Abstract
Introduction
Intracranial aneurysms have a high prevalence in human population. It also has a heavy burden of disease and high mortality rate in the case of rupture. Convolutional neural network(CNN) is a type of deep learning architecture which has been proven powerful to detect intracranial aneurysms.
Methods
Four databases were searched using artificial intelligence, intracranial aneurysms, and synonyms to find eligible studies. Articles which had applied CNN for detection of intracranial aneurisms were included in this review. Sensitivity and specificity of the models and human readers regarding modality, size, and location of aneurysms were sought to be extracted. Random model was the preferred model for analyses using CMA 2 to determine pooled sensitivity and specificity.
Results
Overall, 20 studies were used in this review. Deep learning models could detect intracranial aneurysms with a sensitivity of 90/6% (CI: 87/2–93/2%) and specificity of 94/6% (CI: 0/914–0/966). CTA was the most sensitive modality (92.0%(CI:85/2–95/8%)). Overall sensitivity of the models for aneurysms more than 3 mm was above 98% (98%-100%) and 74.6 for aneurysms less than 3 mm. With the aid of AI, the clinicians’ sensitivity increased to 12/8% and interrater agreement to 0/193.
Conclusion
CNN models had an acceptable sensitivity for detection of intracranial aneurysms, surpassing human readers in some fields. The logical approach for application of deep learning models would be its use as a highly capable assistant. In essence, deep learning models are a groundbreaking technology that can assist clinicians and allow them to diagnose intracranial aneurysms more accurately.
Keywords: Intracranial aneurysms, convolutional neural network, CNN
Introduction
Intracranial aneurysms are a fairly prevalent vascular abnormality with a prevalence of 3.2%. 1 Rupture of intracranial aneurysms may also lead to subarachnoid hemorrhage and be fatal. Aneurysmal subarachnoid hemorrhage is responsible for 85% of all subarachnoid hemorrhage cases. 2 The mortality rate of subarachnoid hemorrhage due to aneurysms is reported to be 23 to 51 percent,2–4 and it leads to 10 to 20 percent disability. 5 However, unruptured intracranial aneurysms are the most prevalent incidental finding in normal individuals. 6 This emphasizes the importance of detection of such lesions since there is an overall 1.4% risk per year for an intracranial aneurysm to rupture.7–10 Increased use of various neuroimaging methods such as CT angiography and MRA has led to an increased detection rate of intracranial aneurysms. 11
Convolutional neural network (CNN), a specific type of deep learning network architecture, has been implemented in recent years and can be applied for classification and detection in medical imaging.12–14 Use of CNN may be particularly easy since it learns the outcome classifications automatically rather than needing any explicit categorization. 15 CNN has been used successfully in detecting various lesions in medical imaging such as pulmonary nodules, glaucoma in retinal fundus images, and even diagnosis of covid-19 in X-rays and CT scans.16–18 These attempts at overcoming difficulties regarding automatic detection and segmentation of medical imaging have proven to be efficient and successful. 19
There have been numerous studies on the use of CNN in detection of intracranial aneurysms that have had controversial results. These studies were done in various methods and different choices of modalities for detection of intracranial aneurysms.20–22 Addressing this vastness in features of studies previously carried out, we not only reviewed the outcome of articles discussing intracranial aneurysms, but also took a step farther and surveyed this issue in the eyes of clinicians.
Methods
Eligibility criteria
Studies which used CNN methods to detect intracranial aneurysms and reported the sensitivity of deep learning (DL) for detection of intracranial aneurysms were included in this study. The exclusion criteria were non-English articles, ongoing trials, modeled articles, abstracts, methodologies, conference papers, case reports and case series, editorials, letter to editors, and review articles.
Information sources
PubMed, Scopus, Cochrane library and Embase were searched by two separate authors simultaneously. References of articles were explored to cover any missed articles. The search process was performed in November 2021.
Search strategy
The related words and synonym used for artificial inelegance were: “Artificial Intelligence, Computational Intelligence, Machine Intelligence, Computer Reasoning, AI, Computer Vision System, Machine Learning, Deep Learning, Supervised Machine Learning, Unsupervised Machine Learning, Intelligence Retrieval and Neural Network. “These words were combined using “OR” and then combined with the words “Intracranial” and “Stenosis” to make the final search strategy. The filters that were used were Title-abstract filter for PubMed and Embase and Title-abstract-keywords for Scopus. No limits were used in the search process. The search strategy was kept broad to not only catch all related articles, but also study other aspects of using DL for vascular lesions of intracranial aneurysms; then, the articles related to the detection of aneurysms were selected. The details of search in each database are provided in supplementary material 1.
Selection and data collection process
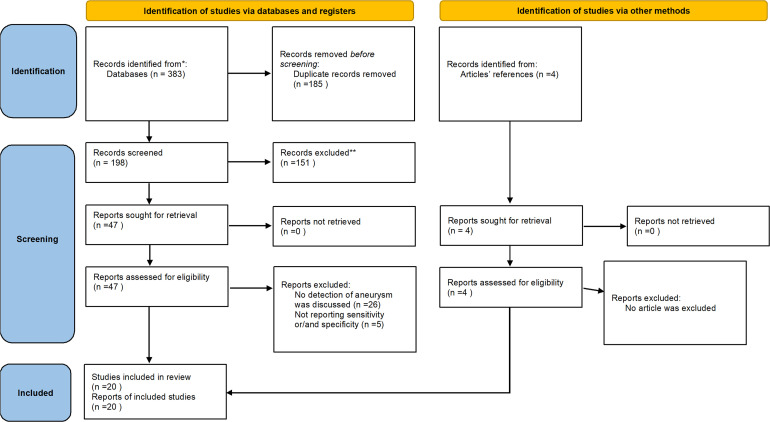
The selection and data collection process were all performed manually by two separate author without using any automation tool in two steps; in case any conflicts were noticed between the researchers, a third author was consulted. All exports from each database was exported in an endnote library(Version 8; 23 ) then, the duplicates were removed. In the first step of selection process, titles and abstracts were read, and the selected articles entered the second step which was reading the full text of articles and checking to see if they were matched to our eligibility criteria. The included articles were discussed in the group, and we then decided which outcomes were needed to be extracted. Figure 1 reflects these processes in a PRISMA flowchart.
Figure 1.
PRISMA flow chart of the included studies determining sensitivity and specificity of clinicians and AI models for detection of intracranial aneurysms .383 articles were identified and after removing duplicates 198 articles were screened. 4 articles were added manually and finally 20 articles were included in this review.
Data items
We sought to extract the demographic data and outcomes from the studies. The year of publication, architecture of machine learning(ML) system, imaging modality, and number of training and test sets were also gathered as complementary data. Overall, location-based and size-based sensitivity and specificity regarding each modality were our main outcomes.
Study risk of bias assessment
Quality and risk of bias were assessed using national institute of health tool for observational studies. Two independent authors did it for each article. This tool consists of 14 questions that assess different parts of studies, and for each question there are three options of “Yes” for being according to that index, “No” for not consider it and cannot reported, or not applicable or not reported for other conditions.
Effect measures and synthesis methods
Effect size was determined using comprehensive meta-analysis version 2. 24 Four different analyses were performed regarding each modality: 1) the overall sensitivity and specificity of ML for detection of aneurysm without considering the place and size of lesions, 2)sensitivity of ML for detection of aneurysm based on the location and size of aneurysm, 3)comparison of sensitivity of ML compared to human readers and to the combination of these two together, and 4) the changes in interrater agreement with and without use of ML model. Final data were reported as event rate (sensitivity, specificity and interrater agreement) in a forest plot. Heterogeneity of data was explored using I2. Duval and Tweedie trim and fill method was used to determine the risk of publication bias. Sensitivity of the results to an article was assessed by dropping out the articles one-by-one and re-analyze the rest of articles.
Results
Study selection
Our search result included 383 articles. After omitting the duplicates, 198 articles remained for screening. Eventually, 16 articles were included for quantitative discussion. 26 articles were excluded because they had assessed other aspects of using ML for intracranial arteries rather than detection of aneurysms, and some were excluded because they had not reported sensitivity and/or specificity. 4 articles that were found from references of articles were added parallelly. PRISMA flowchart comprehensively displays this process (Figure 1).
Study characteristics
7 studies used computed tomography angiography (CTA),25–31 9 used time of flight magnetic resonance angiography (TOF-MRA),22,32–39 and 4 used Digital Subtraction Angiography (DSA).40–43 18 out of 20 articles had retrospective study design, and the two others used randomized crossover 25 and crossover design. 27 6 articles compared the sensitivity and/or specificity of ML model to the human readers.25,27,28,31,32,36 Sensitivity of ML and human reader based on location and size of aneurysms was reported in722,30,33–36,38 and522,26,33,34,36 articles, respectively.
Risk of bias in studies
Risk of bias and quality assessment was found to be desirable.
Results of synthesis and biases
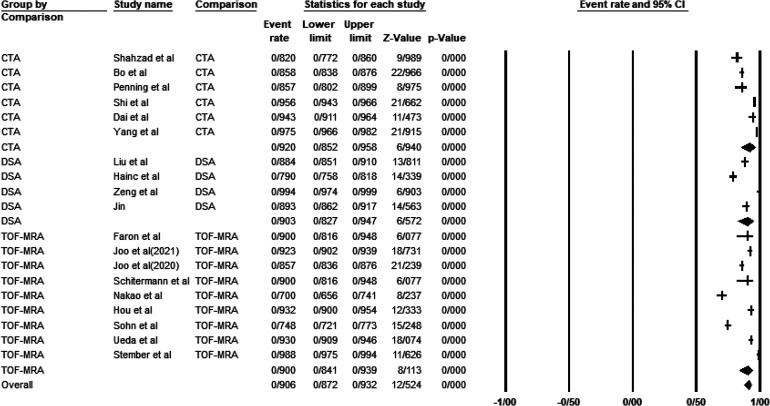
Deep learning models could detect intracranial aneurysms with a lesion-wise sensitivity of 90.6% (CI: 87/2–93/2%) and a patient-wise sensitivity of 96.4%(CI: 95/2–97/3%) and specificity of 94/6%(CI: 0/914–0/966). CTA was the most sensitive and least specific modality for detection of intracranial aneurysms regardless of their size and location with a sensitivity and specificity of 92/0%(CI:85/2–95/8%) and 68/8%(CI: 34/8–90/1%), respectively. The highest specificity was reported in TOF-MRA (96/0%, CI: 93/3–97/6%). Overall sensitivity of the models for aneurysms more than 3 mm was above 98%(98%-100%) and 74/6%(CI: 70/8–78%) for intracranial aneurysms less than 3 mm, and CTA was more sensitive than modalities for intracranial aneurysms less than 3 mm (84/1%(63/1–94/2%). Sensitivity of DL models for posterior circulation aneurysms was the least, while the models detected ACA aneurysms with the most sensitivity(80.6%(CI:73/7–86.0%) versus 94/5(CI:89/9–97.1%). Clinicians diagnosed aneurysms with a lesion-wise and patient-wise sensitivity of 80/9% and 79.8 respectively. With the aid of artificial intelligence (AI), clinicians could mark aneurysms with a sensitivity of 93/7%(CI:0/686–0/990). It is worth mentioning that interrater agreement increased 0/193 unit after applying AI assistance (0/668 vs. 0/862). The details of our analyses are shown in Tables 1 and 2 and Figure 2. More detailed tables are included in supplementary material. Duval and Tweedie trim and fill method revealed no publication bias.
Table 1.
Characteristics and primary outcomes of the included studies determining sensitivity and specificity of clinicians and AI models for detection of intracranial aneurysms.
| Study | Year | Architecture | Modality | Study design | Number of training set/validation/test set | Model | Overall sensitivity of model | Overall specificity of model (Rate) |
|---|---|---|---|---|---|---|---|---|
| Park et al. 25 | 2019 | 3D-CNN | CTA | Randomized Crossover | 611/92/115 | HeadXNet | Patient-wise: 94/9 | Patient-wise: 66/1) |
| Faron et al. 32 | 2020 | 3D-CNN | TOF-MRA | Retrospective | 58/10/17 Five-fold cross validation | DeepMedic | 90 | |
| Joo et al. 22 | 2021 | 3D-CNN | TOF-MRA | Retrospective | 421/47/332 | 3D-ResNet + 3D UNet | Patient-wise: 91.11 Lesion-wise: 92/26 | Patient-wise: 93/91 |
| Joo et al. 33 | 2020 | 3D-CNN | TOF-MRA | Retrospective | 468/-/106/574 | 3D-ResNet | 85/7% | 98% |
| Shahzad et al. 26 | 2020 | 3D-CNN | CTA | Retrospective | 79/215 Five-fold cross validation | 82% | ||
| Bo et al. 27 | 2020 | 3D-CNN | CTA | Crossover | 1186/152 | GLIA-Net | Patient-wise:96/2 lesion-wise:85/8 | Patient-wise:38/9 lesion-wise:19/3 |
| Sichtermann et al. 34 | 2018 | CNN | TOF-MRA | Retrospective | 58/10/17 Five-fold cross validation | DeepMedic | 90 | |
| Liu et al. 40 | 2021 | 3D-CNN | DSA | Retrospective | 347/41/63 | 3D-Dense-UNet | 88.4 | |
| Pennig et al. 28 | 2021 | 3D-CNN | CTA | Retrospective | 79/126 Five-fold cross validation | 85/7 | ||
| Nakao et al. 37 | 2017 | 2D-CNN | TOF-MRA | Retrospective | 300/50/100 | 70 | ||
| Hou et al. 35 | 2020 | 1D-CNN | TOF-MRA | Retrospective | 245/35/70 | 93/16 | 96/87 | |
| Hainc et al. 41 | 2020 | CNN | DSA | Retrospective | 565/141 Cross validation | 79 | 79 | |
| Sohn et al. 36 | 2021 | 3D-CNN | TOF-MRA | Retrospective | 600/110/332 | 3D-ResNet | Patient-lesion- wise :74/8 | 93/9 |
| Shi et al. 29 | 2020 | 3D-CNN | CTA | Retrospective | 927/100/150 | DAResUNet | Patient-wise: 97/3(150) Lesion-wise:95/6 | 89/7 |
| Zeng et al. 43 | 2019 | 3D-CNN | DSA | 300 | 99/38 | 98/19 | ||
| Dai et al. 30 | 2020 | 2D-RCNN | CTA | Retrospective | 208/-/103 | ResNet | 94/3 | |
| Ueda et al. 38 | 2018 | TOF-MRA | Retrospective | 683/-/67Five fold cross validation | ResNet | 93 | ||
| Stember et al. 39 | 2018 | 2D-CNN | MRA + TOF MRA | Retrospective | 250/250/86 | U-Net | 98/8 | |
| Yang et al. 31 | 2021 | 3D-CNN | CTA | Retrospective | 534/534/400 Five fold cross validation | 97/5 | ||
| Jin et al. 42 | 2020 | 2D-CNN | DSA | Retrospective | 249/98/146 | Lesion wise:89/3 Patient wise: 97/7 |
CNN: Convolutional neural network, DLM: Deep learning model, CTA: Computed tomography angiography, TOF-MRA: Time-of-flight magnetic resonance angiography, DSA: Digital subtraction angiography, MRA: magnetic resonance angiography.
Table 2.
Results of meta-analysis of the included studies determining sensitivity and specificity of clinicians and AI models for detection of intracranial aneurysms.
| Analyses | Subgroup | Outcome (% (CI 95%)) |
|---|---|---|
| Overall Sensitivity of Model(lesion-wise) | Overall | 90/6, CI: 87/2- 93/2 |
| CTA | 92/0, CI: 85/2- 95/8 | |
| DSA | 90/2, CI: 82/6- 94/7 | |
| MR | 90/0, CI: 84/1- 93/9 | |
| Overall Sensitivity of Model(Patients-wise) | 96/4, CI: 952- 973 | |
| Overall Sensitivity of Model | Overall | 94/6, CI:91/4–96/6 |
| CTA | 68/8, CI:34/8- 90/1 | |
| DSA | 93/2, CI: 50/3–99/5 | |
| MR | 96/0, CI: 93/3–97/6 | |
| Sensitivity of AI to detection of aneurisms base on their size | <3mm | 74/6, CI: 70/8- 78/0 |
| 3–5 mm | 98/1, CI: 97/3- 98/6 | |
| 3–7 mm | 94/3, CI: 89/7- 96/9 | |
| >5mm | 98/0, CI: 96/6- 98/8 | |
| >10mm | 100, CI: 99/8–100 | |
| Sensitivity of AI to detection of aneurisms base on their size | ICA | 92/8, CI: 92/8- 88/8 |
| MCA | 93/3, CI: 89/7- 95/7 | |
| ACA | 94/5, CI: 89/9- 97/1 | |
| Posterior circulation | 80/6, CI: 73/7- 86/0 | |
| Overall senstivity of clinicians to detect intracranial aneurysms (lesion-wise) | 80/9, CI: 69/5- 88/7 | |
| Overall senstivity of clinicians to detect intracranial aneurysms (patient-wise) | 79/8, CI: 78/6- 81/0 | |
| Sensitivity of clinicians to detection of aneurisms base on their size | <3mm | 82/8, CI: 48/8- 96/0 |
| >3mm | 90/8, CI: 80/5- 95/9 | |
| Sensitivity of clinicians to detection of aneurisms base on their location | ICA | 84/3, CI: 50/3- 96/6 |
| MCA | 94/6, CI: 93/2- 95/8 | |
| ACA | 67/7, CI: 12/3- 96/9 | |
| Posterior circulation | 85/6, CI: 58/2- 96/2 | |
| Overall senstivity of clinicians to detect intracranial aneurysms with the help of AI | 92/2, CI: 87/2- 95/3 | |
| Interrater agreement before AI augmentation | 66/8, CI: 34/4- 88/5 | |
| Interrater agreement after AI augmentation | 86/2, CI: 83/9- 88/2 |
Figure 2.
Overall and based on modality senstivity of AI models to detect intracranial aneurysms (Lesion-wise)(Over:Event rate: 0/906, CI: 0/872–0/932, I2:96/42, Duval and Tweedie's trim and fill model not significant, senstivity analysis not significant, CTA: event rate: 0/920, CI: 0/852–0/958, I2:97/08, DSA: event rate: 0/902, CI: 0/826–0/947, I2:94/11, TOF-MRA: event rate: 0/900, CI: 0/841–0/939, I2:97/19).
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis on the determination of the performance of AI models and how they could aid clinicians for better diagnosis of intracranial aneurysms. In general, deep learning models were able to detect aneurysms regardless of their size and location with an acceptable sensitivity compared to human readers. Additionally, DL models assisted the clinicians to detect more lesions and also increased inter-observer agreement. Our discussion will focus on the interpretation and analysis of our results from a clinical standpoint, rather than details of model development.
Reviewed studies can be categorized into 2 groups: first, studies that investigated the performance of DL models detection of intracanal aneurysms and second the studies which not only performed the previous test, but also compared these models to the performance of human readers. Before discussing the interpretation of these studies in more detail, it should be noted that determining true positive aneurysms (ground truth) was performed by experienced clinicians who were not error-free. An example of this can be found in the Ueda et al.'s study, where the model detected aneurysms that were not detected by the reference model. 38 This would be a double-edged sword. On the one hand, it demonstrates that these models can identify aneurysms that even a highly experienced clinician would not find. On the other hand, it casts doubt on the accuracy of all results. Nonetheless, we cannot make any other choice than accepting the reference clinician's opinion as the ground truth.
The search for additional aneurysms may be stopped because the human reader has already found one lesion, a phenomenon called satisfaction of search. 44 The importance of this will be most apparent in emergency settings when missing a lesion can lead to a catastrophe. Even though this phenomenon may concern human readers, DL models could simply prevent it via searching for an infinite number of lesions. A study only reported patient-wise sensitivity; that is, when the model found a lesion, it stopped searching for additional ones, an observation we would like to call the satisfaction of search for AI model. 25 This is an undesirable feature if we ever want to rely on DL models solely without the supervision of a clinician due to staff shortages. Accordingly, we analyzed the sensitivity of detection of aneurysms both patient-wise and lesion-wise. Our results indicate that machine learning is more sensitive than human readers alone, but when combined altogether, the results will be more promising than either alone.
Size
The diameter of intracranial aneurism is an important determinant of whether an intervention is needed or not. According to the stroke council of the American Heart Association and Greving et al., in patients without a history of SAH with aneurysms below 7 mm, observation is preferred to intervention, but for those above 7 mm and patients who develop symptoms from intradural aneurysms, treatment becomes necessary.45,46 The risk of rupture is estimated to be 0/36% per year for aneurysms with a diameter of 3–4 mm, while treatment is suggested for patients with a high risk of rupture.47–49 While asymptomatic patients may not require urgent intervention for <3 mm aneurysms, analyzing the sensitivity of DL model and comparing it to human readers shed light on the limitations of these models in detecting such aneurysms besides the fact that the incidence of these aneurysms are increasing.49,50 The sensitivity of human readers to detect these aneurysms was pretty more compared to DL models. Clinicians’ detection rate was solely based on MR modality due to limitations of the analyzed studies, where machine learning could detect these tiny aneurysms more accurately if we only consider CTA. The hazard ratio for the risk of rupture for aneurysms 7–9 mm was reported 3/3, 9/1 for 10–24 mm and 76/3 for aneurysm sizes more than 25 mm compared to tiny aneurysms. 51 Our study showed an average sensitivity of 94% for aneurysms above 3 mm of diameter, and it reached a sensitivity of 100% for those greater than 10 mm. These sensitivities were higher than those of the clinicians and demonstrates the power of machine learning.
Location
The international study of unruptured intracranial aneurysms (ISUIA) has stratified the risk of the rapture (RoR) of aneurysms based on the location into low, intermediate, and high. Cavernous carotid artery aneurysms RoR is considered low, anterior circulation and internal carotid arteries are considered intermediate, and posterior circulation high. 52 The neurological approach to aneurysms of the posterior territory is complicated due to their location, size of these aneurysms, and connection of vessels. Aneurysms of the posterior territory account for 15% of intracranial aneurysms.53–55 Pooled results showed less sensitivity of DL models in detection of posterior circulation aneurysms compared to human readers. Sensitivity of DL models in CTA for detection of posterior circulation aneurysms was more than of human readers in the same manner that DL models based on CTA were more successful than human readers in detecting small sized aneurysms. Anterior circulation makes up 85% of total intracranial aneurysms. 56 DL models could detect anterior circulation aneurysms with a higher detection rate compared to those of clinicians and the same result was observed for aneurysm of the ICA either, but human readers showed a more sensitivity rate for detection of MCA aneurysms. These differences can be justified due to various reasons. It can be argued that posterior circulation contains more bony structures, which could decrease the accuracy of CNN models. An aneurysm may be missed or mis-detected in the case of overlap of vessels or curvatures of an artery.
Modality
For unruptured intracranial aneurysms, DSA is considered the diagnostic gold standard since it provides precise information about the size, shape, and the orifice size of the aneurysm and can be used to determine future treatment options. 57 However, the use of CTA or MRA may be preferred due to being less invasive, having lower cost, and being more agile and accessible in versatile facilities. 58 Regardless of their advantages, CTA and MRA come with some limitations that make them an unfitting substitute for DSA, for example, limitation of CTA on detection of small aneurysms or aneurysms in MCA and some parts of ICA59,60 and artifacts of TOF-MRA when there is tortuosity in the vessels or a turbulent flow. 61 These limitations increase the amount of false positive and negative cases which decrease the overall sensitivity and specificity of these modalities regardless of a human's interpretation or that of an AI-based model. Also, contrast material should be injected for CTA and DSA that may be contraindicated for a number of patients where those undergoing MRA should be MRI compatible.57,62,63 Removal of bony parts were used in several studies in the preprocessing step. It should be noted that without a sufficient model or human supervision, some calcified lesions may also be omitted, which leads to misdiagnosis. 57 By and large, the modality with most sensitivity in the detection of aneurysms is desirable. The analysis pointed out that models using CTA had the highest sensitivity and those based on DSA and MRA had approximately the same lower rates. CTA-based model also showed better performance for detection of lesions under 3 mm and in the posterior circulation. It was a question for authors if machine learning can detect previously treated aneurysms that recur. Metal elements used for treated aneurysms produce artifacts that decrease the image quality and make it difficult for the model to detect the regrown aneurysms. 64 Some studies found that the CNN model could successfully find new aneurysms that were previously coiled in CTA.30,33
Aneurysmal subarachnoid hemorrhage
Aneurysmal subarachnoid hemorrhage(aSAH) is a serious complication of intracranial aneurysms rapture. 65 SAH has a high mortality rate both in the short and long term and accounts for a 30-day mortality rate of 45%.66–69 Common signs and symptoms of SAH are headache, nausea, vomiting, mesangial signs and decreased level of consciousness. 70 CT scan without contrast is the best primary workup for diagnosis of SAH and has a sensitivity of 98.7% for detection of SAH in the first 6 h.71,72 It should be noticed that most aneurysms do not rupture, have no symptoms, and sometimes may even be found incidentally; however, in case they rupture and are missed, the results would be fatal. 64 Two studies designed a DL model to detect aneurysms in the context of aneurysmal SAH based on CTA. Both models could detect aneurysms with an acceptable sensitivity and proved that machine learning could be helpful in the emergency setting for detection of such aneurysms.26,28
Morphology of aneurysms
There is another classification for brain aneurysms based on their shape. Aneurysms could be divided into groups, saccular(90%), and fusiform(3–13%).73,74 Fusiform aneurysms have a lower risk of rupture and are mostly located on the posterior circulation. 75 Due to their shape, fusiform aneurysms are difficult to detect by deep learning models. Some studies have even omitted them in their training sets and trained the model just to diagnose saccular ones. Yang et al. examined this issue more deeply and asked whether the sensitivity of the model was affected by saccular or fusiform aneurysms. While the model showed high sensitivity across both classes, it was higher for saccular aneurysms. 31
Limitations
In almost all analyses, there was a high level of heterogeneity. Some explanations may interpret this issue. The first and most important issue is different models used by articles. The second reason may be different inclusion and exclusion criteria and number of data sets. Another reason could be different pre-processing algorithms used before training and testing data such as data augmentation, removal of bony parts, and intensification of relevant parts. We have performed many subgroup analyses to reduce heterogeneity, but it was not successful. Another limitation of our study was the scarcity of modalities in different subgroups. Specificity was not reported by studies based on the location and size of aneurysms to be analyzed.
Conclusion
To address whether or not machine learning is reliable to detect aneurysms in routine clinical practice and emergencies, for a variety of sizes and locations, we found that CNN models had an acceptable sensitivity for detection of intracranial aneurysms, surpassing human readers in some fields. Nevertheless, some studies reported low specificities in CTA models which may limit its usage. Thus, the logical approach will be using machine learning as an assistant, not as a sole decision maker. Moreover, CTA had the best performance among modalities regarding sensitivity and could swiftly detect aneurysms in pitfalls rather than other modalities. In essence, deep learning models are a groundbreaking technology that can assist clinicians and allow them to diagnose intracranial aneurysms more accurately.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199221097475 for Application of convolutional network models in detection of intracranial aneurysms: A systematic review and meta-analysis by Saeed Abdollahifard, Amirmohammad Farrokhi, Fatemeh Kheshti, Mahtab Jalali and Ashkan Mowla in Interventional Neuroradiology
Footnotes
Contribution: The first author position is shared by AS and FA because both contributed equally. All authors contributed in writing the developing the idea, writing the draft and finalizing the final draft.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ashkan Mowla: Speakers Bureau/Consultant to Cerenovus, Stryker, Wallaby Medical, RapidAI , BALT USA,LLC.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Amirmohammad Farrokhi https://orcid.org/0000-0003-0769-9304
Ashkan Mowla https://orcid.org/0000-0001-5352-8747
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Vlak MH, Algra A, Brandenburg Ret al. et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. The Lancet Neurology 2011; 10: 626–636. [DOI] [PubMed] [Google Scholar]
- 2.Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. The Lancet 2007; 369: 306–318. [DOI] [PubMed] [Google Scholar]
- 3.Investigators UJ. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366: 2474–2482. [DOI] [PubMed] [Google Scholar]
- 4.Ingall T, Asplund K, Mähönen Met al. et al. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000; 31: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 5.Hop JW, Rinkel G, Algra Aet al. et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997; 28: 660–664. [DOI] [PubMed] [Google Scholar]
- 6.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357: 1821–1828. [DOI] [PubMed] [Google Scholar]
- 7.Rajabzadeh-Oghaz H, Varble N, Shallwani H, et al. Computer-Assisted three-dimensional morphology evaluation of intracranial aneurysms. World Neurosurg 2018; 119: e541–ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varble N, Rajabzadeh-Oghaz H, Wang Jet al. et al. Differences in morphologic and hemodynamic characteristics for “PHASES-based” intracranial aneurysm locations. AJNR American Journal of Neuroradiology 2017; 38: 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajabzadeh-Oghaz H, Varble N, Davies JM, et al. Computer-Assisted adjuncts for aneurysmal morphologic assessment: toward more precise and accurate approaches. Proc SPIE Int Soc Opt Eng 2017; 10134: 10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greving JP, Wermer MJ, Brown RD, Jr., et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. The Lancet Neurology 2014; 13: 59–66. [DOI] [PubMed] [Google Scholar]
- 11.Chalouhi N, Dumont AS, Randazzo C, et al. Management of incidentally discovered intracranial vascular abnormalities. Neurosurg Focus 2011; 31: E1. [DOI] [PubMed] [Google Scholar]
- 12.Erickson BJ, Korfiatis P, Akkus Zet al. et al. Toolkits and libraries for deep learning. J Digit Imaging 2017; 30: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson DB, Chen MC, Lungren MPet al. et al. Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology 2018; 287: 313–322. [DOI] [PubMed] [Google Scholar]
- 14.Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Adv Neural Inf Process Syst 2012; 25: 1097–1105. [Google Scholar]
- 15.Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal 2017; 42: 60–88. [DOI] [PubMed] [Google Scholar]
- 16.Setio AAA, Ciompi F, Litjens G, et al. Pulmonary nodule detection in CT images: false positive reduction using multi-view convolutional networks. IEEE Trans Med Imaging 2016; 35: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 17.Zilly J, Buhmann JM, Mahapatra D. Glaucoma detection using entropy sampling and ensemble learning for automatic optic cup and disc segmentation. Comput Med Imaging Graph 2017; 55: 28–41. [DOI] [PubMed] [Google Scholar]
- 18.Maghdid HS, Asaad AT, Ghafoor KZ, Sadiq AS, Mirjalili S, Khan MK, editors. Diagnosing COVID-19 pneumonia from X-ray and CT images using deep learning and transfer learning algorithms. Multimodal image exploitation and learning 2021; 2021: International Society for Optics and Photonics. [Google Scholar]
- 19.Sarvamangala D, Kulkarni RV. Convolutional neural networks in medical image understanding: a survey. Evol Intell 2021; 15: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bo Z-H, Qiao H, Tian C, et al. Toward human intervention-free clinical diagnosis of intracranial aneurysm via deep neural network. Patterns 2021; 2: 100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hainc N, Mannil M, Anagnostakou V, et al. Deep learning based detection of intracranial aneurysms on digital subtraction angiography: a feasibility study. Neuroradiol J 2020; 33: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo B, Choi HS, Ahn SS, et al. A deep learning model with high standalone performance for diagnosis of unruptured intracranial aneurysm. Yonsei Med J 2021; 62: 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The EndNote Team. Endnote. EndNote X8 ed. Philadelphia, PA: Clarivate; 2013. [Google Scholar]
- 24.Borenstein M, Hedges L, Higgins Jet al. et al. Comprehensive meta-analysis version 2. Englewood: Biostat, 2011. [Google Scholar]
- 25.Park A, Chute C, Rajpurkar P, et al. Deep learning-assisted diagnosis of cerebral aneurysms using the HeadXNet model. JAMA network Open 2019; 2: e195600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahzad R, Pennig L, Goertz L, et al. Fully automated detection and segmentation of intracranial aneurysms in subarachnoid hemorrhage on CTA using deep learning. Sci Rep 2020; 10: 21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo ZH, Qiao H, Tian C, et al. Toward human intervention-free clinical diagnosis of intracranial aneurysm via deep neural network. Patterns 2021; 2: 100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennig L, Hoyer UCI, Krauskopf A, et al. Deep learning assistance increases the detection sensitivity of radiologists for secondary intracranial aneurysms in subarachnoid hemorrhage. Neuroradiology 2021; 63: 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z, Miao C, Schoepf UJ, et al. A clinically applicable deep-learning model for detecting intracranial aneurysm in computed tomography angiography images. Nat Commun 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X, Huang L, Qian Y, et al. Deep learning for automated cerebral aneurysm detection on computed tomography images. Int J Comput Assist Radiol Surg 2020; 15: 715–723. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Xie M, Hu C, et al. Deep learning for detecting cerebral aneurysms with CT angiography. Radiology 2021; 298: 155–163. [DOI] [PubMed] [Google Scholar]
- 32.Faron A, Sichtermann T, Teichert N, et al. Performance of a deep-learning neural network to detect intracranial aneurysms from 3D TOF-MRA compared to human readers. Clin Neuroradiol 2020; 30: 591–598. [DOI] [PubMed] [Google Scholar]
- 33.Joo B, Ahn SS, Yoon PH, et al. A deep learning algorithm may automate intracranial aneurysm detection on MR angiography with high diagnostic performance. Eur Radiol 2020; 30: 5785–5793. [DOI] [PubMed] [Google Scholar]
- 34.Sichtermann T, Faron A, Sijben Ret al. et al. Deep learning-based detection of intracranial aneurysms in 3D TOF-MRA. AJNR Am J Neuroradiol 2019; 40: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou W, Mei S, Gui Q, et al. 1D CNN-Based intracranial aneurysms detection in 3D TOF-MRA. Complexity 2020; 2020. [Google Scholar]
- 36.Sohn B, Park KY, Choi J, et al. Deep learning-based software improves clinicians’ detection sensitivity of aneurysms on brain TOF-MRA. American Journal of Neuroradiology 2021; 42: 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao T, Hanaoka S, Nomura Y, et al. Deep neural network-based computer-assisted detection of cerebral aneurysms in MR angiography. J Magn Reson Imaging 2018; 47: 948–953. [DOI] [PubMed] [Google Scholar]
- 38.Ueda D, Yamamoto A, Nishimori M, et al. Deep learning for MR angiography: automated detection of cerebral aneurysms. Radiology 2019; 290: 187–194. [DOI] [PubMed] [Google Scholar]
- 39.Stember JN, Chang P, Stember DM, et al. Convolutional neural networks for the detection and measurement of cerebral aneurysms on magnetic resonance angiography. J Digit Imaging 2019; 32: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Feng J, Wu Z, et al. Deep neural network-based detection and segmentation of intracranial aneurysms on 3D rotational DSA. Interv Neuroradiol 2021; 27: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hainc N, Mannil M, Anagnostakou V, et al. Deep learning based detection of intracranial aneurysms on digital subtraction angiography: a feasibility study. Neuroradiology Journal 2020; 33: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin H, Geng J, Yin Y, et al. Fully automated intracranial aneurysm detection and segmentation from digital subtraction angiography series using an end-to-end spatiotemporal deep neural network. J Neurointerv Surg 2020; 12: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 43.Zeng Y, Liu X, Xiao N, et al. Automatic diagnosis based on spatial information fusion feature for intracranial aneurysm. IEEE Trans Med Imaging 2020; 39: 1448–1458. [DOI] [PubMed] [Google Scholar]
- 44.Huynh JD, Rhodes SC, Hatton JFet al. et al. Satisfaction of search in periapical radiograph interpretation. J Endod 2021; 47: 291–296. [DOI] [PubMed] [Google Scholar]
- 45.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke 2000; 31: 2742–2750. [DOI] [PubMed] [Google Scholar]
- 46.Greving JP, Rinkel GJ, Buskens Eet al. et al. Cost-effectiveness of preventive treatment of intracranial aneurysms: new data and uncertainties. Neurology 2009; 73: 258–265. [DOI] [PubMed] [Google Scholar]
- 47.Ikawa F, Morita A, Tominari S, et al. Rupture risk of small unruptured cerebral aneurysms. Journal of Neurosurgery JNS 2020; 132: 69–78. [DOI] [PubMed] [Google Scholar]
- 48.Lee G-J, Eom K-S, Lee Cet al. et al. Rupture of very small intracranial aneurysms: incidence and clinical characteristics. J Cerebrovasc Endovasc Neurosurg 2015; 17: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malhotra A, Wu X, Forman HPet al. et al. Management of tiny unruptured intracranial aneurysms: a comparative effectiveness analysis. JAMA Neurol 2018; 75: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bender MT, Wendt H, Monarch T, et al. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: a 25-year, single institution study. Neurosurgery 2018; 83: 692–699. [DOI] [PubMed] [Google Scholar]
- 51.Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366: 2474–2482. [DOI] [PubMed] [Google Scholar]
- 52.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet (London, England) 2003; 362: 103–110. [DOI] [PubMed] [Google Scholar]
- 53.Gardijan D, Herega T, Premužić V, et al. Comparison between stenting and conservative management of posterior circulation perforator aneurysms: systematic review and case series. Neuroradiology 2021; 63: 639–651. [DOI] [PubMed] [Google Scholar]
- 54.Buell TJ, Ding D, Raper DMS, et al. Posterior circulation perforator aneurysms: a proposed management algorithm. J Neurointerv Surg 2018; 10: 55–59. [DOI] [PubMed] [Google Scholar]
- 55.Tsianaka E, Al-Shawish A, Potapov Aet al. et al. Clipping versus coiling in posterior circulation intracranial aneurysms: a meta-analysis. Chinese Neurosurgical Journal 2019; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schievink WI. Intracranial aneurysms. N Engl J Med 1997; 336: 28–40. [DOI] [PubMed] [Google Scholar]
- 57.Howard BM, Hu R, Barrow JWet al. et al. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurgical Focus FOC 2019; 47: E20. [DOI] [PubMed] [Google Scholar]
- 58.Uricchio M, Gupta S, Jakowenko N, et al. Computed tomography angiography versus digital subtraction angiography for postclipping aneurysm obliteration detection. Stroke 2019; 50: 381–388. [DOI] [PubMed] [Google Scholar]
- 59.Pradilla G, Wicks RT, Hadelsberg U, et al. Accuracy of computed tomography angiography in the diagnosis of intracranial aneurysms. World Neurosurg 2013; 80: 845–852. [DOI] [PubMed] [Google Scholar]
- 60.Chen W, Xing W, He Zet al. et al. Accuracy of 320-detector row nonsubtracted and subtracted volume CT angiography in evaluating small cerebral aneurysms. J Neurosurg 2017; 127: 725–731. [DOI] [PubMed] [Google Scholar]
- 61.Kemmling A, Noelte I, Gerigk Let al. et al. A diagnostic pitfall for intracranial aneurysms in time-of-flight MR angiography: small intracranial lipomas. Am J Roentgenol 2008; 190: W62–WW7. [DOI] [PubMed] [Google Scholar]
- 62.Vasconcelos R, Vrtiska TJ, Foley TA, et al. Reducing iodine contrast volume in CT angiography of the abdominal aorta using integrated tube potential selection and weight-based method without compromising image quality. AJR Am J Roentgenol 2017; 208: 552–563. [DOI] [PubMed] [Google Scholar]
- 63.Nagashima H, Sakamoto H, Sano Yet al. et al. [Fundamental study of DSA images using gadolinium contrast agent]. Nihon Hoshasen Gijutsu Gakkai Zasshi 2002; 58: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 64.Williams LN, Brown RD, Jr. Management of unruptured intracranial aneurysms. Neurol Clin Pract 2013; 3: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Björkman J, Frösen J, Tähtinen O, et al. Irregular shape identifies ruptured intracranial aneurysm in subarachnoid hemorrhage patients with multiple aneurysms. Stroke 2017; 48: 1986–1989. [DOI] [PubMed] [Google Scholar]
- 66.Chan V, Lindsay P, McQuiggan Jet al. et al. Declining admission and mortality rates for subarachnoid hemorrhage in Canada between 2004 and 2015. Stroke 2019; 50: 181–184. [DOI] [PubMed] [Google Scholar]
- 67.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the stroke council of the American heart association. Stroke 2000; 31: 2742–2750. [DOI] [PubMed] [Google Scholar]
- 68.Schatlo B, Fung C, Stienen MN, et al. Incidence and outcome of aneurysmal subarachnoid hemorrhage: the Swiss study on subarachnoid hemorrhage (Swiss SOS). Stroke 2021; 52: 344–347. [DOI] [PubMed] [Google Scholar]
- 69.Lindbohm JV, Kaprio J, Jousilahti Pet al. et al. Risk factors of sudden death from subarachnoid hemorrhage. Stroke 2017; 48: 2399–2404. [DOI] [PubMed] [Google Scholar]
- 70.Petridis AK, Kamp MA, Cornelius JF, et al. Aneurysmal subarachnoid hemorrhage. Dtsch Arztebl Int 2017; 114: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubosh NM, Bellolio MF, Rabinstein AAet al. et al. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47: 750–755. [DOI] [PubMed] [Google Scholar]
- 72.Marcolini E, Hine J. Approach to the diagnosis and management of subarachnoid hemorrhage. West J Emerg Med 2019; 20: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park S-H, Yim M-B, Lee C-Yet al. et al. Intracranial fusiform aneurysms: it’s pathogenesis, clinical characteristics and managements. J Korean Neurosurg Soc 2008; 44: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faluk M and De Jesus O. Saccular Aneurysm. [Updated 2021 Aug 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- 75.Awad AJ, Mascitelli JR, Haroun RRet al. et al. Endovascular management of fusiform aneurysms in the posterior circulation: the era of flow diversion. Neurosurgical Focus FOC 2017; 42: E14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199221097475 for Application of convolutional network models in detection of intracranial aneurysms: A systematic review and meta-analysis by Saeed Abdollahifard, Amirmohammad Farrokhi, Fatemeh Kheshti, Mahtab Jalali and Ashkan Mowla in Interventional Neuroradiology