Abstract
The Percidae family comprises many fish species of major importance for aquaculture and fisheries. Based on three new chromosome-scale assemblies in Perca fluviatilis, Perca schrenkii and Sander vitreus along with additional percid fish reference genomes, we provide an evolutionary and comparative genomic analysis of their sex-determination systems. We explored the fate of a duplicated anti-Mullerian hormone receptor type-2 gene (amhr2bY), previously suggested to be the master sex determining (MSD) gene in P. flavescens. Phylogenetically related and structurally similar amhr2 duplications (amhr2b) were found in P. schrenkii and Sander lucioperca, potentially dating this duplication event to their last common ancestor around 19–27 Mya. In P. fluviatilis and S. vitreus, this amhr2b duplicate has been lost while it was subject to amplification in S. lucioperca. Analyses of the amhr2b locus in P. schrenkii suggest that this duplication could be also male-specific as it is in P. flavescens. In P. fluviatilis, a relatively small (100 kb) non-recombinant sex-determining region (SDR) was characterized on chromosome-18 using population-genomics approaches. This SDR is characterized by many male-specific single-nucleotide variants (SNVs) and no large duplication/insertion event, suggesting that P. fluviatilis has a male heterogametic sex determination system (XX/XY), generated by allelic diversification. This SDR contains six annotated genes, including three (c18h1orf198, hsdl1, tbc1d32) with higher expression in testis than ovary. Together, our results provide a new example of the highly dynamic sex chromosome turnover in teleosts and provide new genomic resources for Percidae, including sex-genotyping tools for all three known Perca species.
Keywords: sex-determination, genome, perches, pikeperches, sex-chromosomes
INTRODUCTION
The percid family (Percidae, Rafinesque) encompasses a large number (over 250) of diverse ecologically and economically important fish species, assigned to 11 genera [1]. Two genera, Perca and Sander are found across both Eurasia and North America, with separate species native to each continent (Eurasia: Perca fluviatilis / Sander lucioperca; North America: Perca flavescens / Sander vitreus). Percids are classically described as typical freshwater species of the Northern hemisphere, even if some species can be regularly found in brackish waters (e.g. Sander lucioperca, Perca fluviatilis). In the context of declining fisheries over the past few decades, but also due to their high value and good market acceptance, four percid species - Perca flavescens (yellow perch) and Sander vitreus (walleye) in North America and P. fluviatilis (European perch) and S. lucioperca (zander) in Eurasia are particularly promising for aquaculture. Rearing these fish in recirculation aquaculture systems (RAS) allows for a control of reproduction and a year-round production of stocking fish [2,3]. Although year-round production represents an important competitive goal, current production targets premium markets and an up-scaling of production faces several bottlenecks [4].
Among these bottlenecks is better control of the sex of developing individuals because in both Perca and Sander genera, females grow faster than males [5,6]. Due to faster female growth (up to 25–50% in Perca, 10% in Sander), all-female stocks are highly desirable. In Perca fluviatilis, sex determination has been assumed to be male heterogametic (XX/XY) based on gynogenesis or hormonal treatment experiments [7,8]. These methodologies also produced genetic female but phenotypic male individuals (neomales) that can be used to produce all-female stocks by crossing normal XX females with these chromosomally XX neomales. This approach would, however, greatly benefit from a reliable sexing method allowing the identification of genetic sex early during development to select rare genetically XX neomales as future breeders in aquaculture. In P. flavescens, an XX female/XY male heterogametic genetic sex determination system has been also recently uncovered, with duplication / insertion of an anti-Mullerian hormone receptor type 2 (amhr2) gene as a potential master sex determining gene [9].
Genes encoding many members of the transforming growth factor beta (TGF-β) gene family, including anti-Mullerian hormone (amh) and anti-Mullerian hormone receptor type-2 (amhr2), have repeatedly and independently evolved as master sex-determining (MSD) genes in vertebrates [10]. For instance, amh has been characterized or suspected to be the MSD gene in pikes [11,12], Nile tilapia [13], lumpfish [14], Sebastes rockfish [15], lingcod [16], and Patagonian pejerrey [17]. The cognate receptor gene of Amh, amhr2, has also been found as a potential MSD gene in Pangasiidae [18], Takifugu [19], Ayu [20], common seadragon and alligator pipefish [21], as well as in yellow perch [9]. The repeated and independent recruitment of TGF-β receptors, including Amhr2, in teleost fish sex determination is even more puzzling as many of these MSD genes, encoding a TGFβ receptor, share a similar N-terminal truncation [9,18,21], supporting their evolution towards a ligand-independent mechanism of action [18]. Therefore, the extent of evolutionary conservation of the Y-linked amhr2bY gene found in yellow perch in closely related species (genus Perca and Sander) is an important question with implications for better sex-control in these aquaculture species and for understanding the evolution of sex linkage and protein structure.
Regarding genomics of Percidae, two long-read reference quality genome assemblies have recently been published for P. flavescens and S. lucioperca [9,22]. While for P. fluviatilis and S. vitreus only draft genomes, generated from short-read sequencing, have been available [23]. Here, we provide three new long-read chromosome-scale genome assemblies for P. fluviatilis, P. schrenkii and Sander vitreus and thus complete genomic resources for the economically most important species of Percidae. These data enabled us to develop PCR-assays for sexing of all three Perca species and shed light on gene gain- and-loss in the evolution of an old MSD gene in Percidae.
MATERIAL AND METHODS
Biological samples
In Perca fluviatilis, high molecular weight (HMW) genomic DNA (gDNA) for genome sequencing was extracted from a blood sample of a male called “Pf_M1” (BioSample ID SAMN12071746) from the aquaculture facility of the University de Lorraine, Nancy, France. Blood (0.5 ml) was sampled and directly stored in 25 ml of a TNES-Urea lysis buffer (TNES-Urea: 4 M urea; 10 mM Tris-HCl, pH 7.5; 125 mM NaCl; 10 mM EDTA; 1% SDS). HMW gDNA was extracted from the TNES-urea buffer using a slightly modified phenol/chloroform protocol as described [12]. For the chromosome contact map (Hi-C), 1.5 ml of blood was taken from the same animal and slowly (1 K/min) cryopreserved with 15 % dimethyl sulfoxide (DMSO) in a Mr. Frosty Freezing Container (ThermoFisher) at −80°C. Additional fin clip samples for RAD-Sequencing (RAD-Seq), Pool-Sequencing (Pool-Seq) or sex-genotyping assays were collected and stored in 90% ethanol, either at the Lucas Perche aquaculture facility (Le Moulin de Cany, 57170 Hampont, France), at Kortowskie Lake in Poland, or at Mueggelsee Lake in Germany.
Samples of Perca schrenkii were obtained for genome sequencing and sex genotyping from male and female wild catches at lake Alakol, Kazakhstan (46.328 N, 81.374 E). Different organs and tissues (brain, liver, muscle, ovary, testis) were sampled for genome and transcriptome sequencing (Biosample ID SAMN15143703) and stored in RNAlater. HMW gDNA for genome sequencing was extracted from brain tissue of the male P. schrenkii individual, using the MagAttract HMW DNA Kit (Qiagen, Germany). Total RNA for transcriptome sequencing was isolated using a standard Trizol protocol, in combination with the RNAeasy Mini Kit (Qiagen, Germany).
For genome sequencing of Sander vitreus a fin clip of a male was sampled by Ohio Department of Natural Resources (Ohio, DNR) in spring 2017 and stored in 96% ethanol. The S. vitreus sample called “19-12246” originated from Maumee River, Ohio [41.554 N; −83.6605W]. DNA was extracted using the DNeasy Tissue Kit (Qiagen). Short DNA fragments were removed/reduced by size-selective, magnetic-bead purification using 0.35x of sample volume AMPure beads (Beckmann-Coulter) and two washing steps with 70% ethanol.
Sequencing
Genomic sequencing of P. fluviatilis was carried out using a combination of 2x250 bp Illumina short-reads, Oxford Nanopore long reads and a chromosome contact map (Hi-C). For long-read sequencing, DNA was sheared to 20 kb using the megaruptor system (Diagenode). ONT (Oxford nanopore technologies) library preparation and sequencing was performed using 5 µg of sheared DNA and ligation sequencing kits SQK-LSK108 or SQK-LSK109, according to the manufacturer's instructions. The libraries were loaded at a concentration of 0.005 to 0.1 pmol and sequenced for 48 h on 11 GridION R9.4 or R9.4.1 flowcells. Short read wgs (whole genome shotgun) sequencing for consensus polishing of noisy long read assemblies was carried out by shearing the HMW DNA to approximately 500 bp fragments and using the Illumina Truseq X kit, according to the manufacturer’s instructions. The library was sequenced using a read length of 250 bp in paired-end mode (HiSeq 3000, Illumina, California, USA). Hi-C library generation for chromosome assembly was carried out according to a protocol adapted from Rao et al. 2014 [24]. The blood sample was spun down, and the cell pellet was resuspended and fixed in 1% formaldehyde. Five million cells were processed for the Hi-C library. After overnight-digestion with HindIII (NEB), DNA-ends were labeled with Biotin-14-DCTP (Invitrogen), using Klenow fragment (NEB) and re-ligated. A total of 1.4 µg of DNA was sheared to an average size of 550 bp (Covaris). Biotinylated DNA-fragments were pulled down using M280 Streptavidin Dynabeads (Invitrogen) and ligated to PE adaptors (Illumina). The Hi-C library was amplified using PE primers (Illumina) with 10 PCR amplification cycles. The library was sequenced using a HiSeq3000 (Illumina, California, USA), generating 150 bp paired-end reads.
Genomic sequencing of P. schrenkii and S. vitreus was carried out using Oxford Nanopore long reads on a MinION nanopore sequencer (Oxford Nanopore Technologies, UK) in combination with the MinIT system. Several libraries were constructed using the tagmentation-based SQK-RAD004 kit with varying amounts of input DNA (0.4 to 1.2 µg) from a male individual or using the ligation approach of the SQK-LSK109 kit (input DNA 2 µg). Libraries were sequenced on R9.4.1 flowcells with variable run times and exonuclease washes by the EXP-WSH003 kit to remove pore blocks and improve the data yield. Short-read wgs-sequencing of P. schrenkii was conducted at BGI (BGI Genomics Co., Ltd.). A P. schrenkii male and a female wgs library (300 bp fragment length) were constructed and paired end reads of 150 bp length were generated on an Illumina Hiseq4000 system. Public short-read wgs data of S. vitreus were obtained from the NCBI Sequence Read Archive (SRA) using the accession SRR9711286. Transcriptome sequencing of six P. schrenkii samples (female brain, male brain, male muscle, female liver, ovary and testis) was conducted at BGI. Transcriptome-sequencing libraries were constructed from total RNA, applying enrichment of mRNA with oligo(dT) hybridization, mRNA fragmentation, random hexamer cDNA synthesis, size selection and PCR amplification. Sequencing of 150 bp paired-end reads was performed by an Illumina HiSeq X Ten system.
Genome assembly of Perca fluviatilis
Residual adaptor sequences in ONT GridION long reads were trimmed and split by Porechop (v0.2.1) [25]. Reads longer than 9999 bp were assembled by SmartDeNovo (May-2017) [26] using default parameters. Long reads were remapped to the SmartDeNovo contigs by Minimap2 (v2.7) [27] and Racon (v1.3.12) [28] was used to polish the consensus sequence. In a second round of polishing, Illumina short-reads were mapped by BWA mem (v0.7.12-r1039) [29] to the contigs, which were subsequently polished by Pilon (v1.223) [30]. The chromosome-scale assembly was performed by mapping Hi-C data to the assembled contigs, using the Juicer pipeline (v1.5.6) [31] and subsequent scaffolding by 3D-DNA (v180114) [32]. Juicebox (v1.8.8) [33] was used to manually review and curate the chromosome-level scaffolds. A final gap-closing step, applying long reads and LR_gapcloser (v1.1, default parameters) [34], further increased contig length. After gap-closing, a final consensus sequence polishing step was performed by mapping short reads to the scaffolds, sequence variants (1/1 genotypes were considered as corrected errors) were detected with Freebayes (v0.9.7) [35] and written to a vcf-file. The final fasta file was then generated by vcf-consensus from Vcftools (v0.1.15, default parameters).
Genome assembly of Perca schrenkii and Sander vitreus
Illumina short reads were trimmed using Trimmomatic (v0.35) [36]. Short reads were assembled using a custom compiled high kmer version of idba-ud (v1.1.1) [37] with kmer size up to 252. The resulting contigs were mapped against available Percidae genomes (P. flavescens, P. fluviatilis and S. lucioperca) by Minimap2 and analysis of overall mapped sequence length resulted in P. schrenkii aligned best with P. flavescens and S. vitreus aligned best with S. lucioperca. According to the benchmarks published in [38], the publicly available chromosome-level assembly of P. flavescens (RefSeq: GCF_004354835.1) could be used to aid the chromosome assembly of P. schrenkii as follows: ONT MinION long reads (male sample) were trimmed and split using Porechop (v0.2.1) [25]. The inhouse developed CSA method (v2.6) [38], was used to assemble the P. schrenkii genome from long-read data and short-read contigs and to infer chromosomal scaffolds using the P. flavescens reference genome. CSA parameters were optimized to account for relatively low long-read sequencing coverage and hybrid assembly of long reads and short-read contigs:
CSA2.6.pl –r longreads.fa.gz –g P.flavescens.fa –k 19 –s 2 –e 2 –l „–i shortreadcontigs.fa –L3000 –A“
Similarly, we assembled S. vitreus, using the S. lucioperca contigs and P. flavescens chromosomes as references for chromosomal assembly. Here, the short-read contigs were treated as long-reads:
CSA2.6c.pl -r longreads+contigs.fa.gz -g sanLuc.CTG.fa.gz,PFLA_1.0_genomic.fna.gz -k 19 -s 2 -e 2
The assemblies were manually curated, and the consensus sequences were polished using long reads and flye (v2.6) [39], with options: --nanoraw --polish-target, followed by two rounds of polishing by Pilon (v1.23) [30], using the short-read data, which had been mapped by Minimap2 (v2.17-r941) [27], to the genome assemblies.
Genome annotation
De novo repeat annotation was performed using RepeatModeler (version open-1.0.8) and Repeat Masker (version open-4.0.7). The P. fluviatilis genome has been assigned to the RefSeq assembly section of NCBI and has been annotated by GNOMON (www.ncbi.nlm.nih.gov/genome/annotation_euk/process), which included evidence from Actinopterygii proteins (n=154,659) and P. fluviatilis RNAseq reads (n = 3,537,868,978) (www.ncbi.nlm.nih.gov/genome/annotation_euk/Perca_fluviatilis/100). To annotate our P. schrenkii and S. vitreus assemblies, we used the high-quality GNOMON annotations from their closest relatives P. flavescens (www.ncbi.nlm.nih.gov/genome/annotation_euk/Perca_flavescens/100) and S. lucioperca (www.ncbi.nlm.nih.gov/genome/annotation_euk/Sander_lucioperca/101), respectively. We performed high-throughput comparative protein coding gene annotation by spliced alignment of GNOMON mRNAs and proteins by Spaln (v2.06f, [40]) to our assemblies and combined the resulting CDS- and UTR-matches into complete gene models by custom scripts. All annotations were benchmarked using BUSCO [41] with the Actinopterygii_odb9 database and obtained highly similar values as the reference annotations used for the comparative annotation approach.
Genome browsers and data availability
We provide UCSC genome browsers [42] for the five available Perca and Sander reference genomes (this study: P. fluviatilis, P. schrenkii, S. vitreus; earlier studies: P. flavescens [9] and S. lucioperca [22] at http://genomes.igb-berlin.de/Percidae/. These genome browsers provide access to genomic sequences and annotations (either public NCBI GNOMON annotations or annotations resulting from our comparative approach). Blat [43] servers for each genome are available to align nucleotide or protein sequences.
Phylogenomics and divergence time estimation
We performed pair-wise whole-genome alignments of 36 teleost genome assemblies as in [44], using Last-aligner and Last-split [45] for filtering 1-to-1 genome matches, Multiz [46] for multiple alignment construction from pairwise alignments and filtered for non-coding sequences to calculate the species tree using iqtree2 and raxml-ng [47,48]. We added the genomes of P. schrenkii, S. vitreus and Etheostoma spectabile (GCF_008692095.1) to this dataset and re-analyzed the highly-supported subclade containing Percidae species using several outgroups (Lates, Oreochromis, Pampus and Thunnus sp.). We estimated divergence times using a large subset of our multiple alignment (106 nt residues) and the approximate method of Mcmctree (Paml package version, [49]). We calibrated 5 nodes of the tree by left or right CI values, obtained from www.timetree.org and applied independent rates or correlated rates clock models and the HKY85 evolutionary model. Approximately 108 samples were calculated, of which we used the top 50% for divergence time estimation. Each calculation was performed in two replicates, which were checked for convergence using linear regression. The final tree was plotted using FigTree (v1.4.4, http://tree.bio.ed.ac.uk/software/figtree).
Perca fluviatilis RAD-Sequencing
Perca fluviatilis gDNA samples from 35 males and 35 females were extracted with the NucleoSpin Kit for Tissue (Macherey-Nagel, Duren, Germany), following the manufacturer’s instructions. Then, gDNA concentrations were quantified with a Qubit3 fluorometer (Invitrogen, Carlsbad, CA) using a Qubit dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA). RAD libraries were constructed from each individual’s gDNA, using a previously described protocol with the single Sbf1 restriction enzyme [50]. These libraries were sequenced on an Illumina HiSeq 2500. Raw reads were demultiplexed using the process_radtags.pl wrapper script of stacks, version 1.44, with default settings [51], and further analyzed with the RADSex analysis pipeline [52] to identify sex-specific markers.
Perca fluviatilis Pool-Sequencing
Sequencing of pooled samples (Pool-Seq) was carried out in Perca fluviatilis to increase the resolution of RAD-Sequencing for the identification of sex-specific signatures characteristic of its sex-determining region. The gDNA samples used for RAD-Sequencing were pooled in equimolar quantities according to their sex. Pooled male and pooled female libraries were constructed using a Truseq nano kit (Illumina, ref. FC-121-4001) following the manufacturer’s instructions. Each library was sequenced in an Illumina HiSeq2500 with 2x 250 reads. Pool-Seq reads were analyzed as previously described [9,11,53–55] with the PSASS pipeline (psass version 2.0.0: https://zenodo.org/record/2615936#.XtyIS3s6_AI) that computes the position and density of single nucleotide variations (SNVs), heterozygous in one sex but homozygous in the other sex (sex-specific SNVs), and the read depths for the male and female pools along the genome to look for sex coverage differences. Psass was run with default parameters except –window-size, which was set to 5,000, and –output-resolution, which was set to 1,000.
PCR-based sex diagnostics
A Perca schrenkii PCR-based sex-diagnostic test was designed based on multiple alignments of the different amhr2 genes in P. fluviatilis (one autosomal gene only), P. flavescens (two genes), and Perca schrenkii (two genes) to target a conserved region for all Perca amhr2 genes, allowing the design of PCR-primers that amplify both the autosomal amhr2a and the male-specific amhr2bY with different and specific PCR-amplicon sizes. Selected PCR primer sequences were forward: 5’-AGTTTATTGTGTTAGTTTGGGCT-3’ and reverse: 5’-CAAATAAATCAGAGCAGCGCATC-3’. PCRs were carried out with 1U Platinum Taq DNA Polymerase and its corresponding Buffer (Thermofisher) supplemented with 0.8 mM dNTPs (0.2mM each), 1.5 mM MgCl2 and 0.2 µM of each primer with the following cycling conditions, 96°C for 3 min; 40 cycles of denaturation (96°C, 30 s), annealing (54°C, 30 s) and extension (72°C, 1 min); final extension (72°C, 5 min); storage at 4°C. PCR amplicons were separated on 1.5% agarose gels (1.5% std. agarose, 1x TBE buffer, 5 V/cm, running time 40 min) and the systematic amplification of the autosomal (amhr2a) amplicon was used as a positive PCR control.
Perca fluviatilis primers were designed to amplify a 27 bp-deletion variant in the third intron of the P. fluviatilis hsdl1 gene, which was identified as a male specific (Y-specific) variation based on the pool-seq analysis. Selected PCR-primer sequences were forward 5’-ACACTGATCAACATTTTCTGTCTCA-3’ and reverse 5’-TGTTAACATTTGAGAATTTTGCCTT-3’. PCRs were carried out as described above with the following cycling conditions: denaturation 96°C for 3 min; 40 cycles of denaturation (96°C, 30 s), annealing (60°C, 30 s) and extension (72°C, 30 min); final extension (72°C, 5 min); storage at 4°C. PCR amplicons were separated on 5% agarose gels (5% Biozym sieve 3:1 agarose, 1x TBE buffer, 5 V/cm, 1 h 40 min running time) and the amplicon derived from the amplification of the X-chromosome allele was used as a positive PCR control. In addition to this classical PCR sex-genotyping method, we also explored the sex-linkage of some sex-specific SNVs in P. fluviatilis using Kompetitive Allele-Specific Polymerase chain reaction (KASPar) assays [56]. Seven sex-specific SNVs were selected at different locations within the P. fluviatilis sex-determining region. Primers (Table 1) were designed using the design service available on the 3CR Bioscience website (www.3crbio.com/free-assay-design). KASPar genotyping assays were carried out with a single end-point measure on a Q-PCR Light Cycler 480 (Roche) using the Agencourt® DNAdvance kit (Beckman), following the manufacturer’s instructions.
Table 1: KASpar allele-specific PCR-primers.
For each allele (AL1 and AL2) primers, the X and Y sex chromosome-specific alleles are provided along with their sequences.
| Primer ID# | Allele (X or Y) | Sequence (5’ – 3’) |
|---|---|---|
| SNV1_AL1 | X | GAAGGTGACCAAGTTCATGCTGACACATATTGTCCATCTGATGTAAATG |
| SNV1_AL2 | Y | GAAGGTCGGAGTCAACGGATTATGACACATATTGTCCATCTGATGTAAATT |
| SNV1_C | Common | CACCACCACTGACTGAAGAATAATATGAA |
| SNV2_AL1 | X | GAAGGTGACCAAGTTCATGCTGGACTGATTGTGCTGCTTCTCTC |
| SNV2_AL2 | Y | GAAGGTCGGAGTCAACGGATTGGACTGATTGTGCTGCTTCTCTT |
| SNV2_C | Common | CAGATGAGGAAGGAGGAGATGCAT |
| SNV3_AL1 | X | GAAGGTGACCAAGTTCATGCTTCACCACCATAGAACCACC |
| SNV3_AL2 | Y | GAAGGTCGGAGTCAACGGATTCGCTTCACCACCATAGAACCACT |
| SNV3_C | Common | GGGATGAGATGCCATTCTTCCAAATAATA |
| SNV4_AL1 | Y | GAAGGTGACCAAGTTCATGCTCGCCCTCAGCCTGGTTGAT |
| SNV4_AL2 | X | GAAGGTCGGAGTCAACGGATTCGCCCTCAGCCTGGTTGAG |
| SNV4_C | Common | TCGTCATGCACTCCTTCACAGCTTT |
| SNV5_AL1 | X | GAAGGTGACCAAGTTCATGCTGGAATTTGCCTGAAATAATGAATGAATATG |
| SNV5_AL2 | Y | GAAGGTCGGAGTCAACGGATTGTGGAATTTGCCTGAAATAATGAATGAATATA |
| SNV5_C | Common | AGGACATTACAGATTGGTCAGACCATATA |
| SNV6_AL1 | X | GAAGGTGACCAAGTTCATGCTTACCCTTCTCACCACCTGTTT |
| SNV6_AL2 | Y | GAAGGTCGGAGTCAACGGATTCTTACCCTTCTCACCACCTGTTG |
| SNV6_C | Common | CATAGTTCTTACCCTTACTGTACCAGATA |
| SNV7_AL1 | Y | GAAGGTGACCAAGTTCATGCTCAGTGAACCTCCCTATGAGGCA |
| SNV7_AL2 | X | GAAGGTCGGAGTCAACGGATTAGTGAACCTCCCTATGAGGCG |
| SNV7_C | Common | CTCGGTACAAGGTTGAAAGATGAAAGATA |
RNAseq expression analyses
Already available gonadal datasets of P. fluviatilis (age 9 month) RNAseq [57] (SRA accessions: SRR14461526 and SRR14461527) were used to compare gene expression between ovary and testes for the gene models annotated in the SD-region. Reads were mapped to our P. fluviatilis reference genome using HISAT2 [58] and transcript assembly and FPKM-values were calculated using STRINGTIE [59].
RESULTS
Genome assemblies of Perca fluviatilis, Perca schrenkii and Sander vitreus
The genome of P. fluviatilis was sequenced to high coverage using Oxford Nanopore long-read sequencing (estimated coverage: 67-fold / N50 read length: 11.9 kbp), Hi-C data was generated to allow for chromosome-level assembly (coverage: 52-fold / alignable pairs 89.1%/ Hi-C map see Suppl. Fig. 1). The final assembly yielded a highly complete reference genome (99.0% of sequence assigned to 24 chromosomes (N50 length: 39.6 Mbp) and highly continuous contigs (N50 length: 4.1 Mbp). Compared to a previously published genome assembly of P. fluviatilis, obtained from “linked-short-reads” (10X Genomics), these numbers represent a 316-fold improvement of contig continuity and a 6.3-fold increase of scaffold continuity. The better continuity resulted in an increased percentage of predictable genes (BUSCO results below). Genome assembly statistics are also highly congruent with the previously published reference quality P. flavescens genome, except for obvious size differences as the P. fluviatilis assembly is about 8.1% larger than the P. flavescens assembly and represents the largest genome known in the genus Perca (Table 2).
Table 2: Genome assembly statistics for P. fluviatilis and P. schrenkii.
For comparison, the table also provides numbers for two recently published Perca sp. assemblies [9,64]. Abbreviations: LR = long reads; HiC = chromosome conformation capture; CG = comparative genomics; 10X = linked-read sequencing; GLM = genetic linkage map.
| this study | earlier studies | |||||
|---|---|---|---|---|---|---|
| Species | P. fluviatilis | P. schrenkii | S. vitreus | P. flavescens | P. fluviatilis | S. lucioperca |
| Strategy | LR+HiC | LR+CG | LR+CG | LR+10X+HiC | 10X | LR+GLM |
| total length | 951,362,726 | 908,224,480 | 791,708,797 | 877,456,336 | 958,225,486 | 901,238,333 |
| longest 24 sequences assembly fraction | 99.0% | 94.7% | 96.5% | 98.8% | 41.4% | 99.5% |
| scaffold/chromosome N50 | 39,550,354 | 36,400,992 | 33,333,317 | 37,412,490 | 6,260,519 | 41,060,379 |
| contig N50 | 4,101,751 | 3,162,456 | 6,206,245 | 4,268,950 | 12,991 | 6,160,542 |
The genome of P. schrenkii was assembled by a hybrid assembly method, which was highly efficient regarding long-read sequencing coverage and read length needed (here only 30-fold / N50 read length: 4.95 kb). De novo assembled contigs from short reads were combined with long reads and scaffolded using our CSA-pipeline [38], with the P. flavescens as the closest reference genome (Fig. 1: divergence time about 7.1 Mya) for genome comparison and inferring chromosomal-level sequences. Using this approach, we were able to assemble the genome of P. schrenkii to similar quality as those obtained for P. flavescens and P. fluviatilis (94.7% assigned to 24 chr. / contig N50 length: 3.2 Mbp; Table 2). The genome assembly size of P. schrenkii was in between the other two Perca sp. genomes (877 Mbp < 908 Mbp < 951 Mbp).
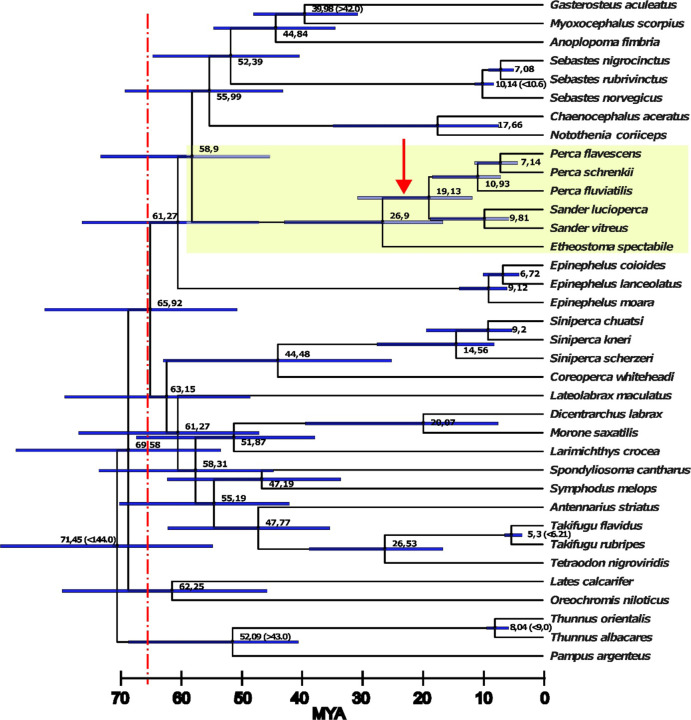
Figure 1:
Time calibrated phylogenomic tree constructed from non-coding alignments of 36 Percomorpha genome assemblies reveals a massive radiation after the Cretaceous–Paleogene (K–Pg) boundary (66 Mya, dotted red line). The family Percidae is highlighted in yellow. Node numbers depict the median ages in Mya calculated by Mcmctree (values in brackets were taken from www.timetree.org and used for calibration). Blue bars depict the 95% confidence intervals for the node ages. Red arrow indicates duplication event of amhr2 in Percidae (more details in Fig. 2a, amhr2 gene tree). All branches of the tree obtained 100% support using the SH-aLRT (Shimodaira–Hasegawa like approximate likelihood ratio test) and UFBS (ultra-fast boostrap) tests.
The genome of Sander vitreus was assembled from long reads (coverage: 12-fold / N50 read length: 10 kb) and short reads similar to procedures for P. schrenkii but we used two reference genomes for chromosomal assembly. First, contigs of S. lucioperca (closest relative, divergence time 9.8 Mya) served to order the S. vitreus contigs (N50: 6.2 Mb), which improved scaffold N50 significantly (result. N50: 16.8 Mb), then these nearly chromosome-scale scaffolds were ordered according to the P. flavescens (div. time 19.1 Mya) Hi-C chromosomes (result. N50: 33.3 Mb / 96.5% assigned to 24 chromosomes). This two-step approach resulted in more consistent results than just using the S. lucioperca chromosomal scaffolds, which were generated by genetic linkage mapping. We observed similar genome size differences as in Perca sp. between both Sander species. The S. vitreus genome assembly (791 Mb) is smaller than the one of S. lucioperca (901 Mb), thus the North American Sander and Perca species tend to have smaller genome sizes than their Eurasian relatives (Table 2).
De novo repeat analysis showed that 60.1% of the genome assembly size difference between Sander vitreus and S. lucioperca could be explained by repeat expansion/reduction. Similarly, for Perca flavescens and P. fluviatilis about 64.8% of the genome size differences were due to repeat expansion/reduction. In both species pairs most repeat expansions/reductions were observed in repeat elements classified as “unknown”. Regarding annotated repeat element classes, L2, DNA, Helitron, CMC-EnSpm, hAT, Rex-Babar, hAT-Charlie and PiggyBac elements expanded the most in both Eurasian species (together adding roughly 20 Mbp to the genomes of S. lucioperca and P. fluviatilis), while a clear expansion of only a single repeat element, called RTE-BovB, was found in both North American species (adding about 3 and 7 Mbp of sequence to S. vitreus and P. flavescens, respectively; Suppl. table 1).
The genome of P. fluviatilis was annotated by NCBI/GNOMON, which included ample public RNAseq data and protein homology evidence. For P. schrenkii and S. vitreus, we transferred the NCBI/GNOMON annotations of P. flavescens and S. lucioperca, respectively. BUSCO analysis (Table 3) revealed values larger than 95.9% for complete BUSCOs (category “C:”) for all annotations. The comparative annotation approach resulted only in small losses (category “M:”) of a few BUSCO genes in the range of 0.4% – 1.1%. In this regard, the S. vitreus assembly performed better than the P. schrenkii assembly, possibly due to the higher N50 read length of the underlying long-read data.
Table 3: BUSCO scoring of annotations of five Percidae genome assemblies.
(P. fluviatilis, P. schrenkii, S. vitreus from this study). The comparative mapping of high quality NCBI/GNOMON annotations onto closely related species’ genome assemblies is a cost-effective and fast procedure to annotate new genomes. Abbreviations used: C = complete; S = single copy; D = duplicated; F = fragmented; M = missing.
| Species | Annotation type | BUSCO scoring code | |
|---|---|---|---|
| # 1 | P. fluviatilis | NCBI/GNOMON | C: 98.5% [S: 95.3%, D: 3.2%], F: 1.0%, M: 0.5%, n: 4584 |
| #2 | P. flavescens | NCBI/GNOMON | C: 99.3% [S: 95.9%, D: 3.4%], F: 0.5%, M: 0.2%, n: 4584 |
| # 3 | P. schrenkii | mapping of annot. #2 | C: 95.9% [S: 92.6%, D: 3.3%], F: 2.8%, M: 1.3%, n: 4584 |
| # 4 | S. lucioperca | NCBI/GNOMON | C: 99.3% [S: 95.9%, D: 3.4%], F: 0.5%, M: 0.2%, n: 4584 |
| # 5 | S. vitreus | mapping of annot. #4 | C: 98.6% [S: 95.5%, D: 3.1%], F: 0.8%, M: 0.6%, n: 4584 |
Percomorpha phylogenomics and divergence time estimation
To calculate the phylogenetic tree of 36 Percomorpha species and their divergence times, we aligned whole genomes and extracted the non-coding alignments (Fig. 1). The use of non-coding sequences is preferable to calculate difficult-to-resolve phylogenetic trees that occur after massive radiations [60–62]. Our multiple alignment consisted of 6,594,104 nt residues (2,256,299 distinct patterns; 1,652,510 parsimony-informative; 1,136,496 singleton sites; 3,805,098 constant sites) and resulted in a highly-supported tree (raxml-ng and IQtree2 topologies were identical; all IQtree2’s SH-aLRT and ultrafast bootstrapping (UFBS) values were 100). According to the divergence time analysis, most Percomorpha orders emerged after the Cretaceous–Paleogene (K-Pg) boundary about 65.9 Mya ago (CI: 51.3 – 83.6). The lineage leading to the Percidae (represented with species of Perca, Sander, and Etheostoma) emerged about 58.9 Mya (CI: 45.8 – 74.2), and the extant Percidae species analyzed in this study diverged from a last common ancestor (LCA) about 26.9 Mya (CI: 16.8 – 43.4). The Perca and Sander genera split about 19.1 Mya (CI: 11.8 –31.1), and S. vitreus and S. lucioperca splitted at 9.8 Mya (CI: 5.7 – 18.9) similar to the divergence of P. fluviatilis from P. flavescens and P. schrenkii 10.9 Mya (CI: 7.1 – 18.6). The closest Perca species are P. flavescens and P. schrenkii which diverged about 7.1 Mya (CI: 4.2 – 11.4), although today both species are occurring in completely different global ranges.
The fate of amhr2 genes during evolution of Perca and Sander species
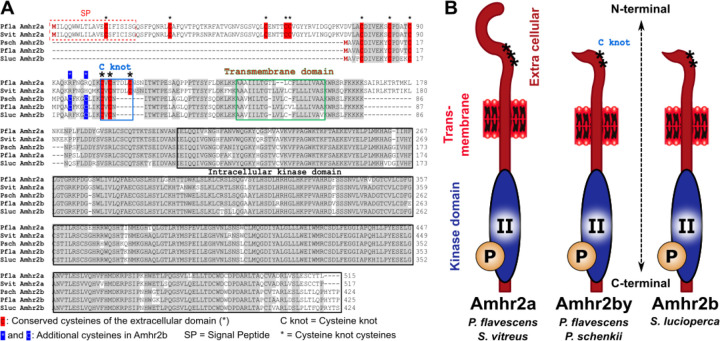
In the genome of P. flavescens, two amhr2 paralogs were previously described, i.e., an autosomal gene, amhr2a, present in both males and females on chromosome 04 (Chr04), and a male-specific duplication on the Y-chromosome (Chr09), amhr2bY [9]. A similar amhr2 gene duplication was also found in our male P. schrenkii assembly, and sequence homologies and conserved synteny analyses show that these two P. schrenkii amhr2 genes are orthologs of P. flavescens amhr2a and amhr2bY, respectively (Fig. 2A and AB). Genotyping of one male and one female also suggests that the amhr2bY gene could be male-specific in P. schrenkii (Suppl. Fig. 2, Table 4), as described in P. flavescens [9]. This potential sex-linkage is also supported by a half coverage of reads in the genomic region containing the amhr2bY locus in our male P. schrenki genome assembly (Suppl. Fig. 3), in agreement with the hemizygosity of a male-specific region on the Y in species with a XX/XY sex determination system. Alignment of the P. schrenkii amhr2bY ortholog shows that its coding sequence (CDS) shares 98% identity with the P. flavescens amhr2bY CDS and 95.7 % identity at the protein level the P. flavescens Amhr2bY. As in P. flavescens [9], the P. schrenkii amhr2bY gene encodes a N-terminal-truncated type II receptor protein that lacks most of the cysteine-rich extracellular part of the receptor, which is crucially involved in ligand-binding specificity [63] (Fig. 3).
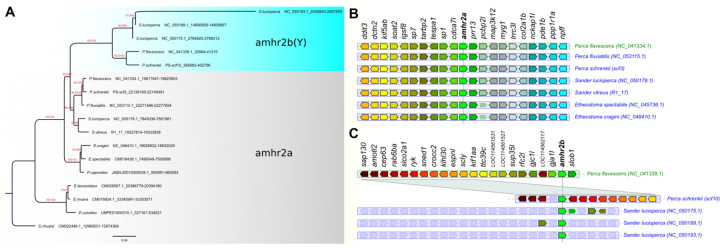
Figure 2: Evolution of amhr2 genes in Percidae.
A. Orthologs of amhr2a were identified in genome assemblies and their gene tree is consistent with the species tree, which was found by the phylogenomics approach (see Figure 1) The amhr2b duplications were only found in the genome assemblies of P. flavescens (single copy, male specific), P. schrenkii (single copy, potentially male specific) and S. lucioperca (three copies, no clear sex linkage) and they clustered together. Thus, amhr2b stems from a gene duplication event that occurred at the origin of Percidae (19–27 Mya) and the absence of amhr2b in P. fluviatilis and S. vitreus suggests a secondary loss event in these species. This tree was calculated on codon position 1 and 2 alignments and achieved the best bootstrap support (red numbers: SH-aLRT / UFBS support values) for the split of the amhr2a and b clades. Trees based on complete CDS, CDS + Introns and amino acid sequences resulted in the same topology albeit with lower bootstrap support for some splits (Suppl. Fig. 8). Numbers after species names depict the coordinates of the respective amhr2 genes in the genome assemblies. B & C. Conserved synteny around the amhr2a (B) and amhr2b (C) loci in some Percidae species. These multiple duplications (in S. lucioperca) or the loss of the amhr2b genes (in P. fluviatilis and S. vitreus) emphasize that amhr2b may be considered a “jumping” gene locus, which is also supported by conserved synteny analysis.
Table 4: Sex-linkage of different sex-markers in Perca fluviatilis and Perca schrenkii.
Associations between each sex-specific marker and sex phenotypes are provided for both males and females (number of positive individuals / total number of individuals) along with the p-value of association with sex that was calculated for each species and population based on the Pearson’s Chi-square test with Yates’ continuity correction. ns = not statistically significant.
| Species | Population | marker | males | females | p value |
|---|---|---|---|---|---|
| Perca schrenkii | Alakol Lake, Kazakhstan | amhr2bY | 1/1 | 0/1 | ns |
| Perca fluviatilis | Mueggelsee Lake Germany | hsdl1 | 10/10 | 0/9 | 9.667e-05 |
| Perca fluviatilis | Lucas Perche, France | SNV1 | 48/48 | 0/47 | < 2.2e-16 |
| Perca fluviatilis | Kortowskie Lake, Poland | SNV1 | 17/17 | 0/20 | 8.83e-09 |
Figure 3:
Alignment and structure of Amhr2a and Amhr2b proteins in Perca flavescens (Pfla) and P. schrenkii (Psch), Sander lucioperca (Sluc) and S. vitreus (Svit). A. Alignment of some Percidae Amhr2a (P fluviatilis and S. vitreus) and Amhr2b (P. schrenkii, P. flavescens and S. lucioperca) proteins. B. Schematic structure of Amhr2a and Amhr2b proteins.
In contrast, in our P. fluviatilis male genome assembly, only one copy of amhr2 could be identified and sequence homologies and conserved synteny analysis (Fig. 2A and 2B) shows that this amhr2 gene is the ortholog of P. flavescens and P. schrenkii amhr2a. In addition, PCR with primers designed to amplify both amhr2a and amhr2bY from P. flavescens and P. schrenkii did not show any sex-differences in P. fluviatilis. Altogether, these results support the absence of an amhr2b gene in P. fluviatilis.
In the Sander lucioperca male genome assembly, four copies of Amhr2 were detected. Using the same primers as those used for the amplification of both amhr2a and amhr2bY in P. flavescens and P. schrenkii, PCR genotyping on males and females of S. lucioperca produced complex amplification patterns with multiple bands and no visible association with sex. In the publicly available genome assembly of Sander vitreus, sequence homologies and /or conserved synteny analysis (Fig. 2A and 2B) allowed the identification of a single autosomal amhr2a gene.
A phylogenetic analysis of the sequences with similarity to amhr2 in Perca and Sander (Fig. 2A and 2B) shows that the different amhr2 genes likely originated from a gene duplication event that happened in the branch leading to the last common ancestor of these species, dated around 19–27 Mya. Since that time, the amhr2bY gene has been conserved in P. schrenkii and P. flavescens, lost in P. fluviatilis and S. vitreus, and amplified in S. lucioperca.
Evolution of sex determination in P. fluviatilis
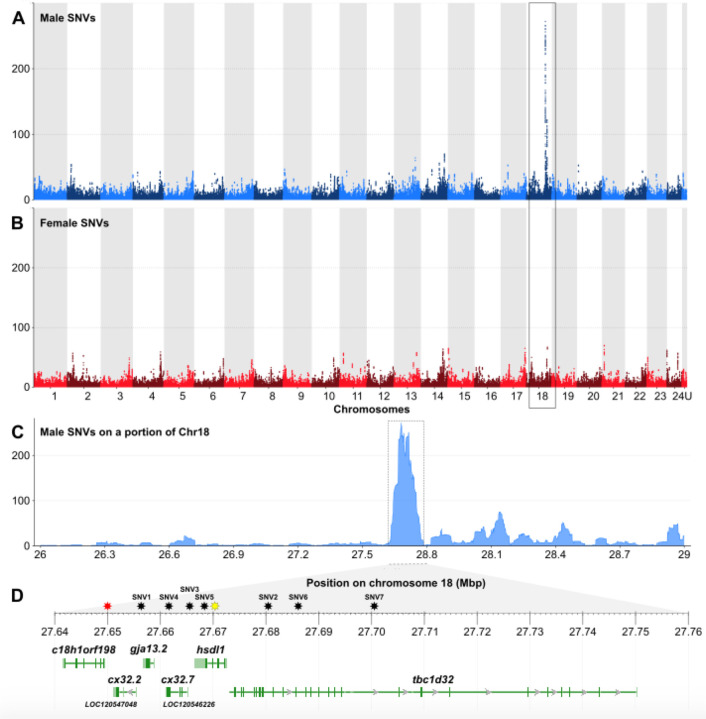
Because P. fluviatilis sex determination does not rely on an amhr2 duplication like what has been found in P. flavescens [9] and P. schrenkii (this study), we used genome-wide approaches to better characterize its sex-determination system. RAD-Seq analysis on 35 males and 35 females of P. fluviatilis, carried out with a minimum read depth of one, allowed the characterization of a single significant sex marker (Suppl. Fig. 4). This 94 bp RAD sequence matches (Blast Identities: 93/95%) a portion of P. fluviatilis chromosome 18 (GENO_Pfluv_1.0, Chr18: 27656212 – 27656305). This RAD-Seq analysis suggests that Chr18 could be the P. fluviatilis sex chromosome, and that its sex-determining region could be very small because we only detected a single significant sex-linked RAD-sequence. To get a better characterization of the P. fluviatilis sex chromosome and sex-determining region, we then used Pool-Sequencing (Pool-Seq) to re-analyze the same samples used for RAD-Seq by pooling together DNA from the males in one pool and DNA from females in a second pool. Using these Pool-Seq datasets, we identified a small 100 kb region on P. fluviatilis Chr18 with a high density of male-specific SNVs (Fig. 4), confirming the RAD-Seq hypothesis that Chr18 is the sex chromosome in that species. No male-specific duplication / insertion event was found in this sex-determining region on Chr18, which contains six annotated genes (Fig. 4D). These genes encode a protein of unknown function (C18h1orf198), three gap-junction proteins (Cx32.2, Gja13.2 and Cx32.7), a protein annotated as inactive hydroxysteroid dehydrogenase-like protein (Hsdl1), and a protein known as protein broad-minded or Tcb1 domain family member 32 protein (Tbc1d32). Three of these six annotated genes, i.e., c18h1orf198, hsdl1 and tbc1d32 display a higher expression in testis than in the ovary (Suppl. Fig. 5).
Figure 4: Chromosome 18 is the sex chromosome of Perca fluviatilis.
(A, B) Genome-wide Manhattan-plots of (A) male- and (B) female-specific single-nucleotide variations (SNVs), showing that chromosome 18 (Chr18) contains a 100 kb region, enriched for male-specific SNVs. Male- and female-specific SNVs are represented as dots (total of SNVs per 50 kb window size) of alternating colors on adjacent chromosomes. (C) Zoomed view of the male-specific SNVs on the sex-biased region of Chr18 with its gene annotation content (D): c18h1orf198 = c18h1orf198 homolog; cx32.2 (LOC120547048) = gap junction Cx32.2 protein-like; gja13.2 = gap junction protein alpha 13.2; cx32.7 (LOC120546226) = gap junction Cx32.7 protein-like; hsdl1 = inactive hydroxysteroid dehydrogenase-like protein 1; tbc1d32 = tcb1 domain family member 32 (also known as protein broad-minded). Stars over the ruler of panel D are the locations of the single male-specific RAD maker (red star) and of the SNVs used for designing KASPar assays (black stars; SNV1-7). The location of the sex-specific intronic indel inside the hsdl1-gene used for sex specific PCR is shown by a yellow star
To provide a better support for the sex-linkage of the male-specific variants found within this sex determining region on Chr18, we designed different types of genotyping assays (classical PCR and KASpar) that have been applied to different P. fluviatilis individuals which were phenotypically sexed with confidence. A classical PCR-assay was first developed based on the detection of a Y-allele-specific 27 bp deletion in the third intron of the P. fluviatilis hsdl1 gene, (Suppl. Fig. 6, Table 4) and this assay successfully identified all males of a Lake Mueggelsee from Germany (10 males and 9 females; p-value of association with sex = 9.667e-05). In addition, KASpar allele-specific PCR-assays were developed based on seven single nucleotide sex-specific variants, located at different positions within the Chr18 sex-determining region of P. fluviatilis (Fig. 3D). Tests of 48 males and 48 females showed that of seven KASpar allele-specific PCR-assays, five resulted in a high proportion of correctly-genotyped individuals with males being heterozygote and females being homozygote (>95%). Two of the targeted SNVs (SNV1 and SNV3) that displayed 100% sex-linkage accuracy (Suppl. Fig. 7, Tables 4 and 5). Sex-linkage of SNV1 was then checked on a wild-type population from Kortowskie Lake in Poland for which the association of male phenotype and SNV1 heterozygosity was also complete (17 males and 20 females; p-value = 8.83e-09, Table 4).
Table 5: KASpar allele-specific PCR assays on seven single sex-specific nucleotide variations (SNV ID#) in P. fluviatilis.
Numbers of homozygote (Ho), heterozygote (He) and uncalled genotypes (U). N = Total numbers of genotyped individuals (M/F: Males/females), %N = percentage of genotyped individuals, % As = percentage of correctly assigned genotypes i.e., male heterozygotes and female homozygotes. The p-value of association with sex was calculated for each SNV based on the Pearson’s Chi-square test with Yates’ continuity correction scoring heterozygote males and homozygote females as positives.
| SNV ID# | N (M/F) | % N | M Ho/He/U | F Ho/He/U | % As | p value |
|---|---|---|---|---|---|---|
| SNV1 | 95 (48/47) | 99,0 | 0/48/0 | 47/0/0 | 100,0 | < 2.2e-16 |
| SNV2 | 96 (48/48) | 100,0 | 8/37/3 | 34/2/12 | 74,0 | 3.175e-11 |
| SNV3 | 93 (45/48) | 96,9 | 0/45/0 | 48/0/0 | 100,0 | < 2.2e-16 |
| SNV4 | 96 (48/48) | 100,0 | 0/46/2 | 46/0/2 | 95,8 | < 2.2e-16 |
| SNV5 | 92 (44/48) | 95,8 | 0/42/2 | 46/0/2 | 95,7 | < 2.2e-16 |
| SNV6 | 93 (48/45) | 96,9 | 1/47/0 | 25/2/18 | 77,4 | 1.964e-14 |
| SNV7 | 95 (47/48) | 99,0 | 1/46/0 | 45/3/0 | 95,8 | < 2.2e-16 |
DISCUSSION
The Percidae family is represented by 239 species and 11 genera. The genera Perca and Sander are especially important for aquaculture and fisheries. By providing new genome sequence assemblies for Perca fluviatilis, P. schrenckii and Sander vitreus, we provide for the first-time access to all economically important species of the Percidae at the DNA-level. Assembly statistics for these three new genome sequence assemblies are reference grade with N50 continuity in the megabase range and chromosomal length scaffolds, obtained either by Hi-C scaffolding or by conserved synteny analysis involving the closest relatives. Importantly completeness on the gene-level is significantly higher than in a short-read based draft genome for P. fluviatilis, published earlier [64], allowing much stronger conclusions to be drawn regarding the presence or absence of possible sex-determining genes.
The origin of Percidae
A phylogenomic approach using aligned non-coding sequences of 36 genomes resulted in a highly-supported tree showing a rapid radiation of fish families. It has recently been shown that phylogenomics based on non-coding sequences may be more reliable at resolving difficult-to-resolve radiations in species trees (i.e. in Aves). According to our time calibration, many taxonomic orders related to the Percidae emerged shortly after the Cretaceous-Paleogene (K-Pg) mass extinction event, about 66 Mya, and gave rise to Percidae about 59 Mya. This is in contrast to many older studies, which have, for example, dated the split of Perca ssp. and Gasterosteus ssp. back to the Cretaceous (73–165 Mya; 18 of 23 studies listed at www.timetree.org). Similar patterns in rapid radiations have been observed in the avian tree of life and have likewise been attributed to the K-Pg mass extinction [61,62]. In context, it has been argued that the so-called “Lilliput effect”, which describes the selection in favor of species with small body sizes and fast generation times after mass extinction events, can lead to an increase in substitution rates and results in overestimations of node-ages for molecular clocks [65].
The evolution of sex determination in Perca and Sander species
Evolution and turnover of Amhr2
Sex determination systems with MSD evolved from duplications of the amh [10–17] or amhr2 [9,18–21] genes have now been characterized in many fish species, all with a male-heterogametic system (XX/XY). In addition, the fact that Amh in monotremes [66], or Amhr2 in some lizards [67] are Y-linked also makes them strong MSD gene candidates in other vertebrate species. In Percidae, sex determination has only been explored in some species of Perca [8,9], and Amhr2 has been characterized as a potential MSD gene in yellow perch, P. flavescens [9]. Our results suggest that this duplication of amhr2 in P. flavescens is also shared by P. schrenkii and S. lucioperca, implying an origin of duplication in their last common ancestor, dated around 19–27 Mya. However, the fate of this duplication seems to be complex - with multiple duplications/insertions on different chromosomes with no clear sex-linkage in S. lucioperca, a secondary loss in P. fluviatilis and S. vitreus, contrasting with a single potentially sex-linked duplication/insertion in P. schrenkii and in P. flavescens. This finding suggests that the shared ancestral amhr2b-duplicated locus (Fig. 2A) might be a jumping locus that has been moving around during its evolution as found for the sdY MSD jumping sex locus in salmonids [68,69]. Additional evidence that these amhr2b genes originated a single ancestor also rely on the fact that the Amhr2b proteins of P. flavescens [9], P. schrenkii and S. lucioperca share a similar gene structure with an N-terminal truncation that results in the absence of the cysteine-rich extracellular part of the receptor. This part of the receptor is known to be crucial for ligand binding [70]. A similar N-terminal truncation of a duplicated amhr2 was also described in catfishes from the Pangasidae family, where this truncation had been hypothetically linked to a potentially new sex-determination function that lacks ligand dependency [18]. In the genus Sander, the situation might be similar to that in Perca regarding the changes of the sex-determination systems between species. In S. lucioperca, amhr2b might still serve as the MSD-gene, but the several recent amhr2b duplications have complicated our analysis so far. Similar to P. fluviatilis, S. vitreus has lost amhr2b and likely another factor took over as a potential MSD-gene.
A new sex-specific locus in P. fluviatilis
The fact that P. fluviatilis sex does not rely on an amhr2-duplication like P. flavescens [9] and P. schrenkii do, indicates that P. fluviatilis evolved a completely different and new MSD-gene. Our results also show that this sex locus on P. fluviatilis Chr18 is very small compared to what is observed in many fish species, with a potentially non-recombining size around 100 kb. This locus however is not the smallest SD-locus described in teleost fish: in the pufferfish, Takifugu rubripes, the sex locus is limited to a few SNPs that differentiate amhr2 alleles on the X- and Y -chromosomes [19]. Because we did not find any sign of a sex chromosome-specific duplication/insertion event in the P. fluviatilis SDR, this sex locus seems to result from pure allelic diversification and is thus in contrast to P. flavescens [9] and probably also P. schrenkii (this study). The P. fluviatilis sex specific-region on Chr18 contains six annotated genes, which encode a protein of unknown function (C18h1orf198), three gap-junction proteins (Cx32.2, Gja13.2 and Cx32.7), a protein annotated as inactive hydroxysteroid dehydrogenase-like protein (Hsdl1) and a protein known as protein broad-minded or Tcb1 domain family member 32 protein (Tbc1d32). Of these genes, hsdl1 and tcb1d32 are interesting potential MSD candidates in P. fluviatilis, based on their potential functions and the fact that both display a higher testicular expression compared to the ovary. The Hsdl1 protein is indeed annotated as “inactive” [71], but this annotation only refers to its lack of enzymatic activity against substrates so far tested, leaving other potential functional roles for a protein that is highly conserved in vertebrates [71]. In Epinephelus coioides, hsdl1 has been shown to be differentially expressed during female-to-male sex-reversal, and its expression profile clustered with hsd17b1 [72], which plays a central role in converting sex steroids and has recently been identified as a potential MSD gene in different species with a female heterogametic (ZZ/ZW) sex determination system, like in oyster pompano, Trachinotus anak [73] and different amberjack species [74,75]. The Tbc1d32 protein has been shown to be required for a high Sonic hedgehog (Shh) signaling in the mouse neural tube [76]. Given the role of Shh signaling downstream of steroidogenic factor 1 (nr5a1) for the proper steroidogenic lineage fate [77] and the importance of steroids in gonadal sex differentiation [78,79], tbc1d32 would be also an interesting candidate as a potential MSD gene in Perca fluviatilis.
CONCLUSIONS
Our study shows that Percidae exhibit a remarkable high variation in sex-determination mechanisms. This variation is connected to deletion or amplification of amhr2bY, which if lost in certain species (like Perca fluviatilis or Sander vitreus) should cause re-wiring of the sex determining pathways and result in the rise of new SD-systems. The mechanisms behind a “jumping” amhr2bY expansion or loss and which genes replace it as the MSD remain to be elucidated. The new Percidae reference sequence assemblies presented here and the highly reliable sex markers developed for P. fluviatilis can now be applied for sex genotyping in basic science as well in aquaculture.
Supplementary Material
ACKNOWLEDGEMENTS
We kindly acknowledge the NCBI/Genbank team for providing a GNOMON annotation of the P. fluviatilis genome. This work was funded by the German Research Foundation (DFG) “Eigene Stelle” grant KU 3596/1-1; project number: 324050651, by the Agence Nationale pour la Recherche (ANR) / DFG PhyloSex project (ANR-13-ISV7-0005), the CRB-Anim “Centre de Ressources Biologiques pour les animaux domestiques” project PERCH'SEX, the FEAMP “Fonds européen pour les affaires maritimes et la pêche” project SEX'NPERCH, NIH (R35 GM139635), and NSF (2232891). The GeT-PlaGe and MGX sequencing facilities were supported by France Génomique National infrastructure as part of the “Investissement d’avenir” program managed by (ANR-10-INBS-09). We thank Dr. Tatjana Dujsebayeva, Salamat Karlybai and his colleague for help during field work in Kazakhstan. We thank Eva Kreuz and Wibke Kleiner for technical assistance. We thank Matt Faust (Ohio, DNR) for help with samples of S. vitreus.
Footnotes
DATA AVAILABILITY
All genome assemblies and raw sequence datasets have been submitted to NCBI/GENBANK under the bioproject accessions: PRJNA549142, PRJNA637487, PRJNA808842 (P. fluviatilis, P. schrenkii, S. vitreus, respectively).
BENEFIT-SHARING STATEMENT
A research collaboration was developed with scientists from the countries providing genetic samples (PE and WL in USA, DZ in Poland and ST in Russia), all collaborators are included as co-authors, the results of research have been shared with the provider communities, and the research addresses a priority concern, in this case the conservation of organisms being studied. More broadly, our group is committed to international scientific partnerships, as well as institutional capacity building.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Stepien CA, Haponski AE. Taxonomy, Distribution, and Evolution of the Percidae. In: Kestemont P, Dabrowski K, Summerfelt RC, editors. Biology and Culture of Percid Fishes: Principles and Practices. Dordrecht: Springer Netherlands; [Google Scholar]
- 2.Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C. Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol. 2011; doi: 10.1016/j.ygcen.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer FJ, Overton JL, Kloas W, Wuertz S. Length rather than year-round spawning, affects reproductive performance of RAS-reared F-generation pikeperch, Sander lucioperca (Linnaeus, 1758) - Insights from practice. J Appl Ichthyol. 2018; doi: 10.1111/jai.13628. [DOI] [Google Scholar]
- 4.Policar T, Schaefer FJ, Panana E, Meyer S, Teerlinck S, Toner D, et al. Recent progress in European percid fish culture production technology-tackling bottlenecks (vol 27, pg 4, 2019). Aquacult Int. 2019; doi: 10.1007/s10499-019-00457-4. [DOI] [Google Scholar]
- 5.Malison JA, Kayes TB, Best CD, Amundson CH, Wentworth BC. Sexual Differentiation and Use of Hormones to Control Sex in Yellow Perch (Perca flavescens). Can J Fish Aquat Sci. 1986; doi: 10.1139/f86-004. [DOI] [Google Scholar]
- 6.Melard C, Kestemont P, Grignard JC. Intensive culture of juvenile and adult Eurasian perch (P-fluviatilis): Effect of major biotic and abiotic factors on growth. J Appl Ichthyol. 1996; doi: DOI 10.1111/j.1439-0426.1996.tb00085.x. [DOI] [Google Scholar]
- 7.Rougeot C, Jacobs B, Kestemont P, Melard C. Sex control and sex determinism study in Eurasian perch, Perca fluviatilis, by use of hormonally sex-reversed male breeders. Aquaculture. 2002; doi: Pii S0044-8486(01)00893-6 Doi 10.1016/S0044-8486(01)00893-6. [DOI] [Google Scholar]
- 8.Rougeot C, Ngingo JV, Gillet L, Vanderplasschen A, Melard C. Gynogenesis induction and sex determination in the Eurasian perch, Perca fluviatilis. Aquaculture. 2005; doi: 10.1016/j.aquaculture.2004.11.004. [DOI] [Google Scholar]
- 9.Feron R, Zahm M, Cabau C, Klopp C, Roques C, Bouchez O, et al. Characterization of a Y-specific duplication/insertion of the anti-Mullerian hormone type II receptor gene based on a chromosome-scale genome assembly of yellow perch, Perca flavescens. Mol Ecol Resour. 2020; doi: 10.1111/1755-0998.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Q, Kay T, Depincé A, Adolfi M, Schartl M, Guiguen Y, et al. Evolution of master sex determiners: TGF-β signalling pathways at regulatory crossroads. Philos Trans R Soc Lond B Biol Sci. 2021; doi: 10.1098/rstb.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q, Feron R, Jouanno E, Darras H, Herpin A, Koop B, et al. The rise and fall of the ancient northern pike master sex-determining gene. Przeworski M, Weigel D, editors. eLife. eLife Sciences Publications, Ltd; 2021; doi: 10.7554/eLife.62858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Q, Feron R, Yano A, Guyomard R, Jouanno E, Vigouroux E, et al. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLOS Genetics. 2019; doi: 10.1371/journal.pgen.1008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Sun Y, Zhao J, Shi H, Zeng S, Ye K, et al. A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia, Oreochromis niloticus. PLOS Genetics. 2015; doi: 10.1371/journal.pgen.1005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holborn MK, Einfeldt AL, Kess T, Duffy SJ, Messmer AM, Langille BL, et al. Reference genome of lumpfish Cyclopterus lumpus Linnaeus provides evidence of male heterogametic sex determination through the AMH pathway. Mol Ecol Resour. 2022; doi: 10.1111/1755-0998.13565. [DOI] [PubMed] [Google Scholar]
- 15.Song W, Xie Y, Sun M, Li X, Fitzpatrick CK, Vaux F, et al. A duplicated amh is the master sex-determining gene for Sebastes rockfish in the Northwest Pacific. Open Biol. 2021; doi: 10.1098/rsob.210063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondeau EB, Laurie CV, Johnson SC, Koop BF. A PCR assay detects a male-specific duplicated copy of Anti-Mullerian hormone (amh) in the lingcod (Ophiodon elongatus). BMC Res Notes. 2016; doi: 10.1186/s13104-016-2030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, et al. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci USA. 2012; doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen M, Pan Q, Jouanno E, Montfort J, Zahm M, Cabau C, et al. An ancient truncated duplication of the anti-Müllerian hormone receptor type 2 gene is a potential conserved master sex determinant in the Pangasiidae catfish family. Mol Ecol Resour. 2022; doi: 10.1111/1755-0998.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 2012; doi: 10.1371/journal.pgen.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamoto M, Uchino T, Koshimizu E, Kuchiishi Y, Sekiguchi R, Wang L, et al. A Y-linked anti-Müllerian hormone type-II receptor is the sex-determining gene in ayu, Plecoglossus altivelis. PLoS Genet. 2021; doi: 10.1371/journal.pgen.1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu M, Liu Y, Zhang Y, Wan S, Ravi V, Qin G, et al. Seadragon genome analysis provides insights into its phenotype and sex determination locus. Sci Adv. 2021; doi: 10.1126/sciadv.abg5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguinkal JA, Brunner RM, Verleih M, Rebl A, de los Rios-Perez L, Schafer N, et al. The First Highly Contiguous Genome Assembly of Pikeperch (Sander lucioperca), an Emerging Aquaculture Species in Europe. Genes-Basel. 2019; doi: ARTN 708 10.3390/genes10090708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmstrom M, Matschiner M, Torresen OK, Jakobsen KS, Jentoft S. Whole genome sequencing data and de novo draft assemblies for 66 teleost species. Sci Data. 2017; doi: ARTN 160132 10.1038/sdata.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foissac S, Djebali S, Munyard K, Vialaneix N, Rau A, Muret K, et al. Multi-species annotation of transcriptome and chromatin structure in domesticated animals. BMC Biology. 2019; doi: 10.1186/s12915-019-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wick RR, Judd LM, Gorrie CL, Holt KE. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom. 2017; doi: 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hailin Liu SW. SMARTdenovo: a de novo assembler using long noisy reads. Gigabyte. 2021; doi: 10.46471/gigabyte.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018; doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017; doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:13033997 [q-bio]. 2013; [Google Scholar]
- 30.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014; doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durand NC, Shamim MS, Machol I, Rao SSP, Huntley MH, Lander ES, et al. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst. 2016; doi: 10.1016/j.cels.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudchenko O, Batra SS, Omer AD, Nyquist SK, Hoeger M, Durand NC, et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science. 2017; doi: 10.1126/science.aal3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson JT, Turner D, Durand NC, Thorvaldsdottir H, Mesirov JP, Aiden EL. Juicebox.js Provides a Cloud-Based Visualization System for Hi-C Data. Cell Syst. 2018; doi: 10.1016/j.cels.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu GC, Xu TJ, Zhu R, Zhang Y, Li SQ, Wang HW, et al. LR_Gapcloser: a tiling path-based gap closer that uses long reads to complete genome assembly. Gigascience. 2019; doi: ARTN giy157 10.1093/gigascience/giy157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrison E MG. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:12073907 [q-bioGN]. 2012; [Google Scholar]
- 36.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Leung HC, Yiu SM, Chin FY. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012; doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 38.Kuhl H, Li L, Wuertz S, Stock M, Liang XF, Klopp C. CSA: A high-throughput chromosome-scale assembly pipeline for vertebrate genomes. Gigascience. 2020; doi: 10.1093/gigascience/giaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019; doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 40.Iwata H, Gotoh O. Benchmarking spliced alignment programs including Spaln2, an extended version of Spaln that incorporates additional species-specific features. Nucleic Acids Res. 2012; doi: ARTN e161 10.1093/nar/gks708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015; doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn RM, Haussler D, Kent WJ. The UCSC genome browser and associated tools. Brief Bioinform. 2013; doi: 10.1093/bib/bbs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kent WJ. BLAT - The BLAST-like alignment tool. Genome Res. 2002; doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He S, Li L, Lv LY, Cai WJ, Dou YQ, Li J, et al. Mandarin fish (Sinipercidae) genomes provide insights into innate predatory feeding. Commun Biol. 2020; doi: ARTN 361 10.1038/s42003-020-1094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frith MC, Kawaguchi R. Split-alignment of genomes finds orthologies more accurately. Genome Biol. 2015; doi: ARTN 106 10.1186/s13059-015-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AF, Roskin KM, et al. Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res. 2004; doi: 10.1101/gr.1933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019; doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol. 2020; doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007; doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 50.Amores A, Catchen J, Ferrara A, Fontenot Q, Postlethwait JH. Genome evolution and meiotic maps by massively parallel DNA sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics. 2011; doi: 10.1534/genetics.111.127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: building and genotyping Loci de novo from short-read sequences. G3 (Bethesda). 2011; doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feron R, Pan Q, Wen M, Imarazene B, Jouanno E, Anderson J, et al. RADSex: A computational workflow to study sex determination using restriction site-associated DNA sequencing data. Mol Ecol Resour. 2021; doi: 10.1111/1755-0998.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imarazene B, Du K, Beille S, Jouanno E, Feron R, Pan Q, et al. A supernumerary “B-sex” chromosome drives male sex determination in the Pachón cavefish, Astyanax mexicanus. Curr Biol. 2021; doi: 10.1016/j.cub.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen M, Pan Q, Larson W, Eché C, Guiguen Y. Characterization of the sex determining region of channel catfish (Ictalurus punctatus) and development of a sex-genotyping test. Gene. 2023; doi: 10.1016/j.gene.2022.146933. [DOI] [PubMed] [Google Scholar]
- 55.Jasonowicz AJ, Simeon A, Zahm M, Cabau C, Klopp C, Roques C, et al. Generation of a chromosome-level genome assembly for Pacific halibut (Hippoglossus stenolepis) and characterization of its sex-determining genomic region. Mol Ecol Resour. 2022; doi: 10.1111/1755-0998.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith SM, Maughan PJ. SNP genotyping using KASPar assays. Methods Mol Biol. 2015; doi: 10.1007/978-1-4939-1966-6_18. [DOI] [PubMed] [Google Scholar]
- 57.Pasquier J, Cabau C, Nguyen T, Jouanno E, Severac D, Braasch I, et al. Gene evolution and gene expression after whole genome duplication in fish: the PhyloFish database. BMC Genomics. 2016; doi: 10.1186/s12864-016-2709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015; doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015; doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braun EL, Kimball RT. Data Types and the Phylogeny of Neoaves. Birds. 2:1–222021; [Google Scholar]
- 61.Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014; doi: 10.1126/science.1253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhl H, Frankl-Vilches C, Bakker A, Mayr G, Nikolaus G, Boerno ST, et al. An Unbiased Molecular Approach Using 3 ’-UTRs Resolves the Avian Family-Level Tree of Life. Mol Biol Evol. 2021; doi: 10.1093/molbev/msaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heldin C-H, Moustakas A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb Perspect Biol. 2016; doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozerov MY, Ahmad F, Gross R, Pukk L, Kahar S, Kisand V, et al. Highly Continuous Genome Assembly of Eurasian Perch (Perca fluviatilis) Using Linked-Read Sequencing. G3: Genes, Genomes, Genetics. 2018; doi: 10.1534/g3.118.200768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berv JS, Field DJ. Genomic Signature of an Avian Lilliput Effect across the K-Pg Extinction. Syst Biol. 2018; doi: 10.1093/sysbio/syx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, et al. Origins and functional evolution of Y chromosomes across mammals. Nature. Nature Publishing Group; 2014; doi: 10.1038/nature13151. [DOI] [PubMed] [Google Scholar]
- 67.Pinto BJ, Gamble T, Smith CH, Wilson MA. A lizard is never late: squamate genomics as a recent catalyst for understanding sex chromosome and microchromosome evolution. bioRxiv; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yano A, Nicol B, Jouanno E, Quillet E, Fostier A, Guyomard R, et al. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol Appl. 2013; doi: 10.1111/eva.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertho S, Herpin A, Schartl M, Guiguen Y. Lessons from an unusual vertebrate sex-determining gene. Philos Trans R Soc Lond B Biol Sci. 2021; doi: 10.1098/rstb.2020.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hart KN, Stocker WA, Nagykery NG, Walton KL, Harrison CA, Donahoe PK, et al. Structure of AMH bound to AMHR2 provides insight into a unique signaling pair in the TGF-β family. Proc Natl Acad Sci U S A. 2021; doi: 10.1073/pnas.2104809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meier M, Tokarz J, Haller F, Mindnich R, Adamski J. Human and zebrafish hydroxysteroid dehydrogenase like 1 (HSDL1) proteins are inactive enzymes but conserved among species. Chemico-biological interactions. Chem Biol Interact; 2009; doi: 10.1016/j.cbi.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 72.Xiao L, Guo Y, Wang D, Zhao M, Hou X, Li S, et al. Beta-Hydroxysteroid Dehydrogenase Genes in Orange-Spotted Grouper (Epinephelus coioides): Genome-Wide Identification and Expression Analysis During Sex Reversal. Front Genet. 2020; doi: 10.3389/fgene.2020.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan B, Xie D, Li Y, Wang X, Qi X, Li S, et al. A single intronic single nucleotide polymorphism in splicing site of steroidogenic enzyme hsd17b1 is associated with phenotypic sex in oyster pompano, Trachinotus anak. Proc Biol Sci. 2021; doi: 10.1098/rspb.2021.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koyama T, Nakamoto M, Morishima K, Yamashita R, Yamashita T, Sasaki K, et al. A SNP in a Steroidogenic Enzyme Is Associated with Phenotypic Sex in Seriola Fishes. Current Biology. 2019; doi: 10.1016/j.cub.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 75.Purcell CM, Seetharam AS, Snodgrass O, Ortega-García S, Hyde JR, Severin AJ. Insights into teleost sex determination from the Seriola dorsalis genome assembly. BMC Genomics. 2018; doi: 10.1186/s12864-017-4403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-Minded Links Cell Cycle-Related Kinase to Cilia Assembly and Hedgehog Signal Transduction. Developmental Cell. 2010; doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.HUANG C-CJ, YAO HH-C. Diverse Functions of Hedgehog Signaling in Formation and Physiology of Steroidogenic Organs. Mol Reprod Dev. 2010; doi: 10.1002/mrd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guiguen Y, Fostier A, Piferrer F, Chang C-F. Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol. 2010; doi: 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Baroiller JF, Guiguen Y. Endocrine and environmental aspects of sex differentiation in gonochoristic fish. EXS. :177–201 2001; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.