Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol 18:703–710. 10.1111/j.1468-1331.2010.03246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y (2012) Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells: relevance to the pathogenesis of Parkinson’s disease. J Neurochem 123(6):932–943. 10.1111/j.1471-4159.2012.07924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y (2013) Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem 126(5):591–603. 10.1111/jnc.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Sharabi Y, Kopin IJ (2014) A vesicular sequestration to oxidative deamination shift in myocardial sympathetic nerves in Parkinson disease. J Neurochem 131:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y (2015a) Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson’s disease. J Neurochem 133(1):14–25. 10.1111/jnc.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Sharabi Y, Mash DC (2015b) Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy. Parkinsonism Relat Disord 21(6):567–572. 10.1016/j.parkreldis.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, Sharabi Y (2016) Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells. J Pharmacol Exp Ther 356(2):484–493. 10.1124/jpet.115.230201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Jinsmaa Y, Sullivan P, Sharabi Y (2017a) N-Acetylcysteine prevents the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochem Res 42(11):3289–3295. 10.1007/s11064-017-2371-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Mash DC, Kopin IJ, Sharabi Y (2017b) Determinants of denervation-independent depletion of putamen dopamine in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord 35:88–91. 10.1016/j.parkreldis.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Lopez GJ, Wu T, Sharabi Y (2018a) Cardiac sympathetic denervation predicts PD in at-risk individuals. Parkinsonism Relat Disord 52:90–93. 10.1016/j.parkreldis.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Lopez GJ, Wu T, Sharabi Y (2018b) Cerebrospinal fluid biomarkers of central dopamine deficiency predict Parkinson’s disease. Parkinsonism Relat Disord. 10.1016/j.parkreldis.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Pekker MJ, Eisenhofer G, Sharabi Y (2019) Computational modeling reveals multiple abnormalities of myocardial noradrenergic function in Lewy body diseases. JCI Insight. 10.1172/jci.insight.130441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group PS (1996) Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP subjects not requiring levodopa. Parkinson Study Group. Ann Neurol 39:29–36 [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Ruder J, Monoranu CM, Riederer P, Youdim MB, Mandel SA (2018) Differential alterations in metabolism and proteolysisrelated proteins in human Parkinson’s disease substantia nigra. Neurotox Res 33(3):560–568. 10.1007/s12640-017-9843-5 [DOI] [PubMed] [Google Scholar]
- Guillot TS, Miller GW (2009) Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol 39(2):149–170. 10.1007/s12035-009-8059-y [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Matsuzaki-Kobayashi M, Takeda A, Sugeno N, Kikuchi A, Furukawa K, Perry G, Smith MA, Itoyama Y (2006) Alphasynuclein facilitates the toxicity of oxidized catechol metabolites: implications for selective neurodegeneration in Parkinson’s disease. FEBS Lett 580(8):2147–2152. 10.1016/j.febslet.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Hastings TG (2009) The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. J Bioenerg Biomembr 41(6):469–472. 10.1007/s10863-009-9257-z [DOI] [PubMed] [Google Scholar]
- Herrera A, Munoz P, Steinbusch HWM, Segura-Aguilar J (2017) Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson’s disease? ACS Chem Neurosci 8(4):702–711. 10.1021/acschemneuro.7b00034 [DOI] [PubMed] [Google Scholar]
- Huenchuguala S, Sjodin B, Mannervik B, Segura-Aguilar J (2019) Novel alpha-synuclein oligomers formed with the aminochrome-glutathione conjugate are not neurotoxic. Neurotox Res 35(2):432–440. 10.1007/s12640-018-9969-0 [DOI] [PubMed] [Google Scholar]
- Isonaka R, Rosenberg AZ, Sullivan P, Corrales A, Holmes C, Sharabi Y, Goldstein DS (2019) Alpha-Synuclein deposition within sympathetic noradrenergic neurons is associated with myocardial noradrenergic deficiency in neurogenic orthostatic hypotension. Hypertension 73(4):910–918. 10.1161/HYPERTENSIONAHA.118.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Goldstein DS (2012) Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 46(3):572–580. 10.1016/j.nbd.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S (2011a) Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochimica et Biophys Acta 1812(6):663–673. 10.1016/j.bbadis.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S (2011b) Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1812(6):663–673. 10.1016/j.bbadis.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS (2014) Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci Lett 569:27–32. 10.1016/j.neulet.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Sharabi Y, Sullivan P, Isonaka R, Goldstein DS (2018) 3,4-Dihydroxyphenylacetaldehyde-induced protein modifications and their mitigation by N-acetylcysteine. J Pharmacol Exp Ther 366(1):113–124. 10.1124/jpet.118.248492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Isonaka R, Sharabi Y, Goldstein DS (2019) 3,4-Dihydroxyphenylacetaldehyde is more efficient than dopamine in oligomerizing and quinonizing alphasynuclein. J Pharmacol Exp Ther. 10.1124/jpet.119.262246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Hague K, Perl D (2001) Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology 56(7):980–981 [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D (2017) Natural history of pure autonomic failure: a United States prospective cohort. Ann Neurol 81(2):287–297. 10.1002/ana.24877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FH, Sen T, Maiti AK, Jana S, Chatterjee U, Chakrabarti S (2005) Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implications for Parkinson’s disease. Biochim Biophys Acta 1741(1–2):65–74. 10.1016/j.bbadis.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318:876–880 [DOI] [PubMed] [Google Scholar]
- Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ (2001) Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med 30(8):924–931 [DOI] [PubMed] [Google Scholar]
- Kumagae Y, Matsui Y, Iwata N (1991) Deamination of norepinephrine, dopamine, and serotonin by type A monoamine oxidase in discrete regions of the rat brain and inhibition by RS-8359. Jpn J Pharmacol 55(1):121–128. 10.1254/jjp.55.121 [DOI] [PubMed] [Google Scholar]
- Kumar VB, Hsu FF, Lakshmi VM, Gillespie KN, Burke WJ (2019) Aldehyde adducts inhibit 3,4-dihydroxyphenylacetaldehyde-induced alpha-synuclein aggregation and toxicity: implication for Parkinson neuroprotective therapy. Eur J Pharmacol 845:65–73. 10.1016/j.ejphar.2018.12.027 [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ (2000a) Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J Neurosci Res 60(4):552–558 [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Nechustan A, Kirk K, Kopin IJ (2000b) 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res 868(2):191–201 [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Hrycyna C, He LP, Nechushtan A, Tjurmina O, Harvey-White J, Eisenhofer G, Rojas E, Kopin IJ (2000c) Acidic dopamine metabolites are actively extruded from PC12 cells by a novel sulfonylurea-sensitive transporter. Naunyn Schmiedebergs Arch Pharmacol 361(6):654–664 [DOI] [PubMed] [Google Scholar]
- Lamotte G, Holmes C, Wu T, Goldstein DS (2019) Long-term trends in myocardial sympathetic innervation and function in synucleinopathies. Parkinsonism Relat Disord 67:27–33. 10.1016/j.parkreldis.2019.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Baek SM, Ho DH, Suk JE, Cho ED, Lee SJ (2011) Dopamine promotes formation and secretion of non-fibrillar alphasynuclein oligomers. Exp Mol Med 43(4):216–222. 10.3858/emm.2011.43.4.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SL, Cappai R, Barnham KJ, Pham CL (2009) Modulation of alpha-synuclein aggregation by dopamine: a review. Neurochem Res 34(10):1838–1846. 10.1007/s11064-009-9986-8 [DOI] [PubMed] [Google Scholar]
- Li SW, Lin TS, Minteer S, Burke WJ (2001) 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res Mol Brain Res 93(1):1–7 [DOI] [PubMed] [Google Scholar]
- Linsenbardt AJ, Wilken GH, Westfall TC, Macarthur H (2009) Cytotoxicity of dopaminochrome in the mesencephalic cell line, MN9D, is dependent upon oxidative stress. Neurotoxicology 30(6):1030–1035. 10.1016/j.neuro.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW (2014) Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci USA 111(27):9977–9982. 10.1073/pnas.1402134111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, Youdim MB (2005) Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann N Y Acad Sci 1053:356–375 [DOI] [PubMed] [Google Scholar]
- Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R (1995) An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration 4(3):271–281 [DOI] [PubMed] [Google Scholar]
- Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G (1987) Postprandial and orthostatic hypotension in Parkinson’s disease. Neurology 37(3):386–393 [DOI] [PubMed] [Google Scholar]
- Molochnikov L, Rabey JM, Dobronevsky E, Bonucelli U, Ceravolo R, Frosini D, Grunblatt E, Riederer P, Jacob C, Aharon-Peretz J, Bashenko Y, Youdim MB, Mandel SA (2012) A molecular signature in blood identifies early Parkinson’s disease. Molec Neurodegen 7:26. 10.1186/1750-1326-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ, Zhong L, Bowen B, Intenzo CM, Iacovitti L, Newberg AB (2016) N-acetyl cysteine may support dopamine neurons in Parkinson’s disease: preliminary clinical and cell line data. PLoS One 11(6):e0157602. 10.1371/journal.pone.0157602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Bazzan AJ, Zhong L, Bowens BK, Chervoneva I, Intenzo C, Newberg AB (2019) N-acetyl cysteine is associated with dopaminergic improvement in Parkinson’s disease. Clin Pharmacol Ther. 10.1002/cpt.1548 [DOI] [PubMed] [Google Scholar]
- Montine TJ, Picklo MJ, Amarnath V, Whetsell WO Jr, Graham DG (1997) Neurotoxicity of endogenous cysteinylcatechols. Exp Neurol 148(1):26–33. 10.1006/exnr.1997.6662 [DOI] [PubMed] [Google Scholar]
- Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ, Doshi S, Gupta P, Grossman JL, Tan VX, Kalb RG, Caldwell KA, Caldwell GA, Wolfe JH, Ischiropoulos H (2017) Dopamine induces soluble alpha-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci 20(11):1560–1568. 10.1038/nn.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D (2009) Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62(2):218–229. 10.1016/j.neuron.2009.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P, Paris I, Sanders LH, Greenamyre JT, Segura-Aguilar J (2012) Overexpression of VMAT-2 and DT-diaphorase protects substantia nigra-derived cells against aminochrome neurotoxicity. Biochim Biophys Acta 1822(7):1125–1136. 10.1016/j.bbadis.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P, Cardenas S, Huenchuguala S, Briceno A, Couve E, Paris I, Segura-Aguilar J (2015) DT-diaphorase prevents aminochrome-induced alpha-synuclein oligomer formation and neurotoxicity. Toxicol Sci 145(1):37–47. 10.1093/toxsci/kfv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T (1997) Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res 29:99–111 [DOI] [PubMed] [Google Scholar]
- Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361(13):1268–1278. 10.1056/NEJMoa0809335 [DOI] [PubMed] [Google Scholar]
- Orimo S, Amino T, Takahashi A, Kojo T, Uchihara T, Mori F, Wakabayashi K, Takahashi H (2006) Cardiac sympathetic denervation in Lewy body disease. Parkinsonism Relat Disord 12(Suppl 2):S99–S105 [Google Scholar]
- Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE (2010) The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One 5(12):e15251. 10.1371/journal.pone.0015251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris I, Lozano J, Perez-Pastene C, Munoz P, Segura-Aguilar J (2009) Molecular and neurochemical mechanisms in PD pathogenesis. Neurotox Res 16(3):271–279. 10.1007/s12640-009-9059-4 [DOI] [PubMed] [Google Scholar]
- Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R (2009) Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol 387(3):771–785. 10.1016/j.jmb.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Plotegher N, Berti G, Ferrari E, Tessari I, Zanetti M, Lunelli L, Greggio E, Bisaglia M, Veronesi M, Girotto S, Dalla Serra M, Perego C, Casella L, Bubacco L (2017) DOPAL-derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci Rep 7:40699. 10.1038/srep40699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047 [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B (1976) Dysautonomia in Parkinsonism: a clinicopathological study. J Neurol Neurosurg Psychiatry 39:1092–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Paul KC, Bronstein JM (2016) Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr Environ Health Rep 3(1):40–52. 10.1007/s40572-016-0083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Khan MAI, Mudhara D, Deep S (2018) Tuning the balance between fibrillation and oligomerization of alpha-synuclein in the presence of dopamine. ACS Omega 3(10):14213–14224. 10.1021/acsomega.8b00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J (2017) On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease. Neural Regen Res 12(6):897–901. 10.4103/1673-5374.208560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J (2019) On the role of aminochrome in mitochondrial dysfunction and endoplasmic reticulum stress in Parkinson’s disease. Front Neurosci 13:271. 10.3389/fnins.2019.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- Staal RG, Sonsalla PK (2000) Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. J Pharmacol Exp Ther 293(2):336–342 [PubMed] [Google Scholar]
- Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J (2002) Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol 63(5):909–920 [DOI] [PubMed] [Google Scholar]
- Su Y, Duan J, Ying Z, Hou Y, Zhang Y, Wang R, Deng Y (2013) Increased vulnerability of parkin knock down PC12 cells to hydrogen peroxide toxicity: the role of salsolinol and NM-salsolinol. Neuroscience 233:72–85. 10.1016/j.neuroscience.2012.12.045 [DOI] [PubMed] [Google Scholar]
- Surh YJ, Kim HJ (2010) Neurotoxic effects of tetrahydroisoquinolines and underlying mechanisms. Exp Neurobiol 19(2):63–70. 10.5607/en.2010.19.2.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA (2011) The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal 14(7):1289–1301. 10.1089/ars.2010.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW (2014) Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology 76 Pt A:97–105. 10.1016/j.neuropharm.2013.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R, Low PA (2004) Autonomic dysfunction in dementia with Lewy bodies. Neurology 62(10):1804–1809. 10.1212/01.wnl.0000125192.69777.6d [DOI] [PubMed] [Google Scholar]
- Vauzour D, Corona G, Spencer JP (2010) Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch Biochem Biophys 501(1):106–111. 10.1016/j.abb.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM (2011) Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 17(10):724–729. 10.1016/j.parkreldis.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SR, Abercrombie ED (1994) L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J Neurochem 63(1):108–117. 10.1046/j.1471-4159.1994.63010108.x [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H (1998) Alphasynuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 249(2–3):180–182 [DOI] [PubMed] [Google Scholar]
- Ward CD (1994) Does selegiline delay progression of Parkinson’s disease? A critical re-evaluation of the DATATOP study. J Neurol Neurosurg Psychiatry 57(2):217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten P, Zhou QY (2001) Protection of intracellular dopamine cytotoxicity by dopamine disposition and metabolism factors. J Neurochem 77(3):776–785 [DOI] [PubMed] [Google Scholar]
- Werner-Allen JW, DuMond JF, Levine RL, Bax A (2016) Toxic dopamine metabolite DOPAL forms an unexpected dicatechol pyrrole adduct with lysines of alpha-synuclein. Angew Chem Int Ed Engl 55(26):7374–7378. 10.1002/anie.201600277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Allen JW, Levine RL, Bax A (2017) Superoxide is the critical driver of DOPAL autoxidation, lysyl adduct formation, and crosslinking of alpha-synuclein. Biochem Biophys Res Commun 487(2):281–286. 10.1016/j.bbrc.2017.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Allen JW, Monti S, DuMond JF, Levine RL, Bax A (2018) Isoindole linkages provide a pathway for DOPAL-mediated cross-linking of alpha-synuclein. Biochemistry 57(9):1462–1474. 10.1021/acs.biochem.7b01164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey M, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R (2012) Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLoS ONE 7:e31522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R (2011) In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA 108(10):4194–4199. 10.1073/pnas.1100976108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YN, Johnson SW (2011) Dopamine oxidation facilitates rotenonedependent potentiation of N-methyl-D-aspartate currents in rat substantia nigra dopamine neurons. Neuroscience 195:138–144. 10.1016/j.neuroscience.2011.08.041 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Riederer PF (2004) A review of the mechanisms and role of monoamine oxidase inhibitors in Parkinson’s disease. Neurology 63(7 Suppl 2):S32–S35. 10.1212/wnl.63.7_suppl_2.s32 [DOI] [PubMed] [Google Scholar]


