Abstract
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that commonly causes medical hardware, wound, and respiratory infections. Temperate filamentous Pf phages that infect P. aeruginosa impact numerous bacterial virulence phenotypes. Most work on Pf phages has focused on strain Pf4 and its host P. aeruginosa PAO1. Expanding from Pf4 and PAO1, this study explores diverse Pf strains infecting P. aeruginosa clinical isolates. We describe a simple technique targeting the Pf lysogeny maintenance gene, pflM (PA0718), that enables the effective elimination of Pf prophages from diverse P. aeruginosa hosts. This study also assesses the effects different Pf phages have on host quorum sensing, biofilm formation, virulence factor production, and virulence. Collectively, this research not only introduces a valuable tool for Pf prophage elimination from diverse P. aeruginosa isolates, but also advances our understanding of the complex relationship between P. aeruginosa and filamentous Pf phages.
Introduction
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that commonly infects medical hardware, diabetic ulcers, burn wounds, and the airways of cystic fibrosis patients (1). P. aeruginosa isolates are often infected by filamentous viruses (phages) called Pf (2–4). Pf phages live a temperate lifestyle and integrate into the bacterial chromosome as a prophage, passively replicating with each bacterial cell division. When induced, the Pf prophage is excised from the chromosome forming a circular double-stranded episome called the replicative form (5). Pf replicative form copy numbers increase in the cytoplasm where they serve as templates for viral transcription and the production of circular single-stranded DNA genomes that are packaged into filamentous virions as they are extruded from the cell by a process that is analogous to type IV pili assembly (3, 6).
Filamentous Pf virions enhance P. aeruginosa virulence potential by promoting biofilm formation (7) and inhibiting phagocytic uptake by macrophages (8, 9). Pf virions also carry a high negative charge density allowing them to sequester cationic antimicrobials such as aminoglycoside antibiotics and antimicrobial peptides (7, 10, 11). Additionally, Pf phages enhance the virulence potential of P. aeruginosa by modulating the secretion of the quorum-regulated virulence factor pyocyanin (12, 13). These properties may explain why the presence of Pf virions at sites of infection is associated with more chronic lung infections and antibiotic resistance in cystic fibrosis patients (8) and why P. aeruginosa strains cured of their Pf infection are less virulent in murine models of pneumonia (14) and wound infection (15).
Most studies to date have focused on interactions between Pf strain Pf4 and its host P. aeruginosa PAO1. (3, 9, 11, 14). Despite the clear link between Pf4 and the virulence of P. aeruginosa PAO1, the effects diverse Pf strains that infect P. aeruginosa clinical isolates have on virulence phenotypes remains unclear. This is in part due to the significant challenge of ‘curing’ clinical isolates of their Pf prophage infections. Prior efforts to delete Pf4 from PAO1 relied on the integration of a selectable marker into the integration site used by Pf4 (14), which precludes complementation studies that re-introduce the Pf4 prophage to the host chromosome. In prior work, we were able to generate a clean Pf4 deletion strain by first deleting the pfiTA toxin-antitoxin module encoded by Pf4 followed by deletion of the rest of the prophage (16).
Here, we find that the Pf4 gene PA0718 maintains Pf4 in a lysogenic state; we therefore refer to PA0718 as the Pf lysogeny maintenance gene pflM. Deletion of PA0718 or homologous alleles from Pf prophages in clinical P. aeruginosa isolates LESB58, CPA0053, CPA0087, and the multidrug resistant strain DDRC3 resulted in the complete loss of Pf prophages from each strain. Furthermore, we observe that some substrains of PAO1 are lysogenized by two Pf phages, Pf4 and Pf6, and we successfully cured PAO1 of both Pf4 and Pf6 prophages. We compare phenotypic differences between wild-type and ΔPf prophage mutants by assessing Las, Rhl, and PQS quorum sensing activity, biofilm formation, and pyocyanin production. We also examine how Pf prophages impact virulence phenotypes in a Caenorhabditis elegans avoidance model. Overall, we present a new methodology for efficiently curing P. aeruginosa strains of their resident Pf prophages and leverage this tool to gain insight into the diverse impacts Pf phages have on their bacterial hosts.
Results
PA0718 (PflM) maintains Pf4 integration in P. aeruginosa PAO1
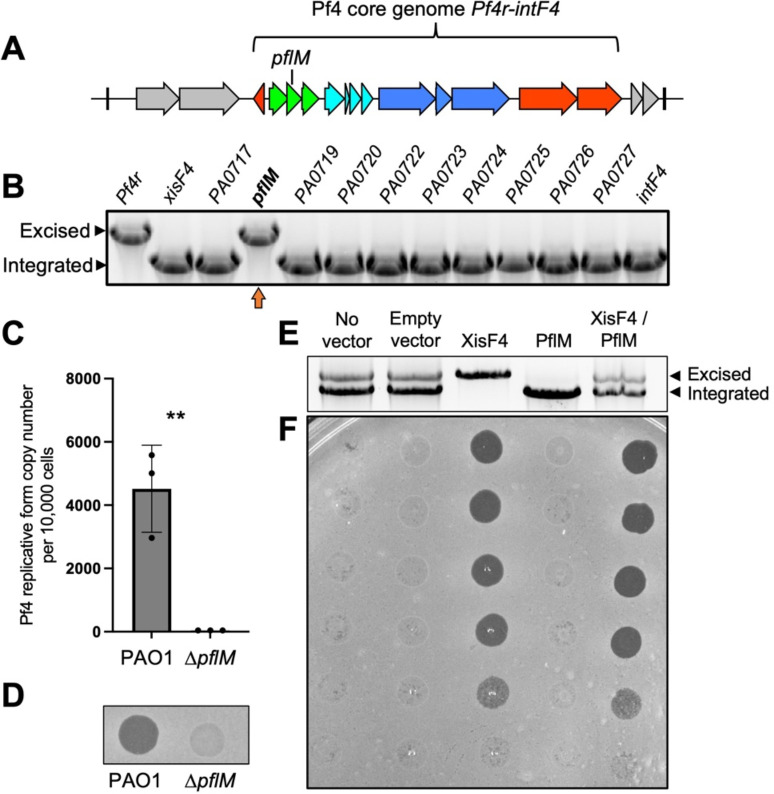
While making single gene deletions from the core Pf4 genome (pf4r-intF4) in P. aeruginosa PAO1 (Fig. 1A), we noted that deleting either the Pf4r repressor or the PA0718 gene results in the complete excision of the Pf4 prophage from the P. aeruginosa chromosome (Fig. 1B, upper bands). Prior work demonstrates that deletion of the Pf4r repressor induces Pf4 prophage excision and virion replication (17), but how PA0718 is involved in Pf4 excision is not known.
Figure 1: PA0718 (PflM) maintains Pf4 lysogeny.
(A) Schematic of the Pf4 prophage (PA0715-PA0729) integrated into the tRNA-Gly gene PA0729.1 in P. aeruginosa PAO1. The core genome that is conserved amongst Pf strains is indicated. (B) Multiplex PCR was used to measure Pf4 prophage integration and excision from the PAO1 chromosome in the indicated Pf4 single-gene mutants. Excision and integration are differentiated by the size of the PCR product produced. Note that that deleting PA0721 (pfsE) from the Pf4 prophage is lethal to P. aeruginosa (16) explaining why pfsE is not included in the assay. A representative gel is shown. (C) Quantitative PCR (qPCR) was used to measure episomal Pf4 replicative form in PAO1 or ΔPA0718 cells after 18 hours of growth in LB broth. Data are the mean of three replicate experiments, ΔPA0718 values were below the limit of detection for the assay (37 copies per microliter of input material). (D) Supernatants obtained from 18 hour-old cultures of PAO1 or ΔPA0718 were spotted onto lawns of P. aeruginosa ΔPf4. A representative image is shown. (E) PflM and/or XisF4 were expressed from inducible plasmids in P. aeruginosa PAO1. After 18 hours of growth in LB broth, Pf4 integration and excision was measured by multiplex PCR. (F) Filtered supernatants collected from the indicated strains were tittered on lawns of PAO1ΔPf4 and imaged after 18 hours of growth.
After excision, Pf4 replicates as a circular episome called the replicative form (5). We used qPCR to measure circular Pf4 replicative form copy number in wild-type and ΔPA0718 cells. In wild-type cells, approximately 4,400 replicative form copies were detected for every 10,000 cells; however, the Pf4 replicative form was not detected in ΔPA0718 cells (Fig. 1C), indicating that Pf4 genome replication is not initiated, and the replicative form is lost as cells divide. Consistently, infectious Pf4 virions are detected in supernatants collected from wild-type cultures but not in supernatants collected from ΔPA0718 (Fig. 1D). These results indicate that PA0718 maintains the Pf4 prophage in a lysogenic state and that deleting PA0718 induces Pf4 prophage excision, but not replication, curing PAO1 of its Pf4 infection. Herein, we refer to PA0718 as the Pf lysogeny maintenance gene pflM.
The observation that 4,400 Pf4 replicative form copies are detected for every 10,000 wild-type cells (Fig. 1C) indicates Pf4 is actively replicating in a subpopulation of cells. We used a multiplex PCR excision assay to measure Pf4 prophage excision and integration in P. aeruginosa populations. In PAO1 populations with no expression vector or those carrying an empty expression vector, both Pf4 prophage integration and excision are observed (Fig. 1E, two bands are present); however, infectious virions were not detected in supernatants by plaque assay (Fig. 1F), suggesting that Pf4 is replicating at low levels during planktonic growth in LB broth, consistent with prior results (18).
The Pf4 excisionase XisF4 regulates Pf4 prophage excision (17) and expressing XisF4 in trans induces complete Pf4 prophage excision (Fig 1E) and robust virion replication (Fig. 1F). In contrast, expressing PflM in trans maintains the entire population in a lysogenic state (Fig. 1E) and virion replication is not detected (Fig. 1F). When PflM and XisF4 are expressed together, both Pf4 integration and excision products are observed (Fig. 1E) and infectious virions are produced at titers comparable to cells where XisF4 was expressed by itself (Fig. 1F). These results indicate that expressing PflM is not sufficient to inhibit XisF4-mediated Pf4 prophage excision and replication, but that PflM can maintain some cells in a lysogenic state during active viral replication.
The targeted deletion of pflM cures diverse P. aeruginosa isolates of their Pf prophages
We hypothesized that deleting pflM would provide a convenient way to cure P. aeruginosa clinical isolates of their Pf prophages. To test this hypothesis, we deleted pflM from the Pf prophages in cystic fibrosis isolate LESB58, two cystic fibrosis isolates from the Stanford Cystic Fibrosis Center (CPA0053 and CPA0087), and the multidrug-resistant urine isolate DDRC3 (Table 1).
Table 1.
P. aeruginosa isolates and Pf prophage characteristics
| Strain | Accession | Source | Pf name / lineage | Pf integration site | Pf prophage length (kb) |
|---|---|---|---|---|---|
| PAO1 | GCF_000006765.1 | Lab strain | Pf4 / I | tRNA-Gly | 12.4 |
| Pf6 / I | tRNA-Met | 12.1 | |||
| LESB58 | FM209186.1 | CF isolate, Liverpool, United Kingdom | Pf-LESB58 / II | Direct repeat | 10.5 |
| CPA0053 | CP137561 | CF isolate, Stanford, CA, USA | Pf-CPA0053/ II | Direct repeat | 10.4 |
| CPA0087 | CP137562 | CF isolate, Stanford, CA, USA | Pf-CPA0087/ II | tRNA-Gly | 11.1 |
| DDRC3 | CP137563 | Urine isolate, Trivandrum, Kerala, India | Pf-DDRC3/ II | tRNA-Gly | 15.5 |
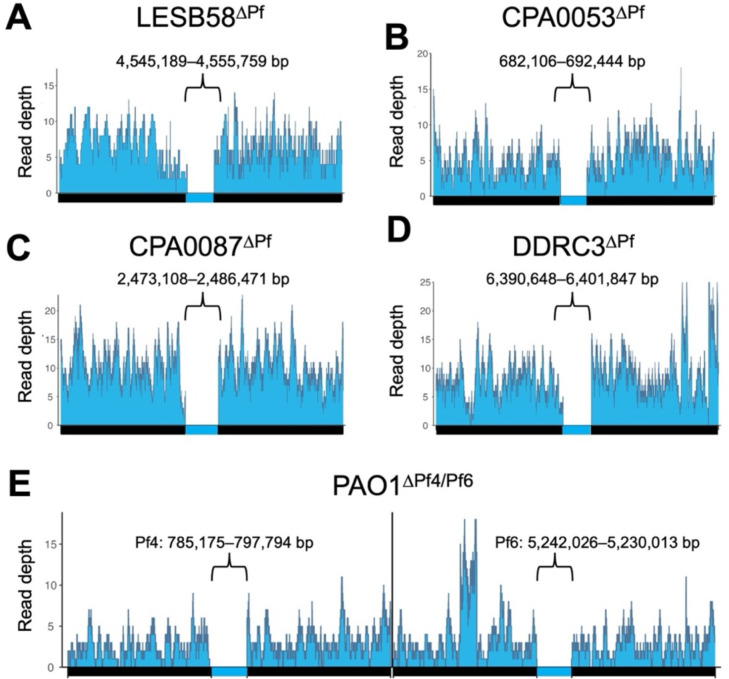
Pf prophage loss was confirmed by long-read whole-genome sequencing. Targeting pflM successfully cured all the above clinical P. aeruginosa isolates of their Pf prophages (Fig. 2A–D). Of the Pf prophages we deleted, four were integrated into tRNA genes (three in tRNA-Gly and one in tRNA-Met) and two were integrated into direct repeats (Table 1). Further, Pf prophages fall into two main lineages (I and II, Table 1) (4) and we were successful in deleting representatives from each lineage. These observations indicate integration site nor lineage have no influence on pflM-mediated Pf prophage deletion.
Figure 2: Targeted deletion of pflM cures diverse P. aeruginosa isolates of their Pf prophage infections.
(A-E) Long-read whole genome sequencing was used to confirm the successful deletion of the indicated Pf prophages. Reads were aligned to 50kb sequences flanking the Pf prophage insertion sites in the parental chromosome. The genomic coordinates for each Pf prophage are shown above each bracket.
Many P. aeruginosa strains are infected by one or more Pf prophages (3). For example, some P. aeruginosa PAO1 sub-isolates are infected by Pf4 and Pf6 (19). Deleting pflM from Pf4 results in the loss of the Pf4 prophage, as does deleting pflM from Pf6 (Fig .2A, B, Fig. S1). Furthermore, we were able to delete Pf6 from ΔPf4, producing a PAO1ΔPf4/Pf6 double mutant (Fig 2E). This observation indicates that pflM from one Pf prophage is specific to that prophage and does not compensate for the loss of pflM from another Pf prophage residing in the same host.
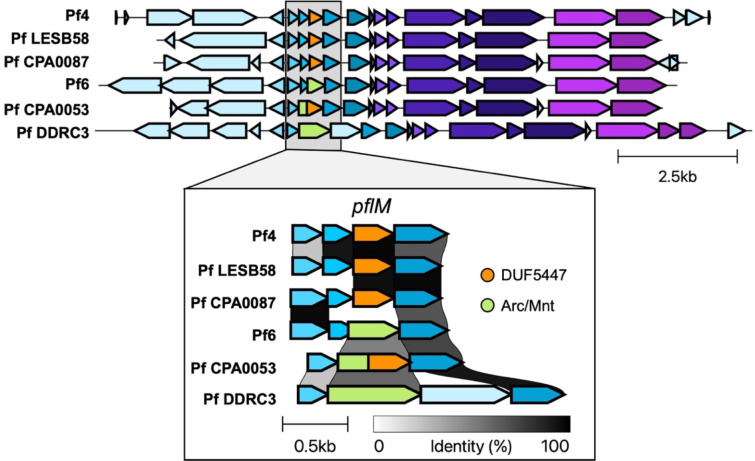
PflM specificity may be explained by the diversity in the operon encoding pflM. In Pf4, pflM is truncated by a 5’ insertion of PA0717 (4) (Fig. 3). The truncated pflM allele in Pf4, Pf LESB58, and Pf CPA0053 contain a predicted DUF5447 domain (pfam17525) whereas the pflM allele in other Pf strains, such as Pf6, Pf CPA0053, and Pf DDRC3, contain an additional Arc/Mnt domain (Fig. 3). Arc/Mnt proteins encoded by Salmonella phage P22 govern lysis-lysogeny decisions by binding phage operator sequences (20, 21), suggesting PflM may regulate Pf lysis-lysogeny decisions by a similar mechanism.
Figure 3.
Variation in the operon encoding pflM in Pf prophages.
Pf phages differentially modulate host quorum sensing
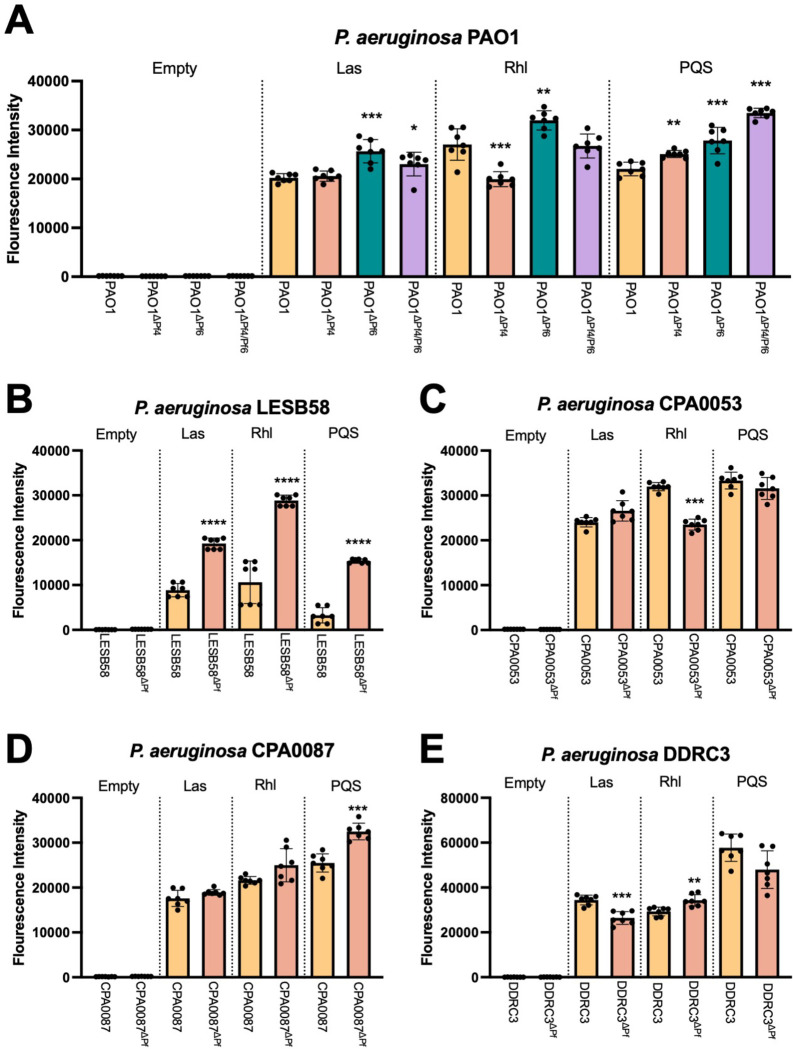
Pf4 is known to suppress PQS and Rhl quorum sensing in P. aeruginosa PAO1 (12, 13, 22). We hypothesized that Pf phages would likewise modulate quorum sensing in the Pf deletion strains we constructed here. To test this, we used fluorescent transcriptional reporters (12) to measure Las (rsaL), Rhl (rhlA), and PQS (pqsA) transcriptional activity in parental strains and Pf mutants.
We find that differences in quorum sensing activity vary by Pf strain and host. In PAO1ΔPf4, Las signaling is not significantly affected, Rhl transcription is downregulated, and PQS is upregulated (Fig. 4A). Pf6 differentially affects host quorum sensing—Las, Rhl, and PQS signaling are all upregulated in PAO1ΔPf6 compared to the parental strain (Fig. 4A). Deleting both Pf4 and Pf6 had no significant impact on Las or Rhl signaling, but PQS signaling was significantly upregulated in PAO1ΔPf4/Pf6 compared to the parental strain (Fig. 4A).
Figure 4: Pf phage differentially modulate P. aeruginosa quorum sensing.
(A-E) GFP fluorescence from the transcriptional reporters PrsaLI-gfp (Las), PrhlA-gfp (Rhl), or PpqsA-gfp (PQS) was measured in the indicated strains after 18 hours of growth. GFP fluorescence intensity was normalized to cell growth (OD600). Data are the mean ±SEM of seven replicates. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Student’s t-test comparing ΔPf strains to the wild-type parent.
PQS transcriptional activity is also significantly (P<0.0001) upregulated in LESB58ΔPf as are Las and Rhl (Fig 4B). In strain CPA0053, Las and PQS signaling is not significantly affected while Rhl transcription is reduced when the Pf prophage is deleted (Fig. 4C). PQS is activated in CPA0087ΔPf while Las and Rhl signaling is not significantly affected (Fig. 4D). Finally, in DDRC3ΔPf, Las is downregulated, Rhl signaling is upregulated, and PQS signaling is not significantly affected compared to the parental DDRC3 strain but is trending downward (Fig. 4E). Taken together, these data indicate that Pf phages have diverse and complex relationships with host quorum sensing systems that vary significantly by strain.
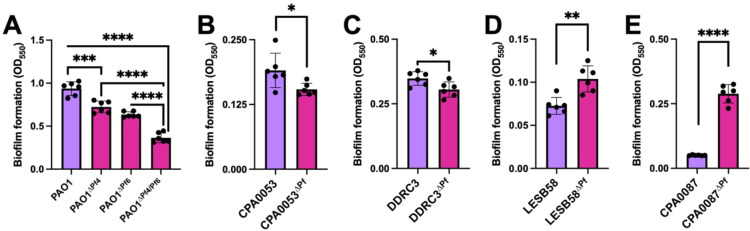
Pf phages have contrasting impacts on P. aeruginosa biofilm formation
Pf4 is known to promote P. aeruginosa PAO1 biofilm assembly and function (3, 5, 7, 11, 14, 23, 24). To test if other strains of Pf affect biofilm formation, we used the crystal violet biofilm assay (25) to measure biofilm formation of lysogenized P. aeruginosa isolates compared to the Pf prophage deletion mutants. In PAO1, deletion of either Pf4 or Pf6 significantly (P<0.001) reduce biofilm formation by 1.79- and 2.33-fold, respectively, while deletion of both Pf4 and Pf6 reduces biofilm formation by 7.14-fold (Fig. 5A). This result indicates both Pf4 and Pf6 contribute to PAO1 biofilm formation, which is consistent with prior observations (5, 7, 11, 14, 23, 24). The clinical isolates in general did not form as robust biofilms as the PAO1 laboratory strain under the in vitro conditions tested. Even so, deleting the Pf prophage from strains CPA0053 and DDRC3 modestly but significantly (P<0.05) reduced biofilm formation (Fig. 5B and C). In contrast, biofilm formation was significantly (P<0.01) increased in strains LESB58ΔPf and CPA0087ΔPf compared to the parental strains (Fig. 5D and E). The variation in biofilm formation phenotypes is perhaps not surprising given the variation in quorum sensing regulation between Pf lysogens and their corresponding Pf prophage mutants (Fig 4).
Figure 5: Pf prophage deletion has significant but variable effects on P. aeruginosa biofilm formation.
Crystal violet biofilm assays were performed to measure biofilm formation of the indicated strains after 48h incubation. Data are the mean ±SEM of six replicates. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Student’s t-test.
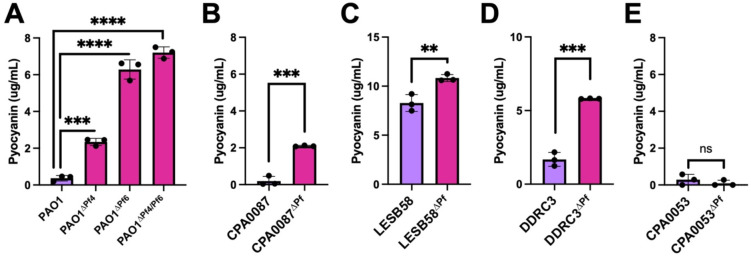
Pf phages suppress P. aeruginosa pyocyanin production
Pyocyanin is a redox-active quorum-regulated virulence factor (26). Deleting the Pf4 prophage from PAO1 enhances pyocyanin production (27). We observed increased pyocyanin production in all ΔPf strains tested except CPA0053, which did not produce much pyocyanin under any condition tested (Fig. 6A–E). These results suggest that Pf prophages encode gene(s) that inhibit host pyocyanin production, which is consistent with recent work indicating that the PfsE protein encoded by Pf phages inhibits PQS signaling by binding to and inhibiting PqsA (13).
Figure 6: Pyocyanin production is enhanced in Pf prophage deletion strains.
(A-E) Pyocyanin was CHCl3-HCl extracted from the supernatants of the indicated cultures after 18h of incubation. Pyocyanin concentration was measured (Abs 520nm). Data are the mean ±SEM of three replicates. **P<0.01, ***P<0.001, ****P<0.0001, Student’s t-test.
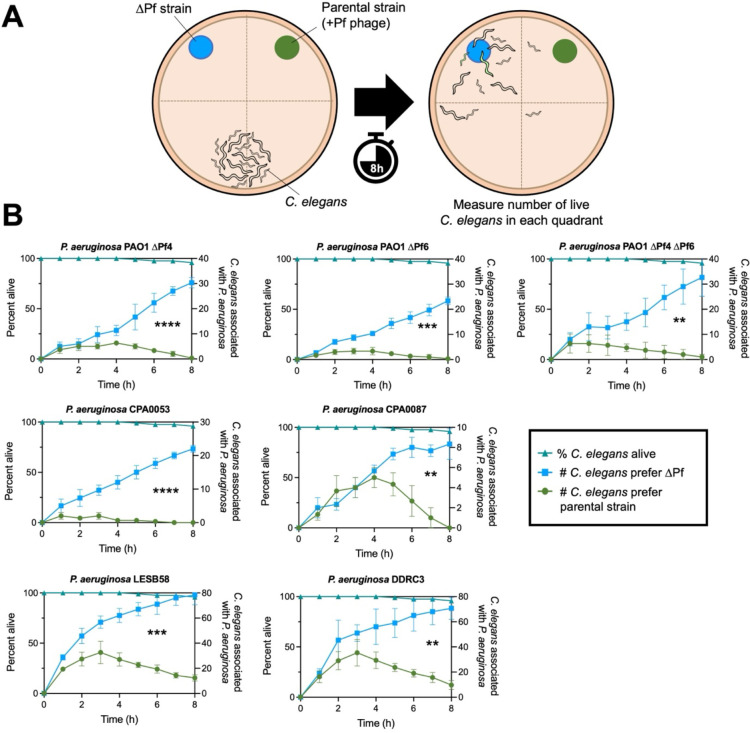
Pf phages induce avoidance behavior in bacterivorous nematodes
In the environment, bacterivores impose high selective pressures on bacteria (28, 29). Pf4 modulation of quorum-regulated virulence factors increases P. aeruginosa fitness against the bacterivorous nematode Caenorhabditis elegans (12). We hypothesized that Pf prophages in P. aeruginosa clinical isolates would similarly protect P. aeruginosa from predation by C. elegans. To test this, we employed C. elegans avoidance assays (30–33) as a metric of bacterial fitness when confronted with nematode predation (Fig. 7A). C. elegans avoided all Pf lysogens, preferring to associate with the ΔPf strains in every case (Fig. 7B). Note that nematode survival was over 95% over the course of the experiment (8 hours) in all experiments (Fig. 7B, triangles). Collectively, our results suggest that Pf modulates P. aeruginosa virulence phenotypes in ways that repel C. elegans.
Figure 7: C. elegans actively avoids P. aeruginosa Pf lysogens.
(A) Experimental design: P. aeruginosa and an isogenic ΔPf mutant were spotted onto NMMG plates with wild-type N2 C. elegans at the indicated locations. C. elegans localization to the indicated quadrants was measured hourly. (B) C. elegans association with P. aeruginosa (circles) or isogenic ΔPf mutants (squares) in the indicated strain backgrounds was measured hourly over eight hours (three experiments with N=30 per replicate [90 animals total]). P values were calculated by two-way ANOVA comparing ΔPf strains to the parental strains using the Šidák correction (95% CI threshold), **P<0.01, ***P<0.001, ****P<0.0001.
Discussion
This study describes a convenient method to cure P. aeruginosa isolates of their Pf prophage infections and explores relationships between diverse Pf phages and their P. aeruginosa hosts. Overall, different Pf strains exhibit varying effects on host quorum sensing and biofilm formation. One commonality between all Pf strains examined is their ability to suppress pyocyanin production and repel C. elegans away from P. aeruginosa, protecting their host from predation.
Our results indicate that PflM maintains Pf in a lysogenic state and that deleting the pflM gene induces Pf prophage excision, but not replication. In Pf4, the site-specific tyrosine recombinase IntF4 catalyzes Pf4 prophage integration into and excision from the chromosome while the Pf4 excisionase XisF4 regulates Pf4 prophage excision by promoting interactions between IntF4 and Pf4 attachment sites as well as inducing the expression of the replication initiation protein PA0727 (4, 17). In response to stimuli such as oxidative stress (34), these coordinated events induce Pf4 prophage excision and initiate episomal replication, allowing Pf4 to complete its lifecycle.
While it is presently not known how PflM maintains Pf lysogeny, it is possible that PflM promotes the integrase activity of IntF or inhibits XisF-mediated excision, causing the Pf prophage to excise from the chromosome without concurrently inducing the replication initiation protein PA0727 (6), thus resulting in Pf prophage excision without initiating episomal Pf replication.
Our study highlights a role for Pf phages in manipulating P. aeruginosa quorum sensing. Pf phages have varying effects on host quorum sensing; broadly, we determine that Pf phage modulate quorum sensing activity and quorum-regulated phenotypes in all strains tested. These findings imply that different Pf strains interact with host quorum sensing networks in diverse ways, indicating a complex interplay between Pf phages and host regulatory systems.
Quorum sensing regulates P. aeruginosa biofilm formation and Pf4 contributes to biofilm formation in PAO1 (5, 7, 14, 18, 35). Consistently, we find that both Pf4 and Pf6 contribute to PAO1 biofilm formation. Interestingly, the impact of Pf prophage deletion on biofilm formation varies among clinical isolates, which may be related to different quorum sensing hierarchies present in clinical P. aeruginosa isolates (36).
Despite differences in interactions between Pf phages and host quorum sensing, deleting Pf prophages from the host chromosome enhances pyocyanin production in all strains tested except for strain CPA0053, which produces low levels of pyocyanin compared to all other strains tested. As pyocyanin is the terminal signaling molecule in P. aeruginosa quorum sensing networks (26), these results suggest that inhibition of pyocyanin production is the ultimate goal of Pf phages and inhibition of pyocyanin production may be beneficial to Pf phages during active replication. Pyocyanin and other redox-active phenazines are toxic to bacteria; it is possible that stress responses that are induced by pyocyanin-producing P. aeruginosa are detrimental to Pf replication.
We recently discovered that Pf phages encode a protein called PfsE (PA0721) that inhibits PQS signaling by binding to the anthranilate-coenzyme A ligase PqsA and that this results in enhanced Pf replication (13). It is possible that the loss of PfsE in the ΔPf strains in this study is responsible for the observed increase in pyocyanin production.
Pf lysogens induce avoidance behavior by C. elegans, which prefers to associate with the ΔPf strains. Strikingly, although strains lacking Pf prophages are less virulent in a nematode infection model, the reduced virulence of ΔPf strains contrasts with their high pyocyanin virulence factor production. This discrepancy may be partly explained by our prior findings that Pf4 suppresses pyocyanin and other bacterial pigment production as a means to avoid detection by innate host immune responses (12) that are regulated by the aryl hydrocarbon receptor (37, 38).
In summary, this research reveals the crucial role of the PflM gene in maintaining Pf lysogeny, demonstrates strain-specific effects on quorum sensing and biofilm formation, reveals the consistent inhibition of pyocyanin production by Pf phages, and suggests a role for Pf phages in protecting P. aeruginosa against nematode predation.
Materials and Methods
Strains, plasmids, primers, and growth conditions
Strains, plasmids, and their sources are listed in Table 2. Unless otherwise indicated, bacteria were grown in lysogeny broth (LB) at 37 °C with 230 rpm shaking and supplemented with gentamicin (Sigma) where appropriate, at either 10 or 30 μg ml−1.
Table 2.
Strains and plasmids used in this study.
| Strain | Description | Source |
|---|---|---|
| Escherichia coli | ||
| DH5α | Cloning strain | New England Biolabs |
| S17 | Donor strain | (39) |
| OP50 | C. elegans food source | PMID 4366476 |
| P. aeruginosa | ||
| PAO1 | Wild type | (14) |
| PAO1 ΔPf4 | Deletion of Pf4 prophage from PAO1 | This study |
| PAO1 ΔPf4 ΔPf6 | Deletion of Pf4 and Pf6 prophage from PAO1 | This study |
| LES B58 | Liverpool Epidemic strain B58 | (40) |
| LES B58 ΔPf | Deletion of Pf prophage from LESB58 | This study |
| CPA0053 | CF clinical isolate | Gift from Paul Bollyky, Stanford University |
| CPA0053 ΔPf | Deletion of Pf prophage from CPA0053 | This study |
| CPA0087 | CF clinical isolate | Gift from Paul Bollyky, Stanford University |
| CPA0087 ΔPf | Deletion of Pf prophage from CPA0087 | This study |
| DDRC3 | MDR clinical isolate | Gift from Geetha Kumar, Amrita University |
| DDRC ΔPf | Deletion of Pf prophage from DDRC3 | This study |
| C. elegans | ||
| N2 | Wild type | Caenorhabditis Genetic Center |
| Plasmids | ||
| CP59 pBBR1-MCS5 rsaL-gfp | GFP lasI transcriptional reporter | (41) |
| CP57 pBBR1-MCS5 rhlA-gfp | GFP rhlA transcriptional reporter | (41) |
| CP53 pBBR1-MCS5 pqsA-gfp | GFP pqsA transcriptional reporter | (42) |
| CP1 pBBR-MCS5- Empty | GFP empty vector control | (41) |
| pHERD30T - pflM | Expression vector with pflM insert | This study |
| pHERD20T – xisF4 | Expression vector with xisF4 insert | (16) |
| pENTRpEX18-Gm:ΔPA0718 | Allelic exchange vector for the deletion of pflM | (43) |
| pLM61 | pENTR221L1L2-RFqPCRstandard | This study |
| pUC57-rplU | qPCR standard for rplU | (44) |
Construction of Deletion Mutants
We used allelic exchange to delete alleles from P. aeruginosa (43). Briefly, to delete pflM (PA0718) upstream and downstream homologous sequences (~500bp) were amplified through PCR from PAO1 genomic DNA using the UP and DOWN primers listed in Table 3. These amplicons were then ligated through splicing-by-overlap extension (SOE)-PCR to construct a contiguous deletion allele. This amplicon was then run on a 0.5% agarose gel, gel extracted (New England Biolabs #T3010L), and cloned (Gateway, Invitrogen) into a pENTRpEX18-Gm backbone to produce the deletion construct. The deletion construct was then transformed into DH5a, mini-prepped (New England Biolabs #T1010L), and sequenced (Plasmidsaurus.com). Sequencing-confirmed vectors were then transformed into E. coli S17 Donor cells for biparental mating with the recipient P. aeruginosa strain. Single crossovers were isolated on VBMM agar supplemented with 30 μg/mL gentamicin followed by selection of double crossovers on no salt sucrose. The final obtained mutants were confirmed by excision assay (see below), Sanger sequencing of excision assay products, and whole genome sequencing.
Table 3.
Primers used in this study.
| Name | Tm° (C) | Sequence |
|---|---|---|
| Construction of pENTRpEX18-Gm:ΔPA0718 | ||
| ΔpflM UPattB1-Fwd | 62.1 | ggggacaagtttgtacaaaaaagcaggcttcCTAATGCCACGAATAGTGACGG |
| ΔpflM UP-Rev | 65.3 | TCAGCCCTCCAGTTGGAATGCGTAGGGACTGGCGGCCAT |
| ΔpflM DOWN-Fwd | 63.2 | GCATTCCAACTGGAGGGCTGA |
| ΔpflM DOWNattB2-Rev | 62.1 | ggggaccactttgtacaagaaagctgggtaAAAGTGATTTGTCGGGCGATCC |
| ΔpflM Seq-Fwd | 57.5 | TTTTTGGGGCCGATTTTCTTG |
| ΔpflM Seq-Rev | 56.3 | ATTGGACCGAGGCGTGA |
| Quantitative PCR | ||
| RF-Fwd | 60.5 | TAGGCATTTCAGGGGCTTGG |
| RF-Rev | 62.5 | GAGCTACGGAGTAAGACGCC |
| rplU-Fwd | 52.4 | CAAGGTCCGCATCATCAAGTT |
| rplU-Rev | 52.6 | GGCCCTGACGCTTCATGT |
| Prophage Mutant Screening | ||
| 16SRNA-F | 59.5 | TGGTTCAGCAAGTTGGATGTG |
| 16SRNA-R | 59.5 | GTTTGCTCCCCACGCTTTC |
| pfsE-F | 56.3 | ATGCTCCGCTATCTCTCG |
| pfsE-R | 58.4 | TCAAACAGCCAGGGAGGC |
| Excision Assays | ||
| Pf4-Fwd1 | 62.5 | GGATATGGAGCGTGGTGGAG |
| Pf4-Fwd2 | 59.9 | AGTGGCGGTTATCGGATGAC |
| Pf4-Rev | 61.4 | TCATTGGGAGGCGCTTTCAT |
| Pf6-Fwd1 | 60.5 | GTGATCCACGTGTCCAACAG |
| Pf6-Fwd2 | 60.5 | CCCAGTGCAGATGACTTGGT |
| Pf6-Rev | 60.5 | CGCCACTGGTCATTGATCCT |
| LES B58-Fwd1 | 59.8 | AGCGACAGCCGCCAGCA |
| LES B58-Fwd2 | 61.6 | GCTTGCCGAACTGCTGGTG |
| LES B58-Rev | 62.5 | CGGGTTTCGTCGGTCATCAC |
| CPA0053-Fwd1 | 52.8 | GCAGGTCGAGGTAGTAG |
| CPA0053-Fwd2 | 60 | TTCGTCGCTGAACATGACCA |
| CPA0053-Rev | 51.6 | CCTCGATCATGTTGAAGT |
| CPA0087-Fwd1 | 52.8 | GCAGGTCGAGGTAGTAG |
| CPA0087-Fwd2 | 60 | TTCGTCGCTGAACATGACCA |
| CPA0087-Rev | 51.6 | CCTCGATCATGTTGAAGT |
| DDRC3gly-Fwd1 | 60.1 | GCTTTCTACTCCTGAGCATGTA |
| DDRC3gly-Fwd2 | 59.8 | CGCTGCGGAACACCGTG |
| DDRC3gly-Rev | 59.5 | ACCGTGAAGTACCTGCAGC |
Excision Assays
Excision assays were designed as described previously (45). Briefly, a multiplex PCR assay was designed to produce amplicons of distinct sizes if the Pf prophage was integrated (primers Fwd_1 and Rev produce a smaller band) or excised (primers Fwd_2 and Rev produce a larger band) using Phusion Plus PCR Mastermix (Thermo Scientific # F631L). Primers were used at a final concentration of 0.5μM and are listed in (Table 3).
Plaque assays
Plaque assays were performed using ΔPf4 as the indicator strain grown on LB plates. Phage in filtered supernatants were serially diluted 10x in PBS and spotted onto lawns of PAO1ΔPf4. Plaques were imaged after 18h of growth at 37 °C. PFUs/mL were then calculated.
Quantitative PCR (qPCR)
Cultures were grown overnight in LB broth with shaking at 37°C. Following 18h incubation, cultures were pelleted at 16,000xg for 5 minutes, washed 3x in 1X PBS, and treated with DNase at a final concentration of 0.1 mg/mL. qPCR was performed using SsoAdvanced Universal SYBR Green Supermix (BioRad #1725270) on the BioRad CFX Duet. For the standard curves, the sequence targeted by the primers were inserted into vectors pLM61 and pUC57-rplU, respectively, and 10-fold serial dilutions of the standard were used in the qPCR reactions with the appropriate primers (Table 3) to construct standard curves. Normalization to chromosomal copy number was performed as previously described (44) using 50S ribosomal protein gene rpIU.
Pyocyanin extraction and measurement
Pyocyanin was measured as previously described (46, 47). Briefly, 18-hour cultures were treated with chloroform at 50% vol/vol. Samples were vortexed vigorously and the organic phase separated by centrifuging samples at 6,000×g for 5 minutes. The chloroform layer was removed to a fresh tube and 20% the volume of 0.1 N HCl was added and the mixture vortexed vigorously. Once separated, the aqueous fraction was aliquoted to a 96-well plate and absorbance measured at 520 nm. The concentration of pyocyanin, expressed as μg/ml, was obtained by multiplying the OD520 nm by 17.072, as described previously (47).
Quorum sensing reporters
Competent P. aeruginosa cells were prepared by washing overnight cultures in 300 mM sucrose followed by transformation by electroporation (48) with the plasmids CP1 PBBR-MCS5 Empty, CP53 PBBR1-MCS5 pqsA-gfp, CP57 PBBR1-MCS5 rhlA-gfp, CP59 PBBR1-MCS5 rsaL-gfp listed in (Table 2). Transformants were selected by plating on the appropriate antibiotic selection media. The indicated strains were grown in buffered LB containing 50 mM MOPS and 100 μg ml−1 gentamicin for 18 hours. Cultures were then sub-cultured 1:100 into fresh LB MOPS buffer and grown to an OD600 of 0.3. To measure reporter fluorescence, each strain was added to a 96-well plate containing 200 μL LB MOPS with a final bacterial density of OD600 0.1 and incubated at 37°C in a CLARIOstar BMG LABTECH plate reader. Prior to each measurement, plates were shaken at 230 rpm for a duration of two minutes. A measurement was taken every 15 minutes for both growth (OD600) or fluorescence (excitation at 485–15 nm and emission at 535–15 nm). End-point measurements at 18h were normalized to cell density.
C. elegans Growth Conditions
Synchronized adult N2 C. elegans were propagated on Normal Nematode Growth Medium (NNGM) agar plates with E. coli OP50 as a food source.
C. elegans Avoidance Assays
C. elegans avoidance assays were performed as previously described (32). Briefly, synchronized adult N2 worms were propagated at 24°C on 3.5 cm NNGM agar plates with E. coli OP50 for 48h, collected, and washed 4x to remove residual OP50. NNGM agar was spotted with 20 μL of P. aeruginosa (Pf lysogens and their isogenic ΔPf mutant) overnight cultures (LB broth) as shown in Fig 7A and grown for 18 hours at 37°C. Worms were plated in in triplicate and incubated at 24°C. C. elegans migration was monitored hourly for 8h.
Whole Genome Sequencing & Annotation
Whole genome sequencing was performed by Plasmidsaurus. Reads were filtered using Filtlong (v0.2.1), assembled using Flye (v2.9.1) and/or Velvet (v7.0.4). Contigs polished using Medaka (v1.8.0), and annotated using Bakta (v1.6.1), Bandage (v0.8.1), and RAST (https://rast.nmpdr.org/). Domain analysis was performed using PfamScan (https://www.ebi.ac.uk/Tools/pfa/pfamscan/) against the library of Pfam HMM using an e-value cutoff of 0.01. Supporting domain models were obtained from Conserved Domain Database, and Defense Finder (49). Raw sequencing reads and assemblies for parental strains introduced in this study (Table 2) have been deposited as part of BioProject PRJNA1031220 in the NCBI SRA database.
Statistical analyses
Differences between data sets were evaluated with a Student’s t-test (unpaired, two-tailed), or two-way ANOVA using the Šidák correction (95% CI threshold) where appropriate. P values of < 0.05 were considered statistically significant. GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, CA) was used for all analyses.
Supplementary Material
Importance.
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that is frequently infected by filamentous Pf phages (viruses) that integrate into its chromosome, affecting behavior. While prior work has focused on Pf4 and PAO1, this study investigates diverse Pf strains in clinical isolates. A simple method targeting the deletion of the Pf lysogeny maintenance gene pflM (PA0718) effectively eliminates Pf prophages from clinical isolates. The research evaluates the impact Pf prophages have on bacterial quorum sensing, biofilm formation, and virulence phenotypes. This work introduces a valuable tool to eliminate Pf prophages from clinical isolates and advances our understanding of P. aeruginosa and filamentous Pf phage interactions.
Acknowledgements
This work was supported by NIH grants R01AI138981 and P20GM103546 to PRS. DRF was supported by NSF GRFP grant 366502. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest.
References
- 1.Hauser AR, Jain M, Bar-Meir M, McColley SA. 2011. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev 24:29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knezevic P, Voet M, Lavigne R. 2015. Prevalence of Pf1-like (pro)phage genetic elements among Pseudomonas aeruginosa isolates. Virology 483:64–71. [DOI] [PubMed] [Google Scholar]
- 3.Secor PR, Burgener EB, Kinnersley M, Jennings LK, Roman-Cruz V, Popescu M, Van Belleghem JD, Haddock N, Copeland C, Michaels LA, de Vries CR, Chen Q, Pourtois J, Wheeler TJ, Milla CE, Bollyky PL. 2020. Pf Bacteriophage and Their Impact on Pseudomonas Virulence, Mammalian Immunity, and Chronic Infections. Front Immunol 11:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiedoruk K, Zakrzewska M, Daniluk T, Piktel E, Chmielewska S, Bucki R. 2020. Two Lineages of Pseudomonas aeruginosa Filamentous Phages: Structural Uniformity over Integration Preferences. Genome Biol Evol 12:1765–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb JS, Lau M, Kjelleberg S. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. Journal of bacteriology 186:8066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez E, Campos-Gomez J. 2016. Pf Filamentous Phage Requires UvrD for Replication in Pseudomonas aeruginosa. mSphere 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secor PR, Sweere JM, Michaels LA, Malkovskiy AV, Lazzareschi D, Katznelson E, Rajadas J, Birnbaum ME, Arrigoni A, Braun KR, Evanko SP, Stevens DA, Kaminsky W, Singh PK, Parks WC, Bollyky PL. 2015. Filamentous Bacteriophage Promote Biofilm Assembly and Function. Cell Host Microbe 18:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgener EB, Sweere JM, Bach MS, Secor PR, Haddock N, Jennings LK, Marvig RL, Johansen HK, Rossi E, Cao X, Tian L, Nedelec L, Molin S, Bollyky PL, Milla CE. 2019. Filamentous bacteriophages are associated with chronic Pseudomonas lung infections and antibiotic resistance in cystic fibrosis. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secor PR, Michaels LA, Smigiel KS, Rohani MG, Jennings LK, Hisert KB, Arrigoni A, Braun KR, Birkland TP, Lai Y, Hallstrand TS, Bollyky PL, Singh PK, Parks WC. 2017. Filamentous Bacteriophage Produced by Pseudomonas aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection In Vivo. Infect Immun 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janmey PA, Slochower DR, Wang YH, Wen Q, Cebers A. 2014. Polyelectrolyte properties of filamentous biopolymers and their consequences in biological fluids. Soft Matter 10:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarafder AK, von Kugelgen A, Mellul AJ, Schulze U, Aarts D, Bharat TAM. 2020. Phage liquid crystalline droplets form occlusive sheaths that encapsulate and protect infectious rod-shaped bacteria. Proc Natl Acad Sci U S A 117:4724–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartzkopf CM, Robinson AJ, Ellenbecker M, Faith DR, Schmidt AK, Brooks DM, Lewerke L, Voronina E, Dandekar AA, Secor PR. 2023. Tripartite interactions between filamentous Pf4 bacteriophage, Pseudomonas aeruginosa, and bacterivorous nematodes. PLoS Pathog 19:e1010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartzkopf CM, Taylor VL, Groleau MC, Faith DR, Schmidt AK, Lamma TL, Brooks DM, Deziel E, Maxwell KL, Secor PR. 2023. Inhibition of PQS signaling by the Pf bacteriophage protein PfsE enhances viral replication in Pseudomonas aeruginosa. bioRxiv doi: 10.1101/2023.08.25.554831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Tay M, Hauser A, McDougald D, Webb JS, Kjelleberg S. 2009. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. The ISME journal 3:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, Kaber G, Manasherob R, Suh GA, Cao X, de Vries CR, Lam DN, Marshall PL, Birukova M, Katznelson E, Lazzareschi DV, Balaji S, Keswani SG, Hawn TR, Secor PR, Bollyky PL. 2019. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt AK, Fitzpatrick AD, Schwartzkopf CM, Faith DR, Jennings LK, Coluccio A, Hunt DJ, Michaels LA, Hargil A, Chen Q, Bollyky PL, Dorward DW, Wachter J, Rosa PA, Maxwell KL, Secor PR. 2022. A Filamentous Bacteriophage Protein Inhibits Type IV Pili To Prevent Superinfection of Pseudomonas aeruginosa. mBio doi: 10.1128/mbio.02441-21:e0244121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Liu X, Tang K, Wang P, Zeng Z, Guo Y, Wang X. 2019. Excisionase in Pf filamentous prophage controls lysis-lysogeny decision-making in Pseudomonas aeruginosa. Mol Microbiol 111:495–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Secor PR, Sass G, Nazik H, Stevens DA. 2017. Effect of acute predation with bacteriophage on intermicrobial aggression by Pseudomonas aeruginosa. PLoS One 12:e0179659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, Larbig KD, Heeb S, Schock U, Pohl TM, Wiehlmann L, Tummler B. 2010. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol 192:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breg JN, van Opheusden JH, Burgering MJ, Boelens R, Kaptein R. 1990. Structure of Arc repressor in solution: evidence for a family of beta-sheet DNA-binding proteins. Nature 346:586–9. [DOI] [PubMed] [Google Scholar]
- 21.Vershon AK, Youderian P, Susskind MM, Sauer RT. 1985. The bacteriophage P22 arc and mnt repressors. Overproduction, purification, and properties. J Biol Chem 260:12124–9. [PubMed] [Google Scholar]
- 22.Tortuel D, Tahrioui A, David A, Cambronel M, Nilly F, Clamens T, Maillot O, Barreau M, Feuilloley MGJ, Lesouhaitier O, Filloux A, Bouffartigues E, Cornelis P, Chevalier S. 2022. Pf4 Phage Variant Infection Reduces Virulence-Associated Traits in Pseudomonas aeruginosa. Microbiology Spectrum 10:e01548–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secor PR, Jennings LK, Michaels LA, Sweere JM, Singh PK, Parks WC, Bollyky PL. 2015. Biofilm assembly becomes crystal clear - filamentous bacteriophage organize the Pseudomonas aeruginosa biofilm matrix into a liquid crystal. Microb Cell 3:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElroy KE, Hui JG, Woo JK, Luk AW, Webb JS, Kjelleberg S, Rice SA, Thomas. 2014. Strain-specific parallel evolution drives short-term diversification during Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences of the United States of America 111:E1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Toole GA. 2011. Microtiter dish biofilm formation assay. Journal of visualized experiments : JoVE doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–21. [DOI] [PubMed] [Google Scholar]
- 27.Schwartzkopf CM, Robinson AJ, Ellenbecker M, Faith DR, Schmidt AK, Brooks DM, Lewerke L, Voronina E, Dandekar AA, Secor PR. 2023. Tripartite interactions between filamentous Pf4 bacteriophage, Pseudomonas aeruginosa, and bacterivorous nematodes. PLOS Pathogens 19:e1010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. 2007. Environmental predators as models for bacterial pathogenesis. Environ Microbiol 9:563–75. [DOI] [PubMed] [Google Scholar]
- 29.Schulenburg H, Felix MA. 2017. The Natural Biotic Environment of Caenorhabditis elegans. Genetics 206:55–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filipowicz A, Lalsiamthara J, Aballay A. 2021. TRPM channels mediate learned pathogen avoidance following intestinal distention. eLife 10:e65935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaletsky R, Moore RS, Vrla GD, Parsons LR, Gitai Z, Murphy CT. 2020. C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature 586:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh DD, Lee D, Jin X, Horvitz HR, Nitabach MN. 2021. C. elegans discriminates colors to guide foraging. Science 371:1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao Y, Yang W, Ren J, Hall Q, Zhang Y, Kaplan JM. 2018. Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. eLife 7:e36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Q, Minh PN, Dotsch A, Hildebrand F, Panmanee W, Elfarash A, Schulz S, Plaisance S, Charlier D, Hassett D, Haussler S, Cornelis P. 2012. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res 40:4320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mooij MJ, Drenkard E, Llamas MA, Vandenbroucke-Grauls CM, Savelkoul PH, Ausubel FM, Bitter W. 2007. Characterization of the integrated filamentous phage Pf5 and its involvement in small-colony formation. Microbiology 153:1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asfahl KL, Smalley NE, Chang AP, Dandekar AA. 2022. Genetic and Transcriptomic Characteristics of RhlR-Dependent Quorum Sensing in Cystic Fibrosis Isolates of Pseudomonas aeruginosa. mSystems 7:e0011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moura-Alves P, Puyskens A, Stinn A, Klemm M, Guhlich-Bornhof U, Dorhoi A, Furkert J, Kreuchwig A, Protze J, Lozza L, Pei G, Saikali P, Perdomo C, Mollenkopf HJ, Hurwitz R, Kirschhoefer F, Brenner-Weiss G, Weiner J 3rd, Oschkinat H, Kolbe M, Krause G, Kaufmann SHE. 2019. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science 366. [DOI] [PubMed] [Google Scholar]
- 38.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, Skrahina T, Guhlich-Bornhof U, Klemm M, Koehler AB, Bandermann S, Goosmann C, Mollenkopf HJ, Hurwitz R, Brinkmann V, Fillatreau S, Daffe M, Tummler B, Kolbe M, Oschkinat H, Krause G, Kaufmann SH. 2014. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512:387–92. [DOI] [PubMed] [Google Scholar]
- 39.Castang S, Dove SL. 2012. Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. Journal of bacteriology 194:5101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winstanley C, Langille MG, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F, Thomson NR, Winsor GL, Quail MA, Lennard N, Bignell A, Clarke L, Seeger K, Saunders D, Harris D, Parkhill J, Hancock RE, Brinkman FS, Levesque RC. 2009. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome research 19:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feltner JB, Wolter DJ, Pope CE, Groleau MC, Smalley NE, Greenberg EP, Mayer-Hamblett N, Burns J, Deziel E, Hoffman LR, Dandekar AA. 2016. LasR Variant Cystic Fibrosis Isolates Reveal an Adaptable Quorum-Sensing Hierarchy in Pseudomonas aeruginosa. mBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smalley NE, Schaefer AL, Asfahl KL, Perez C, Greenberg EP, Dandekar AA. 2022. Evolution of the Quorum Sensing Regulon in Cooperating Populations of Pseudomonas aeruginosa. mBio 13:e00161–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS, Lin C, Irie Y, Storek KM, Yang JJ, Siehnel RJ, Howell PL, Singh PK, Tolker-Nielsen T, Parsek MR, Schweizer HP, Harrison JJ. 2015. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nature protocols 10:1820–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgener EB, Secor PR, Tracy MC, Sweere JM, Bik EM, Milla CE, Bollyky PL. 2020. Methods for Extraction and Detection of Pf Bacteriophage DNA from the Sputum of Patients with Cystic Fibrosis. Phage: Therapy, Applications, and Research 1:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKitterick AC, Seed KD. 2018. Anti-phage islands force their target phage to directly mediate island excision and spread. Nat Commun 9:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiedoruk K, Zakrzewska M, Daniluk T, Piktel E, Chmielewska S, Bucki R. 2020. Two Lineages of Pseudomonas aeruginosa Filamentous Phages: Structural Uniformity over Integration Preferences. Genome Biology and Evolution 12:1765–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi K-H, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: Application for DNA fragment transfer between chromosomes and plasmid transformation. Journal of Microbiological Methods 64:391–397. [DOI] [PubMed] [Google Scholar]
- 49.Tesson F, Hervé A, Mordret E, Touchon M, d’Humières C, Cury J, Bernheim A. 2022. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nature Communications 13:2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.