Abstract
Pyrobaculum aerophilum, a hyperthermophilic archaeon, can respire either with low amounts of oxygen or anaerobically with nitrate as the electron acceptor. Under anaerobic growth conditions, nitrate is reduced via the denitrification pathway to molecular nitrogen. This study demonstrates that P. aerophilum requires the metal oxyanion WO42− for its anaerobic growth on yeast extract, peptone, and nitrate as carbon and energy sources. The addition of 1 μM MoO42− did not replace WO42− for the growth of P. aerophilum. However, cell growth was completely inhibited by the addition of 100 μM MoO42− to the culture medium. At lower tungstate concentrations (0.3 μM and less), nitrite was accumulated in the culture medium. The accumulation of nitrite was abolished at higher WO42− concentrations (<0.7 μM). High-temperature enzyme assays for the nitrate, nitrite, and nitric oxide reductases were performed. The majority of all three denitrification pathway enzyme activities was localized to the cytoplasmic membrane, suggesting their involvement in the energy metabolism of the cell. While nitrite and nitric oxide specific activities were relatively constant at different tungstate concentrations, the activity of nitrate reductase was decreased fourfold at WO42− levels of 0.7 μM or higher. The high specific activity of the nitrate reductase enzyme observed at low WO42− levels (0.3 μM or less) coincided with the accumulation of nitrite in the culture medium. This study documents the first example of the effect of tungstate on the denitrification process of an extremely thermophilic archaeon. We demonstrate here that nitrate reductase synthesis in P. aerophilum occurs in the presence of high concentrations of tungstate.
Tungsten is a trace element that stimulates the growth of a variety of microorganisms. With the exception of some methylotrophs (9), most organisms that benefit nutritionally from tungsten additions are strict anaerobes. These include bacteria, such as certain acetogenic clostridia, Eubacterium acidaminophilum, Desulfovibrio sp., Pelobacter acetylenicus, and the thermophilic bacterium Thermotoga maritima (1, 2, 11, 13, 22, 25, 27, 28, 33). In addition, a variety of archaea benefit from tungsten supplementation, including some methanogenic species and the extreme thermophiles Thermococcus litoralis and Pyrococcus furiosus (4, 12, 14, 15, 17, 18, 24, 29–32). Most of these microbes can also grow without the addition of tungstate to the culture medium. However, growth yields or certain enzyme activities are greatly improved upon the addition of tungstate to the medium. The role of tungsten in biological systems and the properties of tungstoenzymes have recently been reviewed extensively by Kletzin and Adams (16).
In contrast to the relatively ubiquitous abundance of molybdenum in mesophilic soil, freshwater, and seawater environments, the levels of tungsten in these environments are much lower (16). However, high concentrations of tungsten are found in selected environments, such as hot springs and deep-sea hydrothermal vents (16). These are the ecological niches that are the natural environments of hyperthermophilic microbes.
Pyrobaculum aerophilum was isolated from a hot spring in Italy (26). The organism grows optimally at a temperature of 100°C. Unlike its strictly anaerobic, sulfur-reducing relatives, P. aerophilum utilizes nitrate and oxygen at a low partial pressure (0.6 to 1% O2 in the gas phase) as the electron acceptors for growth (26). Lack of production of ammonium and the demonstration that molecular nitrogen is formed from nitrate suggest that nitrate reduction in P. aerophilum occurs via the denitrification pathway (26). The first step in denitrification is catalyzed by the enzyme nitrate reductase. This enzyme was found to contain a molybdenum cofactor in all microbes from which it was isolated (34). In general, molybdoenzymes such as nitrate reductase are rendered inactive by the addition of tungstate to the culture medium (6).
With the goal of studying what effect tungstate might have on the ability of P. aerophilum to respire anaerobically with nitrate, growth studies with various tungstate concentrations were performed.
MATERIALS AND METHODS
Strain.
P. aerophilum (DSM 7523) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen in Braunschweig, Germany.
Culture conditions.
P. aerophilum was cultured anaerobically in marine medium as previously described by Völkl et al. (26) with the following modifications: the NaCO3-CO2 buffer system was replaced by a 20 mM PIPES (piperazine-N,N′-bis(2-ethanesulfonic acid)-HCl buffer, pH 7.0, and reducing agents were omitted from the culture medium. Molybdate and tungstate were excluded. Where indicated, either oxyanion was added to give the final concentrations given in the text and figures. The medium contained 0.05% yeast extract and 0.05% peptone (both from Difco) as carbon and electron sources. Nitrate served as the electron acceptor at an initial concentration of 10 mM. Anaerobic conditions were established by the Hungate technique. The cultures were grown in anaerobic serum vials with N2 gas in the headspace at a temperature of 96°C. Growth experiments were performed with 125-ml acid-washed, anaerobic serum vials containing 50 ml of the medium with the indicated additions. Each vial was inoculated with a 10% volume of a culture previously grown at the same tungstate or molybdate concentration. When nitrogen gas production was followed, argon was used as the headspace gas in the vials to prepare the medium. Cell densities were monitored with a Beckman DU 640 spectrophotometer.
Multiple attempts to adapt P. aerophilum to microaerophilic growth conditions or to anaerobic growth with propionate or acetate as an alternative electron donor in place of yeast extract and peptone for nitrate respiration were unsuccessful. Therefore, the effect of tungstate under these reported growth conditions could not be evaluated.
Preparation of cells for enzyme assays.
Cultures grown with the indicated tungstate or molybdate additions were harvested in late logarithmic growth phase. The cells were washed once in a buffer containing 100 mM Tricine-KOH (pH 7.0)–300 mM NaCl–15 mM MgCl2 and then were resuspended in 0.1 ml of 100 mM Tricine-KOH (pH 7.0).
Preparation of membrane and soluble fractions.
After being harvested, the cell pellet was resuspended in 20 mM potassium phosphate buffer, pH 7.0, and the cells were disrupted by sonication. Whole cells were removed by centrifugation at 2,000 × g for 5 min at 4°C. The membrane fraction was separated from the soluble fraction by ultracentrifugation at 100,000 × g for 60 min at 4°C and resuspended in 20 mM potassium phosphate buffer, pH 7.0.
Enzyme assays.
Nitrate reductase activity was determined anaerobically with reduced benzyl viologen as the electron donor. The assay was performed at 85°C in an anaerobic 100 mM potassium phosphate buffer, pH 7.0, containing 0.3 mM benzyl viologen. Anaerobic glass cuvettes were stoppered with butyl rubber stoppers, flushed with N2 gas for 5 min, and filled with 1.7 ml of the anaerobic buffer. To prevent breakage due to temperature fluctuations, it was essential to keep the cuvettes in an 85°C water bath at all times. Benzyl viologen was reduced with 10 μl of 40 mM Na2S2O4. Washed cells (5 to 15 μl) were added to the assay mixture, and the reaction was initiated by the addition of nitrate to give a final concentration of 10 mM. The oxidation of reduced benzyl viologen was monitored at 598 nm.
Nitrite reductase activity was measured as described above for nitrate reductase except that the buffer was 50 mM Tricine-KOH (pH 7.5), 2 mM dithiothreitol, and 1 mM benzyl viologen. Nitrate and nitrite reductase specific activities are expressed as micromoles of nitrate or nitrite reduced per minute (U) per milligram of cell protein.
Nitric oxide reductase activity was determined at 75°C with reduced N-methylphenazonium methosulfate (PMS) as the electron donor. The assay buffer (1 ml) contained 20 mM potassium phosphate buffer, pH 6.5, and 0.1 mM reduced PMS. Washed cells (5 to 15 μl) were added to the cuvettes, and the reaction was initiated by the addition of 0.15 ml of NO-saturated water to give an approximate final concentration of 0.2 mM NO. Reduced PMS was prepared under anaerobic conditions by the addition of 100 mM sodium ascorbate to 20 mM oxidized PMS (10). The resulting reduced PMS formed a white precipitate, which was washed twice with 10 ml of H2O. The reduced PMS was then resuspended in 2% Tween 20 (in H2O), and its concentration was determined spectroscopically. The oxidation of reduced PMS was monitored at 386 nm. NO reductase activity is expressed as micromoles of reduced PMS oxidized per minute (U) per milligram of protein.
Protein measurement.
The protein concentration was determined by using Petersen’s modification of the Lowry procedure (Sigma). Bovine serum albumin was used as the reference protein.
Analytical assays.
Nitrate and nitrite were measured on a Water’s 625 high-performance liquid chromatography system with a Partishere strong anion exchanger column (Whatman) and 50 mM potassium phosphate buffer, pH 3, as the carrier phase. Nitrite was also measured with sulfanilamide (21). The concentrations of tungsten and molybdenum in the culture medium were analyzed by inductively coupled plasma mass spectroscopy. The analysis was performed by the Soil and Plant Analysis Laboratory, University of Wisconsin—Madison/Extension. N2 gas was measured by gas chromatography with a HP5MS 60-cm by 250-μm (inside diameter) column with helium as the carrier gas at −10°C.
RESULTS
Anaerobic growth of P. aerophilum on nitrate requires tungstate.
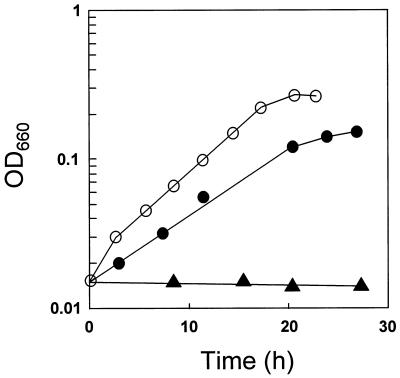
Tungsten is an essential trace element for certain hyperthermophilic microorganisms. To test whether tungsten is required for the cultivation of P. aerophilum, cells were transferred into a medium containing 0.3 μM WO42− but no molybdate and then used to inoculate media that either lacked or contained added WO42−. After the third serial transfer into medium lacking tungstate, P. aerophilum cells ceased to grow, whereas control cultures that contained 0.3 or 1.5 μM WO42− grew normally (Fig. 1). Inductively coupled plasma mass spectroscopy analysis of the basal medium without added tungstate revealed a residual WO42− concentration of 5 pM. Thus, 5 pM of WO42− is not sufficient to sustain growth of P. aerophilum; a significantly higher WO42− concentration is required.
FIG. 1.
Growth of P. aerophilum at various WO42− concentrations. P. aerophilum was grown anaerobically with 0.05% peptone, 0.05% yeast extract, and 10 mM NO3− in the absence (▴) or the presence of 0.3 (•) or 1.5 (○) μM WO42−. OD660, optical density at 660 nm.
Cell growth rate is tungstate dependent.
To determine the optimal WO42− concentration for anaerobic growth of P. aerophilum, cells were grown in media containing 0.1, 0.3, 0.7, 1.5, or 5.0 μM WO42−. Doubling times were determined after at least three transfers under identical conditions. The fastest doubling time, 6 h, occurred at WO42− concentrations of 0.7 and 1.5 μM (Fig. 1 and data not shown). Doubling times of about 8 h were obtained for cells grown in a medium containing either 0.1 or 0.3 μM WO42−. At 5 μM WO42−, the doubling time was slowed to about 10 h, suggesting that this concentration was somewhat inhibitory. The WO42− concentration had only little effect on the final cell density of P. aerophilum cultures: cell densities were slightly higher when 0.7 μM or more WO42− was present. Based on the observed doubling times for P. aerophilum, the optimal WO42− concentration for anaerobic growth of P. aerophilum is between 0.7 and 1.5 μM WO42−.
Molybdate does not replace tungstate for cell growth.
To determine whether MoO42− could replace WO42− as the growth supplement, P. aerophilum was grown anaerobically in a medium containing 1 μM MoO42− but no added WO42−. No growth occurred after the second transfer, indicating that MoO42− did not substitute for WO42−. The concentration of residual molybdenum in the medium lacking added MoO42− was determined to be less than 5 pM. This result confirms that P. aerophilum cells specifically require WO42− as a growth supplement. Conversely, in the absence of MoO42− but with 0.7 μM WO42− present in the culture medium, cells could be transferred more then 10 times with no difference seen in the growth rate compared to that in medium containing 1 μM MoO42− in addition to WO42− (data not shown). Therefore, additional MoO42− is not required to support anaerobic growth of P. aerophilum. While 1 μM MoO42− did not affect cell growth, an addition of 100 μM MoO42− resulted in reduced cell growth after the first transfer and complete inhibition of cell growth after the second transfer.
At low tungstate concentrations, nitrite accumulates in the culture medium.
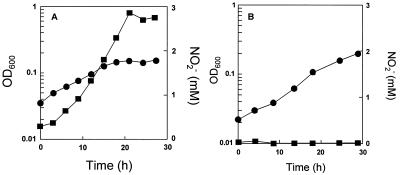
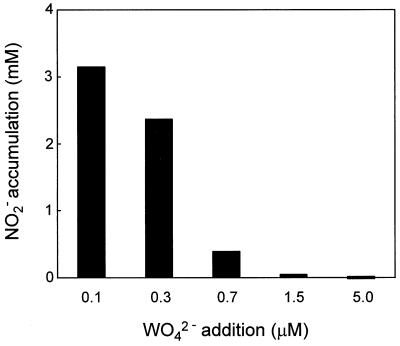
Denitrifying bacteria often excrete nitrite into the medium, which eventually inhibits cell growth (34). To test whether nitrite was produced during P. aerophilum growth, the nitrite concentration was determined in cultures that were grown with either 0.3 or 1.5 μM WO42− (Fig. 2). In the medium that contained 0.3 μM WO42−, nitrite accumulated to a final concentration of 3 mM (Fig. 2A). The amount of nitrite formed corresponded to the amount of nitrate which had been consumed by the culture. Very little molecular nitrogen was detected in the headspace of the vial (i.e., 4.5% N2). In contrast, when P. aerophilum cells were grown in the presence of 1.5 μM WO42−, no nitrite was accumulated in the medium (Fig. 2B). Under these conditions, dinitrogen gas accumulated in the headspace to 30.5% by the end of logarithmic growth, suggesting that nitrate was completely reduced to nitrogen gas at the higher WO42− concentration. To further define the effect of WO42− anions on the accumulation of nitrite, P. aerophilum was grown with different concentrations of WO42−. A marked decrease of nitrite accumulation was found in cultures grown at 0.7 μM WO42− or higher (Fig. 3). At WO42− concentrations of 1.5 μM or higher, nitrate at concentrations less than 0.05 mM accumulated. The addition of 1.0 μM molybdate to the culture medium containing the various WO42− concentrations had no effect on the formation of nitrite during anaerobic growth on nitrate (data not shown).
FIG. 2.
Accumulation of NO2− by P. aerophilum. P. aerophilum was grown anaerobically with 0.05% peptone, 0.05% yeast extract, and 10 mM NO3− in the presence of 0.3 (A) and 1.5 (B) μM WO42− in the culture medium. The optical density of the culture at 600 nm (OD600) (•) and the concentration of NO2− (■) were determined.
FIG. 3.
Effects of different WO42− concentrations on NO2− accumulation in the medium. P. aerophilum was grown anaerobically with 0.05% peptone, 0.05% yeast extract, 10 mM NO3−, and the different WO42− concentrations indicated. The concentration of NO2− was determined when the cultures reached stationary phase. The results represent the means of more than eight independent growth experiments with cells that had been previously adapted to the various tungstate concentrations.
Nitrate reductase activity is inhibited but not abolished by elevated tungstate concentrations.
Nitrate reductase enzymes isolated from a number of mesophilic denitrifiers and ammonifiers contain molybdopterin as the cofactor (3, 7). Since WO42− is an essential growth factor for P. aerophilum, we wished to determine if elevated WO42− concentrations in the medium might affect the specific activity of nitrate reductase in the cells. A nitrate reductase activity assay was performed with intact P. aerophilum cells with reduced benzyl viologen as the electron donor. The assay was stable up to a temperature of 95°C but was routinely performed at 85°C. The addition of 0.1% Triton X-100 did not increase the activity, indicating that the substrates, nitrate and reduced benzyl viologen, are readily accessible to the active site of the enzyme. This result was confirmed by comparing the nitrate reductase specific activities of broken and unbroken cells. The specific activities were found to be identical (data not shown). To determine if nitrate reductase specific activity varied in cells grown with different WO42− concentrations, these cells were harvested in late logarithmic growth phase, washed, and used directly in the nitrate reductase assay. Nitrate reductase specific activity was consistently highest in cells that were grown in the presence of 0.1 to 0.3 μM WO42− (Table 1). When 0.7 μM or more WO42− was present in the medium, the nitrate reductase activity was reduced fourfold. Nitrate reductase activity was not affected by the addition of 5 μM WO42− to the cuvette assay (data not shown), suggesting that the reduced levels of nitrate reductase activity mentioned above were not due to simple inhibition. The high specific activity of nitrate reductase seen in cells grown with 0.1 or 0.3 μM WO42− coincided with the high levels of nitrite formed under these conditions (Fig. 3). Interestingly, at 1.5 μM WO42− the levels of nitrite in the medium were lower than at 0.7 μM WO42−, although the nitrate reductase activity of the cells was the same under both conditions. This suggests that tungstate may play a complex role in the denitrification pathway.
TABLE 1.
Specific activities of nitrate, nitrite, and nitric oxide reductases assayed in intact cells of P. aerophilum grown at different WO42− concentrations
| WO42− (μM) | Specific activity (U/mg of protein)a
|
||
|---|---|---|---|
| Nitrate reductase | Nitrite reductase | Nitric oxide reductase | |
| 0.1 | 1.36 ± 0.15 | 1.39 ± 0.32 | NDb |
| 0.3 | 1.10 ± 0.24 | 1.41 ± 0.34 | 0.15 ± 0.03 |
| 0.7 | 0.30 ± 0.04 | 1.70 ± 0.06 | 0.15 ± 0.02 |
| 1.5 | 0.35 ± 0.07 | 1.41 ± 0.08 | 0.13 ± 0.02 |
| 5.0 | 0.33 ± 0.07 | 1.95 ± 0.41 | 0.17 ± 0.03 |
Specific enzyme activities represent averages taken from six cultures that were grown independently in the presence of different WO42− concentrations.
ND, not determined.
Level of tungstate in the medium has no effect on nitrite reductase and nitric oxide reductase activities.
The accumulation of nitrite seen at low WO42− concentrations could be the result of reduced nitrite reductase activity. To explore this possibility, nitrite reductase activity was measured in whole cells with reduced benzyl viologen as the electron donor. In control experiments, all nitrite reductase assay components were shown to be stable at the assay temperature of 85°C. Nitrite reductase specific activity was constant in P. aerophilum cells pregrown over the range of tungstate concentrations examined (Table 1). The activity was not altered by the addition of 0.1% Triton X-100. Broken and unbroken cells contained the same specific activities, indicating that the substrates are readily available at the enzyme active site.
The cd1-type nitrite reductases from mesophilic bacteria are known to be inhibited by their end product, nitric oxide (NO) (23). NO could accumulate if the synthesis of nitric oxide reductase was reduced in the presence of elevated levels of tungstate in the medium. Therefore, nitric oxide activity was measured by using reduced PMS as the electron donor. The NO reductase assay mixture contained 0.2% Tween 20, which may be sufficient to permeabilize the cytoplasmic membrane of P. aerophilum for reduced PMS. The addition of 0.1% Triton X-100 did not result in a further increase of the enzyme activity. Like the nitrite reductase activity, the specific activity of nitric oxide reductase was unchanged in cells grown with different tungstate concentrations (Table 1). Therefore, the accumulation of nitrite in the culture medium of cells grown with 0.1 or 0.3 μM tungstate was not due to the absence or reduced levels of nitrite and nitric oxide reductase enzymes in these cells.
Cellular localization of nitrate, nitrite, and nitric oxide reductase enzymes.
To determine the cellular localization of the three denitrification pathway enzymes, P. aerophilum cells were disrupted by sonication. After removal of unbroken cells, the membrane fraction was separated from the soluble fraction by ultracentrifugation. The majority of all three enzyme activities was found to be associated with the membrane fraction (Table 2).
TABLE 2.
Cellular localization of nitrate, nitrite, and nitric oxide reductase activities in P. aerophilum
| Enzyme preparation | Specific activity [U (%)]a
|
||
|---|---|---|---|
| Nitrate reductase | Nitrite reductase | Nitric oxide reductase | |
| Cell extract | 268 (100) | 90 (100) | 5.4 (100) |
| Soluble fraction | 25 (9) | 12 (13) | 0.9 (16) |
| Membrane fraction | 206 (77) | 72 (72) | 4.2 (79) |
U, total activity in micromoles of substrate reduced per minute.
DISCUSSION
P. aerophilum is the only hyperthermophilic archaeon yet isolated that reduces nitrate via the denitrification pathway to dinitrogen gas (26). In general, the denitrification pathway involves four enzymes, which catalyze the complete reduction of nitrate to N2 (34). The activities of three of the denitrification pathway enzymes from P. aerophilum—the nitrate, nitrite, and nitric oxide reductases—were localized to the membrane fractions of P. aerophilum cells. This result suggests a participation of each enzyme in the energy metabolism of P. aerophilum. Whereas a membrane-bound localization is common to all dissimilatory nitrate and nitric oxide reductases that have been isolated, most nitrite reductases are soluble and have been localized to the periplasms of denitrifying organisms (34). The direct contribution of these soluble nitrite reductases and the recently characterized periplasmically located nitrate reductases to the energy generation is unclear (34).
The environments from which extreme thermophiles are isolated typically contain elevated levels of tungsten but very little molybdenum (16). The stimulatory effect of tungsten on cell growth has been observed for some hyperthermophilic euryarchaeota (16). However, tungsten was also reported to stimulate the growth of several mesophilic methanogens and some mesophilic and thermophilic bacteria (reviewed in reference 16). Most of these organisms, however, can also grow in the absence of tungstate. We report here the obligate requirement of tungstate for the anaerobic growth of the extremely thermophilic archaeon P. aerophilum. When tungstate was omitted from the culture medium, P. aerophilum was unable to grow on the residual tungsten present in the medium (ca. 5 pM). This is the first example of a tungsten-dependent archaeon belonging to the crenarchaeota. The dependency of the organism’s growth rate on tungsten suggests the involvement of tungstoenzymes in essential metabolic pathways of this hyperthermophile. The fastest doubling time was achieved in the presence of 0.7 to 1.5 μM tungstate. Higher tungstate concentrations (5 μM) were inhibitory, and the cell doubling time increased from 6 to 10 h. However, final cell densities were not significantly changed by the various tungstate concentrations, suggesting that factors other than tungsten limited the growth of P. aerophilum. This is in contrast to Methanobacterium wolfei, where the addition of tungstate resulted in proportionally higher cell densities (29). Like that of P. aerophilum, M. wolfei cell growth was inhibited by high tungstate concentrations in the medium (29). However, inhibition of growth in M. wolfei occurred at a threefold-higher tungstate concentration (15 μM) and resulted in a decrease in final cell density rather than a decrease in growth rate.
Since P. aerophilum utilizes tryptone and yeast extract as sources for electrons and cell carbon, possible candidates for tungstoenzymes could potentially be found among the enzymes that are involved in the oxidation of amino acids. The tungsten-containing aldehyde ferredoxin oxidoreductase (AOR) isolated from P. furiosus and other thermophilic archaea is implied to be involved in the oxidation of aldehydes generated from 2-keto acids in the amino acid oxidation pathway (18). The presence of the aor gene in P. aerophilum was recently established as part of the P. aerophilum genome sequencing project (8). The P. aerophilum aor gene product has significant homology with the tungsten-containing AOR protein isolated from P. furiosus, suggesting that AOR in P. aerophilum might be one of the enzymes responsible for the strict tungsten requirement of the organism. Since attempts to adapt P. aerophilum to other growth conditions, such as microaerophilic cell growth and anaerobic growth with acetate or propionate in place of yeast extract and peptone, were unsuccessful, a tungsten requirement under these growth conditions could not be evaluated.
Molybdate was not required as a growth supplement for P. aerophilum. This, however, does not imply that this metal is not essential for P. aerophilum. Bacteria, such as Escherichia coli, have been demonstrated to exhibit high-affinity uptake systems capable of extracting traces of molybdenum from their environment (5, 20). On the other hand, the addition of 100 μM molybdate resulted in the complete inhibition of P. aerophilum growth after the second cell transfer. It is possible that molybdate acts as an antagonist to tungstate either by interfering with the uptake of tungstate into the cell or by poisoning tungstoenzymes that are most likely present in P. aerophilum. The fact that some growth is still sustained after the first transfer of the culture is most likely due to residual tungsten that is present inside of the cells. The inhibitory effect of molybdate on P. aerophilum is analogous to the inhibition of growth and enzyme activities by tungstate in molybdenum-requiring mesophilic organisms (6). Due to their similar chemical properties, tungsten is incorporated in place of molybdenum into the pterin cofactor of the nitrate reductase and formate dehydrogenase enzymes in E. coli. This renders both enzymes inactive (6). In contrast, molybdenum apparently does not replace tungsten in the pterin cofactor of tungstoenzymes such as AOR, formaldehyde ferredoxin oxidoreductase (FOR), and glyceraldehyde-3-phosphate oxidoreductase (GAPOR) in P. furiosus (19). No molybdenum was detected in these enzymes purified from cells grown with molybdate in the absence of added tungstate. This suggests that molybdenum is not incorporated into the pterin cofactor in place of tungsten. The addition of increasing levels of tungstate to P. aerophilum resulted in a decrease in its nitrate reductase specific activity, suggesting that this enzyme might be a molybdenum-containing nitrate reductase (Table 1). However, in contrast to that of E. coli, the nitrate reductase activity of P. aerophilum was never abolished. Even at a high tungstate concentration of 5 μM the specific activity of nitrate reductase was sufficient to allow for the utilization of nitrate as the terminal electron acceptor for growth. Therefore, the ability of P. aerophilum to respire with nitrate in the presence of high tungstate levels may reflect an adaptation of the organism to a high-tungsten environment by a yet unknown mechanism.
Coinciding with the increased nitrate reductase activity in cells grown at low tungstate concentrations was the accumulation of nitrite in the culture medium. This accumulation of nitrite was not due to the absence or reduced levels of nitrite reductase under these conditions, since nitrite reductase activity was constant in cells grown at the various tungstate concentrations. The nitrite reductases of mesophilic bacteria have been shown to be both substrate and end product inhibited and thus were suggested to comprise the rate-limiting step in the denitrification pathway (23). The substrate, nitrite, and the end product, NO, are both reactive oxyanions whose reactivity might be intensified at high temperatures. It might, therefore, be essential to limit the production of nitrite by nitrate reductase by lowering its activity and/or controlling its level of synthesis. Whether tungsten somehow regulates the activity or synthesis of the denitrification pathway enzymes in P. aerophilum is not clear but is under investigation in our laboratory.
ACKNOWLEDGMENTS
This work was supported by grants from the National Science Foundation (MCB-9631006) and the University of California Biotechnology Research and Education program (95-1730).
REFERENCES
- 1.Andreesen J R, El Ghazzawi E, Gottschalk G. The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Microbiol. 1974;96:103–118. doi: 10.1007/BF00590167. [DOI] [PubMed] [Google Scholar]
- 2.Andreesen J R, Ljungdahl L G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973;116:867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berks B C, Ferguson S J, Moir J W B, Richardson D J. Enzymes and associated electron transport systems that catalyze the respiratory reduction of nitrogen oxides and oxyanions. Biochim Biophys Acta. 1995;1232:97–173. doi: 10.1016/0005-2728(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 4.Bryant F O, Adams M W W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989;264:5070–5079. [PubMed] [Google Scholar]
- 5.Corcuera G L, Bastidas M, Dubourdieu M. Molybdenum uptake in Escherichia coli K12. J Gen Microbiol. 1993;139:1869–1875. doi: 10.1099/00221287-139-8-1869. [DOI] [PubMed] [Google Scholar]
- 6.Enoch H G, Lester R L. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzyme systems in Escherichia coli. J Bacteriol. 1972;110:1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson S J. Denitrification: a question of the control and organization of electron and ion transport. Trends Biochem Sci. 1987;12:354–357. [Google Scholar]
- 8.Fitz-Gibbon S, Choi A J, Miller J H, Stetter K O, Simon M I, Swanson R, Kim U-J. A fosmid-based genomic map and identification of 474 genes of the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles. 1997;1:36–51. doi: 10.1007/s007920050013. [DOI] [PubMed] [Google Scholar]
- 9.Girio F M, Marcos J C, Amaral-Collaco M T. Transition metal requirement to express high level NAD-dependent formate dehydrogenase from a serine-type methylotrophic bacterium. FEMS Microbiol Lett. 1992;97:161–166. [Google Scholar]
- 10.Hakimian S. Undergraduate research thesis. Los Angeles: University of California; 1997. [Google Scholar]
- 11.Hensgens C M H, Nienhuiskuiper M E, Hansen T A. Effects of tungsten on the growth of Desulfovibrio gigas NCIMB 9332 and other sulfate reducing bacteria with ethanol as a substrate. Arch Microbiol. 1994;162:143–147. [Google Scholar]
- 12.Hochheimer A, Schmitz R A, Thauer R K, Hedderich R. The tungsten formylmethanofuran dehydrogenase from Methanobacterium thermoautotrophicum contains sequence motifs characteristic for enzymes containing molybdopterin dinucleotide. Eur J Biochem. 1995;234:910–920. doi: 10.1111/j.1432-1033.1995.910_a.x. [DOI] [PubMed] [Google Scholar]
- 13.Huber C, Skopan H, Feicht R, White H, Simon H. Pterin cofactor, substrate specificity, and observations on the kinetics of the reversible tungsten-containing aldehyde oxidoreductase from Clostridium thermoaceticum. Arch Microbiol. 1995;164:110–118. [Google Scholar]
- 14.Jones J B, Stadtman T C. Methanococcus vanielii: culture and effects of selenium and tungsten on growth. J Bacteriol. 1977;130:1404–1406. doi: 10.1128/jb.130.3.1404-1406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juszczak A, Aono S, Adams M W W. The extremely thermophilic eubacterium, Thermotoga maritima, contains a novel iron-hydrogenase whose cellular activity is dependent on tungsten. J Biol Chem. 1991;266:13834–13841. [PubMed] [Google Scholar]
- 16.Kletzin A, Adams M W W. Tungsten in biological systems. FEMS Microbiol Rev. 1996;18:5–63. doi: 10.1016/0168-6445(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 17.May H D, Patel P S, Ferry J G. Effect of molybdenum and tungsten on synthesis and composition of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1988;170:3384–3389. doi: 10.1128/jb.170.8.3384-3389.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukund S, Adams M W W. Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Thermococcus litoralis. A role for tungsten in peptide metabolism. J Biol Chem. 1993;268:13592–13600. [PubMed] [Google Scholar]
- 19.Mukund S, Adams M W W. Molybdenum and vanadium do not replace tungsten in the catalytically active forms of the three tungstoenzymes in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1996;178:163–167. doi: 10.1128/jb.178.1.163-167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rech S, Wolin C, Gunsalus R P. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J Biol Chem. 1996;271:2557–2562. doi: 10.1074/jbc.271.5.2557. [DOI] [PubMed] [Google Scholar]
- 21.Rider B F, Mellon M G. Colorimetric determination of nitrite. Ind Eng Chem. 1946;18:96–98. [Google Scholar]
- 22.Rosner B M, Schink B. Purification and characterization of acetylene hydratase of Pelobacter acetylenicus, a tungsten iron-sulfur protein. J Bacteriol. 1995;177:5767–5772. doi: 10.1128/jb.177.20.5767-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schichman S A, Meyer T E, Gray H B. Kinetics of electron transfer in Pseudomonas aeruginosa cytochrome cd1-nitrite reductase. Inorg Chim Acta. 1996;243:25–31. [Google Scholar]
- 24.Schicho R N, Snowden L J, Mukund S, Park J-B, Adams M W W, Kelly R M. Influence of tungsten on metabolic patterns in Pyrococcus furiosus, a hyperthermophilic archaeon. Arch Microbiol. 1993;159:380–385. [Google Scholar]
- 25.Taya M, Hinoki H, Kobayashi T. Tungsten requirement of an extremely thermophilic cellulolytic anaerobe (strain NA 10) Agric Biol Chem. 1985;49:2513–2515. [Google Scholar]
- 26.Völkl P, Huber R, Drobner E, Rachel R, Burggraf S, Trincone A, Stetter K O. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl Environ Microbiol. 1993;59:2918–2926. doi: 10.1128/aem.59.9.2918-2926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner R, Andreesen J R. Accumulation and incorporation of 185W-tungsten into protein of Clostridium acidurici and Clostridium cylindrosporum. Arch Microbiol. 1987;147:295–299. [Google Scholar]
- 28.Widdel F. Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol. 1986;51:1056–1062. doi: 10.1128/aem.51.5.1056-1062.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter J, Lerp C, Zabel H-P, Wildenauer F X, Koenig H, Schindler F. Methanobacterium wolfei, sp. nov., a new tungsten-requiring, thermophilic, autotrophic methanogen. Syst Appl Microbiol. 1984;5:457–466. [Google Scholar]
- 30.Zellner G, Alten C, Stackebrandt E, Conway de Macario E, Winter J. Isolation and characterization of Methanocorpusculum parvum, gen. nov., spec. nov., a new tungsten requiring, coccoid methanogen. Arch Microbiol. 1987;147:13–20. [Google Scholar]
- 31.Zellner G, Sleytr U B, Messner P, Kneifel H, Winter J. Methanogenium liminatans spec. nov., a new coccoid, mesophilic methanogen able to oxidize secondary alcohols. Arch Microbiol. 1990;153:287–293. [Google Scholar]
- 32.Zellner G, Winter J. Growth promoting effect of tungsten on methanogens and incorporation of tungsten-185 into cells. FEMS Microbiol Lett. 1987;40:81–87. [Google Scholar]
- 33.Zindel U, Freudenberg W, Rieth M, Andreesen J R, Schnell J, Widdel F. Eubacterium acidaminophilum sp. nov., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Description and enzymatic studies. Arch Microbiol. 1988;150:254–266. [Google Scholar]
- 34.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]