Abstract
Metformin is a classical drug used to treat type 2 diabetes. With the development of research on metformin, it has been found that metformin also has several advantages aside from its hypoglycemic effect, such as anti-inflammatory, anti-aging, anti-cancer, improving intestinal flora, and other effects. The prevention of inflammation is critical because chronic inflammation is associated with numerous diseases of considerable public health. Therefore, there has been growing interest in the role of metformin in treating various inflammatory conditions. However, the precise anti-inflammatory mechanisms of metformin were inconsistent in the reported studies. Thus, this review aims to summarize various currently known possible mechanisms of metformin involved in inflammatory diseases and provide references for the clinical application of metformin.
Keywords: metformin, AMPK, inflammation, mechanisms
Introduction
In the 19th century, metformin (1,1-dimethylbiguanide hydrochloride) was discovered, which is a biguanide derivative extracted from the plant Galega officinalis (French lilac).1 By promoting the binding of insulin and receptors, metformin accelerates glucose utilization in the body, reduces the output of liver sugar, improves insulin resistance, and achieves the therapeutic effect of controlling blood sugar.2 With its economy, safety, and effectiveness advantages, it has become the first-line drug for type II diabetes. In recent years, several experimental studies and epidemiological data have shown that, in addition to hypoglycemic effects, long-term use of metformin may exert anti-inflammation,3 avoid age-related diseases,4 prevent tumors,5 and reduce the occurrence and development of various metabolic diseases.6 Notably, given its potential anti-inflammatory properties, metformin is now receiving increasing attention.
Inflammation is a defense mechanism against various noxious stimuli triggered by exogenous stimuli, such as infections7 or endogenous sterile injuries.8 Overactive inflammatory responses, however, can be harmful to the body. Studies have found that inflammation is related to the development of numerous diseases. Chronic inflammation can induce senescence,9 tissue destruction,10 immunosuppression,11 and cancer.12
Although metformin is widely used in the clinic, especially for controlling inflammatory diseases, and has made promising progress, the mechanisms behind its anti-inflammatory effects are still poorly understood. Hence, in this review, to clear the way for possible targets for therapeutic use, we will concentrate on current developments in research on the function of metformin in various inflammatory illnesses and its underlying mechanisms of action.
Adenosine Monophosphate-Activated Protein Kinase (AMPK)-Dependent Pathways
Metformin is a medication often prescribed for individuals with diabetes. It works to reduce the production of glucose in the liver through both AMPK-dependent and AMPK-independent pathways.13 Similarly, metformin also inhibits inflammation in an AMPK-dependent14 and AMPK-independent manner.
AMPK is a heterotrimeric serine/threonine protein kinase consisting of three subunits. It serves as a crucial metabolic sensor linking cellular energy homeostasis to the regulation of inflammatory signaling.15–17 Indeed, AMPK is activated when energy is deficient, with an increase in the adenosine monophosphate (AMP)/ adenosine triphosphate (ATP) ratio being the activation signal.18 To ensure metformin’s effectiveness, activating the AMPK system is vital. By inhibiting complex I of the mitochondrial respiratory chain, also known as the electron transport chain (ETC), metformin inhibits transmembrane electron flow and membrane potential formation, thereby reducing mitochondrial oxygen consumption and inhibiting the production of ATP, increasing the AMP/ATP ratio and thereby activating AMPK.19,20
Through activation of AMPK, energy homeostasis is restored by promoting catabolic pathways, which cause ATP synthesis, and inhibiting anabolic ways that consume it. In addition, metformin suppressed inflammatory cytokine production by promoting macrophage polarization to the anti-inflammatory M2 type via activating AMPK-1α phosphorylation.21 Besides, its anti-inflammatory properties appear related to its inhibitory effect on nuclear factor-kappaB (NF-κB), the most common transcription factor for inflammatory responses. Moreover, activating AMPK facilitates the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and increases the expression of heme oxygenase-1 (HO-1) to counteract lipopolysaccharide (LPS)-induced cellular damage.22,23
To date, various models have been used to assess the metformin effects on renal inflammation. AMPK activation appears to be a key mechanism behind many of these renoprotective effects.24–29 Moreover, in an experimental study of myocardial ischemia-reperfusion (I/R) injury, the metformin treatment increased the phosphorylation of AMPK, decreased pro-inflammatory cytokines, and suppressed NLRP3 inflammasome activation. Then all led to a significant reduction in myocardial infarction size, apoptosis attenuation, and myocardial fibrosis inhibition. Significantly, the observed effects were reversed upon inhibition of AMPK phosphorylation by Compound C (CC).30 As shown in a mouse model, metformin significantly reduced lung inflammation in SiO2-instilled mice at the fibrotic stages, which can be exacerbated by inflammatory mediators, including interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α).31 Besides, metformin attenuated oxidative stress-induced cell toxicity, the epithelial-mesenchymal transition process in epithelial cells, and inhibited inflammation response in macrophages via an AMPK-dependent pathway.32 Another AMPK-dependent effect of metformin shown in research was interrupting the priming signal for NLRP3 inflammasome activation via blockade of Toll-like receptor 4 (TLR4)/NF-κB signaling pathway, resulting in the inhibition of bioactive forms IL-1β and IL-18 as well as the pyroptosis process.33
Additionally, it was discovered that metformin could induce autophagy through AMPK activation, resulting in an increase of Beclin-1 and a decrease of p62/SQSTM1, ultimately leading to the degradation of a critical inflammatory mediator, NLRP3.33 This is similar to the findings of Fei et al.34 In another study, metformin could decrease STAT3 phosphorylation through an increase in AMPK activity, which in turn inhibited differentiation of monocyte to macrophage and eventually suppressed proinflammatory responses. Moreover, by this mechanism, metformin attenuated aortic atherosclerotic lesions induced by AngII and aneurysm in ApoE−/− mice.35
Collectively, evidence suggested that metformin exerts remarkable anti-inflammatory and immunosuppressive effects in inflammatory/autoimmune models. A vital component of this process was enhancing the AMPK pathway.
Liver Kinase B1 (LKB1)
LKB1 is a serine/threonine kinase encoded by the tumor suppressor STK11 gene, and it plays a vital role in energy metabolism through phosphorylating AMPK at position Thr 172 as a major upstream kinase of AMPK.36,37 Recent studies have shown that LKB1 exerts a certain anti-inflammatory activity.38,39 For instance, after the knockout of LKB1, levels of inflammatory cytokines, including TNF-α, IL-1β, major intrinsic proteins 2 (MIP2), and interferon-beta (IFN-β) induced by LPS, significantly increased in bone marrow-derived macrophages.40 In T cells, LKB1 loss led to decreased AMPK phosphorylation and increased mammalian target of rapamycin complex 1 (mTORC1) activation, leading to T-cell activation and high levels of inflammatory cytokine.41
Metformin induces the activation of LKB1. Subsequently, it enhances the LKB1/AMPK pathway, which plays a vital role in various physiopathological processes.42 In streptozotocin (STZ) and LPS-induced inflammation in mouse lungs,43 metformin increased the expression of LKB1, phosphor-AMPK, and phosphor-ACC. It markedly decreased the levels of pro-inflammatory cytokines such as TNF-α, IL-lβ, monocyte chemotactic protein-1 (MCP-1), and interleukin-1β (IL-6). In addition, to investigate whether this protective effect is mediated explicitly via the AMPK pathway, another AMPK activator, AICAR, was introduced into LPS-induced inflammatory peritoneal macrophages under high glucose conditions. It also inhibited the generation of inflammatory cytokines.
Collectively, the aforementioned studies suggest that the LKB1-AMPK-dependent pathway may play a role in mediating metformin’s anti-inflammatory effects.
Acetyl-CoA Carboxylase (ACC)
ACC, one of the target molecules of AMPK, is a rate-limiting enzyme in fatty acid synthesis.44 The AMPK/ACC signaling pathway plays a crucial role in regulating fatty acid oxidation, according to several studies.45,46
In TNF-α-induced lipid accumulation in human hepatoma G2 (HepG2) cells, co-treatment with metformin or AICAR decreased the intracellular triglyceride and increased AMPK and ACC phosphorylation.47 In obese mice, metformin increased levels of phosphorylated AMPK and its downstream target ACC and attenuated pulmonary eosinophilic inflammation.48 High-fat diets induced metabolic disturbances, renal damage, increased adipokine expression, and macrophage infiltration in mice. Metformin administration exerts protective effects via augmentation of AMPK and ACC activation.49 Chung et al50 also verified that metformin alleviated AGE-induced inflammatory responses through AMPK-associated suppression of the inhibitor of kappaB kinase (IKK), NF-κB, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) levels, and it negatively regulated the expression of inflammatory cytokine expression in human neural stem cells (hNSCs), and the transcript and protein expression levels of ACC were also rescued.
Mammalian Target of Rapamycin (mTOR)
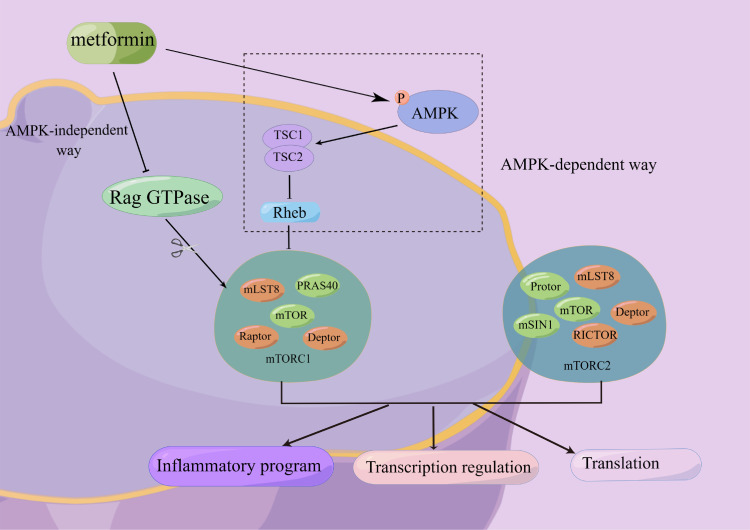
mTOR is a serine/threonine protein kinase found in two complexes, mTORC1 and mTORC2. The mTOR signaling pathway is implicated in cell growth, metabolism, energy balance, protein turnover, and autophagy and plays a critical role in inflammatory diseases, autoimmune disorders, and diabetes.51–53 There is some evidence that metformin inhibits the mTOR pathway both with and without activating AMPK.54,55 The latter will be discussed in a later section (Figure 1). Metformin inhibits mTORC1 activity dependent on AMPK, tuberous sclerosis complex 2 (TSC2), and AMPK-mediated phosphorylation of Raptor.56
Figure 1.
Metformin inhibits mTOR both with and without activating AMPK. There are two ways that metformin could inhibit mTORC1 downstream: (1) After AMPK activity is activated, the TSC1/TSC2 complex (which can inhibit the activity of Rheb) is activated, and then the action of mTORC1 activity is inhibited. (2) Metformin inhibited Rag GTPase activity in an AMPK-independent manner, thereby inhibiting mTORC1. mTOR has two complexes, mTORC1 and mTORC2, which have important regulatory effects on inflammation, gene transcription, protein translation, etc.
Abbreviations: AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian target of rapamycin; PRAS40, proline-rich Akt substrate 40; TSC, tuberous sclerosis complex; RICTOR, rapamycin-insensitive companion of mTOR; Rheb, ras homolog enriched in the brain; mLST8, mammalian lethal with sec-13 protein 8; Raptor, regulatory associated protein of mTOR; Deptor, DEP domain-containing mTOR-interacting protein; mSIN1, mammalian stress-activated protein kinase (SAPK)-interacting protein 1.
Among patients with polycystic ovary syndrome (PCOS), metformin reduced the high levels of TNF-α in B cells. Also, it improved PCOS phenotypes induced by dehydroepiandrosterone (DHEA) in mice through the AMPK/PI3K/mTOR signaling pathway.57 One recent study by Mohamed et al58 shows that metformin treatment of diabetic epileptic rats reduced the rise in pro-inflammatory cytokines and apoptotic markers and improved the histopathological results of model rats. Such effects were closely correlated with increased ATP/ADP ratio and reduced death-associated protein (DAP) and mTOR. Besides, a previous clinical experiment demonstrated that metformin had positive effects in reducing inflammation in the colons of people living with HIV (PLWH) undergoing antiretroviral therapy (ART). This effect is achieved through the inhibition of mTOR phosphorylation.59 Altogether, these suggest the pharmacologic intervention of mTOR may have therapeutic value in diseases characterized by inflammatory responses (Table 1).
Table 1.
Effects of Metformin Targeting mTOR on Inflammatory Diseases
| Disease | Subjects | Pathways | Effects | Reference |
|---|---|---|---|---|
| Scleroderma | Mice | mTOR-STAT3 signaling | ↓IL-6, IL-1β, IL-17, TNF-α, TGF-β, Col1a, Th17 ↑p-AMPK |
[60] |
| Silica-induced pulmonary fibrosis | Human and rats | AMPK-mTOR signaling | ↓TGF-β1, TNF-α, IL-1β, α-SMA, p62 ↑E-cadherin, p-AMPK, autophagy |
[61] |
| Thyroid-associated ophthalmopathy | Human | AMPK-mTOR signaling | ↓IL6, IL-8, CXCL1, CXCL2, CCL2, HA,α-SMA, ITGA5, COL1A1, COL2A1, COL3A1, FN1, ITGB1, and p-Smad2 ↑Autophagy |
[62] |
| Gout | Human | mTOR signaling | ↓The rate of monocyte death, inflammation, and frequency of gout flares ↑Autophagy |
[63] |
| Polycystic ovary syndrome | Human and mice | AMPK-PI3K-mTOR signaling | ↓TNF-α, mitochondrial membrane potential (MMP), ROS Reverse ovulatory dysfunction, disturbed estrous cycle, and abnormal ovarian morphology, and restore the impaired glucose tolerance |
[57] |
| Psoriasis | HaCaT cells | mTOR signaling | ↓Proliferation, p-p70S6K, IL-6, TNF-α, and VEGF ↑Apoptosis |
[64] |
| Liver injury | Rats | mTOR-HIF-1α signaling | ↓α-SMA, TIMP-1, hs-CRP, TNF-α, IL-6,ALT and AST | [65] |
| Spinal cord injury | Rats and microglial cells | AMPK-mTOR signaling | ↓Apoptosis, inflammation ↑Axon regeneration, M1 to M2 phenotype polarization, and autophagy |
[66] |
| Intestinal injury | HUVECs, HIECs, and BALB/c mice | mTOR signaling | ↓Senescence, intestinal canal inflammation, and oxidative stress | [67] |
| Sepsis-associated myocardial injury | C57BL/6J mice and H9c2 cells | AMPK-mTOR signaling | ↓Cardiac dysfunction, myocardial enzymes, and cardiac hydroncus ↑Autophagy |
[68] |
NF-κB
NF-κB is a major transcription factor that regulates inflammatory processes, and AMPK modulates NF-κB-dependent inflammation as well.69,70 In a wide variety of inflammatory disorders, such as atherosclerosis, osteoarthritis, and rheumatoid arthritis (RA), NF-κB is activated.71,72 Transfection of AMPK α1 siRNA in vascular endothelial cells significantly suppressed AMPK expression and reversed inhibition of NF-κB activation and various adhesion molecules expression induced by vaspin. All these data strongly suggested that AMPK activation is responsible for the inhibition of NF-κB activation.73
Activation of the NF-κB signaling pathway contributes to neuronal pathology in response to LPS and cognitive impairments by accumulating inflammatory mediators. It could be achieved through metformin blocking NF-κB pathway.74 In a study of the streptozotocin-induced-induced diabetic rat models accompanied by evident infiltration of inflammatory cells, phosphor-AMPK was upregulated, and NF-κB p65, as well as the anti-oxidation factors, such as Nrf2, HO-1, and SOD2, were downregulated as compared to model rats not receiving metformin.75 Notably, no significant drop in blood glucose level was observed after metformin treatment, suggesting that metformin ameliorated meibomian gland dysfunction (MGD) by regulating the AMPK/NF-κB axis instead of reducing blood glucose.
Signal Transducer and Activator of Transcription 3 (STAT3)
A master transcriptional regulator named STAT3 plays an essential role in cell survival, proliferation, differentiation, and inflammatory signaling cascade.76 Persistent STAT3 activation has been observed in several inflammatory diseases.77–79 Therefore, the most effective way of targeting STAT3 might be an attractive therapeutic strategy for suppressing the inflammatory response and keeping homeostasis. Through STAT3 inhibition and promoting STAT5 signaling, metformin could alleviate T-cell-mediated inflammation by regulating the balance between Th17s and Tregs.80,81 More specifically, this may be due to STAT3 and STAT5 competing for the same IL-17 promoter binding site.82 However, another study showed that inflammatory injury during sepsis in mice could not be relieved or even worsened after STAT3 inhibition, and obesity amplified this effect.76 One possible explanation for the mechanism is the interaction between STAT3 and leptin, which are affected during sepsis. Future studies are required to understand the impact of STAT3 activation on inflammatory diseases. Overall, the current understanding of the mechanism by which metformin regulates T cell function in autoimmune diseases is partly through AMPK/STAT3 signaling.
Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
An essential transcription factor, Nrf2, is a basic leucine zipper transcription factor.83 As well documented, Nrf2, a central factor for regulating the antioxidant and cytoprotective gene expression, is indispensable for controlling inflammatory responses.84,85 The loss of Nrf2 in the skin prolonged the inflammatory response after wounding, and Nrf2-knockout mice were significantly more likely to develop multi-tissue inflammation than normal mice.86,87 In line with the unequivocal role of the Nrf2-pathway in reactive oxygen species (ROS) detoxifying and inflammation suppression, the potential crosstalk between AMPK and Nrf2 signaling pathways has been noted.17 A natural antioxidant has been reported to provide therapeutic neuroprotection against inflammation and oxidative stress caused by cerebral ischemia-reperfusion via activating the AMPK/Nrf-2 pathway.88
Thus, what is the exact role of the Nrf2-related pathway in metformin-induced effects? In primary bovine mammary epithelial cells (PBMECs), metformin was shown to reduce proinflammatory cytokine secretion. In addition to the anti-inflammatory effect, metformin exhibited antioxidant properties through activation of the Nrf2 pathway, and this activation is significantly AMPK-dependent.89 Moreover, metformin could dramatically improve the condition of chronic obstructive pulmonary disease (COPD) by reducing the inflammatory response and oxidative stress as well as reducing pathological injury. The molecular mechanism may be related to the Nrf2/HO-1 activation and subsequently activating its downstream target MRP1, then alleviating the glucocorticoid resistance.90
AMPK-Independent Pathways
However, several studies suggested that AMPK is not the only pathway for metformin’s beneficial anti-inflammation effects.91,92 Several lines of evidence indicate that some kinases and redox-related transcription factors are regulated by metformin via an AMPKα-independent pathway, including STAT3, mTOR, hypoxia-inducible factor-1α (HIF-1α), and SIRT1.93–96
For example, a study performed in murine burn models demonstrated that metformin prevented pathological browning and lipolysis, improving outcomes for burn patients. The reason for this could be metformin’s tissue-specific actions on murine and human burn white adipose tissue (WAT), which functioned via an AMPK-independent mechanism through protein phosphatase 2A (PP2A) activation. However, AICAR, another potent AMPK agonist, did not show this effect. In the adipocytes culture experiment, metformin was shown to significantly decrease the phosphorylated c-Jun N-terminal kinase (JNK) p46 and the mRNA levels of TNFα, IL-1β, and IL-6, as well as increased adipocyte expression of PFKFB3/iPFK2. Notably, treatment of adipocytes with metformin did not significantly alter the phosphorylation state of AMPK and failed to suppress the intracellular proinflammatory responses in PFKFB3/iPFK2-knockdown adipocytes effectively.97 These findings were consistent with a previous study.98 That is, metformin appears to suppress proinflammatory responses of adipocytes in a manner independent of AMPK.
It is widely recognized that the AMPK-mTOR pathway is indispensable in metformin exerts anti-inflammatory effects. Many reports still show that metformin inhibits inflammation by modulating mTOR independently of the AMPK pathway. Metformin has been reported to directly inhibit mTORC1 activation in a Rag GTPase-dependent manner rather than dependent on TSC1/2 or AMPK. These observations were consistent with two distinct pre-clinical models of cancer and diabetes. Thus, direct inhibition of mTORC1 inhibits inflammatory response.94 In HepG2 cells transfected with DN-AMPK, metformin was still effective in reducing ROS production, although not to the same extent as in control cells. It indicates metformin may work independently of AMPK as well as through it.99
In primary murine bone marrow-derived macrophages (BMDMs), metformin inhibited LPS-induced production of ROS and IL-1β and elevated levels of the anti-inflammatory cytokine IL-10. Furthermore, metformin could also exert a protective effect in AMKPα1- or AMPKβ1-deficient cells. These effects did not depend on AMPK activation and were possibly attributed to the inhibition of reverse electron transport.100
Similar effects have been demonstrated in wild-type (WT) and AMPKα2−/− mice exposed to PM2.5 every other day and subsequently received metformin. However, metformin therapy effectively attenuated lung injury and cardiac dysfunction caused by PM2.5 in both the WT and AMPKα2−/− mice. Furthermore, systemic and pulmonary inflammation, pulmonary and myocardial fibrosis, and oxidative stress were reduced in two mice models, suggesting that these effects are AMPK-independent.101
Mitochondrial Dysfunction
Mitochondria are the cell’s power stations and the source of various pro-inflammatory signals that activate the immune system and generate inflammatory responses. Mitochondrial damage can lead to mitochondrial dysfunction, oxidative stress, inflammation, inhibiting respiration, and decreasing membrane potential.102 It is also the primary subcellular target of metformin. Multiple studies have demonstrated that metformin can reduce mitochondrial respiration via inhibiting complex I of the electron transport chain, leading to an increase in the AMP/ATP ratio and activating AMPK, thereby alleviating the mitochondrial fragmentation, blocking the production of ROS produced by mitochondria, lessening activation of NLRP3 inflammasomes and the cytoplasmic release of mitochondrial DNA (mtDNA), thus reducing inflammation by downregulating the expression of inflammatory cytokines such as TNF-α, IL-6 and IL-1β.54,100,103,104
NLRP3
A growing body of evidence indicates that NLRP3 inflammasome regulates the innate immune system and induces inflammatory responses, which consists of caspase adaptor (ASC), pro-caspase-1, and NLRP3 protein, to activate caspase-1 and thus produce high numbers of mature IL-1β.105,106 Several studies have confirmed that mitochondrial dysfunction and consequent overproduction of ROS are necessary to activate the NLRP3 inflammasome.107,108
Yang et al109 have shown that metformin could inhibit the NLRP3 inflammasome by activating the AMPK/mTOR axis in diabetic mice and high glucose-treated cardiomyocytes, and AMPK inhibitor reversed the upregulation of NLRP3, caspase-1, and IL1-β expression. Besides that, another study also drew a similar conclusion.30 It reported that metformin inhibited NLRP3 by activating phosphorylated AMPK. At the same time, the expression levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β as well as NLRP3, were decreased when treated with metformin, a special stimulator of NLRP3 (nigericin), abolished the protective effects of metformin in neonatal rat ventricular myocytes. Additional studies have indicated that the NLRP3 inflammasome is an essential inflammatory target in several brain disorders, including depression.110,111 A recent study found that metformin could act as an antidepressant partly by suppressing peripheral and central NLRP3 inflammasome activation, subsequent caspase-1 cleavage, and interleukin-1β secretion.112
Oxidative Stress
Oxidative stress is an imbalance between the formation of oxidative free radicals, also known as reactive oxygen species (ROS), and defense mechanisms by antioxidants.113 It is well-recognized that inflammation is closely associated with oxidative stress. Oxidative stress can cause the activation of a variety of transcription factors, such as NF-κB, protein-1 activator (AP-1), and hypoxia-inducible factor-1 (HIF-1), leading to the expression of various genes involved in inflammatory pathways.114–116 The inflammation triggered by constant oxidative stress is the cause of the development of many chronic diseases. During atherosclerosis, oxidative damage increases endothelial permeability, resulting in lipoproteins being released into the subendothelial space and inflammation.117
Metformin has been proposed to exert an anti-inflammatory effect through its antioxidant activity.118 Metformin could suppress the CEBP-homologous protein (CHOP), an endoplasmic reticulum stress (ERS) marker, thus serving cardioprotective roles by attenuating ERS.119 Araújo et al120 found that metformin restored the marker of antioxidant genes expression such as malondialdehyde (MDA), a major end product of lipid peroxidation, and superoxide dismutase (SOD) (involved in the neutralization of ROS) in a rat model of periodontitis, implied a strong antioxidant effect, thus caused significant reductions in the expression of pro-inflammatory factors such as IL-1β and TNF-α as well as COX-2 immunostaining. In vitro and in vivo experiments indicated that metformin ameliorated aldo+salt-induced oxidative stress by inhibiting TRAF3IP2 activation, which is an oxidative stress-responsive cytoplasmic adapter molecule, and finally inhibited inflammatory cell infiltration and the pro-inflammatory factors expression such as IL-6, IL-17, and IL-18.121 Metformin relieved oxidative stress by reducing ICAM1 and COX-2 expression and reducing prostaglandin E2 (PGE2) and endogenous mitochondrial ROS production at μM concentrations, thereby inhibiting subsequent inflammatory signaling and metastatic progression in breast cancer cells.122 Moreover, a randomized clinical trial indicated that metformin was more efficient in reducing oxidative stress and restoring antioxidant reserves than lifestyle modification alone in patients newly diagnosed with type 2 diabetes.123
Nevertheless, numerous studies have suggested that high doses of metformin may compromise mitochondrial function and induce oxidative stress.124,125 As a case in point, Picone et al125 observed that ROS production level increased in the human lymphocytes by treatment of metformin at a range of concentrations (5, 10, 20 mM), higher than the regular dose. Hence, different concentrations of metformin application may lead to other, even divergent results. For this reason, concomitant medication should be strictly controlled in future studies.
Mitochondrial DNA (mtDNA)
In mitochondria, extrachromosomal circular DNA is found as mtDNA.126 In recent years, mtDNA has been recognized as a biomarker of oxidative stress.127 Increased oxidative stress directly attacks mtDNA, compromising mitochondrial physiology and leading to mitochondrial dysfunction, resulting in an inflammatory response.128,129 Mutations, deletions, and depletions of mtDNA have been observed in several diseases and pathological disorders.130,131 The released mtDNA could act as endogenous damage-associated molecular patterns (DAMPs) and induce subsequent inflammation by directly activating the inflammasomes such as NLRP3 and AIM2.129,132,133 The above research suggested that the accumulation of dysfunctional mitochondria and the release of mtDNA after mitochondria damage play a critical role in the inflammatory response.
Due to the inhibition of mtATP production and the LPS-induced ATP-dependent synthesis of the NLRP3 ligand mtDNA, metformin could reduce Ox-mtDNA generation, thus attenuating acute respiratory distress syndrome (ARDS), which is an inflammatory condition and is associated with high mortality rates.92 Moreover, metformin treatment profoundly abrogated mtDNA-ATP-induced mitochondrial ROS production, NLRP3 and caspase-1 activation, the release of IL-1β and IL-18 in the RAW264. 7 macrophages.34
Immune Cell Populations
Monocytes-Macrophages
Macrophages are well known that play a pivotal role in inflammatory responses. They recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) via pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) to initiate rapid protective immune responses, serving as the first line of defense against invading pathogens.134,135 However, when macrophages are activated, excessive inflammatory factors are released, aggravating the injured tissues and triggering inflammation.136
Metformin has anti-angiogenic and antitumor properties, and these effects have been demonstrated to be closely associated with the suppression of M2-like polarization of tumor-associated macrophages (TAMs).137 It has been previously shown that in the intestine, the ability of monocytes differentiate into macrophages was inhibited by a supra-pharmacological dose of metformin via AMPK induction, resulting in decreased inflammatory cytokine production. In addition, it activated macrophage polarization into anti-inflammatory phenotypes of M2.138 It has similarly been shown in ApoE−/− mice as an atherosclerosis model.35 In addition, metformin has been reported to improve inflammation at a low level in obesity by modulating macrophage polarization to anti-inflammatory M2 type partly via activation of the AMPK pathway.139 Similar findings have been demonstrated in mice with the multiple sclerosis model autoimmune encephalomyelitis. In addition, another clinical study concerning pancreatic cancer also found that metformin could alter the tumor-educated macrophage polarisation and reduce the tumor cell invasion.140 Numerous studies have indicated that metformin alleviates inflammatory response in macrophages, generally through AMPK activation, with subsequent inhibition of the production of mitochondrial ROS (mtROS).141,142 While apart from affecting macrophage polarization, metformin could play an anti-inflammatory role by inhibiting the fatty acid synthesis and Akt palmitoylation in macrophages.143
Neutrophils
Neutrophils are critical inflammatory cells in the early inflammatory response of innate immunity. Neutrophils can be preferentially recruited to inflamed sites to exert their essential function.144 Activated neutrophils could release neutrophil extracellular traps (NETs) (which contain antimicrobial proteins and chromatin) as a form of non-apoptotic cell death termed “NETosis”,145 which is considered to be a host defense mechanism.146 NETosis not only rap and eliminates diverse pathogens1, but also degrades pro-inflammatory mediators, preventing the inordinate inflammatory response.147 Indeed, NETosis-impaired individuals exhibit an overshooting inflammatory response.147 Excessive NETosis is also a hallmark of sepsis and may exert tissue damage and exacerbate pathological conditions.148,149
Metformin may alleviate inflammatory diseases with the mechanism by blunting the NETosis has been reported. For example, a randomized controlled trial by Menegazzo et al150 reported that metformin exerted a protective effect against NETosis to reverse the diabetic inflammatory milieu in vitro and in vivo. In some cases, the production of ROS is required for NETosis.151 By inhibiting complex I of the mitochondrial electron transport chain, metformin decreases ROS production.152 A significant role for NETs has been found in systemic lupus erythematosus (SLE) recently, as they promoted plasmacytoid dendritic cells (pDCs) activation and differentiation as well as mediated activation of the pDC-IFN-α pathway, which led to potentiate the autoinflammatory feedback loop.153,154 Metformin has been found to decrease PMA-induced NETosis, the generation of interferon-alpha (IFN-ɑ), and the NET-mtDNA copies, which are highly pro-inflammatory.155 Ultimately, metformin reduced disease activity through this treatment and improved survival in SLE patients.156
T Cells, B Cells, and Dendritic Cells
Dysregulated immune cells such as T lymphocytes, B lymphocytes, and dendritic cells (DCs) cause autoimmune diseases, which have characteristics of both autoimmune diseases and autoinflammatory diseases.157
T cells are crucial in host immune defense and immune-mediated inflammatory diseases.158,159 Trafficking of T cells from peripheral blood and lymphoid tissues to sites of inflammation is a key regulatory component.160 The high-fat diet reduced T cell recruitment to the liver, but metformin treatment reversed this effect, and diet-enhanced inflammation was alleviated.161 Moreover, Th17 cells are a subset of CD4T cells that secrete proinflammatory cytokines, including IL-17.162 In a mouse model, metformin ameliorated scleroderma by inhibiting Th17 cells through mTOR-STAT3 signaling.60 Similar findings have been demonstrated in a murine model with acute graft-versus-host disease (aGVHD). Researchers found that metformin could reduce the number of Th1 and Th17 cells and reciprocally upregulate Th2 cells and Treg. The outcomes were achieved via inhibiting the mTOR/STAT3 pathway and inducing autophagy.163
Apart from affecting T cells, metformin was also reported to protect women with polycystic ovary syndrome (PCOS) and PCOS mice induced by DHEA via inhibiting TNF-α production in pathological B cells.57 It was also shown in this study that metformin triggered metabolic reprogramming in B cells of PCOS patients, leading to alterations in mitochondrial morphology, a reduction in mitochondrial membrane potential (MMP), decreased generation of ROS, and reduced glucose uptake. Furthermore, metformin altered metabolic intermediates in splenic B cells from PCOS-model mice.
Accumulating evidence has indicated that autoantibodies derived from abnormal activated B cells contribute to the onset of SLE.164,165 Interestingly, a study in Roquinsan/san mice, a murine model of SLE, showed that metformin inhibited systemic autoimmunity by suppressing B cells differentiation into plasma cells and decreasing the number and size of germinal centers.166 Similar results were obtained in murine Sjögren’s syndrome.13
Dendritic cells (DCs) are the most efficient antigen-presenting cells (APCs) that can initiate and regulate T cells, B cells, and natural killer cells, leading to an adaptive immune response.167 Metformin has been demonstrated to reduce major histocompatibility complex (MHC) class I- and class II-restricted presentation of ovalbumin by decreasing co-stimulatory molecule expression.168
Gut Microbiota
The gut microbiota is a complex microbial community consisting of more than 100 trillion microorganisms in the gastrointestinal tract of humans, including bacteria, viruses, fungi, and protozoa.169 Mounting studies have demonstrated that the alteration in gut microbiota composition has been correlated not only with the intestinal inflammatory disorder but also with numerous diseases characterized by systemic chronic inflammation and metabolic disorders,170 such as allergy, asthma, obesity, and atherosclerosis.171–175 The induction of inflammatory processes perhaps linked to the translocation of microbial products into circulation.176 Recently, Yan et al177 discovered that the bacteria proportions were closely related to inflammatory indicators. The result was that the relative abundance of Firmicutes was positively correlated with TNF-α, whereas Bacteroidetes was inversely correlated with plasma TNF-α, aorta IL-1β, and IL-6. Moreover, the abundance of Proteobacteria was positively correlated with the levels of plasma TNF-α, IL-6, and aorta IL-6. Akkermansia was negatively related to plasma LPS and aorta IL-6. Similarly, a negative correlation existed between Bifidobacterium and plasma LPS, TNF-α, and aorta IL-1β. In contrast, a positive correlation existed between Romboutsia and plasma indicators of inflammation such as IL-6, IL-1β, and TNF-α resistance. Alternatively, the study revealed that the primary reason for the effectiveness of metformin treatment in anti-atherosclerosis might be alleviating macrophage-mediated inflammation via rectification of gut dysbiosis.
Chronic low-grade inflammatory response involves metabolites such as endotoxin derived from the gut flora.178 It has been discovered that non-alcoholic fatty liver disease (NAFLD), which includes a variety of pathological changes such as no-alcoholic steatohepatitis (NASH), is linked to an increase in gut permeability that is associated with increased levels of small intestine bacterial overgrowth in humans.179 Along a similar line, endogenous gut-derived bacterial endotoxin is considered a vital cofactor mediating the pathogenesis of NASH.180,181 Thus, the primary source of circulating endotoxin among nonseptic individuals is bacterial endotoxin translocation through a weakened gut barrier. In one study, mice’s fat-, fructose- and cholesterol-rich diet (FFC) feeding were used to model NAFLD.182 The mice were fed a metformin diet for six weeks, and the markers of inflammation, liver health, intestinal barrier function, and microbiota composition were analyzed. The markers of the inflammation and lipid peroxidation were significantly decreased following metformin treatment. Compared to the NAFLD mice model, intestinal microbiota composition in the proximal small intestine was also changed in metformin-fed mice. These data confirm that metformin exerts anti-inflammatory effects partly by regulating gut microbiota function.
In a dextran sulfate sodium-induced ulcerative colitis (UC) murine model, metformin treatment exerted anti-inflammatory and mucus-protective effects by changing the gut microbiota by increasing the abundance of Akkermansia muciniphila and Lactobacillus, both of which possess anti-inflammatory property.183 Additionally, a clinical study confirmed that metformin could regulate the gut microbiota in patients with type 2 diabetes, which may explain the beneficial pleiotropic effects of metformin.184 Moreover, AMPKα1 activation by metformin has been shown to counteract the effect of the intestinal barrier dysfunction in promoting inflammation which was triggered by C-Jun N-terminal kinase (JNK).185
Autophagy
Autophagy is a multi-step, intracellular lysosomal degradation pathway that degrades or removes damaged proteins and organelles in the cytoplasm.186 Thus, steady-state autophagy is essential for preserving cell homeostasis, while additional autophagy induced by environmental stress may be beneficial for protecting cells.187,188 Some studies have shown that inflammation is tightly related to autophagy, and enhanced autophagy can regulate inflammatory reaction.189
Autophagy can be categorized into macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). Among these, macroautophagy (hereafter autophagy), mediated by organelles known as autophagosomes, is the most well-studied and most relevant to inflammasome studies.190,191 Most reported inhibitory effects on inflammation have been associated with macroautophagy, while the implications of other types of autophagy remain unclear.192 The multi-step process of autophagy requires the collaboration of a variety of autophagic proteins. So far, over 30 autophagy-related proteins (ATGs) are involved in autophagy regulation.193 Among these, microtubule-associated protein light chain 3 (LC3), Beclin1, and autophagy-degrading substrate SQSTM1/p62 are the most widely studied autophagy-related proteins.194 It has been suggested that autophagy-related proteins regulate NALP3-dependent inflammation by maintaining mitochondrial integrity.195 Mechanistically, autophagy could decrease NLRP3 activation via diverse mechanisms, including increasing the p-AMPK levels196 and suppressing the mTOR signaling pathway,197 and continuously clearing various endogenous inflammasome agonists and components, such as ROS and damaged mitochondria.107,198 Kabat et al199 also found that selective deletion of Atg16l1 in T cells in mice resulted in increased pro-inflammatory cytokine production in the gut. As a result, the mice developed spontaneous intestinal inflammation and secreted antibodies against gut bacteria and food, indicating the critical role of autophagy in suppressing intestinal inflammation.
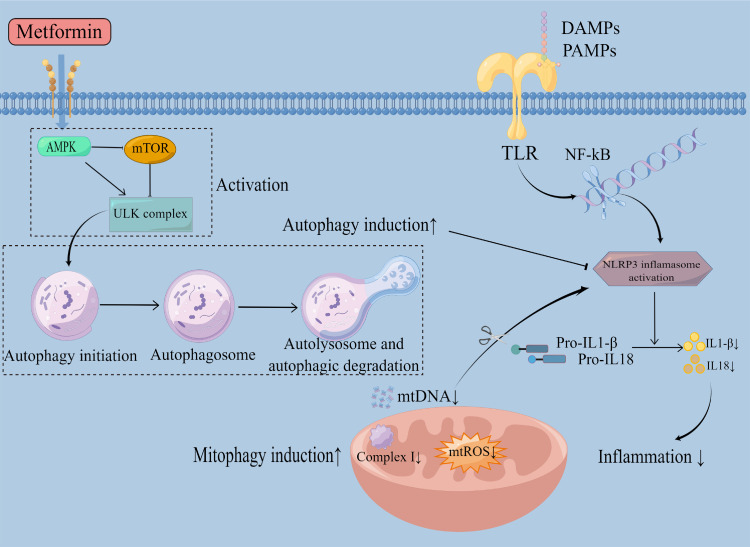
Pharmacological modulation of autophagy is an attractive therapeutic target against inflammatory diseases with metformin treatment (Figure 2). In a Kawasaki disease (KD) vasculitis model, cardiovascular lesions were associated with activation of NLRP3, high ROS levels, and increased systemic 8-OHdG release. Damage induced by ROS and impaired autophagy/mitophagy deteriorated the development of murine KD. In this study, metformin treatment relieved inflammatory response from lactobacillus casei cell wall extract (LCWE), reduced cardiovascular lesions, and enhanced autophagic flux significantly, indicating its potential in treating inflammation diseases. These effects were realized by metformin reducing ROS and activating autophagy through the AMPK axis.200
Figure 2.
Metformin reduces inflammation by regulating the autophagy process. TLR family members activated by PAMPs or DAMPs initiate the innate immune response as a response to infection or injury. Then, it activates NF-kB and further activates the NLRP3 inflammasome. Upon NLRP3 activation, pro-IL1-β and pro-IL18 were cleaved into mature and active IL1-β and IL18. These cytokines are subsequently released, initiating an inflammatory response. Metformin activates AMPK, inhibits mTOR, and activates the ULK complex, thus inducing autophagy and further blocking the activation of NLRP3, inhibiting inflammation. Autophagy involves the formation of autophagosomes, fusion of autophagosomes with lysosomes, and degradation of the autophagy-lysosomes. Metformin could also scavenge mtDNA and mtROS through mitophagy induction via the inhibition of mitochondrial complex I, inhibiting NLRP3 inflammasome activation, thus eventually inhibiting inflammation.
The expression of p-AMPK and the autophagy-related protein LC3 II and p62 were upregulated, and mTOR levels were downregulated by metformin treatment.34 These changes were reversed after treatment with AMPK inhibitor Compound C. What’s more, using the autophagy inhibitors chloroquine (CQ) and 3-methyladenine (3-MA) and knocking down or knocking out Atg5 (autophagy-related gene 5) led to the counteraction of the inhibitory effects of metformin on NLRP3 inflammasome activation induced by hydrogen peroxide and mtDNA-ATP, as well as the release of IL-1β, IL-18 and ROS production in RAW264. 7 macrophages.34
Autophagy has a crucial role in cardiovascular homeostasis.201 Accumulating evidence indicated that inflammatory mediators could be therapeutic targets for heart failure treatment.202,203 Further, a growing body of research suggests that metformin exerts cardiovascular protection irrespective of glucose change and associated effects.109,204–206 Metformin has been reported to regulate autophagy in various cardiovascular diseases, including heart failure, I/R injury, and diabetic cardiomyopathy. However, it has been demonstrated that autophagy activated by I/R appears to act as a compensatory protective mechanism for I/R injury.207
Interestingly, there were almost opposite conclusions about autophagy’s value in I/R injury. A previous study reported that the metformin-driven cardioprotection function involves a decreased level of autophagy and restores the impairment of autophagosome processing, ultimately reducing myocardial inflammation. The study further indicated that metformin’s beneficial effect was mediated by down-regulating the autophagy caused by the Akt signaling pathway.208 Obviously, there is still controversy regarding the role of autophagy in I/R injury.209–211
Of note, over-induction of autophagy leads to inflammatory increases have also been reported. A recent study on periodontal ligament stem cells (PDLSCs) suggested that force-stimulated PDLSC autophagy differentiates macrophages into the M1 phenotype by suppressing the AKT signaling pathway, aiding the inflammatory bone remodeling and tooth movement process.212 Besides, another study indicated that ER stress-induced autophagy may exacerbate the severity of inflammatory bowel diseases.213 Moreover, a recent randomized controlled clinical study found that inhibiting macrophage autophagy by combining corticosteroids and statins enhanced IL-10 production, thereby controlling asthma inflammation.214 Therefore, autophagy is a “double-edged sword” in inflammation.215 Metformin has been shown to down-regulate autophagy by reducing glutamine metabolism and ammonia accumulation at micromolar concentrations.216
However, most of the literature focused on the promoting effect of metformin in autophagy. There are studies indicating otherwise. Whatever the case, metformin’s effect on autophagy activation could be dose-dependent and cell-type specific. More research is needed to elucidate the role of metformin in autophagy further.
Aging
Aging is often accompanied by a state of chronic, low-grade systemic inflammation, which is a major risk factor associated with high levels of mortality and morbidity.217 Many age-related diseases, such as atherosclerosis,218,219 rheumatoid arthritis,220 and Alzheimer’s disease,221 are associated with senescence-associated inflammation caused by pro-inflammatory molecules. Senescent cells occur and accumulate in-vivo, which could increase the production of pro-inflammatory cytokines, including TNFα and IL-6, and matrix metalloproteins, as well as angiogenic factors, creating a secretory profile called Senescence-Associated Secretory Phenotype (SASP), which could result in immune system dysregulation and induce inflammation and senescence.222–225 Therefore, treatment strategies can target cellular senescence to relieve inflammation.
Accumulating evidence demonstrates an anti-inflammatory role of metformin in aging-related inflammation via removing senescent cells and regulating the SASP. Metformin was shown to trigger a DNA damage response that induced senescent cells’ immune clearance via the activation of ATM.226,227 Moreover, mechanisms that metformin accelerate self-renewal include autophagy and prevent several pathologic and destructive changes. In many senescence models, metformin alleviated cellular senescence in a DICER1-dependent mechanism. It reduced the expression of the p16 and p21 proteins and the content of the inflammatory cytokines that are hallmarks of the SASP.228 Similarly, it has been shown that the effects of metformin on preterm birth due to LPS could be alleviated through slowing premature decidual senescence that led to the loss of placenta/fetus attachment to the maternal decidua.229 Furthermore, a randomized controlled trial by Long et al230 demonstrated that metformin could enhance the response of older adults to resistance exercise training by alleviating inflammation in muscle tissue, highlighting the role of metformin in inhibiting aging-related inflammation.
Although studies have shown that elderly patients, especially those with inflammatory diseases, appear less sensitive to metformin, there are some additional benefits. Additional studies are needed to determine whether metformin could reduce inflammation and relieve symptoms of inflammation-associated diseases by reversing the aging processes.
Conclusion
Metformin has been used as an anti-diabetic drug for decades.231 These years, abundant clinical and basic studies have shed light on various critical roles of metformin in various inflammatory diseases. Most studies have focused on targeting AMPK and inhibiting mitochondrial ETC complex I.232 There are still many other possible mechanisms to investigate. Here, we summarize the latest research on the potential mechanisms of metformin in different inflammatory disorders. Gaining more insight into how metformin regulates inflammatory responses and characterizing the exact targets may be helpful in personalized therapy and the clinical use of metformin. The specific role and precise mechanisms of metformin under different physiological or pathological conditions need further study, and these efforts should be the focus of future research.
Funding Statement
This study was supported by the Program of National Natural Science Foundation of China(Nos. 81960352, 82160376, and 81760342); Youth Science Foundation of Jiangxi Province of China (No. 20192BAB215029); the Key Research and Development Plan of the Science and Technology Department of Jiangxi Province of China (No. 20171ACG70004); the Natural Science Foundation of Jiangxi Province (No. 20212ACB214002); Jiangxi Provincial Department of Science and Technology, Gan Po Talents 555 project leader talent training program (No. 1210013001).
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.Marciano O, Mehazri L, Shpungin S, Varvak A, Zacksenhaus E, Nir U. Fer and FerT Govern mitochondrial susceptibility to metformin and hypoxic stress in colon and lung carcinoma cells. Cells. 2021;10(1):97. doi: 10.3390/cells10010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosi E. Metformin--The gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes Metab. 2009;11 Suppl 2:3–8. doi: 10.1111/j.1463-1326.2008.01031.x [DOI] [PubMed] [Google Scholar]
- 3.Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320(5):C873–c879. doi: 10.1152/ajpcell.00604.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Gan D, Lin S, et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics. 2022;12(6):2722–2740. doi: 10.7150/thno.71360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamarudin MNA, Sarker MMR, Zhou JR, Parhar I. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res. 2019;38(1):491. doi: 10.1186/s13046-019-1495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo F, Das A, Chen J, Wu P, Li X, Fang Z. Metformin in patients with and without diabetes: a paradigm shift in cardiovascular disease management. Cardiovasc Diabetol. 2019;18(1):54. doi: 10.1186/s12933-019-0860-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaría E, Letelier NA, Valledor AF. Integrating the roles of liver X receptors in inflammation and infection: mechanisms and outcomes. Curr Opin Pharmacol. 2020;53:55–65. doi: 10.1016/j.coph.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847 [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Chen Z, Shen W, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther. 2021;6(1):245. doi: 10.1038/s41392-021-00646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makino Y, Fujikawa K, Matsuki-Fukushima M, Inoue S, Nakamura M. Role of innate inflammation in the regulation of tissue remodeling during tooth eruption. Dent J (Basel). 2021;9(1). doi: 10.3390/dj9010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi: 10.1097/CCM.0000000000002074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Kim SM, Park JS, et al. Metformin improves salivary gland inflammation and hypofunction in murine Sjögren’s syndrome. Arthritis Res Thera. 2019;21(1):136. doi: 10.1186/s13075-019-1904-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29(2):143–150. doi: 10.1016/j.joca.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Wang L, Zhou XE, et al. Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res. 2015;25(1):50–66. doi: 10.1038/cr.2014.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Kim SJ, Yu KY, Jeong SI, Kim SY. Anti-hyperglycemic effects and signaling mechanism of Perilla frutescens sprout extract. Nutr Res Pract. 2018;12(1):20–28. doi: 10.4162/nrp.2018.12.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Wang M, Ju J, et al. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 2019;11(1):199–209. [PMC free article] [PubMed] [Google Scholar]
- 18.González A, Hall MN, Lin SC, Hardie DG. AMPK and TOR: the Yin and Yang of cellular nutrient sensing and growth control. Cell Metab. 2020;31(3):472–492. doi: 10.1016/j.cmet.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 19.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223 [DOI] [PubMed] [Google Scholar]
- 20.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3(Pt 3):607–614. doi: 10.1042/bj3480607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Z, Tian X, Zhu J, et al. Metformin suppresses UHMWPE particle-induced osteolysis in the mouse calvaria by promoting polarization of macrophages to an anti-inflammatory phenotype. Mol Med. 2018;24(1):20. doi: 10.1186/s10020-018-0013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421(2):163–169. doi: 10.1042/BJ20090613 [DOI] [PubMed] [Google Scholar]
- 23.Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2(4):e001076. doi: 10.1136/bmjopen-2012-001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcum ZA, Forsberg CW, Moore KP, et al. Mortality associated with metformin versus sulfonylurea initiation: a cohort study of veterans with diabetes and chronic kidney disease. J Gen Intern Med. 2018;33(2):155–165. doi: 10.1007/s11606-017-4219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanami D, Takashi Y, Tanabe M. Significance of Metformin Use in Diabetic Kidney Disease. Int J Mol Sci. 2020;21(12):4239. doi: 10.3390/ijms21124239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Ma XY, Han JY, et al. Metformin regulates inflammation and fibrosis in diabetic kidney disease through TNC/TLR4/NF-κB/miR-155-5p inflammatory loop. World J Diabetes. 2021;12(1):19–46. doi: 10.4239/wjd.v12.i1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T, Liu J, Xie C, Yang J, Zhao L, Yang J. Metformin attenuates diabetic renal injury via the AMPK-autophagy axis. Exp Ther Med. 2021;21(6):578. doi: 10.3892/etm.2021.10010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen XC, Wu D, Wu HL, et al. Metformin improves renal injury of MRL/lpr lupus-prone mice via the AMPK/STAT3 pathway. Lupus Sci Med. 2022;9(1):e000611. doi: 10.1136/lupus-2021-000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Huang L, Shi X, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020;12(23):24270–24287. doi: 10.18632/aging.202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santana PT, Luna-Gomes T, Rangel-Ferreira MV, et al. P2Y(12) receptor antagonist clopidogrel attenuates lung inflammation triggered by Silica particles. Front Pharmacol. 2020;11:301. doi: 10.3389/fphar.2020.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng D, Xu Q, Wang Y, et al. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J Transl Med. 2021;19(1):349. doi: 10.1186/s12967-021-03036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saber S, El-Kader EMA. Novel complementary coloprotective effects of metformin and MCC950 by modulating HSP90/NLRP3 interaction and inducing autophagy in rats. Inflammopharmacology. 2021;29(1):237–251. doi: 10.1007/s10787-020-00730-6 [DOI] [PubMed] [Google Scholar]
- 34.Fei Q, Ma H, Zou J, et al. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J Mol Cell Cardiol. 2020;145:1–13. doi: 10.1016/j.yjmcc.2020.05.016 [DOI] [PubMed] [Google Scholar]
- 35.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64(6):2028–2041. doi: 10.2337/db14-1225 [DOI] [PubMed] [Google Scholar]
- 36.Chen YC, Lee SD, Kuo CH, Ho LT. The effects of altitude training on the AMPK-related glucose transport pathway in the red skeletal muscle of both lean and obese Zucker rats. High Alt Med Biol. 2011;12(4):371–378. doi: 10.1089/ham.2010.1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MJ, Nagy LE, Park PH. Globular adiponectin inhibits ethanol-induced reactive oxygen species production through modulation of NADPH oxidase in macrophages: involvement of liver kinase B1/AMP-activated protein kinase pathway. Mol Pharmacol. 2014;86(3):284–296. doi: 10.1124/mol.114.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang WP, Deng JS, Huang SS, et al. Sanghuangporus sanghuang mycelium prevents paracetamol-induced hepatotoxicity through regulating the MAPK/NF-κB, Keap1/Nrf2/HO-1, TLR4/PI3K/Akt, and CaMKKβ/LKB1/AMPK pathways and suppressing oxidative stress and inflammation. Antioxidants (Basel). 2021;10(6):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen J, Xu B, Sun Y, et al. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res. 2019;146:104308. doi: 10.1016/j.phrs.2019.104308 [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Zhang W, Zhang M, Zhu H, Moriasi C, Zou MH. Liver kinase B1 suppresses lipopolysaccharide-induced nuclear factor κB (NF-κB) activation in macrophages. J Biol Chem. 2015;290(4):2312–2320. doi: 10.1074/jbc.M114.616441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacIver NJ, Blagih J, Saucillo DC, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187(8):4187–4198. doi: 10.4049/jimmunol.1100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Dong R, Hu D, et al. Liver kinase B1/AMP-activated protein kinase pathway activation attenuated the progression of endotoxemia in the diabetic mice. Cell Physiol Biochem. 2017;42(2):761–779. doi: 10.1159/000478068 [DOI] [PubMed] [Google Scholar]
- 44.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50 Suppl(Suppl):S138–143. doi: 10.1194/jlr.R800079-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zhao H, Yang L, et al. The role of CD36-Fabp4-PPARγ in skeletal muscle involves insulin resistance in intrauterine growth retardation mice with catch-up growth. BMC Endocr Disord. 2022;22(1):10. doi: 10.1186/s12902-021-00921-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lingesh A, Paul D, Naidu V, Satheeshkumar N. AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells. J Ethnopharmacol. 2019;233:123–130. doi: 10.1016/j.jep.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 47.Lv Q, Zhen Q, Liu L, et al. AMP-kinase pathway is involved in tumor necrosis factor alpha-induced lipid accumulation in human hepatoma cells. Life Sci. 2015;131:23–29. doi: 10.1016/j.lfs.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 48.Calixto MC, Lintomen L, André DM, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS One. 2013;8(10):e76786. doi: 10.1371/journal.pone.0076786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D, Lee JE, Jung YJ, et al. Metformin decreases high-fat diet-induced renal injury by regulating the expression of adipokines and the renal AMP-activated protein kinase/acetyl-CoA carboxylase pathway in mice. Int J Mol Med. 2013;32(6):1293–1302. [DOI] [PubMed] [Google Scholar]
- 50.Chung MM, Nicol CJ, Cheng YC, et al. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352(1):75–83. doi: 10.1016/j.yexcr.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 51.Zhou M, Xu W, Wang J, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–360. doi: 10.1016/j.ebiom.2018.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, Li J, Wang PH, et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim Biophys Acta Mol Basis Dis. 2021;1867(12):166260. doi: 10.1016/j.bbadis.2021.166260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi W, Gupta S, Ricker E, et al. The mTORC1-4E-BP-eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat Commun. 2017;8(1):254. doi: 10.1038/s41467-017-00348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu Z, Ji S, Zheng S. BRAF controls the effects of metformin on neuroblast cell divisions in C. elegans. Int J Mol Sci. 2020;22(1):178. doi: 10.3390/ijms22010178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue W, Zheng X, Lin Y, et al. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget. 2015;6(25):21208–21224. doi: 10.18632/oncotarget.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Nostrand JL, Hellberg K, Luo EC, et al. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes Dev. 2020;34(19–20):1330–1344. doi: 10.1101/gad.339895.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao N, Wang J, Wang T, et al. Metformin abrogates pathological TNF-α-producing B cells through mTOR-dependent metabolic reprogramming in polycystic ovary syndrome. Elife. 2022;11:e74713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohamed MAE, Abdel-Rahman RF, Mahmoud SS, Khattab MM, Safar MM. Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav. 2020;104(Pt A):106893. doi: 10.1016/j.yebeh.2019.106893 [DOI] [PubMed] [Google Scholar]
- 59.Planas D, Pagliuzza A, Ponte R, et al. LILAC pilot study: effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. EBioMedicine. 2021;65:103270. doi: 10.1016/j.ebiom.2021.103270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moon J, Lee SY, Choi JW, et al. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J Transl Med. 2021;19(1):192. doi: 10.1186/s12967-021-02860-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li SX, Li C, Pang XR, et al. Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front Pharmacol. 2021;12:719589. doi: 10.3389/fphar.2021.719589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Z, Ye H, Xiao W, et al. Metformin attenuates inflammation and fibrosis in thyroid-associated ophthalmopathy. Int J Mol Sci. 2022;23(24):15508. doi: 10.3390/ijms232415508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazirpanah N, Ottria A, van der Linden M, et al. mTOR inhibition by metformin impacts monosodium urate crystal-induced inflammation and cell death in gout: a prelude to a new add-on therapy? Ann Rheum Dis. 2019;78(5):663–671. doi: 10.1136/annrheumdis-2018-214656 [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Yang F, Ma W, Sun Q. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytes in vitro via mTOR-signaling pathway. Pharm Biol. 2016;54(7):1173–1178. doi: 10.3109/13880209.2015.1057652 [DOI] [PubMed] [Google Scholar]
- 65.Al-Hashem F, Al-Humayed S, Amin SN, et al. Metformin inhibits mTOR-HIF-1α axis and profibrogenic and inflammatory biomarkers in thioacetamide-induced hepatic tissue alterations. J Cell Physiol. 2019;234(6):9328–9337. doi: 10.1002/jcp.27616 [DOI] [PubMed] [Google Scholar]
- 66.Wu YQ, Xiong J, He ZL, et al. Metformin promotes microglial cells to facilitate myelin debris clearance and accelerate nerve repairment after spinal cord injury. Acta Pharmacol Sin. 2022;43(6):1360–1371. doi: 10.1038/s41401-021-00759-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia J, Chen J, Vashisth MK, et al. Metformin ameliorates 5-fluorouracil-induced intestinal injury by inhibiting cellular senescence, inflammation, and oxidative stress. Int Immunopharmacol. 2022;113(Pt A):109342. doi: 10.1016/j.intimp.2022.109342 [DOI] [PubMed] [Google Scholar]
- 68.Gao Y, Liu J, Li K, et al. Metformin alleviates sepsis-associated myocardial injury by enhancing AMP-activated protein kinase/mammalian target of rapamycin signaling pathway-mediated autophagy. J Cardiovasc Pharmacol. 2023;82(4):308–317. doi: 10.1097/FJC.0000000000001463 [DOI] [PubMed] [Google Scholar]
- 69.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi: 10.1038/nature11862 [DOI] [PubMed] [Google Scholar]
- 70.de Mingo Á, de Gregorio E, Moles A, et al. Cysteine cathepsins control hepatic NF-κB-dependent inflammation via sirtuin-1 regulation. Cell Death Dis. 2016;7(11):e2464. doi: 10.1038/cddis.2016.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–1722. doi: 10.1172/JCI118598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ziadlou R, Barbero A, Martin I, et al. Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules. 2020;10(6):932. doi: 10.3390/biom10060932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung CH, Lee MJ, Kang YM, et al. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol. 2014;13:41. doi: 10.1186/1475-2840-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou C, Peng B, Qin Z, Zhu W, Guo C. Metformin attenuates LPS-induced neuronal injury and cognitive impairments by blocking NF-κB pathway. BMC Neurosci. 2021;22(1):73. doi: 10.1186/s12868-021-00678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, Zhang H, Zhao Z, et al. Hyperglycemia induces meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2022;63(1):30. doi: 10.1167/iovs.63.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williamson L, Ayalon I, Shen H, Kaplan J. Hepatic STAT3 inhibition amplifies the inflammatory response in obese mice during sepsis. Am J Physiol Endocrinol Metab. 2019;316(2):E286–e292. doi: 10.1152/ajpendo.00341.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Mahdy NA, El-Sayad ME, El-Kadem AH, Abu-Risha SE. Metformin alleviates inflammation in oxazolone induced ulcerative colitis in rats: plausible role of sphingosine kinase 1/sphingosine 1 phosphate signaling pathway. Immunopharmacol Immunotoxicol. 2021;43(2):192–202. [DOI] [PubMed] [Google Scholar]
- 78.Geraghty P, Wyman AE, Garcia-Arcos I, Dabo AJ, Gadhvi S, Foronjy R. STAT3 modulates cigarette smoke-induced inflammation and protease expression. Front Physiol. 2013;4:267. doi: 10.3389/fphys.2013.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front Oncol. 2019;9:48. doi: 10.3389/fonc.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Son HJ, Lee J, Lee SY, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. doi: 10.1155/2014/973986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee SY, Lee SH, Yang EJ, et al. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One. 2015;10(9):e0135858. doi: 10.1371/journal.pone.0135858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu HC, Cheng MJ, Yen CH, et al. Chemical constituents with GNMT-promoter-enhancing and NRF2-reduction activities from Taiwan Agarwood excoecaria formosana. Molecules. 2020;25(7):1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 Signaling pathway and its role in inflammation. Molecules. 2020;25(22):5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braun S, Hanselmann C, Gassmann MG, et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22(15):5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishii Y, Itoh K, Morishima Y, et al. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175(10):6968–6975. doi: 10.4049/jimmunol.175.10.6968 [DOI] [PubMed] [Google Scholar]
- 88.Zhou J, Ma X, Cui Y, et al. Methyleugenol protects against t-BHP-triggered oxidative injury by induction of Nrf2 dependent on AMPK/GSK3β and ERK activation. J Pharmacol Sci. 2017;135(2):55–63. doi: 10.1016/j.jphs.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 89.Arbab AAI, Lu X, Abdalla IM, et al. Metformin inhibits lipoteichoic acid-induced oxidative stress and inflammation through AMPK/NRF2/NF-κB signaling pathway in bovine mammary epithelial cells. Front Vet Sci. 2021;8:661380. doi: 10.3389/fvets.2021.661380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tao F, Zhou Y, Wang M, et al. Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway. Korean J Physiol Pharmacol. 2022;26(2):95–111. doi: 10.4196/kjpp.2022.26.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.L A, Zou T, He J, et al. Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front Mol Neurosci. 2019;12:102. doi: 10.3389/fnmol.2019.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xian H, Liu Y, Rundberg Nilsson A, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54(7):1463–1477.e1411. doi: 10.1016/j.immuni.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin CC, Yeh HH, Huang WL, et al. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2013;49(2):241–250. doi: 10.1165/rcmb.2012-0244OC [DOI] [PubMed] [Google Scholar]
- 94.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou X, Chen J, Yi G, et al. Metformin suppresses hypoxia-induced stabilization of HIF-1α through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget. 2016;7(1):873–884. doi: 10.18632/oncotarget.6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song YM, Lee YH, Kim JW, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59. doi: 10.4161/15548627.2014.984271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qi T, Chen Y, Li H, et al. A role for PFKFB3/iPFK2 in metformin suppression of adipocyte inflammatory responses. J Mol Endocrinol. 2017;59(1):49–59. doi: 10.1530/JME-17-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woo SL, Xu H, Li H, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9(3):e91111. doi: 10.1371/journal.pone.0091111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nelson LE, Valentine RJ, Cacicedo JM, Gauthier MS, Ido Y, Ruderman NB. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. Am J Physiol Cell Physiol. 2012;303(1):C4–c13. doi: 10.1152/ajpcell.00296.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelly B, Tannahill GM, Murphy MP, O’Neill LA. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290(33):20348–20359. doi: 10.1074/jbc.M115.662114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao J, Yuan J, Wang Q, et al. Metformin protects against PM(2.5)-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2. Redox Biol. 2020;28:101345. doi: 10.1016/j.redox.2019.101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan S, Lu Q, Shu Y, Sun Y, Chen F, Tang L. Iridoid glycosides fraction isolated from Veronica ciliata Fisch. Protects against Acetaminophen-induced liver injury in mice. Evid Based Complement Alternat Med. 2017;2017:6106572. doi: 10.1155/2017/6106572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Algire C, Moiseeva O, Deschênes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). 2012;5(4):536–543. doi: 10.1158/1940-6207.CAPR-11-0536 [DOI] [PubMed] [Google Scholar]
- 104.Rymut SM, Lu B, Perez A, et al. Acetyl-CoA carboxylase inhibition regulates microtubule dynamics and intracellular transport in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2019;316(6):L1081–l1093. doi: 10.1152/ajplung.00369.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin M, Chen WP, Yin XP, Tu JL, Hu N, Li ZY. LncRNA TUG1 demethylated by TET2 promotes NLRP3 expression, contributes to cerebral ischemia/reperfusion inflammatory injury. ASN Neuro. 2021;13:17590914211003247. doi: 10.1177/17590914211003247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang CS, Shin DM, Jo EK. The role of NLR-related protein 3 inflammasome in host defense and inflammatory diseases. Int Neurourol J. 2012;16(1):2–12. doi: 10.5213/inj.2012.16.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 108.Chen K, Dai H, Yuan J, et al. Optineurin-mediated mitophagy protects renal tubular epithelial cells against accelerated senescence in diabetic nephropathy. Cell Death Dis. 2018;9(2):105. doi: 10.1038/s41419-017-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang F, Qin Y, Wang Y, et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–1019. doi: 10.7150/ijbs.29680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du RH, Tan J, Sun XY, Lu M, Ding JH, Hu G. Fluoxetine Inhibits NLRP3 Inflammasome Activation: implication in Depression. Int J Neuropsychopharmacol. 2016;19(9):pyw037. doi: 10.1093/ijnp/pyw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ka SM, Kuoping Chao L, Lin JC, et al. A low toxicity synthetic cinnamaldehyde derivative ameliorates renal inflammation in mice by inhibiting NLRP3 inflammasome and its related signaling pathways. Free Radic Biol Med. 2016;91:10–24. doi: 10.1016/j.freeradbiomed.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 112.Du RW, Bu WG. Metformin improves depressive-like symptoms in mice via inhibition of peripheral and central NF-κB-NLRP3 inflammation activation. Exp Brain Res. 2020;238(11):2549–2556. doi: 10.1007/s00221-020-05911-x [DOI] [PubMed] [Google Scholar]
- 113.Cenni V, Squarzoni S, Loi M, Mattioli E, Lattanzi G, Capanni C. Emerin phosphorylation during the early phase of the oxidative stress response influences emerin-BAF interaction and BAF nuclear localization. Cells. 2020;9(6):1415. doi: 10.3390/cells9061415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gandellini P, Giannoni E, Casamichele A, et al. miR-205 hinders the malignant interplay between prostate cancer cells and associated fibroblasts. Antioxid Redox Signal. 2014;20(7):1045–1059. doi: 10.1089/ars.2013.5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Y, Liu H, Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J Nanobiotechnology. 2020;18(1):145. doi: 10.1186/s12951-020-00703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi HJ, Alam MB, Baek ME, Kwon YG, Lim JY, Lee SH. Protection against UVB-induced photoaging by Nypa fruticans via inhibition of MAPK/AP-1/MMP-1 signaling. Oxid Med Cell Longev. 2020;2020:2905362. doi: 10.1155/2020/2905362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335(1):191–203. doi: 10.1007/s00441-008-0678-5 [DOI] [PubMed] [Google Scholar]
- 118.Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192(3):233–242. doi: 10.1016/j.cbi.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 119.Yang X, Xu Z, Zhang C, Cai Z, Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1984–1990. doi: 10.1016/j.bbadis.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 120.Araújo AA, Pereira A, Medeiros C, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One. 2017;12(8):e0183506. doi: 10.1371/journal.pone.0183506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mummidi S, Das NA, Carpenter AJ, et al. Metformin inhibits aldosterone-induced cardiac fibroblast activation, migration and proliferation in vitro, and reverses aldosterone+salt-induced cardiac fibrosis in vivo. J Mol Cell Cardiol. 2016;98:95–102. doi: 10.1016/j.yjmcc.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 122.Schexnayder C, Broussard K, Onuaguluchi D, et al. Metformin Inhibits migration and invasion by suppressing ROS production and COX2 expression in MDA-MB-231 Breast cancer cells. Int J Mol Sci. 2018;19(11). doi: 10.3390/ijms19113692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esteghamati A, Eskandari D, Mirmiranpour H, et al. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin Nutr. 2013;32(2):179–185. doi: 10.1016/j.clnu.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 124.Müller S, Versini A, Sindikubwabo F, et al. Metformin reveals a mitochondrial copper addiction of mesenchymal cancer cells. PLoS One. 2018;13(11):e0206764. doi: 10.1371/journal.pone.0206764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Picone P, Nuzzo D, Caruana L, et al. Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-κB activation: use of insulin to attenuate metformin’s effect. Biochim Biophys Acta. 2015;1853(5):1046–1059. doi: 10.1016/j.bbamcr.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 126.Wong C, Li Y, Lee C, Huang CH. Ensemble learning algorithms for classification of mtDNA into haplogroups. Brief Bioinform. 2011;12(1):1–9. doi: 10.1093/bib/bbq008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu Y, Lindh CH, Jönsson BAG, Broberg K, Albin M. Occupational exposure to asphalt mixture during road paving is related to increased mitochondria DNA copy number: a cross-sectional study. Environ Health. 2018;17(1):29. doi: 10.1186/s12940-018-0375-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu MH, Chen IC, Lee CH, et al. Anti-neuroinflammation ameliorates systemic inflammation-induced mitochondrial DNA impairment in the nucleus of the solitary tract and cardiovascular reflex dysfunction. J Neuroinflammation. 2019;16(1):224. doi: 10.1186/s12974-019-1623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou X, Backman LJ, Danielson P. Activation of NF-κB signaling via cytosolic mitochondrial RNA sensing in kerotocytes with mitochondrial DNA common deletion. Sci Rep. 2021;11(1):7360. doi: 10.1038/s41598-021-86522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paunel-Görgülü A, Wacker M, El Aita M, et al. cfDNA correlates with endothelial damage after cardiac surgery with prolonged cardiopulmonary bypass and amplifies NETosis in an intracellular TLR9-independent manner. Sci Rep. 2017;7(1):17421. doi: 10.1038/s41598-017-17561-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mangold A, Alias S, Scherz T, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–1192. doi: 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 132.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu L, Zhou J, Che J, et al. Mitochondrial DNA enables AIM2 inflammasome activation and hepatocyte pyroptosis in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2021;320(6):G1034–g1044. doi: 10.1152/ajpgi.00431.2020 [DOI] [PubMed] [Google Scholar]
- 134.Nishimoto S, Fukuda D, Sata M. Emerging roles of Toll-like receptor 9 in cardiometabolic disorders. Inflamm Regen. 2020;40:18. doi: 10.1186/s41232-020-00118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cai J, Li J, Zhou Y, et al. Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux. J Cell Mol Med. 2020;24(6):3460–3468. doi: 10.1111/jcmm.15027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang M, Xue Y, Li J, et al. PKCδ/MAPKs and NF-κB pathways are involved in the regulation of ingenane-type diterpenoids from euphorbia neriifolia on macrophage function. J Inflamm Res. 2021;14:2681–2696. doi: 10.2147/JIR.S306846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ding L, Liang G, Yao Z, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6(34):36441–36455. doi: 10.18632/oncotarget.5541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xue Y, Zhang H, Sun X, Zhu MJ. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS One. 2016;11(12):e0168670. doi: 10.1371/journal.pone.0168670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jing Y, Wu F, Li D, Yang L, Li Q, Li R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol. 2018;461:256–264. doi: 10.1016/j.mce.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 140.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol. 2014;92(6):543–552. doi: 10.1038/icb.2014.22 [DOI] [PubMed] [Google Scholar]
- 141.Nassif RM, Chalhoub E, Chedid P, et al. Metformin Inhibits ROS Production by Human M2 Macrophages via the Activation of AMPK. Biomedicines. 2022;10(2):319. doi: 10.3390/biomedicines10020319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin CF, Young KC, Bai CH, et al. Blockade of reactive oxygen species and Akt activation is critical for anti-inflammation and growth inhibition of metformin in phosphatase and tensin homolog-deficient RAW264.7 cells. Immunopharmacol Immunotoxicol. 2013;35(6):669–677. doi: 10.3109/08923973.2013.837059 [DOI] [PubMed] [Google Scholar]
- 143.Xiong W, Sun KY, Zhu Y, Zhang X, Zhou YH, Zou X. Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death Dis. 2021;12(10):934. doi: 10.1038/s41419-021-04235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Filtjens J, Roger A, Quatrini L, et al. Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection. Nat Commun. 2021;12(1):2936. doi: 10.1038/s41467-021-22841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gavillet M, Martinod K, Renella R, et al. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am J Hematol. 2015;90(12):1155–1158. doi: 10.1002/ajh.24185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Navrátilová A, Bečvář V, Baloun J, et al. S100A11 (calgizzarin) is released via NETosis in rheumatoid arthritis (RA) and stimulates IL-6 and TNF secretion by neutrophils. Sci Rep. 2021;11(1):6063. doi: 10.1038/s41598-021-85561-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen K, Geng S, Yuan R, Diao N, Upchurch Z, Li L. Super-low dose endotoxin pre-conditioning exacerbates sepsis mortality. EBioMedicine. 2015;2(4):324–333. doi: 10.1016/j.ebiom.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sofoluwe A, Bacchetta M, Badaoui M, Kwak BR, Chanson M. ATP amplifies NADPH-dependent and -independent neutrophil extracellular trap formation. Sci Rep. 2019;9(1):16556. doi: 10.1038/s41598-019-53058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan B, Alam HB, Chong W, et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci Rep. 2017;7(1):8972. doi: 10.1038/s41598-017-09337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Menegazzo L, Scattolini V, Cappellari R, et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018;55(6):593–601. doi: 10.1007/s00592-018-1129-8 [DOI] [PubMed] [Google Scholar]
- 151.Shaban L, Nguyen GT, Mecsas-Faxon BD, Swanson KD, Tan S, Mecsas J. Yersinia pseudotuberculosis YopH targets SKAP2-dependent and independent signaling pathways to block neutrophil antimicrobial mechanisms during infection. PLoS Pathog. 2020;16(5):e1008576. doi: 10.1371/journal.ppat.1008576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mogavero A, Maiorana MV, Zanutto S, et al. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci Rep. 2017;7(1):15992. doi: 10.1038/s41598-017-16149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soni C, Reizis B. Self-DNA at the epicenter of SLE: immunogenic forms, regulation, and effects. Front Immunol. 2019;10:1601. doi: 10.3389/fimmu.2019.01601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parackova Z, Zentsova I, Vrabcova P, et al. Neutrophil extracellular trap induced dendritic cell activation leads to Th1 polarization in type 1 diabetes. Front Immunol. 2020;11:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang LY, Shen XT, Sun HT, Zhu WW, Zhang JB, Lu L. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic. J Cancer. 2022;13(4):1261–1271. doi: 10.7150/jca.64170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015;67(12):3190–3200. doi: 10.1002/art.39296 [DOI] [PubMed] [Google Scholar]
- 157.Cai Q, Huo GW, Zhu FY, Yue P, Yuan DQ, Chen P. Safety and efficacy of immune checkpoint inhibitors in advanced cancer patients with autoimmune disease: a meta-analysis. Hum Vaccin Immunother. 2022;18(7):2145102. doi: 10.1080/21645515.2022.2145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ledderose C, Liu K, Kondo Y, et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest. 2018;128(8):3583–3594. doi: 10.1172/JCI120972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28(4):163–171. doi: 10.1093/intimm/dxw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loza MJ, Foster S, Bleecker ER, Peters SP, Penn RB. Asthma and gender impact accumulation of T cell subtypes. Respir Res. 2010;11(1):103. doi: 10.1186/1465-9921-11-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.de Oliveira S, Houseright RA, Graves AL, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70(4):710–721. doi: 10.1016/j.jhep.2018.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Noguchi M, Nomura A, Doi S, et al. Ternary crystal structure of human RORγ ligand-binding-domain, an inhibitor and corepressor peptide provides a new insight into corepressor interaction. Sci Rep. 2018;8(1):17374. doi: 10.1038/s41598-018-35783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park MJ, Lee SY, Moon SJ, et al. Metformin attenuates graft-versus-host disease via restricting mammalian target of rapamycin/signal transducer and activator of transcription 3 and promoting adenosine monophosphate-activated protein kinase-autophagy for the balance between T helper 17 and Tregs. Transl Res. 2016;173:115–130. doi: 10.1016/j.trsl.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 164.Li S, Liu S, Chen F, et al. Link-polymorphism of 5-HTT promoter region is associated with autoantibodies in patients with systemic lupus erythematosus. J Immunol Res. 2016;2016:3042726. doi: 10.1155/2016/3042726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo Q, Li X, Fu B, et al. Decreased expression of TIGIT in NK cells correlates negatively with disease activity in systemic lupus erythematosus. Int J Clin Exp Pathol. 2018;11(5):2408–2418. [PMC free article] [PubMed] [Google Scholar]
- 166.Lee SY, Moon SJ, Kim EK, et al. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol. 2017;198(7):2661–2670. doi: 10.4049/jimmunol.1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- 168.Shin S, Hyun B, Lee A, et al. Metformin suppresses MHC-restricted antigen presentation by inhibiting co-stimulatory factors and MHC molecules in APCs. Biomol Ther (Seoul). 2013;21(1):35–41. doi: 10.4062/biomolther.2012.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moschen AR, Wieser V, Tilg H. Dietary factors: major regulators of the gut’s microbiota. Gut Liver. 2012;6(4):411–416. doi: 10.5009/gnl.2012.6.4.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liso M, De Santis S, Scarano A, et al. A bronze-tomato enriched diet affects the intestinal microbiome under homeostatic and inflammatory conditions. Nutrients. 2018;10(12):1862. doi: 10.3390/nu10121862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang X, Yi W, He L, et al. Abnormalities in Gut microbiota and metabolism in patients with chronic spontaneous urticaria. Front Immunol. 2021;12:691304. doi: 10.3389/fimmu.2021.691304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51(4):167–174. doi: 10.1097/NT.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Castaner O, Goday A, Park YM, et al. The Gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:4095789. doi: 10.1155/2018/4095789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen ML, Yi L, Zhang Y, et al. Resveratrol attenuates trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. 2016;7(2):e02210–02215. doi: 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu SK, Ma LB, Yuan Y, et al. Alanylglutamine relieved asthma symptoms by regulating gut microbiota and the derived metabolites in mice. Oxid Med Cell Longev. 2020;2020:7101407. doi: 10.1155/2020/7101407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Poroyko VA, Carreras A, Khalyfa A, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. doi: 10.1038/srep35405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yan N, Wang L, Li Y, et al. Metformin intervention ameliorates AS in ApoE-/- mice through restoring gut dysbiosis and anti-inflammation. PLoS One. 2021;16(7):e0254321. doi: 10.1371/journal.pone.0254321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu QJ, Wang YQ, Qi YX. The protective effect of procyanidin against LPS-induced acute gut injury by the regulations of oxidative state. Springerplus. 2016;5(1):1645. doi: 10.1186/s40064-016-3306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Adar T, Ben Ya’acov A, Lalazar G, et al. Oral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clin Exp Immunol. 2012;167(2):252–260. doi: 10.1111/j.1365-2249.2011.04511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18(21):2609–2618. doi: 10.3748/wjg.v18.i21.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dornas W, Lagente V. Intestinally derived bacterial products stimulate development of nonalcoholic steatohepatitis. Pharmacol Res. 2019;141:418–428. doi: 10.1016/j.phrs.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 182.Brandt A, Hernández-Arriaga A, Kehm R, et al. Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci Rep. 2019;9(1):6668. doi: 10.1038/s41598-019-43228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu Z, Liao W, Zhang Z, et al. Metformin affects gut microbiota composition and diversity associated with amelioration of dextran sulfate sodium-induced colitis in mice. Front Pharmacol. 2021;12:640347. doi: 10.3389/fphar.2021.640347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Napolitano A, Miller S, Nicholls AW, et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9(7):e100778. doi: 10.1371/journal.pone.0100778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deng J, Zeng L, Lai X, et al. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med. 2018;22(1):546–557. doi: 10.1111/jcmm.13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L. Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/Autophagy signaling pathway. Nutrients. 2019;11(11):2794. doi: 10.3390/nu11112794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Janssen AFJ, Katrukha EA, van Straaten W, Verlhac P, Reggiori F, Kapitein LC. Probing aggrephagy using chemically-induced protein aggregates. Nat Commun. 2018;9(1):4245. doi: 10.1038/s41467-018-06674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen RJ, Lee YH, Yeh YL, Wang YJ, Wang BJ. The roles of autophagy and the inflammasome during environmental stress-triggered skin inflammation. Int J Mol Sci. 2016;17(12):2063. doi: 10.3390/ijms17122063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo W, Liu J, Li W, et al. Niacin alleviates dairy cow mastitis by regulating the GPR109A/AMPK/NRF2 signaling pathway. Int J Mol Sci. 2020;21(9):3321. doi: 10.3390/ijms21093321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maulucci G, Chiarpotto M, Papi M, Samengo D, Pani G, De Spirito M. Quantitative analysis of autophagic flux by confocal pH-imaging of autophagic intermediates. Autophagy. 2015;11(10):1905–1916. doi: 10.1080/15548627.2015.1084455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rodgers MA, Bowman JW, Liang Q, Jung JU. Regulation where autophagy intersects the inflammasome. Antioxid Redox Signal. 2014;20(3):495–506. doi: 10.1089/ars.2013.5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Into T, Horie T, Inomata M, et al. Basal autophagy prevents autoactivation or enhancement of inflammatory signals by targeting monomeric MyD88. Sci Rep. 2017;7(1):1009. doi: 10.1038/s41598-017-01246-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Role of autophagy in osteosarcoma. J Bone Oncol. 2019;16:100235. doi: 10.1016/j.jbo.2019.100235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lv S, Liu H, Wang H. Exogenous hydrogen sulfide plays an important role by regulating autophagy in diabetic-related diseases. Int J Mol Sci. 2021;22(13):6715. doi: 10.3390/ijms22136715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. doi: 10.18632/aging.102512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vinaik R, Barayan D, Jeschke MG. NLRP3 inflammasome in inflammation and metabolism: identifying novel roles in postburn adipose dysfunction. Endocrinology. 2020;161(9). doi: 10.1210/endocr/bqaa116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He C, Liu G, Zhuang S, et al. Yu Nu compound regulates autophagy and apoptosis through mTOR in vivo and vitro. Diabetes Metab Syndr Obes. 2020;13:2081–2092. doi: 10.2147/DMSO.S253494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456(7219):264–268. doi: 10.1038/nature07383 [DOI] [PubMed] [Google Scholar]
- 199.Kabat AM, Harrison OJ, Riffelmacher T, et al. The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife. 2016;5:e12444. doi: 10.7554/eLife.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marek-Iannucci S, Ozdemir AB, Moreira D, et al. Autophagy-mitophagy induction attenuates cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight. 2021;6(18). doi: 10.1172/jci.insight.151981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang W, Chen L, Shang C, et al. miR-145 inhibits the proliferation and migration of vascular smooth muscle cells by regulating autophagy. J Cell Mol Med. 2020;24(12):6658–6669. doi: 10.1111/jcmm.15316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Iskandar R, Liu S, Xiang F, et al. Expression of pericardial fluid T-cells and related inflammatory cytokines in patients with chronic heart failure. Exp Ther Med. 2017;13(5):1850–1858. doi: 10.3892/etm.2017.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bilchick K, Kothari H, Narayan A, et al. Cardiac resynchronization therapy reduces expression of inflammation-promoting genes related to interleukin-1β in heart failure. Cardiovasc Res. 2020;116(7):1311–1322. doi: 10.1093/cvr/cvz232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082 [DOI] [PubMed] [Google Scholar]
- 205.Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C. The effects of metformin on autophagy. Biomed Pharmacother. 2021;137:111286. doi: 10.1016/j.biopha.2021.111286 [DOI] [PubMed] [Google Scholar]
- 206.Niu C, Chen Z, Kim KT, et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15(5):843–870. doi: 10.1080/15548627.2019.1569913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun B, Ou H, Ren F, et al. Propofol inhibited autophagy through Ca(2+)/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol Med. 2018;24(1):58. doi: 10.1186/s10020-018-0054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang KY, Que JQ, Hu ZS, et al. Metformin suppresses inflammation and apoptosis of myocardiocytes by inhibiting autophagy in a model of ischemia-reperfusion injury. Int J Biol Sci. 2020;16(14):2559–2579. doi: 10.7150/ijbs.40823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Peng C, Rao W, Zhang L, et al. Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cell Physiol Biochem. 2018;46(6):2311–2324. doi: 10.1159/000489621 [DOI] [PubMed] [Google Scholar]
- 210.Yin Y, Sun G, Li E, Kiselyov K, Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev. 2017;34:3–14. doi: 10.1016/j.arr.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu Y, Xue X, Zhang H, et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy. 2019;15(3):493–509. doi: 10.1080/15548627.2018.1531196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jiang N, He D, Ma Y, et al. Force-induced autophagy in periodontal ligament stem cells modulates M1 macrophage polarization via AKT signaling. Front Cell Dev Biol. 2021;9:666631. doi: 10.3389/fcell.2021.666631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang YS, Wang F, Cui SX, Qu XJ. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther. 2018;19(8):735–744. doi: 10.1080/15384047.2018.1453971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maneechotesuwan K, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Role of autophagy in regulating interleukin-10 and the responses to corticosteroids and statins in asthma. Clin Exp Allergy. 2021;51(12):1553–1565. doi: 10.1111/cea.13825 [DOI] [PubMed] [Google Scholar]
- 215.Chen Y, Liang Y, Luo X, Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020;11(4):291. doi: 10.1038/s41419-020-2488-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Saladini S, Aventaggiato M, Barreca F, et al. Metformin impairs glutamine metabolism and autophagy in tumour cells. Cells. 2019;8(1):49. doi: 10.3390/cells8010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Frasca D, Diaz A, Romero M, Blomberg BB. Ageing and obesity similarly impair antibody responses. Clin Exp Immunol. 2017;187(1):64-70. doi:10.1111/cei.12824 [DOI] [PMC free article] [PubMed]
- 218.Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18(1):58–68. doi: 10.1038/s41569-020-0431-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56(4):390–403. doi: 10.1159/000268620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li D, Liu Q, Lu X, et al. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging (Albany NY). 2019;11(23):11084–11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lau L, Porciuncula A, Yu A, Iwakura Y, David G. Uncoupling the senescence-associated secretory phenotype from cell cycle exit via Interleukin-1 inactivation unveils its protumorigenic role. Mol Cell Biol. 2019;39(12). doi: 10.1128/MCB.00586-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sung JY, Kim SG, Kim JR, Choi HC. Prednisolone suppresses Adriamycin-induced vascular smooth muscle cell senescence and inflammatory response via the SIRT1-AMPK signaling pathway. PLoS One. 2020;15(9):e0239976. doi: 10.1371/journal.pone.0239976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Beek AA, Hugenholtz F, Meijer B, et al. Frontline science: tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1(-/Δ7) mice. J Leukoc Biol. 2017;101(4):811–821. doi: 10.1189/jlb.1HI0216-062RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Aguayo-Mazzucato C, Andle J, Lee TB, et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129–142.e124. doi: 10.1016/j.cmet.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Alfaras I, Mitchell SJ, Mora H, et al. Health benefits of late-onset metformin treatment every other week in mice. NPJ Aging Mech Dis. 2017;3:16. doi: 10.1038/s41514-017-0018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Noren Hooten N, Martin-Montalvo A, Dluzen DF, et al. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell. 2016;15(3):572–581. doi: 10.1111/acel.12469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sun X, Tavenier A, Deng W, Leishman E, Bradshaw HB, Dey SK. Metformin attenuates susceptibility to inflammation-induced preterm birth in mice with higher endocannabinoid levels. Biol Reprod. 2018;98(2):208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Long DE, Peck BD, Martz JL, et al. Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials. 2017;18(1):192. doi: 10.1186/s13063-017-1932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Giaccari A, Solini A, Frontoni S, Del Prato S. Metformin benefits: another example for alternative energy substrate mechanism? Diabetes Care. 2021;44(3):647–654. doi: 10.2337/dc20-1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15–30. doi: 10.1016/j.cmet.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]