Abstract
Background and Purpose
Flow diversion of intracranial aneurysms with the Pipeline Embolization Device (PED) is frequently performed, but the outcomes of retreatment for aneurysms that failed to occlude after prior treatment with PED have not been well studied. Here, we report the safety and efficacy of PED retreatment after initial failure to occlude.
Materials and Methods
Clinical and angiographic data from eligible patients were retrospectively assessed for demographics, aneurysm occlusion status, and clinical outcomes. Patients were included in this study if they underwent PED retreatment to treat an aneurysm that had previously been treated with PED.
Results
Retreatment of previously flow-diverted aneurysms with PED was performed in 42 cases. At final angiographic follow-up, angiographic improvement was observed after 45% (19/42) of retreatments and complete aneurysm occlusion was observed following 26% (11/42). Significant clinical complications occurred in 10% (4/42) of PED retreatments.
Conclusions
Retreatment of intracranial aneurysms with PED following initial failure to achieve aneurysm occlusion has a low rate of subsequent complete aneurysm occlusion.
Keywords: Aneurysm, flow diversion, pipeline
Introduction
Flow diversion with the Pipeline Embolization Device (PED) (Medtronic Neurovascular, Irvine, CA, USA) is commonly performed to treat intracranial aneurysms.1–4 While most uses of PED involve placement of a single device across an aneurysm, multiple devices may be needed on occasion. A particularly important use is for retreatment of aneurysms that fail to occlude after previous flow diversion.5,6
Retreatment of brain aneurysms that were previously treated but inadequately occluded has been important to mitigate long-term risk of rupture, particularly in the context of recurrence after endovascular coiling.7–10,11 Incomplete aneurysm occlusion can also occur after flow diversion, but due to the very low rate of aneurysm recurrence after complete occlusion, 12 most cases involve residual aneurysm remnants. The origin of these remnants may be related to technical factors or the endothelialization process of flow-diverted aneurysms, but how to treat these remnants is not completely understood.13,14 While several studies have examined the use of multiple PEDs to treat a single aneurysm, few have specifically examined the clinically important scenario of unplanned PED retreatment of aneurysms that failed to completely occluded with prior flow diversion.15–17 Here, we seek to quantify the success rate of retreatment of a previously flow-diverted but incompletely occluded aneurysm using an additional PED.
Methods
Patient selection
Institutional review board approval was obtained for this study. Consecutive patients undergoing endovascular aneurysm treatment with PED at a single, urban, high-volume neurovascular center from July 2011 to December 2020 were identified from a prospectively maintained neurointerventional database. Patients were included if they underwent treatment of an aneurysm with two or more PEDs placed in separate treatment sessions.
Treatment
Informed consent for each procedure was obtained per clinical routine. Patients were prescribed dual antiplatelet therapy that generally comprised aspirin plus clopidogrel, prasugrel, or ticagrelor with dose titration based on a VerifyNow assay (Accumetrics, San Diego, CA). 18 Intraprocedural systemic heparinization was provided to attain elevation of activated clotting time of approximately 2.5 times above baseline. Patients underwent embolization via a transfemoral or transradial route in the supine position in a biplane neurointerventional suite. Post-procedural care, including the use of closure devices and access site monitoring, was performed per clinical routine. Dual antiplatelet therapy was usually continued for 6–12 months, followed by indefinite aspirin monotherapy. Presence of in-stent stenosis at 6 months typically prompted continuation of dual antiplatelet therapy for 12–18 months post-treatment, or until angiographic resolution of in-stent stenosis.
Data acquisition and classification
Patient characteristics (age, sex, major comorbidities), aneurysm characteristics (location, size, morphology, presentation), treatment details (previous treatment status, device size, number of devices used), and angiographic follow-up (aneurysm occlusion, time of follow-up) were collected. Assessed comorbidities included hypertension, diabetes mellitus, hyperlipidemia, recent smoking history, cardiovascular disease, stroke or transient ischemic attack, and intracranial hemorrhage. Patients who quit smoking more than 6 months prior to first PED placement did not have smoking recorded as a comorbidity. Aneurysm size was reported based on its largest dimension recorded prior to treatment. The appearance of treated aneurysms on follow-up angiography was classified using the O’Kelly-Marotta scale 19 to determine complete aneurysm occlusion as well as angiographic improvement. Time of angiographic follow-up taken as the interval from PED treatment to final angiographic follow-up or subsequent retreatment, whichever came first
Rates are reported as raw counts and percentages, and continuous data are reported as median (IQR). Rates of aneurysm occlusion and angiographic improvement were calculated per PED treatment.
Results
Patients and aneurysms
A total of 416 aneurysms in 330 patients were treated with PED during the study period at our institution (Figure 1). Of these, 29 aneurysms in 28 patients underwent retreatment with multiple PEDs, comprising 42 retreatments of previously flow-diverted aneurysms with PED. 40 retreatments were performed with a single PED, and 2 were performed using 2 PEDs. Retreatments involved aneurysms located in the posterior circulation in 38% (11/29) of cases. Additional details of patients and aneurysms included in our cohort are provided in Table 1.
Figure 1.
Flow diagram of aneurysms included in this study for undergoing retreatment with the Pipeline Embolization Device (PED).
Table 1.
Patients and aneurysms in our cohort. Count data are reported as % (n). Continuous data are reported as median (IQR).
| Patients (N = 28) | ||
| Age | 60 (52–66.5) years | |
| Female sex | 96% (27) | |
| Comorbidities | ||
| Diabetes | 11% (3) | |
| Hypertension | 57% (16) | |
| Hyperlipidemia | 36% (10) | |
| Smoker | 32% (9) | |
| Cardiovascular disease | 21% (6) | |
| Prior intracranial hemorrhage | 25% (7) | |
| Prior stroke or transient ischemic attack | 29% (8) | |
| Aneurysms (N = 29) | ||
| Size | 12.5 (8.0–21.0) mm | |
| Morphology | ||
| Saccular | 66% (19) | |
| Fusiform | 31% (9) | |
| Other | 3% (1) | |
| Location | ||
| Internal carotid artery | 55% (16) | |
| Anterior cerebral artery | 0% (0) | |
| Middle cerebral artery | 7% (2) | |
| Posterior circulation | 38% (11) | |
| Treatment prior to initial flow diversion | ||
| Coiling | 10% (3) | |
| Clipping | 3% (1) | |
| Coiling and clipping | 3% (1) | |
| Adjunctive coiling during initial flow diversion | 17% (5) | |
| Number of re-treatments | ||
| 1 | 83% (24) | |
| 2 | 3% (1) | |
| 3 | 3% (1) | |
| 4 | 7% (2) | |
| 5 | 3% (1) | |
Angiographic outcomes
Angiographic follow-up was available for all treated aneurysms, with a median follow-up time of 14 months (IQR 8-25). Complete aneurysm occlusion was observed following 26% (11/42) of PED retreatments, and angiographic improvement was observed following 45% (19/42) of retreatments. For aneurysms located in the anterior circulation, complete aneurysm occlusion was observed following 31% (8/26) of retreatments and angiographic improvement was observed following 58% (15/26) of retreatments. For aneurysms located in the posterior circulation, complete aneurysm occlusion was observed following 19% (3/16) of retreatments and angiographic improvement was observed following 25% (4/16) of retreatments. Pre-, intra-, and post-operative imaging for a representative patient is shown in Figure 2.
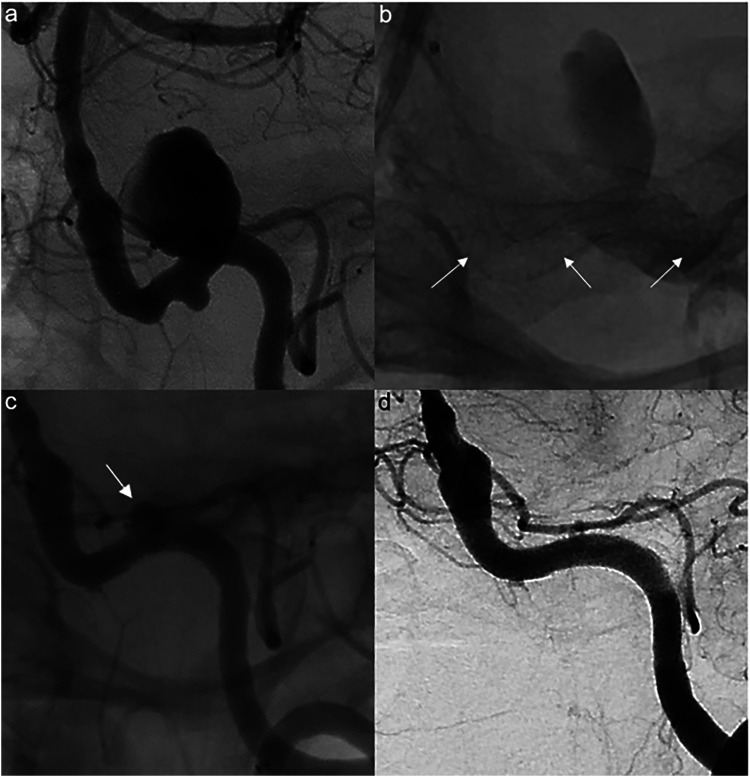
Figure 2.
Preoperative angiogram showing a 15 mm saccular aneurysm in the distal left vertebral artery (a), post-treatment spot image showing a 4.5 × 20 mm PED (arrows) (b), 24-month follow-up angiography showing subtotal aneurysmal filling (arrow) immediately prior to treatment with an additional 4.75 × 20 mm PED (c), and follow-up angiography 6 months after PED retreatment showing complete aneurysm occlusion (d).
Complications
Clinical complications occurred in 10% (4/42) of PED retreatments, comprising 7% (3/42) ipsilateral ischemic strokes and 2% (1/42) subarachnoid hemorrhage. One ischemic stroke and one subarachnoid hemorrhage occurred following retreatment of aneurysms in the anterior circulation, while two ischemic strokes occurred following retreatment of aneurysms in the posterior circulation. The subarachnoid hemorrhage was the result of balloon angioplasty following PED deployment and resulted in patient mortality.
Discussion
In this study, we reviewed a cohort of patients who underwent retreatment of intracranial aneurysms with PED following previous failure to achieve aneurysm occlusion with flow diversion. Angiographic improvement was observed in 45% of aneurysms and complete occlusion was observed in 26% of aneurysms following retreatments. Clinically significant complications occurred in 10% of retreatments.
With a 5-year aneurysm occlusion rate of 95% in early trials of PED, 20 flow diversion is well-established for the treatment of intracranial aneurysms. Aneurysm occlusion following treatment with PED demonstrates high durability, and therefore the rate of recurrence after occlusion is exceedingly low. 12 However, aneurysms remnants after flow diversion are not uncommon. 21 While the long-term rupture risk of these remnants is not well understood and may differ from untreated aneurysms, retreatment may be necessary to reduce risk of later hemorrhage. 9
Past studies quantifying the safety and efficacy of aneurysm retreatment have largely focused on previously coiled aneurysms and consistently reported that retreatment with coiling, stent-assisted coiling, microsurgical clipping, or flow diversion is safe and effective.7,8,10,22,23 Fewer options are available in the aftermath of flow diversion, as the high metal coverage of these devices precludes endosaccular treatments such as coiling, while microsurgical clipping after flow diversion carries a high mortality risk.21,24 For these reasons, additional flow diversion treatment is often the only practical option for retreatment.
Some studies have examined the use of multiple PEDs placed in a single treatment session, which remains controversial since some authors report a higher rate of ischemic complications in such cases and comparable efficacy relative to single device treatment.15–17 In our study, we identified a 7% rate of ipsilateral ischemic stroke following retreatment, nearly twice that reported in the IntrePED study of over 900 aneurysms treated with PED. 25 We also observed angiographic improvement in 45% of aneurysms and complete occlusion in only 26% of aneurysms following retreatment with PED, even lower than a recent study by Salem et al. that reported occlusion or near-complete occlusion in 67% of re-treated aneurysms.5,21 While the precise reasons for this observed low efficacy are unclear, risk factors such as a “collar sign” of radiolucency parallel to the base of nonoccluded aneurysms may predict increased likelihood of treatment failure when pursuing repeated flow diversion. 26 Despite this low rate of efficacy, repeat flow diversion may still be reasonable due to the frequent lack of suitable alternative strategies for retreatment.
This study has several limitations. First, the small sample size precludes robust statistical analysis, especially related to safety as there were only four safety-related events. Second, while 14 months of angiographic follow-up is sufficient for the majority of flow-diverted aneurysms to occlude, it is possible that some aneurysms that failed to occlude during this time went on to occlude later on. Finally, this study suffers from the inherent limitations of a retrospective, observational study, including heterogeneity in PED sizing and delivery systems, follow-up times, and threshold for treatment.
Conclusion
Retreatment of intracranial aneurysms that do not occlude following treatment with a single PED has a low rate of subsequent aneurysm occlusion. Further study is necessary to better define when this approach is appropriate and when alternative treatments should be pursued.
Footnotes
Authors’ contribution: All authors have met ICMJE criteria for authorship, and all authors have read and approved the submitted manuscript. Study conception: DCL and APK. Data collection: DCL, SJC. Data analysis: DCL and APK. Manuscript writing: DCL and APK. Critical revision: SJC, JWO, ARC, CJM, APK. Final approval: DCL, SJC, JWO, ARC, CJM, APK.
JWO is a consultant for Medtronic and Microvention. CJM is a consultant for Medtronic, Cerenovus, Microvention, Stryker, and Balt. APK is a consultant for Penumbra and Microvention.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
IRB approval: Washington University School of Medicine Institutional Review Board ID 202012039.
ORCID iD: David C. Lauzier https://orcid.org/0000-0003-2825-3360
References
- 1.Saatci I, Yavuz K, Ozer Cet al. et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg 2020; 12: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 5.Salem MM, Sweid A, Kuhn AL, et al. Repeat flow diversion for cerebral aneurysms failing prior flow diversion: safety and feasibility from multicenter experience. Stroke 2021; 53(4): 1178–1189. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro M, Becske T, Nelson PK. Learning from failure: persistence of aneurysms following pipeline embolization. J Neurosurg 2017; 126: 578–585. [DOI] [PubMed] [Google Scholar]
- 7.Ringer AJ, Rodriguez-Mercado R, Veznedaroglu E, et al. Defining the risk of retreatment for aneurysm recurrence or residual after initial treatment by endovascular coiling: a multicenter study. Neurosurgery 2009; 65: 311–315; discussion 5. [DOI] [PubMed] [Google Scholar]
- 8.Renowden SA, Koumellis P, Benes Vet al. et al. Retreatment of previously embolized cerebral aneurysms: the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol 2008; 29: 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol 2009; 8: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daou B, Chalouhi N, Starke RM, et al. Clipping of previously coiled cerebral aneurysms: efficacy, safety, and predictors in a cohort of 111 patients. J Neurosurg 2016; 125: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 11.Daou B, Starke RM, Chalouhi N, et al. The use of the pipeline embolization device in the management of recurrent previously coiled cerebral aneurysms. Neurosurgery 2015; 77: 692–697. discission 7. [DOI] [PubMed] [Google Scholar]
- 12.Lauzier DC, Cler SJ, Chatterjee ARet al. et al. The value of long-term angiographic follow-up following pipeline embolization of intracranial aneurysms. J Neurointerv Surg 2021. [DOI] [PubMed] [Google Scholar]
- 13.Moran CJ. The collar sign in pipeline embolization device-treated aneurysms. AJNR Am J Neuroradiol 2020; 14(6): 585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griessenauer CJ, Gupta R, Shi S, et al. Collar sign in incompletely occluded aneurysms after pipeline embolization: evaluation with angiography and optical coherence tomography. AJNR Am J Neuroradiol 2017; 38: 323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalouhi N, Tjoumakaris S, Phillips JL, et al. A single pipeline embolization device is sufficient for treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2014; 35: 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tejada JG, Lopez GV, Koovor JMet al. et al. Mid-term follow-up of staged bilateral internal carotid artery aneurysm treatment with pipeline embolization. Interv Neuroradiol 2019; 25: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waqas M, Vakharia K, Gong AD, et al. One and done? The effect of number of pipeline embolization devices on aneurysm treatment outcomes. Interv Neuroradiol 2020; 26: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambu N, Curzen N. Monitoring the effectiveness of antiplatelet therapy: opportunities and limitations. Br J Clin Pharmacol 2011; 72: 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Kelly C J, Krings T, Fiorella Det al. et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 21.Goertz L, Hesse N, Liebig T, et al. Retreatment strategies for recurrent and residual aneurysms after treatment with flow-diverter devices. Neuroradiology 2020; 62: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Gao BL, Li CH, et al. Endovascular retreatment of cerebral aneurysms previously treated with endovascular embolization. J Neurol Surg A Cent Eur Neurosurg 2020; 81: 207–212. [DOI] [PubMed] [Google Scholar]
- 23.Bender MT, Vo CD, Jiang B, et al. Pipeline embolization for salvage treatment of previously stented residual and recurrent cerebral aneurysms. Interv Neurol 2018; 7: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding D, Starke RM, Liu KC. Microsurgical strategies following failed endovascular treatment with the pipeline embolization device: case of a giant posterior cerebral artery aneurysm. J Cerebrovasc Endovasc Neurosurg 2014; 16: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinjikji W, Lanzino G, Cloft HJ, et al. Risk factors for ischemic complications following pipeline embolization device treatment of intracranial aneurysms: results from the IntrePED study. AJNR Am J Neuroradiol 2016; 37: 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Paz S, Akamatsu Y, Moore JMet al. et al. Implications of the collar sign in incompletely occluded aneurysms after pipeline embolization device implantation: a follow-up study. AJNR Am J Neuroradiol 2020; 41: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]